Translate this page into:
Pentafluorophenylammonium triflate: An effective and reusable organocatalyst for the one-pot preparation of 2,4-diaryl-5H-indeno[1,2-b]pyridin-5-one derivatives
*Corresponding author. Tel.: +91 9944093020 smansoors2000@yahoo.co.in (S. Sheik Mansoor)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 5 September 2013
Peer review under responsibility of King Saud University.

Abstract
A new one-pot and efficient three-component condensation of 1,3-diphenyl-2-propen-1-ones (chalcones), 1,3-indandione, and ammonium acetate using pentafluorophenylammonium triflate (PFPAT) as catalyst for the synthesis of substituted 2,4-diaryl-5H-indeno[1,2-b]pyridin-5-one is described. The present methodology offers several advantages, such as good yields, atom economy, short reaction times and a recyclable catalyst with a very easy work up.
Keywords
Pentafluorophenylammonium triflate (PFPAT)
Chalcones
Indeno[1,2-b]pyridines
1,3-Indandione
1 Introduction
5H-Indeno[1,2-b]pyridin-5-ones (4-azafluorenones) are the naturally occurring alkaloids isolated from the root of the plant Polyalthia debilis (Pierre) belonging to the family of Annonaceae. Azaflurenone, being the core structural unit in a wide range of natural products, has attracted much research in recent times (Mueller et al., 2009). Representative members of this class of compounds are onychine (a), polyfothine (b), isoursuline (c), and cyathocaline (d) (Fig. 1). These compounds show powerful antimicrobial, DNA damaging, and anti-malarial effects against Plasmodium falciperum and also acts as a DNA modifying agent (Kraus and Kempema, 2010; Wijeratne et al., 1995). Azafluorenone derivatives have been reported to possess activities of aldose reductase inhibition (Wang and Wei, 2012) and cytotoxic activity (Prachayasittikul et al., 2009). Roots of Polyalthia cerasoides has led to the isolation of the new compound, 6,8-dihydroxy-7-methoxy-1-methyl-azafluorenone. This compound exhibited potent cytotoxic activities (Pumsalid et al., 2010). Koyama et al. (2005) reported structure–activity relations of azafluorenone and azaanthraquinone as antimicrobial compounds. Correlations among reduction potential, hydrophobic parameter, and antimicrobial activity were discussed. Due to their crucial importance in chemistry, biology and industry, a general and flexible method of preparation of azafluorenones is important.
Some naturally occurring bioactive azafluorenones.
Several methods are available describing the synthesis of 5H-indeno[1,2-b]pyridin-5-ones: Dhara et al. (2013) synthesized azafluorenone via oxidative intramolecular Heck cyclization using Pd(0). Tapaswi and Mukhopadhyay, 2011 reported the synthesis of indeno[1,2-b]pyridines using ceric ammonium nitrate as catalyst. Marquise et al. (2013) reported the synthesis of azafluorenones and related compounds by a route involving cyclization of the ketones. Li et al. (2013) reported a novel three-component [5+1] heterocyclization leading to 2-azafluorenone synthesis and its ployfunctionalisations. Tu et al. (2007) developed the synthesis of substituted indeno[1,2-b]pyridines by the multi-component reaction of aldehydes, 1,3-indandione and aromatic ketones in the presence of ammonium acetate under microwave irradiation. Addla et al. (2012) reported the synthesis of novel 1-benzyl 2-butyl-4-chloroimidazole embodied 4-azafluorenones via the molecular hybridization approach in very good yields using one pot condensation of 1-benzyl-2-butyl-4-chloroimidazole-5-carboxaldehyde, 1,3-indanedione, aryl/heteroaryl methyl ketones and ammonium acetate and studied their antimicrobial evaluation. Mukhopadhyay et al. (2010) reported the synthesis of several indeno[1,2-b]pyridine derivatives by the multi-component reaction of 1,3-indandione, aldehydes, 2-acetylthiophene or 2-acetylfluorene and ammonium acetate at room temperature using l-proline (15 mol%) as catalyst. Padwa et al. (2000) reported the synthesis of several alkaloids employing the Pummerer reaction of imidosulfoxides bearing tethered alkenyl groups. Most of these methodologies suffer from one or more serious drawbacks such as laborious and complex work-up and purification, strong basic and acidic conditions, multistep reactions and occurrence of side reactions, low yields, and the use of expensive reagents. In addition, most of the earlier-reported methodologies require elevated temperature created by microwave-oven irradiation.
Considering the novel route for the synthesis of heterocyclic compounds based on the development of environmentally friendly procedures for the synthesis of biologically active molecules, such as 4,6-diphenyl-3,4dihydropyrimidine-2(H)-thione (Aswin et al., 2013), 1,4-dihydropyridine (Mansoor et al., 2013), polyhydroquinoline derivatives (Mansoor et al., 2012a) and 2-amino-4,6-diphenylnicotinonitrile (Mansoor et al., 2012b) by multicomponent reactions, we now describe the synthesis of 2,4-diaryl-5H-indeno[1,2-b]pyridin-5-one derivatives using PFPAT as an efficient organo-catalyst. These compounds were synthesized using chalcones, 1,3-indandione and ammonium acetate (Scheme 1).![Synthesis of various 2,4-diaryl-5H-indeno[1,2-b]pyridin-5-one derivatives.](/content/185/2014/26/3/img/10.1016_j.jksus.2013.08.007-fig2.png)
Synthesis of various 2,4-diaryl-5H-indeno[1,2-b]pyridin-5-one derivatives.
2 Experimental
2.1 Apparatus and analysis
Chemicals were purchased from Merck, Fluka and Aldrich Chemical Companies. All yields refer to isolated products unless otherwise stated. 1H NMR (500 MHz) and 13C NMR (125 MHz) spectra were obtained using Bruker DRX-500 Avance at an ambient temperature, using TMS as internal standard. FT-IR spectra were obtained as KBr disks on Shimadzu spectrometer. Mass spectra were determined on a Varion – Saturn 2000 GC/MS instrument. Elemental analysis was measured by means of Perkin Elmer 2400 CHN elemental analyzer flowchart.
2.2 Preparation of the catalyst (PFPAT)
To a solution of 2,3,4,5,6-pentafluoroaniline (25 mmol) in toluene (25 mL), CF3SO3H (25 mmol) was added at 0–5 °C. The reaction mixture was stirred at the same temperature for 30 min. After this time, the solvent was evaporated in vacuo, the crude product was collected and washed with hexane to give the pure catalyst in 92% yield (Funatomi et al., 2006).
2.3 General experimental procedure for the synthesis of indeno[1,2-b]pyridine derivatives
In a 25 mL round-bottomed flask, 1,3-diphenyl-2-propen-1-ones (1 mmol), 1,3-indandione (1 mmol), and ammonium acetate (1.3 mmol) were stirred in the presence of 10 mol% of PFPAT in ethanol (5 mL) at 60 °C for the stipulated time. The progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was diluted with water (10 mL) and extracted with ethyl acetate (3 × 10 mL). The organic layer was dried over anhydrous Na2SO4, concentrated and recrystallized from hot ethanol to afford pure product.
3 Results
To find out the suitable conditions for the reaction, we studied the standard reaction of 1,3-diphenyl-2-propen-1-one (1a), 1,3-indandione (2), and ammonium acetate (3) as a model reaction using PFPAT (10 mol%) as catalyst (Scheme 1). In order to optimize the conditions, we studied the reaction in various conditions.
