Translate this page into:
Pharmacological activities of allylbenzene and allylanisole phenylpropanoids: Inhibition of antibiotic resistance targets and toxicity profile in a Drosophila melanogaster model
⁎Corresponding authors. hdmcoutinho@urca.br (Henrique Douglas Melo Coutinho), asiyadatpanah@yahoo.com (Abolghasem Siyadatpanah), polrat.wil@mahidol.ac.th (Polrat Wilairatana)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Introduction
The rapid emergence of multidrug-resistant bacteria has demanded the discovery of new drugs capable of inhibiting bacterial resistance mechanisms, such as those mediated by efflux proteins and β-lactamases.
Objective
Considering the evidence indicating that phenylpropanoids are effective substances against bacterial resistance, the present study aimed to evaluate the antibacterial activity of compounds allylbenzene and allylanisol against the Staphylococcus aureus strain SA-K4414, which carries genes encoding the β-lactamase and QacA/B proteins.
Methods
The Minimum Inhibitory Concentrations (MICs) were determined by the broth microdilution method. The expression of β-lactamase was verified through the association of the inhibitor sulbactam with the antibiotic ampicillin, while its inhibition was analyzed through the reduction of the penicillin MIC in the presence of phenolic compounds. The efflux pump inhibition was analyzed by the reduction in the MIC of the pump substrate ethidium bromide. The toxicity against Drosophila melanogaster was assessed by evaluating mortality and negative geotaxis through the fumigation method.
Results
The results demonstrated that the phenylpropanoids failed to show relevant direct antibacterial activity, with MIC values above 1024 µg/mL. The association of allylbenzene and allylanisole with penicillin potentiated its antibacterial effect, reducing the antibiotic MIC (512 µg/mL) to 128 µg/mL and 256 µg/mL, respectively. The compounds also reduced the MIC of EtBr, indicating a possible inhibitory effect against the β-lactamase enzyme and the QacA/B efflux protein. Regarding toxicity, allylbenzene (after 12 h) and allylanisole (after 3 h) presented EC50 of 19.21 μL/mL and 11.07 μL/mL, respectively. These compounds also caused damage to the locomotor system of flies, which was potentiated following increasing concentrations and exposure period.
Conclusion
It is concluded that the compounds allylbenzene and allylanisole can inhibit the resistance mechanisms mediated by the β-lactamase enzyme and the efflux protein QacA/B. However, due to the significant toxicity observed, further research is required to assess the safety of these compounds.
Keywords
Phenolic compounds
Antibiotic resistance
Allylbenzene
Allylanisole
Toxicity
Alternative methods
- MRSA
-
Methicillin-Resistant S. aureus
- PMF
-
Proton-motive force
- MFS
-
Major Facilitator Superfamily
- EPIs
-
efflux pump inhibitors
- EtBr
-
ethidium bromide
- CCCP
-
Carbonyl cyanide 3-chlorophenylhydrazone
- CPMZ
-
chlorpromazine
- DMSO
-
dimethyl sulfoxide
Abbreviations
1 Introduction
Staphylococcus aureus is a major pathogen associated with serious infections. This bacterium has notable virulence factors that contribute to the development of manifestations such as toxic shock syndrome, food poisoning, and soft tissue wound infections (Olatunji et al., 2020). In recent decades, great concern has been raised in the hospital environment regarding this microorganism, due to its ability to acquire resistance to several antimicrobials, as observed in infections caused by the Methicillin-Resistant S. aureus (MRSA) (Tsai et al., 2020), which demonstrates the need to search for new antibacterial compounds.
In this context, medicinal plants are promising sources of bioactive molecules, chemically and structurally diverse, with multiple pharmacological properties (Fernández et al., 2021; Rodrigues et al., 2016). Importantly, in addition to having direct antibacterial activity, these secondary metabolites have demonstrated the ability to potentiate the activity of antibiotics, acting as bacterial resistance modulators (Cappiello et al., 2020).
Allylbenzene is a phenylpropanoid present in several species of medicinal plants, being widely used as components of insecticides, food additives and drugs (Delaforge et al., 1980). On the other hand, the phenylpropanoid allylanilose is found in the composition of essential oils and plant extracts have proven antibacterial, antioxidant, and anti-inflammatory activity (Alves Júnior et al., 2020).
Antibiotic resistance in S. aureus strains is mediated by several mechanisms, which include alteration of extracellular membrane permeability, production of enzymes (such as β-lactamases), change of drug target, and active efflux of the antibiotic (Blair et al., 2015). Among these mechanisms, the enzymatic degradation by β-lactamases is a remarkable mechanism associated with bacterial resistance to β-lactam antibiotics such as penicillin. These enzymes act by degrading the β-lactam ring through hydrolytic catalysis, thus decreasing the effective concentration of the antibiotic, which results in the loss of the antibacterial effect (ur Rahman et al., 2018).