Among various catalysts tested, PFPAT (10 mol%) was found to be an effective catalyst wherein the product was obtained in good yield under mild conditions (Table 1). With PFPAT (10 mol%) as catalyst, next the effect of various solvents was screened (Table 2). Ethanol was found to be the best solvent under these conditions (Table 2, entry 5). Under the optimized set of reaction conditions a number of 1,3-diaryl-2-propen-1-one derivatives (1) were allowed to react with 1,3-indandione (2) and ammonium acetate (3) in the presence PFPAT (10 mol%) as catalyst (Scheme 1). The results are given in Table 3. The IR, 1H NMR, 13C NMR, mass and elemental analysis data of the synthesized compounds are given below.
Entry
Catalyst
Amount of catalyst (mol%)
Time (h)
Yield (%)b
1
None
–
3.0
32
2
FeCl3
20
3.0
65
3
InCl3
20
3.0
68
4
BiCl3
10
3.0
72
5
La(OTf)3
10
3.0
76
6
Nd(OTf)3
10
3.0
60
7
Yb(OTf)3
10
3.0
80
8
PFPAT
10
1.5
92
9
PFPAT
5
1.5
81
10
PFPAT
2
1.5
72
11
PFPAT
15
1.5
90
Entry
Solvent
Amount of catalyst (mol%)
Time (h)
Yield (%)b
1
Acetonitrile
10
1.5
68
2
1,4-Dioxane
10
1.5
63
3
Cyclohexane
10
1.5
48
4
Methanol
10
1.5
84
5
Ethanol
10
1.5
92
6
None
10
1.5
58
Entry
Chalcones
Product
Time (h)
Yield (%)b
Mp (°C)
1
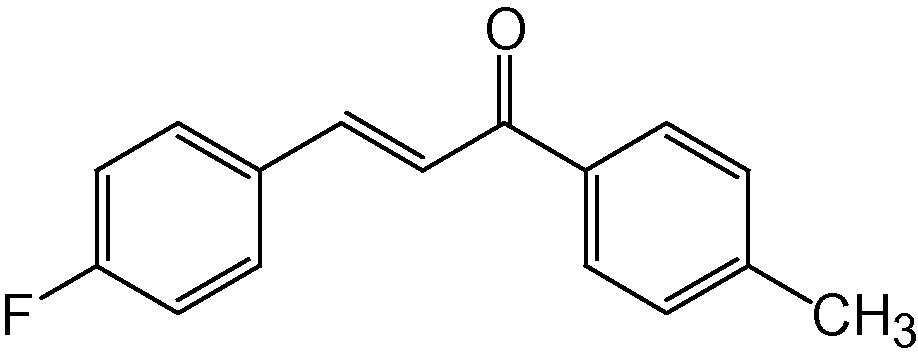
4a
1.5
92
236–238
2
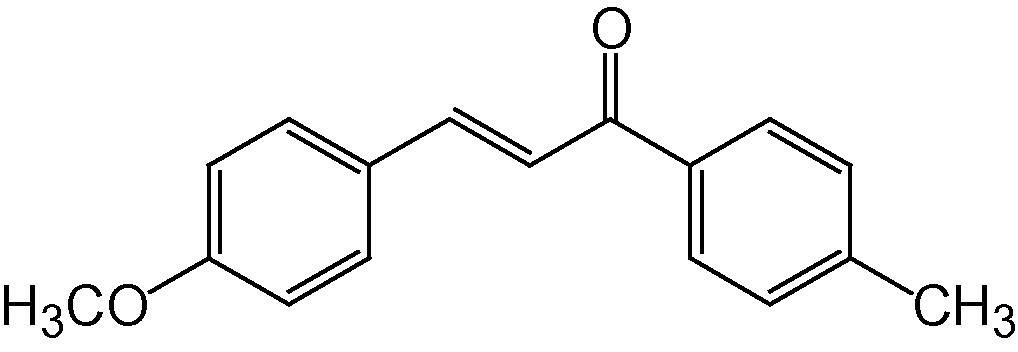
4b
2.0
83
224–226
3
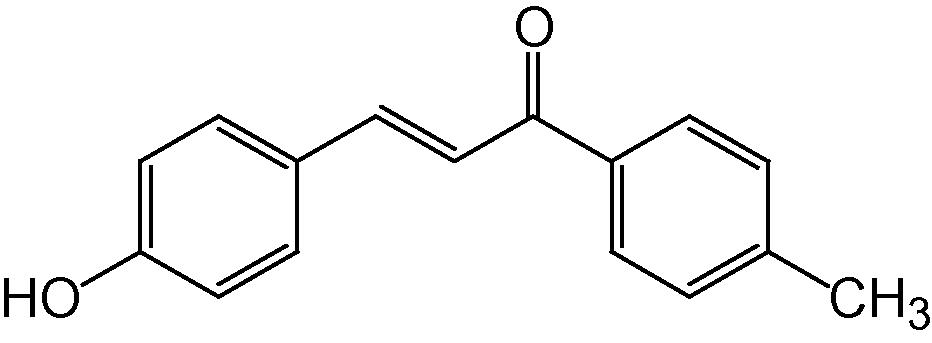
4c
1.5
93
244–246
4
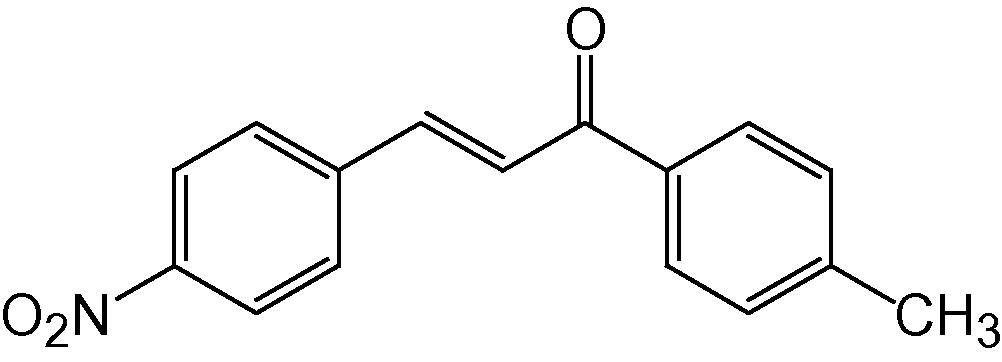
4d
1.5
94
217–219
5
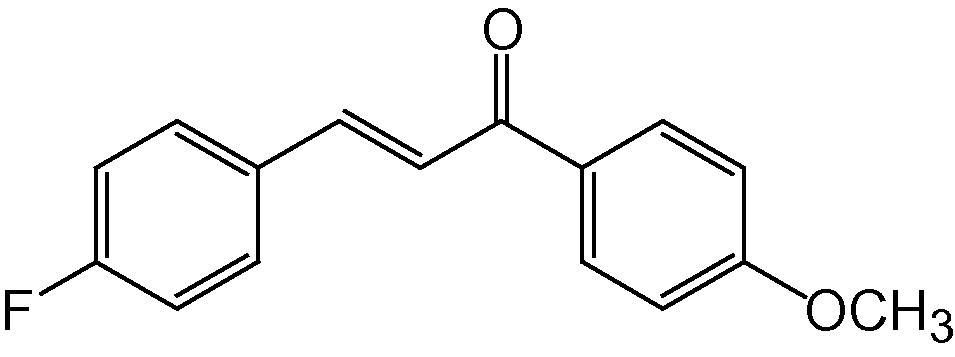
4e
1.5
92
228–230
6
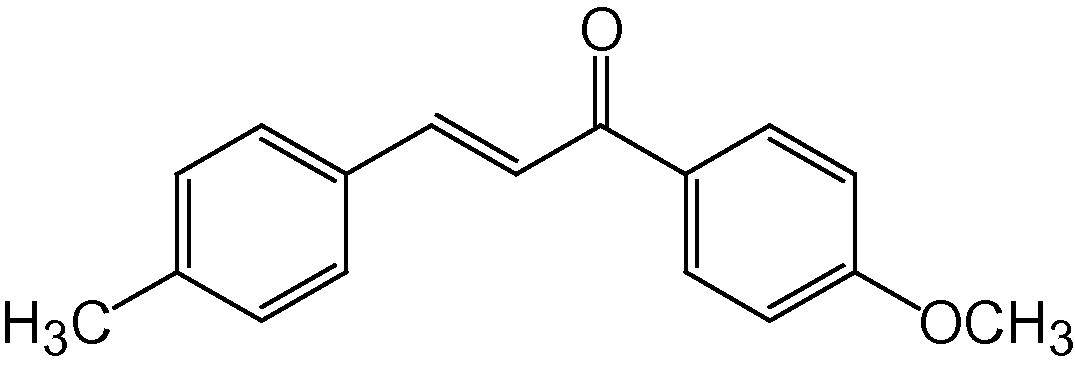
4f
2.0
83
206–208
7
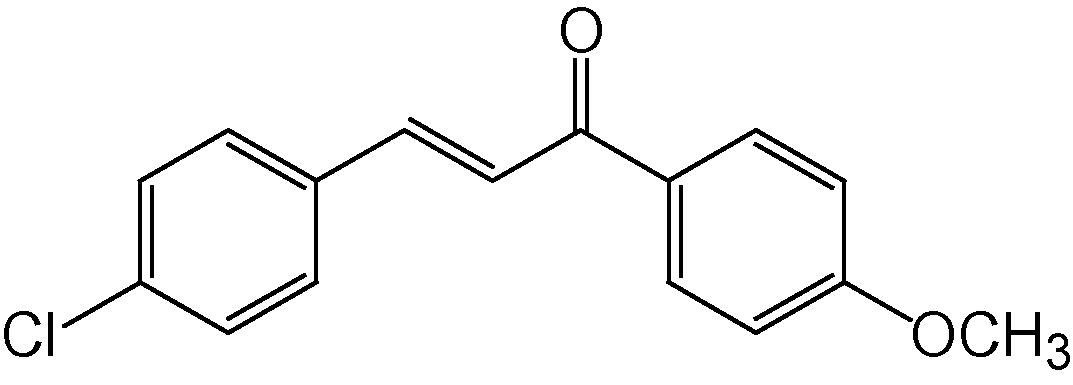
4g
1.5
89
202–204
8
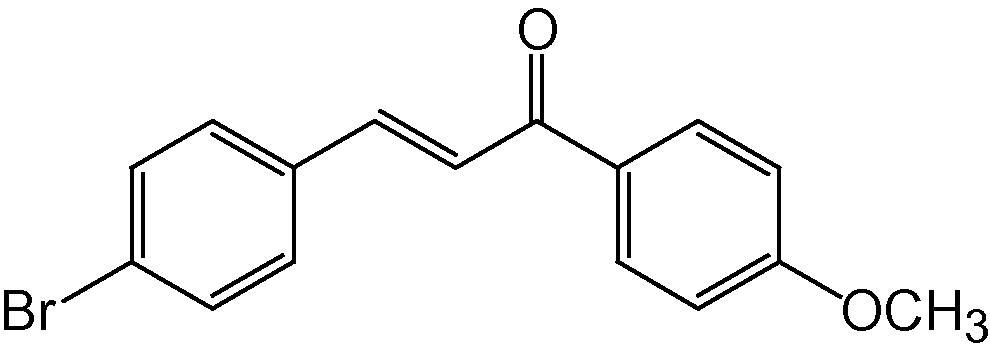
4h
1.5
88
214–216
9
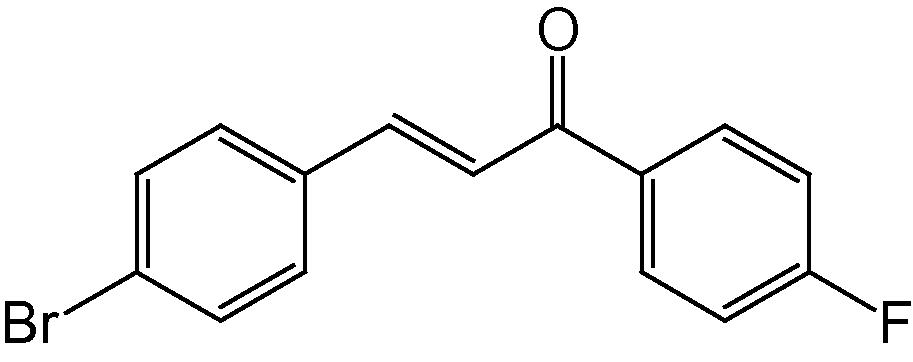
4i
1.5
89
198–200
10
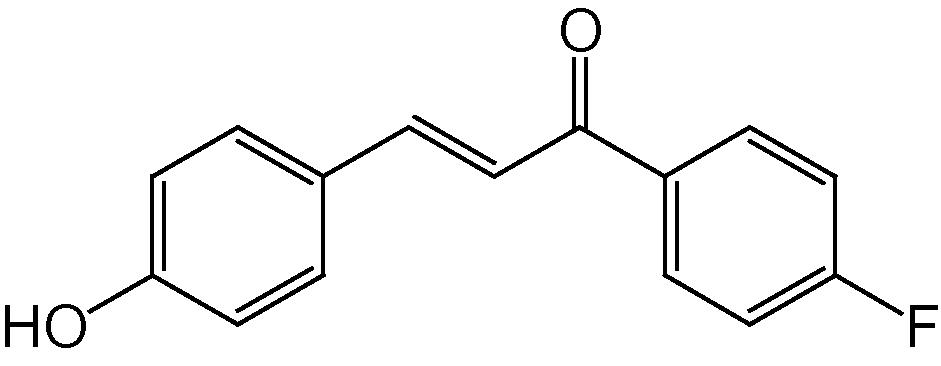
4j
1.5
91
226–228
11
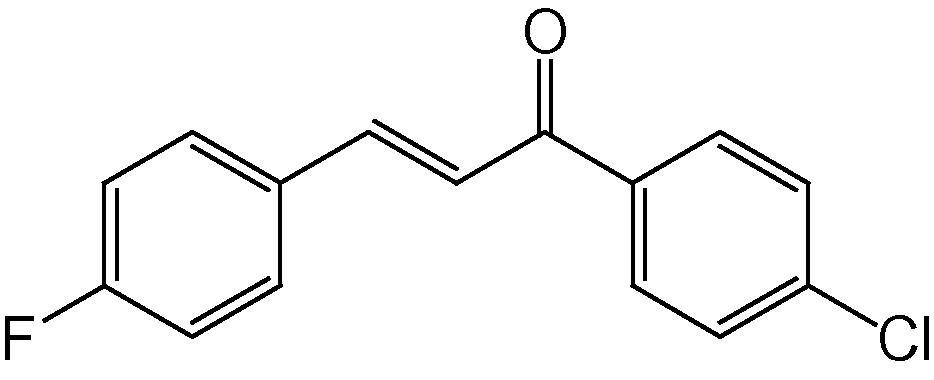
4k
1.5
91
216–218
12
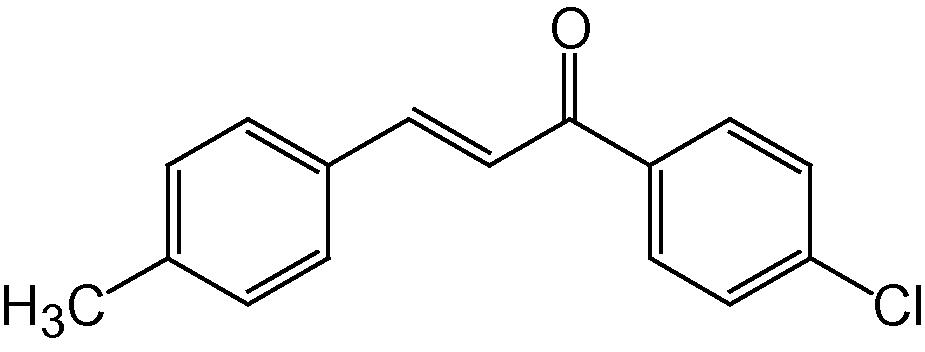
4l
2.0
84
210–212
13
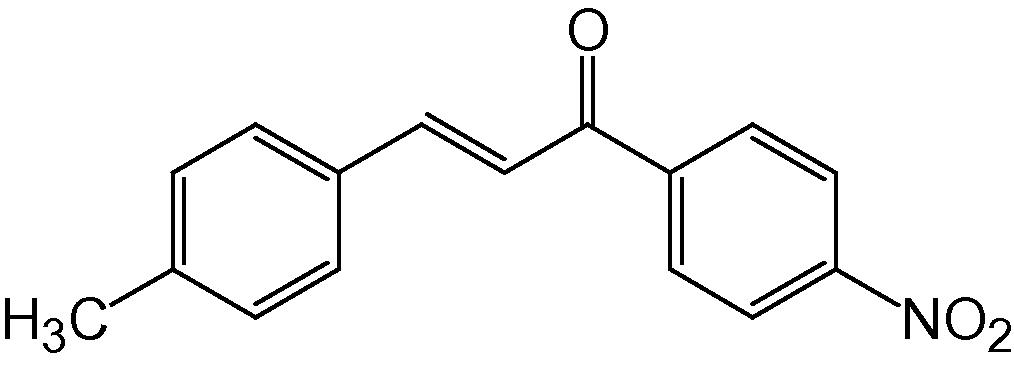
4m
2.0
83
202–204
14
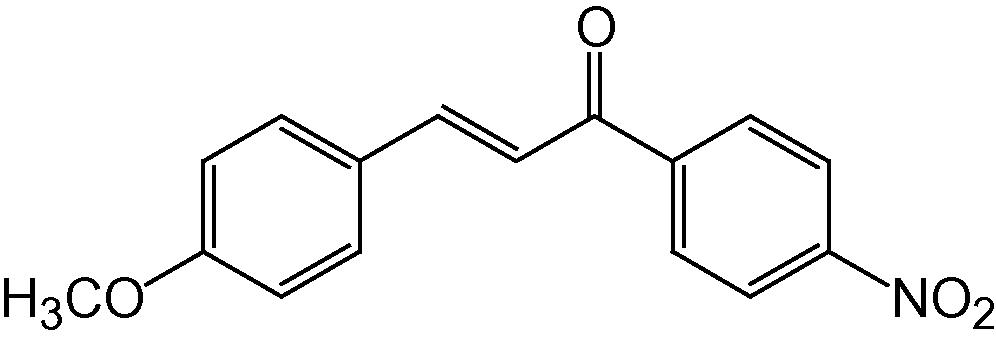
4n
2.0
82
246–248
15
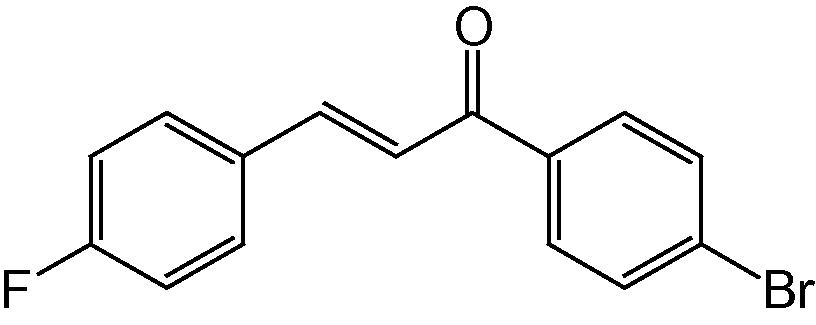
4o
1.5
88
196–198
16
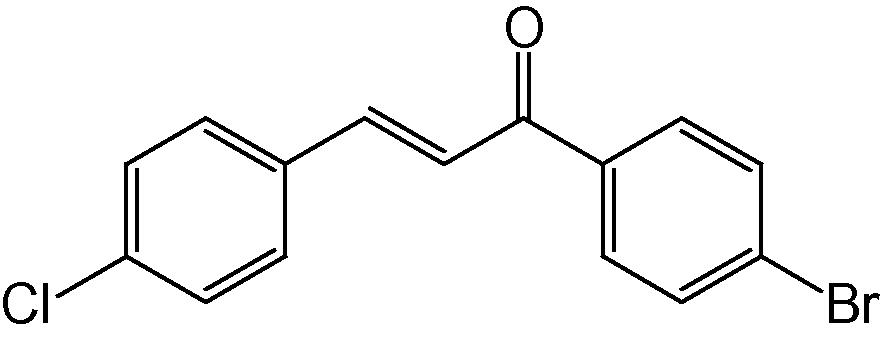
4p
1.5
89
206–208
17
4q
1.5
95
234–236
18
4r
2.0
84
218–220
3.1 Spectral data for the synthesized compounds are presented below (4a–r)
3.1.1 4-(4-Fluorophenyl)-2-(4-methylphenyl)-5H-indeno[1,2-b]pyridin-5-one (4a)
IR (KBr, cm−1): 3051, 2948, 2839, 1714, 1600, 1508, 1362, 1252, 1155, 828, 757; 1H NMR (500 MHz, CDCl3) δ: 2.16 (s, 3H, CH3), 7.08–7.33 (m, 8H, ArH), 7.60 (s, 1H, Py–H), 7.72–7.88 (m, 4H, ArH) ppm; 13C NMR (125 MHz, CDCl3) δ: 18.0, 121.1, 123.3, 123.6, 123.9, 127.4, 128.3, 128.5, 129.0, 129.5, 129.7, 131.4, 131.9, 135.4, 135.7, 139.9, 141.4, 142.2, 142.8, 146.4, 147.4, 162.5, 163.9, 191.6 ppm; MS(ESI): m/z 366 (M+H)+; Anal. Calcd for C25H16FNO: C, 82.19; H, 4.38; N, 3.83%. Found: C, 82.11; H, 4.35; N, 3.80%.