The active extrusion of antibiotics mediated by efflux pumps (EPs) is also a significant mechanism of bacterial resistance. These membrane proteins are physiologically responsible for the elimination of harmful substances, which is achieved through primary transport with the expenditure of ATP, or secondary transport using the proton-motive force (PMF). However, overexpression of these proteins has a significant impact on multidrug resistance, as it impairs the intracellular activity of antibiotics (Troncoso et al., 2017).
The QacA/B efflux protein, expressed by the strain SA-K4414 of S. aureus, belongs to the Major Facilitator Superfamily (MFS) of transporters, which have a plasmid-encoded transcription. The transcription of this protein is regulated by the QacR suppressor, which is responsible for the extrusion of hydrophobic drugs such as tetracycline and other structurally distinct substances. Accordingly, the QacA/B efflux protein can be classified as an MDR EP (Chatterjee et al., 2016).
Considering the importance of investigating new compounds to combat bacterial resistance, this research aimed to investigate the activity of phenylpropanoids against an SA-K4414 strain expressing simultaneously the β-lactamase and QacA/B genes, as well as to evaluate their toxicity using the D. melanogaster model.
2 Methodology
2.1 Bacterial strain
The antibacterial tests were performed using the SA-K4414 strain of S. aureus, which presents resistance to β-lactam antibiotics and ethidium bromide mediated by the expression of β-lactamase and QacA/B, respectively. This strain was provided by Prof. Glenn Kaatz (Wayne State University) and maintained in a blood-agar culture medium (Difco laboratories Ltda., Brazil). Prior to the experiments, the cells were grown in Heart Infusion Agar (HIA, Difco) for 24 h at 36 °C.
2.2 Culture media
The tests were performed using 10% Brain Heart Infusion (BHI, Acumedia Manufacturers Inc.) and Heart Infusion Agar (HIA, Difcolaboratories Ltda.) prepared according to the manufacturer's instructions and.
2.3 Chemicals
Antibiotics (penicillin and ampicillin + sulbactam), ethidium bromide (EtBr), and m-Carbonyl cyanide 3-chlorophenylhydrazone (CCCP), as well as allylbenzene and allylanisole, were obtained from Sigma Aldrich Co. Ltd., while chlorpromazine (CPMZ) was obtained from the Aché laboratory. The antibiotics, as well as the phenylpropanoids, were dissolved in Dimethylsulfoxide (DMSO) and sterile water to a concentration of 1024 µg/mL. CPMZ and EtBr were dissolved in sterile distilled water, while CCCP was dissolved in methanol/water (1:3, v/v). All substances were diluted to a concentration of 1024 µg/mL.
2.4 Minimum inhibitory concentration determination (Mic)
The MIC was determined by the broth microdilution method. Following an incubation period of 24 h, stock samples of strains were transferred to test tubes containing 6 mL of sterile saline solution, and the inoculum was prepared by adjusting the turbidity according to the 0.5 value on the McFarland scale, which corresponds to 108 CFU. Then, 160 µL of the bacterial inoculum was transferred to eppendorf® tubes containing 1440 µL of BHI. Subsequently, the wells on a microdilution plate were filled with 100 µL of the adjusted inoculum solution. The Phenylpropanoids (100 µL) were then added to these wells at concentrations ranging from 512 µg/mL to 0.5 µg/mL. The plates were incubated in the oven at 36 °C for 24 h. After this period, the wells were added with 20 µL resazurin and the readings were determined by observing the change in the color of the medium. The MIC was defined as the lowest concentration capable of completely inhibiting bacterial growth (CLSI, 2015).
2.5 Analisis of Β-lactamase activity
The analysis of β-lactamase activity was determined by establishing the decrease in the MIC of ampicillin following its combination (1/1) with sulbactam (a β-lactamase inhibitor). According to this method, a three-fold decrease in the MIC of ampicillin following the combination with the β-lactamase inhibitor indicates the presence and activity of the resistance enzyme. In this test, CPMZ and CCCP were used as control drugs to verify the activation of efflux mechanisms (CLSI, 2015).
2.6 Evaluation of β-lactamase activity inhibition
To evaluate β-lactamase activity inhibition, we analyzed the ability of allylbenzene and allylanisole (tested at sub-inhibitory concentrations, MIC/8), to decrease the MIC of penicillin against the SA-K4414 strain. Briefly, 170 μL of bacterial inoculum suspended in sterile saline solution was added to test tubes containing the phenylpropanoids diluted in BHI medium at a concentration of 128 μg/mL and a final volume of 1700 μL. This solution was transferred to the wells on a microdilution plate, followed by the addition of penicillin (100 µL) at concentrations ranging from 512 µg/mL to 0.5 µg/mL. After 24 h, the readings were performed as previously described (CLSI, 2015).
2.7 Evaluation of efflux pump inibition
Efflux pump (QacA/B) inhibition was determined by evaluating the ability of allylbenzene and allylanisole (at the concentrations equivalent to their MIC/8), to decrease the MIC of EtBr against the SA-K4414 strain (CLSI, 2015). As for the analysis β-lactamase activity inhibition, 170 μL of bacterial inoculum suspended in sterile saline solution was added to test tubes containing the phenylpropanoids diluted in BHI medium at a concentration of 128 μg/mL and a final volume of 1700 μL. This solution was transferred to the wells on a microdilution plate, followed by the addition of penicillin (100 µL) at concentrations ranging from 512 µg/mL to 0.5 µg/mL. After 24 h, the readings were performed as previously described. The control drugs CCCP and CPMZ were used as standard EP inhibitors at the concentrations of 1.41 μg/mL and 128 μg/mL, respectively, which correspond to their MIC/8.
2.8 Toxicological evaluation
2.8.1 Drosophila melanogaster culture
The Harwich strain of Drosophila melanogaster was obtained from the National Species Stock Center (Bowling Green, OH). The flies were bred according to a previously described methodology (Da Cunha et al., 2015). The medium was prepared in glass containers containing 83% corn mass, 4% sugar, 4% freeze-dried milk, 4% soy bran, 4% wheat bran, and 1% salt in a final volume of 340 mL. The mixture was cooked and then, 1 g of Nipagin (Methylparaben) was added. After cooling in the growth flasks, 1 mL of a solution containing Saccharomyces cerevisiae was added. The flies were kept at a temperature of 25 °C ± 1 °C and a relative humidity of 60% in a BOD oven with a 12:12 h light–dark cycle.
2.8.2 Mortality determination
Adult flies (male and female) were placed in 130 mL glass containers (6 cm high and 6.5 cm in diameter), with a filter paper on the top. The compounds were added to the filter paper and volatilized using the fumigation technique. In the control group, 1 mL of a 20% sucrose solution in distilled water was added to the filter paper. For the test groups, in addition to the sucrose solution, different concentrations of allylbenzene (1 mg/mL, 5 mg/mL, and 10 mg/mL) and allylanisole (1 mg/mL, 2 mg/mL, and 3 mg/mL) were added. These procedures were carried out at a controlled temperature (25 °C ± 1 °C) and 60% relative humidity in a BOD oven with a light: dark cycle of 12:12 h. The experiments were carried out in triplicates where each “n” was composed of two containers, containing 20 flies (male and female) each. Readings for verification of mortality were taken after 1, 3, 5, and 7 h in the allylanisole group, and after 3, 6, 9, 12, 24, 36, and 48 h in the allylbenzene group (Da Cunha et al., 2015).
2.8.3 Negative geotaxis assay
This test was used to assess possible locomotor damage caused by the treatment with the phenylpropanoids, as established by Coulom and Birman (2004). Therefore, each group of live flies was exposed to the compounds, and the analyzes were performed at the same time intervals described in the previous session. Then, the flies were driven to the bottom of the containers and after one minute, the number of flies that reached 4 cm in height of the container was counted. Every analysis was repeated twice at one-minute intervals.
2.9 Statistical analysis
The test results were expressed as the geometric mean. Statistical hypothesis analysis will be applied using Two-Way ANOVA, followed by Bonferroni’s post hoc test, any discrepancies were submitted to Student’s T test to validate the result using the GraphPad Prism 7.0 software.
3 Results and discussion
3.1 Allylbenzene e allylanisole present weak antibacterial effects
The antibacterial analysis revealed that both phenylpropanoids evaluated in this study had MIC values above 1024 µg/mL, while the standard antibiotic penicillin had a MIC of 512 µg/mL. These results indicate that allylbenzene and allylanisole do not have clinically relevant antibacterial activity. The findings of the present study corroborate with Muniz et al. (2021), in which allylbenzene and allylanisole obtained a MIC ≥ 1024 against S. aureus strains 1199 and 1199B, carrying the NorA Efflux pump. However, the antibacterial properties of phenylpropanoids have been reported in the literature (Nogueira et al., 2021). With synthetic polyphenols showed significant antibacterial activity, which was attributed to the inhibition of ATP synthase.
3.2 Assessment of Β-lactamase activity
The results of the present study demonstrated that the combination of sulbactam and ampicillin caused an approximately 5-fold reduction in the MIC of the antibiotic (645.08 µg/mL to 80.63 µg/mL) against the SA-K4414 strain of S. aureus, indicating that this strain presents bacterial resistance mediated by the enzymatic activity of β-lactamase. On the other hand, the association of ampicillin with CMPZ had no significant effect on the MIC, however, when ampicillin was associated with CCCP, there was a potentiation of the antibiotic, verified through the reduction of the MIC of the association (T-student test; p = 0 0.0109) (Fig. 1).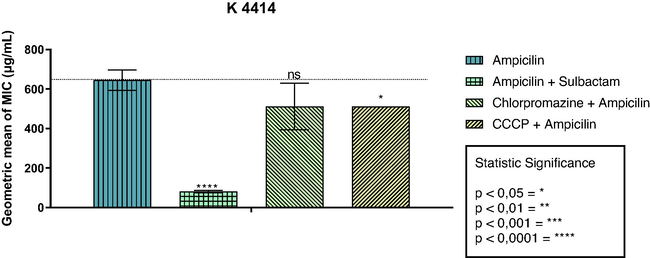
Assessment of β-lactamase activity in S. aureus strain K4414.The values represent the geometric means ± S.E.M. (standard error of mean).