3.1.2 3.1.2.4-(4-Methoxyphenyl)-2-(4-methylphenyl)-5H-indeno[1,2-b]pyridin-5-one (4b)
IR (KBr, cm−1): 3049, 2953, 2844, 1718, 1604, 1509, 1365, 1254, 1154, 826, 755; 1H NMR (500 MHz, CDCl3) δ: 2.20 (s, 3H, CH3), 3.72 (s, 3H, OCH3), 7.13–7.29 (m, 8H, ArH), 7.74 (s, 1H, Py–H), 7.85–7.96 (m, 4H, ArH) ppm; 13C NMR (125 MHz, CDCl3) δ: 18.2, 55.0, 121.5, 123.7, 123.9, 123.9, 127.9, 128.6, 129.4, 129.8, 129.9, 131.1, 135.3, 135.6, 139.4, 141.7, 142.5, 146.7, 147.8, 162.2, 163.6, 191.3 ppm; MS(ESI): m/z 378 (M+H)+; Anal. Calcd for C26H19NO2: C, 82.76; H, 5.04; N, 3.71%. Found: C, 82.72; H, 5.00; N, 3.66%.
3.1.3 4-(4-Hydroxyphenyl)-2-(4-methylphenyl)-5H-indeno[1,2-b]pyridin-5-one (4c)
IR (KBr, cm−1): 3362, 3045, 2942, 2833, 1707, 1604, 1512, 1366, 1256, 1151, 824, 753; 1H NMR (500 MHz, CDCl3) δ: 2.26 (s, 3H, CH3), 7.18–7.45 (m, 8H, ArH), 7.69 (s, 1H, Py–H), 7.79–7.92 (m, 4H, ArH), 9.22 (s, 1H, OH) ppm; 13C NMR (125 MHz, CDCl3) δ: 18.7, 121.0, 123.4, 123.5, 123.9, 128.0, 128.7, 129.1, 129.5, 129.7, 131.6, 135.2, 135.7, 139.0, 141.6, 142.4, 146.6, 147.6, 162.0, 163.4, 191.0 ppm; MS(ESI): m/z 364 (M+H)+; Anal. Calcd for C25H17NO2: C, 82.64; H, 4.68; N, 3.86%. Found: C, 82.60; H, 4.64; N, 3.84%.
3.1.4 4-(4-Nitrophenyl)-2-(4-methylphenyl)-5H-indeno[1,2-b]pyridin-5-one (4d)
IR (KBr, cm−1): 3042, 2939, 2830, 1706, 1602, 1504, 1369, 1250, 1154, 827, 756; 1H NMR (500 MHz, CDCl3) δ: 2.19 (s, 3H, CH3), 7.22–7.40 (m, 8H, ArH), 7.76 (s, 1H, Py–H), 7.84–7.97 (m, 4H, ArH) ppm; 13C NMR (125 MHz, CDCl3) δ: 17.6, 120.9, 123.3, 123.5, 123.7, 127.8, 128.8, 129.2, 129.5, 129.9, 131.7, 135.7, 135.7, 139.7, 142.1, 142.9, 147.1, 148.1, 162.0, 163.1, 191.2 ppm; MS(ESI): m/z 393 (M + H)+; Anal. Calcd for C25H16N2O3: C, 76.53; H, 4.08; N, 7.14%. Found: C, 76.43; H, 4.02; N, 7.10%.
3.1.5 4-(4-Fluorophenyl)-2-(4-methoxyphenyl)-5H-indeno[1,2-b]pyridin-5-one (4e)
IR (KBr, cm−1): 3049, 2946, 2837, 1713, 1611 1513, 1367, 1245, 1152, 822, 752; 1H NMR (500 MHz, CDCl3) δ: 3.66 (s, 3H, OCH3), 7.06–7.33 (m, 8H, ArH), 7.62 (s, 1H, Py–H), 7.73–7.85 (m, 4H, ArH) ppm; 13C NMR (125 MHz, CDCl3) δ: 55.9, 121.1, 123.1, 123.4, 123.8, 127.6, 128.1, 128.6, 128.9, 129.6, 129.9, 131.2, 131.7, 135.4, 135.6, 139.8, 142.3, 142.7, 143.1, 147.3, 148.3, 162.1, 163.8, 191.8 ppm; MS(ESI): m/z 382 (M+H)+; Anal. Calcd for C25H16FNO2: C, 78.74; H, 4.20; N, 3.67%. Found: C, 78.76; H, 4.24; N, 3.69%.
3.1.6 4-(4-Methylphenyl)-2-(4-methoxyphenyl)-5H-indeno[1,2-b]pyridin-5-one (4f)
IR (KBr, cm−1): 3040, 2937, 2828, 1704, 1608, 1509, 1365, 1257, 1147, 821, 750; 1H NMR (500 MHz, CDCl3) δ: 2.22 (s, 3H, CH3), 3.74 (s, 3H, OCH3), 7.28–7.55 (m, 8H, ArH), 7.71 (s, 1H, Py–H), 7.80–7.94 (m, 4H, ArH) ppm; 13C NMR (125 MHz, CDCl3) δ: 17.9, 55.2, 121.5, 123.6, 123.7, 123.9, 127.5, 128.5, 129.0, 129.6, 129.8, 131.5, 135.0, 135.5, 139.5, 142.0, 142.9, 147.2, 148.3, 161.8, 162.9, 192.5 ppm; MS(ESI): m/z 378 (M+H)+; Anal. Calcd for C26H19NO2: C, 82.76; H, 5.04; N, 3.71%. Found: C, 82.79; H, 5.00; N, 3.74%.
3.1.7 4-(4-Chlorophenyl)-2-(4-methoxyphenyl)-5H-indeno[1,2-b]pyridin-5-one (4g)
IR (KBr, cm−1): 3051, 2949, 2844, 1719, 1602, 1511, 1366, 1255, 1144, 817, 746; 1H NMR (500 MHz, CDCl3) δ: 3.70 (s, 3H, OCH3), 7.20–7.48 (m, 8H, ArH), 7.68 (s, 1H, Py–H), 7.77–7.93 (m, 4H, ArH) ppm; 13C NMR (125 MHz, CDCl3) δ: 55.5, 121.7, 123.5, 123.9, 124.2, 128.2, 128.8, 129.3, 129.4, 129.9, 131.3, 135.3, 135.6, 139.6, 142.1, 143.1, 147.3, 148.1, 161.7, 163.5, 192.2 ppm; MS(ESI): m/z 398 (M+H)+; Anal. Calcd for C25H16ClNO2: C, 75.48; H, 4.02; N, 3.52%. Found: C, 75.38; H, 4.05; N, 3.56%.