As demonstrated in the present study, the SA-K4414 strain showed greater resistance to penicillin through the expression of β-lactamases enzymes, however the results also demonstrate the expression of the QacA/B efflux pump. Studies report that resistance to β-lactam in Gram-positive bacteria occurs through the acquisition of “replacement” Penicillin-Binding Proteins (PBPs) that are intrinsically less reactive to a β-lactam site, often with restricted access to active PBP, or revert to PBPs that remodel the biosynthesis of peptidoglycan in a manner that avoids the β-lactam mimicry of the peptide–d-Al-d-Terminus Ala, otherwise used in the PBP-dependent cross-linking of peptidoglycan. In S. aureus both β-lactamase and PBP2a expression are induced by exposure to β-lactams (Llarrull et al., 2010).
However, S. aureus strains carry resistance plasmids, which may express a single resistance determinant, or, in the case of multidrug resistance plasmids, carry multiple resistance determinants (Orlović et al., 2016). In fact, wild-type S. aureus strains generally have multiple resistance mechanisms due to their ability to share different phenotypic and genotypic characteristics associated with multidrug resistance, which is favored by the existence of regulatory systems that modulate gene expression according to substrate specificity (Uddin and Ahn, 2017). In the present study, penicillin resistance is mainly conferred by β-lactamases, whose expression is regulated by genes such as BlaZ, blaRI and bla (Khan, 2020).
3.3 Inhibition of Β-lactamase activity by allylanisole and allylbenzene
As observed in Fig. 2, the combination of the phenylpropanoids with penicillin reduced the MIC of this antibiotic from 512 µg/mL to 256 µg/mL and 128 µg/mL, respectively. According to these results, the phenylpropanoids potentiated the antibacterial effect of penicillin, suggesting that they can act as potential inhibitors of the resistance mechanism mediated by the enzymatic activity of β-lactamases.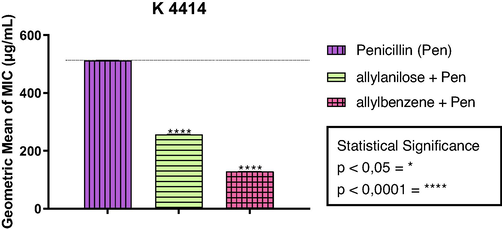
Inhibition of β-lactamase activity by phenylpropanoids allylbenzene and allylanisole in association with penicillin. The values represent the geometric means ± S.E.M. (standard error of mean).
Recent research has identified natural products with significant inhibitory activity against bacterial strains carrying the β-lactamase gene, including phenylpropanoids such as catechin gallate, epicatechin gallate, and epigallocatechin gallate, which demonstrated potent inhibition of the β-lactamase activity (Elfaky et al., 2020).
Trabelsi et al. (2020) identified in the essential oil of the Punica granatum phenylpropanoids that showed synergistic antibacterial effects in association with the β-lactam antibiotic amoxicillin in S. aureus cultures, reducing the antibiotic MIC from 512 mg/mL to 64 mg/mL. The authors proposed that phenylpropanoids can cause changes in the bacterial membrane permeabilization as well as enzymatic inhibition, resulting in a potentiated antibiotic activity. Another study by Scherf et al. (2020), demonstrated that the combination of terpinolene with oxacillin against the S. aureus strain K4100 reduced the antibiotic MIC from 161.26 μg/mL to 71.83 μg/mL, indicating enhanced antibiotic activity, possibly due to β-lactamase inhibition.
Phenylpropanoids such as Verbascoside, forsythoside B, arenarioside showed antibacterial activity against a methicillin-resistant strain of S. aureus (MRSA) with a MIC of 128 µg/mL (Didry et al., 1999). The antibacterial action of compounds of the phenylpropanoid class is associated with the inhibition of the synthesis of elements that are directly related to the functioning of the plasma membrane, causing cell damage to the membrane, and modifying its selective permeability (Nogueira et al., 2021). This occurs because the hydrocarbon portion of the compounds of the phenylpropanoid class have high affinity with the lipid region of plasma membranes, since they are essentially lipophilic substances (Saad et al., 2013). These studies corroborate with the present work, indicating a possible way of action of allylbenzene and allylanisole, by compromising the integrity of the bacterial membrane, however, more studies will be necessary to verify the possible mechanisms of action of these substances.
3.4 Inhibition of the Qaca/B efflux pump by the phenolic compounds
In this study, we demonstrated that the combination with the standard inhibitors CMPZ and CCCP resulted in a reduction in the of EtBr from 512 µg/mL to 256 µg/mL and 17 µg/mL respectively, proving the importance of the efflux mechanism mediated by QacA/B in the bacterial resistance of S. aureus strain K4414 (Fig. 3). Accordingly, the association with allylanisole and allylbenzene reduced the MIC of EtBr from 512 µg/mL to 406 µg/mL and 256 µg/mL, respectively. These findings suggest that both phenylpropanoids may act as inhibitors of the QacA/B efflux pump.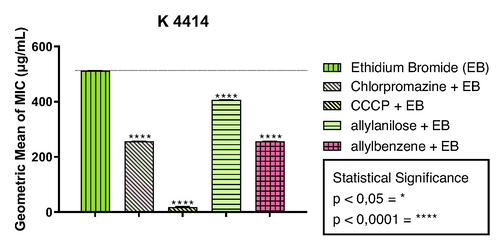
Inhibition of the QacA/B efflux pump by the phenylpropanoids allylbenzene and alylanisole in association with EtBr against the MDR strain SA-K4414 of S. aureus. The values represent the geometric means ± S.E.M. (standard error of mean).
Multi-resistance efflux pumps (MDR) are found in all bacterial species, conferring levels of resistance to almost all antibiotics in clinical use (Lamut et al., 2019). Consequently, several scientific studies have been carried out, with the aim of providing alternatives to inhibit this resistance mechanism.
In the study carried out by Muniz et al. (2021), it was observed that the association of 4-allyl-2,6-dimethoxyphenol, eugenol, and isoeugenol potentiated the activity of the antibiotic norfloxacin against the SA 1199B strain of S. aureus, which expresses the NorA efflux protein. Research carried out by dos Santos Barbosa et al. (2021) using thymol and carvacrol demonstrated that the association of these compounds with norfloxacin showed a synergistic effect against the strains SA-1199 (wild type) and SA-1199B, reducing the MIC of the antibiotic from 64 µg/mL to 32 µg/mL. Also, a study by dos Santos et al. (2018), suggested that that the phenylpropanoids caffeic acid and gallic acid can inhibit the NorA efflux protein expressed by the S. aureus strain 1199B, corroborating the findings of the present research since both studies investigated compounds belonging to the same chemical class against efflux proteins of the same family as that described in this study.
The mechanism of action of phenylpropanoids as EP inhibitors is still poorly understood. Nevertheless, Tintino et al. (2017) suggested that the action of tannic acid against the TetK protein strain expressed by the IS-58 strain of S. aureus involves a mechanism that depends on iron chelation, which prevents the use of this ion as a cofactor to maintain the strength of the proton matrix force (PMF) of this efflux pump.
The ethidium bromide assay demonstrated that the association of this substrate with phenylpropanoids resulted in a potentiated antibacterial effect, which indicates an increase in the intracellular concentration and, therefore, inhibition of the QacA/B EP. With the above-described results, it is possible to observe that the phenylpropanoids allylanilose and allylbenzene showed significant modulatory activity, reducing the MIC of the standard inhibitor sulbactam and the EP substrate EtBr, suggesting that these phenylpropanoids could be inhibiting either gene expression or the activity of β-lactamase and QacA/B. However, further research needs to be carried out to better investigate the molecular mechanisms underlying the pharmacological effects described in this study.
3.5 Effects on mortality and negative geotaxy in the Drosophila melanogaster model
The analysis of mortality in D. melanogaster cultures revealed that allylbenzene had an LC50 of 19.2 µL/mL while allylanisole had an LC50 of 11.07 µL/mL (not shown). It is noteworthy that these values were determined at different exposure times, corresponding to 12 h and 3 h, respectively, and therefore, are not directly comparable.
The highest tested concentration of allylbenzene (10 µL/mL) showed significant mortality from the third hour of exposure. On the other hand, the concentration of 1 µL/mL did not induce significant mortality (Fig. 4). Therefore, we can state that the mortality rate related to this compound increases in a concentration-dependent manner.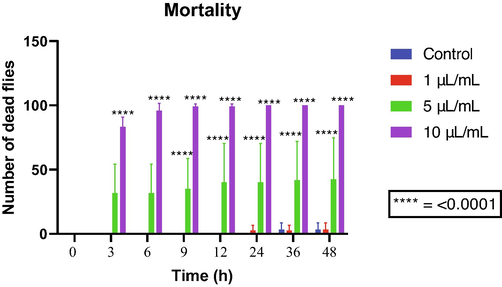
D. melanogaster mortality following exposure to allylbenzene.
About the effect of allylanisole on D. melanogaster mortality, all tested concentrations caused significant mortality after the third hour of exposure (Fig. 5), showing a time- and concentration-dependent toxicity.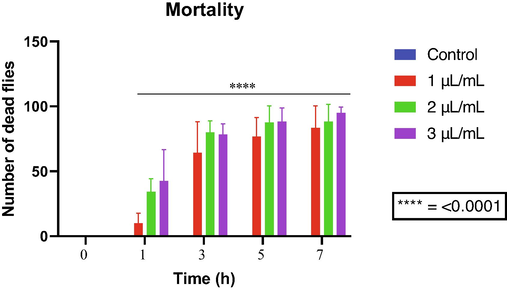
D. melanogaster mortality following exposure to allylanisole.
Drosophila melanogaster is an alternative eukaryotic model that has been widely used to verify the toxicity of substances due to its characteristics that favor its use, among which we can mention its sensitivity to low concentrations of substances, its easy maintenance in the laboratory, the reproductive period is short, and the number of offspring is high (Tiwari et al., 2011).