3.1.8 4-(4-Bromophenyl)-2-(4-methoxyphenyl)-5H-indeno[1,2-b]pyridin-5-one (4h)
IR (KBr, cm−1): 3053, 2954, 2846, 1712, 1609, 1515, 1365, 1255, 1147, 820, 749; 1H NMR (500 MHz, CDCl3) δ: 3.67 (s, 3H, OCH3), 7.30–7.52 (m, 8H, ArH), 7.62 (s, 1H, Py–H), 7.83–7.99 (m, 4H, ArH) ppm; 13C NMR (125 MHz, CDCl3) δ: 55.0, 121.2, 123.3, 123.8, 123.9, 127.9, 128.4, 129.4, 129.6, 129.8, 131.7, 135.7, 135.9, 139.5, 142.2, 142.9, 147.3, 147.5, 162.0, 163.0, 191.1 ppm; MS(ESI): m/z 442.9 (M+H)+; Anal. Calcd for C25H16BrNO2: C, 67.88; H, 3.62; N, 3.17%. Found: C, 67.78; H, 3.58; N, 3.14%.
3.1.9 4-(4-Bromophenyl)-2-(4-fluorophenyl)-5H-indeno[1,2-b]pyridin-5-one (4i)
IR (KBr, cm−1): 3041, 2940, 2831, 1711, 1603, 1503, 1365, 1253, 1145, 819, 747; 1H NMR (500 MHz, CDCl3) δ: 7.14–7.44 (m, 8H, ArH), 7.72 (s, 1H, Py–H), 7.85–7.98 (m, 4H, ArH) ppm; 13C NMR (125 MHz, CDCl3) δ: 121.7, 123.6, 123.7, 123.8, 127.2, 128.3, 128.8, 129.2, 129.4, 129.7, 130.8, 131.4, 134.5, 135.3, 139.5, 141.3, 142.3, 142.6, 146.3, 147.5, 162.6, 163.8, 191.9 ppm; MS(ESI): m/z 430.9 (M+H)+; Anal. Calcd for C24H13BrFNO: C, 66.99; H, 3.02; N, 3.26%. Found: C, 66.84; H, 3.02; N, 3.20%.
3.1.10 4-(4-Hydroxyphenyl)-2-(4-fluorophenyl)-5H-indeno[1,2-b]pyridin-5-one (4j)
IR (KBr, cm−1): 3358, 3048, 2945, 2836, 1719, 1608, 1508, 1367, 1258, 1143, 820, 750; 1H NMR (500 MHz, CDCl3) δ: 7.19–7.39 (m, 8H, ArH), 7.64 (s, 1H, Py–H), 7.73–7.93 (m, 4H, ArH), 9.28 (s, 1H, OH) ppm; 13C NMR (125 MHz, CDCl3) δ: 121.9, 123.0, 123.5, 123.8, 127.2, 127.7, 128.7, 129.3, 129.6, 130.3, 130.9, 131.6, 135.4, 135.8, 139.9, 141.2, 142.3, 142.7, 146.4, 147.5, 162.4, 163.5, 191.4 ppm; MS(ESI): m/z 368 (M+H)+; Anal. Calcd for C24H14FNO2: C, 78.47; H, 3.81; N, 3.81%. Found: C, 78.40; H, 3.80; N, 3.84%.
3.1.11 4-(4-Fluorophenyl)-2-(4-chlorophenyl)-5H-indeno[1,2-b]pyridin-5-one (4k)
IR (KBr, cm−1): 3045, 2944, 2833, 1714, 1602, 1512, 1364, 1254, 1152, 825, 754; 1H NMR (500 MHz, CDCl3) δ: 7.15–7.40 (m, 8H, ArH), 7.73 (s, 1H, Py–H), 7.80–7.95 (m, 4H, ArH) ppm; 13C NMR (125 MHz, CDCl3) δ: 121.3, 123.4, 123.7, 123.9, 127.4, 128.0, 128.5, 129.2, 129.4, 129.7, 130.5, 131.8, 135.6, 135.7, 139.9, 141.6, 142.4, 142.8, 146.6, 147.6, 162.1, 163.7, 192.0 ppm; MS(ESI): m/z 386 (M + H)+; Anal. Calcd for C24H13ClFNO: C, 74.72; H, 3.37; N, 3.63%. Found: C, 74.66; H, 3.32; N, 3.58%.
3.1.12 4-(4-Methylphenyl)-2-(4-chlorophenyl)-5H-indeno[1,2-b]pyridin-5-one (4l)
IR (KBr, cm−1): 3054, 2955, 2845, 1718, 1606, 1504, 1366, 1260, 1156, 829, 758; 1H NMR (500 MHz, CDCl3) δ: 2.23 (s, 3H, CH3), 7.23–7.52 (m, 8H, ArH), 7.63 (s, 1H, Py–H), 7.70–7.88 (m, 4H, ArH) ppm; 13C NMR (125 MHz, CDCl3) δ: 17.6, 122.0, 123.4, 123.7, 124.2, 127.3, 128.4, 129.4, 129.6, 130.5, 131.4, 135.4, 135.6, 139.6, 141.9, 142.9, 146.9, 147.8, 162.6, 163.6, 191.6 ppm; MS(ESI): m/z 382 (M+H)+; Anal. Calcd for C25H16ClNO: C, 78.65; H, 4.19; N, 3.67%. Found: C, 78.55; H, 4.15; N, 3.65%.
3.1.13 4-(4-Methylphenyl)-2-(4-nitrophenyl)-5H-indeno[1,2-b]pyridin-5-one (4m)
IR (KBr, cm−1): 3047, 2942, 2835, 1715, 1607, 1507, 1365, 1255, 1155, 822, 758; 1H NMR (500 MHz, CDCl3) δ: 2.22 (s, 3H, CH3), 7.28–7.49 (m, 8H, ArH), 7.75 (s, 1H, Py–H), 7.79–7.97 (m, 4H, ArH) ppm; 13C NMR (125 MHz, CDCl3) δ: 18.3, 121.7, 123.5, 123.8, 123.9, 128.0, 128.6, 129.5, 129.7, 129.9, 131.3, 135.3, 135.6, 139.9, 141.5, 142.5, 146.4, 147.9, 162.3, 163.3, 191.3 ppm; MS(ESI): m/z 393 (M+H)+; Anal. Calcd for C25H16N2O3: C, 76.53; H, 4.08; N, 7.14%. Found: C, 76.50; H, 4.04; N, 7.10%.
3.1.14 4-(4-Methoxyphenyl)-2-(4-nitrophenyl)-5H-indeno[1,2-b]pyridin-5-one (4n)
IR (KBr, cm−1): 3050, 2949, 2839, 1719, 1602, 1514, 1370, 1262, 1146, 818, 745; 1H NMR (500 MHz, CDCl3) δ: 3.66 (s, 3H, OCH3), 7.30–7.47 (m, 8H, ArH), 7.65 (s, 1H, Py–H), 7.73–7.94 (m, 4H, ArH) ppm; 13C NMR (125 MHz, CDCl3) δ: 55.0, 121.0, 123.0, 123.5, 123.9, 127.8, 128.8, 129.3, 129.7, 129.9, 131.0, 135.0, 135.3, 139.9, 142.0, 142.8, 147.1, 148.5, 162.2, 163.4, 191.6 ppm; MS(ESI): m/z 409 (M + H)+; Anal. Calcd for C25H16N2O4: C, 73.53; H, 3.92; N, 6.86%. Found: C, 73.44; H, 3.90; N, 6.84%.