In the literature, there are several studies that associate the antibacterial activity and the toxicity of substances using D. melanogaster because of the factors mentioned above (Bezerra et al., 2022; Kvitek et al., 2011). Behavioral assays such as the verification of mortality and negative geotaxis are indicators of toxicity of chemical substances, as they show physiological changes because of the behavior being integrated into the subcellular and cellular processes of these organisms (Eom et al., 2017).
Associated with these behavioral tests, biochemical tests can be performed to verify the physiological changes triggered by the substances in this alternative model. In this study, biochemical tests were not performed due to limitations in technical facilities, but in the literature, oxidative stress is identified as the main factor that is correlated with toxicity, which triggers an imbalance in the oxidant and antioxidant system of these organisms (Oboh et al., 2021).
Despite their importance in the industrial context, evidence has indicated that phenolic compounds can be significantly toxic to eukaryotic cells and, therefore, toxicological studies are essential to establish the risks associated with the use of these compounds. Thus, the Drosophila melanogaster model has been shown an excellent tool for the investigation of genetic mechanisms and toxicity profiles of substances for human use and environmental exposure (Talyn et al., 2019).
Ebadollahi (2020) carried out research with the essential oil obtained from the medicinal species Satureja hortensis, which has estragole (synonym allylanisole) as a major constituent. It was demonstrated that this compound also presented significant toxicity against Rhyzopertha dominica after a 72-h exposure period. However, the authors assumed the high toxicity resulted from the synergistic interactions between the main constituent estragole and other phenylpropanoids identified in the plant.
Accordingly, the negative geotaxis test revealed that both compounds caused damage to the flies' locomotor system. The highest concentration of allylbenzene impaired locomotion from the first reading to the third hour of exposure, while the intermediate concentration only affected the locomotion from the sixth hour of exposure. On the other hand, the lowest concentration showed no considerable toxicity (Fig. 6).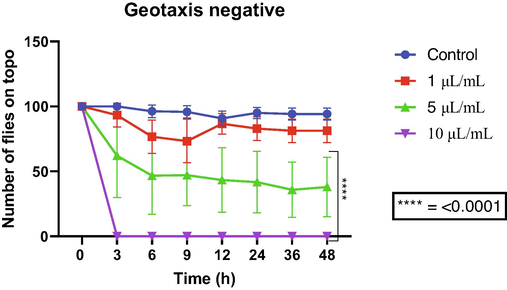
Effects of allylbenzene on the locomotor capacity of D. melanogaster.
These findings are in agreement with those observed by Silveira et al. (2020), who demonstrated that the phenylpropanoids thymol and carvacrol caused damage to the locomotor system of adult D. melanogaster flies at the highest concentration used from the third hour but did not cause significant changes when tested at the lowest concentrations.
On the other hand, allylanilose impaired the locomotion of flies from the first hour at all tested concentrations, with a maximum effect at the concentration of 2 µL/mL. However, statistical significance was demonstrated only from the third hour of exposure (Fig. 7).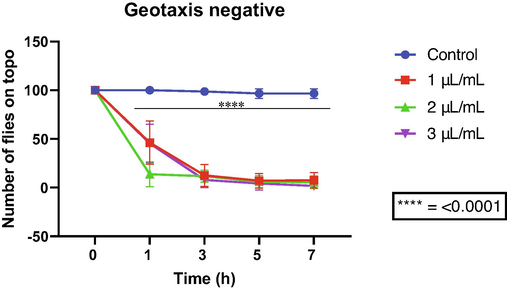
Effects of allylanilose on the locomotor capacity of D. melanogaster.
In a study by Bezerra et al. (2021), the isolated compound estragole showed toxicity to the locomotor system of adult D. melanogaster flies after the first hour of exposure at all tested concentrations. Evidence indicates that chemical compounds and secondary metabolites of medicinal plants affect the locomotor system mainly through mechanisms that lead to the reduction of acetylcholinesterase (AChE), resulting in prolonged neuronal activation and death (Hu et al., 2019).
In the present study, it was observed that both phenylpropanoids presented significant toxicity. Although these substances are structurally related, they differ in the presence of some functional groups such as the carbonyl group, present in allybenzene, but absent in aylanisole. However, from a chemical point of view, the presence of a carbonyl group usually confers toxic and mutagenic activity to many compounds (Semchyshyn, 2014).
4 Conclusion
The present research showed that the phenylpropanoids allylbenzene and allylanisole did not present direct antibacterial activity against the K4414 strain of S. aureus, however they potentiated the antibacterial effect of penicillin, which is supposedly associated with the ability of phenylpropanoids to inhibit the enzymatic activity of β- lactamases. It was also possible to observe that the compounds used reduced the MIC of the standard substrate (EtBr), indicating that they probably also act as inhibitors of the QacA/B efflux protein. On the other hand, toxicity tests in a model of D. melanogaster revealed that both compounds present significant toxicity, causing both mortality and locomotor damage in these organisms. These data stimulate further studies to elucidate the molecular mechanisms of interaction with bacterial targets associated with antibiotic resistance, as well as to characterize the toxicological profile of these compounds in eukaryotic cells.