3.1.15 4-(4-Fluorophenyl)-2-(4-bromophenyl)-5H-indeno[1,2-b]pyridin-5-one (4o)
IR (KBr, cm−1): 3044, 2948, 2838, 1714, 1600, 1500, 1355, 1244, 1149, 825, 751; 1H NMR (500 MHz, CDCl3) δ: 7.35–7.51 (m, 8H, ArH), 7.69 (s, 1H, Py–H), 7.79–7.96 (m, 4H, ArH) ppm; 13C NMR (125 MHz, CDCl3) δ: 121.5, 123.5, 123.8, 124.4, 127.0, 127.6, 128.6, 129.6, 129.8, 130.0, 131.2, 131.7, 135.2, 135.7, 139.6, 141.4, 142.7, 143.0, 146.5, 148.4, 162.4, 163.6, 191.8 ppm; MS(ESI): m/z 430.9 (M+H)+; Anal. Calcd for C24H13BrFNO: C, 66.99; H, 3.02; N, 3.26%. Found: C, 66.94; H, 3.06; N, 3.29%.
3.1.16 4-(4-Chlorophenyl)-2-(4-bromophenyl)-5H-indeno[1,2-b]pyridin-5-one (4p)
IR (KBr, cm−1): 3046, 2947, 2836, 1710, 1606, 1502, 1356, 1248, 1145, 822, 751; 1H NMR (500 MHz, CDCl3) δ: 7.10–7.36 (m, 8H, ArH), 7.71 (s, 1H, Py–H), 7.83–7.96 (m, 4H, ArH) ppm; 13C NMR (125 MHz, CDCl3) δ: 121.8, 123.6, 123.7, 123.9, 128.2, 128.8, 129.0, 129.4, 129.7, 131.5, 135.0, 135.7, 139.7, 142.3, 143.0, 146.6, 147.6, 162.0, 163.0, 191.0 ppm; MS(ESI): m/z 447 (M+H)+; Anal. Calcd for C24H13BrClNO: C, 64.52; H, 2.91; N, 3.14%. Found: C, 64.57; H, 2.90; N, 3.12%.
3.1.17 4-(4-Nitrophenyl)-2-(4-hydroxyphenyl)-5H-indeno[1,2-b]pyridin-5-one (4q)
IR (KBr, cm−1): 3364, 3042, 2943, 2834, 1712, 1602, 1511, 1364, 1256, 1149, 822, 755; 1H NMR (500 MHz, CDCl3) δ: 7.19–7.40 (m, 8H, ArH), 7.67 (s, 1H, Py–H), 7.78–7.98 (m, 4H, ArH), 9.44 (s, 1H, OH) ppm; 13C NMR (125 MHz, CDCl3) δ: 121.2, 123.2, 123.6, 123.8, 127.9, 128.5, 129.0, 129.5, 129.7, 131.7, 135.6, 135.9, 139.4, 141.9, 142.4, 146.4, 148.4, 161.5, 162.5, 191.9 ppm; MS(ESI): m/z 395 (M+ H)+; Anal. Calcd for C24H14N2O4: C, 73.09; H, 3.55; N, 7.11%. Found: C, 73.00; H, 3.54; N, 7.08%.
3.1.18 4-(4-Methylphenyl)-2-(4-hydroxyphenyl)-5H-indeno[1,2-b]pyridin-5-one (4r)
IR (KBr, cm−1): 3359, 3043, 2941, 2830, 1707, 1604, 1509, 1363, 1253, 1148, 824, 759; 1H NMR (500 MHz, CDCl3) δ: 2.27 (s, 3H, CH3), 7.17–7.37 (m, 8H, ArH), 7.63 (s, 1H, Py–H), 7.77–7.97 (m, 4H, ArH), 9.36 (s, 1H, OH) ppm; 13C NMR (125 MHz, CDCl3) δ: 18.0, 121.1, 123.3, 123.6, 123.9, 128.3, 128.8, 129.3, 129.6, 129.8, 130.7, 134.5, 135.3, 139.5, 141.7, 142.9, 146.7, 147.7, 162.6, 163.3, 191.9 ppm; MS(ESI): m/z 364 (M+H)+; Anal. Calcd for C25H17NO2: C, 82.64; H, 4.68; N, 3.86%. Found: C, 82.56; H, 4.64; N, 3.84%.
4 Discussion
A novel and efficient procedure for the synthesis of 2,4-diaryl-5H-indeno[1,2-b]pyridin-5-one derivatives by the reaction of 1,3-diphenyl-2-propen-1-one (1a), 1,3-indandione (2), and ammonium acetate (3) in the presence of PFPAT as a novel organo-catalyst is described.
Our initial work started with screening of catalyst loading so as to identify optimal reaction conditions for the synthesis of 2,4-diaryl-5H-indeno[1,2-b]pyridin-5-one derivatives. First of all, a number of Lewis acid catalysts such as FeCl3, InCl3, BiCl3, La(OTf)3, Nd(OTf)3, Yb(OTf)3 and PFPAT have been screened using the model reaction in ethanol (Table 1). PFPAT was found to be the best catalyst under these conditions. We have studied the amount of PFPAT required for the reaction. It was found that when decreasing the amount of the catalyst from 10 to 5 mol%, the yield decreased from 92% to 81% (Table 1, entry 9). When increasing the amount of the catalyst from 10 to 15 mol%, there is no change in the yield (Table 1, entry 11). The use of 10 mol% of PFPAT maintains the yield at 92%, so this amount is sufficient to promote the reaction. In the presence of more than this amount of the catalyst, neither the yield nor the reaction time was improved (entries 10 and 11).
The reaction was performed in various solvents to identify the best solvent condition. A range of solvents like acetonitrile, 1,4-dioxane, cyclohexane, methanol and ethanol were examined (Table 2, entries 1–5). The reaction without any solvent at 60 °C was not very successful (Table 2, entry 6). Ethanol was found to be the best solvent under these conditions (Table 2, entry 5).
Encouraged by this successful three-component reaction, synthesis of diverse indeno[1,2-b]pyridine derivatives 4a–r was undertaken. The chalcones bearing electron-withdrawing and electron donating groups were found to be equally effective to produce 2,4-diaryl-5H-indeno[1,2-b]pyridin-5-ones 4a–r in very good yields (Table 3). As expected, satisfactory results were observed, and the results are summarized in Table 3. It is shown that in general a wide range of chalcones could react with 1,3-indandione and ammonium acetate smoothly to give 4a–r in good to excellent yields (Table 3, entries 1–18). It is also notable that the electronic property of the aromatic ring of chalcones has some effects on the rate of the condensation process. The results summarized in Table 3 reveals that the reaction gave higher yields of 2,4-diaryl-5H-indeno[1,2-b]pyridin-5-ones and also shorter reaction time was needed when the aromatic aldehyde part of chalcones bear an electron-withdrawing substituents probably because the Michael addition is easier (Table 3, entries 1, 3–5, 7–11, 15–17). On the other hand, when the aromatic aldehyde part of chalcones bearing the electron-donating groups can afford the corresponding products with almost equally satisfactory yields a slightly longer reaction period was necessary to complete the reaction (Table 3, entries 2, 6, 12–14, 18).
To explain the formation of 2,4-diaryl-5H-indeno[1,2-b]pyridin-5-ones via the one-pot multi-component reaction, we have proposed a plausible reaction mechanism, which is illustrated in Scheme 2. According to the reported literatures (Shafiee et al., 2011; Shinde et al., 2012), two courses are possible:
-
First the equilibrium of diketone with its enolic form was established under acidic condition (intermediate a). With the same manner, the catalytic coupling of amine with α,β-unsaturated ketone by Brønsted acid was performed to form intermediate (b) which is equilibrated with its enamine form. In continuation the formation of intermediate (d) resulting from the condensation of intermediate (a) with intermediate (c) was established. Finally the intramolecular coupling of intermediate (d) followed by air oxidation resulting in the synthesis of 2,4-diaryl-5H-indeno[1,2-b]pyridin-5-ones (Scheme 2).
-
First the catalytic condensation of (a) and (b) was performed to form intermediate (e). In continuation the intermediate (f) was formed from the catalytic coupling of ammonium acetate with (e). Intermediate (f) is equilibrated with its enamine form (intermediate d). The intramolecular coupling followed by air oxidation resulting in the targeted molecules (Scheme 2).
![Mechanism for the formation of 2,4-diaryl-5H-indeno[1,2-b]pyridin-5-one derivatives.](/content/185/2014/26/3/img/10.1016_j.jksus.2013.08.007-fig3.png)
- Mechanism for the formation of 2,4-diaryl-5H-indeno[1,2-b]pyridin-5-one derivatives.
The possibility of recycling the catalyst was examined using the model reaction under the optimized conditions. Upon completion of the reaction, the mixture was filtered and then washed with warm ethanol. The product was recrystallized from hot ethanol. The recovered catalyst was dried and reused for subsequent runs. The recycled catalyst could be reused four times without any additional treatment or appreciable reduction in the catalytic activity (Fig. 2). The yield of product 4a obtained from the recycled catalyst for the four runs at 1.5 h are 92%, 90%, 87% and 86% respectively.![Recyclability of PFPAT for the synthesis of 4-(4-fluorophenyl)-2-(4-methylphenyl)-5H-indeno[1,2-b]pyridin-5-one.](/content/185/2014/26/3/img/10.1016_j.jksus.2013.08.007-fig4.png)
Recyclability of PFPAT for the synthesis of 4-(4-fluorophenyl)-2-(4-methylphenyl)-5H-indeno[1,2-b]pyridin-5-one.
5 Conclusion
In conclusion, we successfully developed a facile and efficient method for preparing a variety of 2,4-diaryl-5H-indeno[1,2-b]pyridin-5-ones from the reactions of different chalcones, 1,3-indandione, and ammonium acetate in the presence of a catalytic amount of PFPAT at 60 °C in ethanol. The catalytic activity of PFPAT is remarkable and the use of the environmentally benign, non-toxic PFPAT as catalyst in the synthesis of 2,4-diaryl-5H-indeno[1,2-b]pyridin-5-ones in good yield is also significant. The present method has many obvious advantages compared to those reported in the previous literatures, including the cost-effectiveness, eco-friendly nature, simplicity of the methodology and recycling of the catalyst.
References
- Design, synthesis and antimicrobial evaluation of novel 1-benzyl 2-butyl-4-chloroimidazole embodied 4-azafluorenones via molecular hybridization approach. Bioorg. Med. Chem. Lett.. 2012;22:7475-7480.
- [Google Scholar]
- Aswin, K., Mansoor, S.S., Logaiya, K., Sudhan, S.P.N., 2014. Triphenylphosphine: an efficient catalyst for the synthesis of 4,6-diphenyl-3,4-dihydropyrimidine-2(1H)-thione under thermal conditions. J. King Saud University – Sci. 26, 141–148.
- Synthesis of azafluorenone via oxidative intramolecular Heck cyclization. Tetrahedron Lett.. 2013;54:63-65.
- [Google Scholar]
- Pentafluorophenylammonium triflate (PFPAT): an efficient, practical, and cost-effective catalyst for esterification, thio esterification, trans esterification and macrolactoneformation. Green Chem.. 2006;8:1022-1027.
- [Google Scholar]
- Structure–activity relations of azafluorenone and azaanthraquinone as antimicrobial compounds. Bioorg. Med. Chem. Lett.. 2005;15:1079-1082.
- [Google Scholar]
- A novel three-component[5 + 1] heterocyclization leading to 2-azafluorenone synthesis and its ployfunctionalisations. Org. Biomol. Chem.. 2013;11:2417-2420.
- [Google Scholar]
- An efficient one-pot multi component synthesis of polyhydroquinoline derivatives through Hantzsch reaction catalysed by Gadolinium triflate. Arab. J. Chem. 2012
- [CrossRef] [Google Scholar]
- Aqueous media preparation of 2-amino-4,6-diphenylnicotinonitrile using cellulose sulfuric acid. Res. Chem. Intermed. 2012
- [CrossRef] [Google Scholar]
- Mansoor, S.S., Aswin, K., Logaiya, K., Sudhan, S.P.N., 2013. Melamine Trisulfonic acid as an efficient catalyst for the synthesis of 2,6-dimethyl-4-substituted-1,4-dihydropyridine-3,5-diethyl/dimethylcarboxylate derivatives via Hantzsch reaction in solvent free condition. J. King Saud University – Sci., 25, 191–199.
- Efficient two-step access to azafluorenones and related compounds. Tetrahedron Lett.. 2013;54:3154-3157.
- [Google Scholar]
- Antimalarial activity of azafluorenone alkaloids from the Australian tree Mitrephora diversifolia. J. Nat. Prod.. 2009;72:1538-1540.
- [Google Scholar]
- l-Proline-catalyzed one-pot expeditious synthesis of highly substituted pyridines at room temperature. Tetrahedron Lett.. 2010;51:1797-1802.
- [Google Scholar]
- Using the Pummerer cyclization–deprotonation–cycloaddition cascade of imidosulfoxides for alkaloid synthesis. J. Org. Chem.. 2000;65:2368-2378.
- [Google Scholar]
- Bioactive azafluorenone alkaloids from Polyalthia debilis (Pierre) Finet & Gagnep. Molecules. 2009;14:4414-4424.
- [Google Scholar]
- A new azafluorenone from the roots of Polyalthia cerasoides and its biological activity. Nat. Prod. Commun.. 2010;12:1931-1934.
- [Google Scholar]
- Solvent-free preparation of 2,4,6-triaryl pyridines using silver(i) nitrate adsorbed on silica gel nanoparticles (AgNO3–Nano SiO2) as an efficient catalyst. Lett. Org. Chem.. 2011;8:717-721.
- [Google Scholar]
- Bismuth triflate catalyzed solvent-free synthesis of 2,4,6-triaryl pyridines and an unexpected selective acetalization of tetrazolo[1,5-a]-quinoline-4-carbaldehydes. Tetrahedron Lett.. 2012;53(12):1523-1527.
- [Google Scholar]
- Ceric ammonium nitrate (CAN) catalyzed one-pot synthesis of fully substituted new indeno[1,2-b]pyridines at room temperature by a multi-component reaction. ARKIVOC. (x):287-298.
- [Google Scholar]
- An efficient and expeditious microwave-assisted synthesis of 4-azafluorenones via a multi-component reaction. Tetrahedron Lett.. 2007;48:1369-1374.
- [Google Scholar]
- Effects of 2-azafluorenones on phosphatidyl–inositol specific phospholipase C activation in C6 Glioma cells. Chin. J. Physiol.. 2012;55:101-107.
- [Google Scholar]
- Cyathocaline, an azafluorenone alkaloid from Cyathocalyx zeylanica. J. Nat. Prod.. 1995;58:459-462.
- [Google Scholar]