Funding
This research received no external funding.
CRediT authorship contribution statement
Thais Pereira Lopes: Methodology. Cristina Rodrigues dos Santos Barbosa: Methodology. Jackelyne Roberta Scherf: Methodology. Nair Silva Macêdo: First draft of manuscript. Thiago Sampaio de Freitas: Software. Maria Apoliana Costa dos Santos: Resources. Jaime Ribeiro-Filho: First draft of manuscript. Francisco Assis Bezerra da Cunha: Supervision. Henrique Douglas Melo Coutinho: Coordination of Project. Abolghasem Siyadatpanah: Resources. Polrat Wilairatana: Conceptualization, Coordination of Project. lysson Pontes Pinheiro: Supervision, Final draft.
Acknowledgements
The authors are thankful to the Ceará Foundation for Support to Scientific and Technological Development- FUNCAP (BPI 02/2020 NÚMERO: BP4-0172-00168.01.00 / 20 SPU N°: 09673071/2020); Coordination for the Improvement of Higher Education Personnel - CAPES; National Council for Scientific and Technological Development - CNPq.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Estragole prevents gastric ulcers via cytoprotective, antioxidant and immunoregulatory mechanisms in animal models. Biomed. Pharmacother.. 2020;130:110578.
- [CrossRef] [Google Scholar]
- Effect of estragole over the RN4220 Staphylococcus aureus strain and its toxicity in Drosophila melanogaster. Life Sci.. 2021;264:118675.
- [CrossRef] [Google Scholar]
- Antibacterial activity of eugenol on the IS-58 strain of Staphylococcus aureus resistant to tetracycline and toxicity in Drosophila melanogaster. Microb. Pathog.. 2022;164:105456.
- [CrossRef] [Google Scholar]
- Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol.. 2015;13(1):42-51.
- [CrossRef] [Google Scholar]
- The Revaluation of Plant-Derived Terpenes to Fight Antibiotic-Resistant Infections. Antibiotics. 2020;9:325.
- [CrossRef] [Google Scholar]
- In vitro inhibition of cholera toxin production in vibrio cholerae by methanol extract of sweet fennel seeds and its components. Jpn. J. Infect. Dis.. 2016;69:384-389.
- [CrossRef] [Google Scholar]
- CLSI, 2015. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement Clinical and Laboratory Standards Institute, CLSI document M100-S16CLSI, Wayne, PA.
- Chronic exposure to rotenone models sporadic Parkinson’s disease in Drosophila melanogaster. J. Neurosci.. 2004;24:10993-10998.
- [CrossRef] [Google Scholar]
- Eugenia uniflora leaves essential oil induces toxicity in Drosophila melanogaster: Involvement of oxidative stress mechanisms. Toxicol. Res. (Camb). 2015;4(3):634-644.
- [Google Scholar]
- Biotransformation of allylbenzene analogues in vivo and in vitro through the epoxide-diol pathway. Xenobiotica. 1980;10(10):737-744.
- [CrossRef] [Google Scholar]
- Isolation and antibacterial activity of phenylpropanoid derivatives from Ballota nigra. J. Ethnopharmacol.. 1999;67(2):197-202.
- [CrossRef] [Google Scholar]
- Effect of Carvacrol and Thymol on NorA efflux pump inhibition in multidrug-resistant (MDR) Staphylococcus aureus strains. J. Bioenerg. Biomembr.. 2021;53(4):489-498.
- [CrossRef] [Google Scholar]
- In vitro e in silico evaluation of the inhibition of Staphylococcus aureus efflux pumps by caffeic and gallic acid. Comp. Immunol. Microbiol. Infect. Dis.. 2018;57:22-28.
- [CrossRef] [Google Scholar]
- Estragole-rich essential oil of summer savory (Satureja hortensis L.) as an eco-friendly alternative to the synthetic insecticides in management of two stored-products insect pests. Acta Agric. Slov.. 2020;115:307-314.
- [CrossRef] [Google Scholar]
- Bioassay Guided Isolation and Docking Studies of a Potential β-Lactamase Inhibitor from Clutia myricoides. Molecules. 2020;25:1-13.
- [CrossRef] [Google Scholar]
- Inhalation toxicity of indoor air pollutants in Drosophila melanogaster using integrated transcriptomics and computational behavior analyses. Sci. Rep.. 2017;7(1)
- [CrossRef] [Google Scholar]
- Antiproliferative and palliative activity of flavonoids in colorectal cancer. Biomed. Pharmacother.. 2021;143:112241.
- [CrossRef] [Google Scholar]
- Ecotoxicology and Environmental Safety E ff ects of cadmium on fecundity and defence ability of Drosophila melanogaster. Ecotoxicol. Environ. Saf.. 2019;171:871-877.
- [CrossRef] [Google Scholar]
- Phenotypic and Genotypic Characterization of Beta-lactams Resistant Staphylococcus aureus Isolates from Bovine Mastitis and its Zoonotic Implications. Pak. Vet. J.. 2020;40(04):523-526.
- [Google Scholar]
- Antibacterial activity and toxicity of silver - Nanosilver versus ionic silver. J. Phys. Conf. Ser. IOP Publ.. 2011;304:012029.
- [CrossRef] [Google Scholar]
- Efflux pump inhibitors of clinically relevant multidrug resistant bacteria. Med. Res. Rev.. 2019;39(6):2460-2504.
- [CrossRef] [Google Scholar]
- The future of the β-lactams. Curr. Opin. Microbiol.. 2010;13(5):551-557.
- [CrossRef] [Google Scholar]
- In vitro and in silico inhibitory effects of synthetic and natural eugenol derivatives against the NorA efflux pump in Staphylococcus aureus. Food Chem.. 2021;337:127776.
- [CrossRef] [Google Scholar]
- Mechanism of action of various terpenes and phenylpropanoids against Escherichia coli and Staphylococcus aureus. FEMS Microbiol. Lett.. 2021;368(9)
- [CrossRef] [Google Scholar]
- Anticholinesterase activity and antioxidant properties of Heinsia crinita and Pterocarpus soyauxii in Drosophila melanogaster model. J. Ayurveda Integr. Med.. 2021;12(2):254-260.
- [CrossRef] [Google Scholar]
- Structures of lipoprotein signal peptidase II from Staphylococcus aureus complexed with antibiotics globomycin and myxovirescin. Nat. Commun.. 2020;11:140.
- [CrossRef] [Google Scholar]
- Resistance in staphylococcus Aureus: The never-ending story. Acta Fac. Medicae Naissensis. 2016;33:153-162.
- [CrossRef] [Google Scholar]
- Rodrigues, L.B., Oliveira Brito Pereira Bezerra Martins, A., Cesário, F.R.A.S., Ferreira e Castro, F., de Albuquerque, T.R., Martins Fernandes, M.N., Fernandes da Silva, B.A., Quintans Júnior, L.J., da Costa, J.G.M., Melo Coutinho, H.D., Barbosa, R., Alencar de Menezes, I.R., 2016. Anti-inflammatory and antiedematogenic activity of the Ocimum basilicum essential oil and its main compound estragole: In vivo mouse models. Chem. Biol. Interact. 257, 14–25. https://doi.org/10.1016/j.cbi.2016.07.026.
- Major bioactivities and mechanism of action of essential oils and their components. Flavour Fragr. J.. 2013;28(5):269-279.
- [CrossRef] [Google Scholar]
- Effect of terpinolene against the resistant Staphylococcus aureus strain, carrier of the efflux pump QacC and β-lactamase gene, and its toxicity in the Drosophila melanogaster model. Microb. Pathog.. 2020;149:104528.
- [CrossRef] [Google Scholar]
- Reactive Carbonyl Species In Vivo : Generation and Dual Biological Effects. Sci. World J.. 2014;14:27-31.
- [CrossRef] [Google Scholar]
- Silveira, Z.D.S., Freitas, T.S. De, Douglas, H., Coutinho, M., Balbino, V.Q., 2020. Molecules Evaluation of the Antibacterial Activity and E ffl ux Pump Reversal of Thymol and Carvacrol against Staphylococcus aureus and Their Toxicity in 1–10.
- Roundup®, but not Roundup-ready® corn, increases mortality of Drosophila melanogaster. Toxics. 2019;7(3):38.
- [CrossRef] [Google Scholar]
- Tannic acid affects the phenotype of Staphylococcus aureus resistant to tetracycline and erythromycin by inhibition of efflux pumps. Bioorg. Chem.. 2017;74:197-200.
- [CrossRef] [Google Scholar]
- Environmental chemical mediated male reproductive toxicity: Drosophila melanogaster as an alternate animal model. Theriogenology. 2011;76(2):197-216.
- [Google Scholar]
- Phytochemical Study and Antibacterial and Antibiotic Modulation Activity of Punica granatum (Pomegranate) Leaves. Scientifica. 2020;2020:1-7.
- [Google Scholar]
- Structural and physiological implications of bacterial cell in antibiotic resistance mechanisms. Int. J. Morphol.. 2017;35:1214-1223.
- [CrossRef] [Google Scholar]
- Multidrug-resistance in methicillin-resistant Staphylococcus aureus (MRSA) isolated from a subtropical river contaminated by nearby livestock industries. Ecotoxicol. Environ. Saf.. 2020;200:110724.
- [CrossRef] [Google Scholar]
- Associations between resistance phenotype and gene expression in response to serial exposure to oxacillin and ciprofloxacin in Staphylococcus aureus. Lett. Appl. Microbiol.. 2017;65(6):462-468.
- [CrossRef] [Google Scholar]
- The Growing Genetic and Functional Diversity of Extended Spectrum Beta-Lactamases. Biomed Res. Int.. 2018;2018:1-14.
- [CrossRef] [Google Scholar]







