Translate this page into:
Partial characterization of phenoloxidase enzyme in the hemocytes of Helicoverpa armigera Hübner (Lepidoptera: Noctuidae)
*Corresponding author. Tel.: +98 5612254046 m-saadati@birjand.ac.ir (Mohammad Saadati)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 28 August 2013
Peer review under responsibility of King Saud University.

Abstract
Phenoloxidase (PO) activity plays a key role in the innate immune responses of insects, which catalyzes the biosynthesis of quinones and other reactive intermediates to eliminate invading pathogens and parasites. This study was conducted to characterize the biochemical properties of the PO enzyme from the hemocytes of the cotton bollworm Helicoverpa armigera Hübner. The maximum activity of PO occurred at pH 6 and at 30 °C and it was stable for 12–24 h. The Michaelis–Menten constant (Km) and the maximal velocity (Vmax) were determined as 1.86 mM and 0.35 U/mg protein, respectively. This is the first report of PO characterization in H. armigera from Iran that provided biochemical optimized conditions for its activity, and with these ongoing studies, our aim will be to develop new strategies for cotton bollworm control using disruptors of the immune system.
Keywords
Phenoloxidase
Helicoverpa armigera
Hemocyte
Innate immune
1 Introduction
Cellular immunity covers nodule formation, encapsulation and phagocytosis process, and humeral immunity contains enzymatic process like the proPO cascade (Ashida and Brey, 1998; Cerenius and Soderhall, 2004). Phenoloxidase (PO) (EC 1.14.18.1) is an important enzyme in the immune system that is effective in the melanin pathway (Soderhall and Cerenius, 1998). Microorganisms, animals and plants use this enzyme in the innate defense reactions (Sánchez-Ferrer et al., 1995; Chase et al., 2000). Active phenoloxidase carries out hydroxylation of monophenols to produce diphenols and then oxidizes them to quinones (Sugumaran, 2002; Nappi and Christensen, 2005). Enzymatic reactions like PO cascade and some of the non-enzymatic reactions lead to melanin formation from quinone at the final step of nodulation and encapsulation. Also, phenoloxidase plays a key role in melanin production during cuticle sclerotization at external wound sites and during defense responses, i.e., nodulation (Mason, 1955; Ratcliffe et al., 1984; Cerenius et al., 2008).
Helicoverpa armigera (Lepidoptera: Noctuidae) is a serious pest with a broad distributionworldwide which causes damage to many agricultural crops (Zalucki et al., 1986; Fitt, 1989). Some of plant hosts for this pest contain cotton, tomato, pigeon pea, chickpea, groundnut, sorghum, pearl millet etc., (Raheja, 1996). Nowadays, application of conventional insecticides is recommended as commonplace in Iran. Overusing of these compounds causes concern about creature environmental pollution and destruction of natural enemies in agronomy ecosystems (Saadati et al., 2012). Hence, alternative methods such as host plant resistance, biological control, development of transgenic plants and using of entomopathogenic agents are needed in the struggle to manage H. armigera (Pauchet et al., 2008). The use of the entomopathogenic fungus such as Metarhizium anisopliae, Beauveria bassiana and Paecilomyces fumosoroseus as potential biocontrol factors were recommended against H. armigera larvae and pupae as spray and soil treatment, respectively (Nguyen et al., 2007). The characterization of the PO enzyme as a main part of immune system improved the efficiency of B. bassiana treatments against sunn pest adults (Zibaee and Bandani, 2010).
The study of biochemical details of the PO enzyme would be necessary to discuss the molecular and physiological mechanisms of the immune system (Beckage, 2008). The current study demonstrates the partial characterization of the PO enzyme in H. armigera hemocytes for the first time in Iran. This investigation was aimed to elucidate optimized conditions of PO activity as a part of immunity reactions in cotton bollworm. Also, we will attempt to understand the biochemical reactions of PO enzyme after wounding in hemolymph collection from H. armigera.
2 Materials and methods
2.1 Insects
Larvae of H. armigera Hübner (Lepidoptera: Noctuidae) was collected from the Moghan cotton farms and reared on artificial diet in the laboratory at 27 ± 2 °C Temp., Light 16 h: Dark 8 h (Photoperiod) and 50 ± 5% relative humidity.
2.2 Collection of hemolymph
Last instar larvae were selected as enzyme sources of hemolymph. To collect hemolymph, prolegs were removed and hemolymph was allowed to drip into microtubes. Approximately 40 μl of hemolymph was obtained from each individual. It was mixed with anticoagulant solution in the ratio 4:5, immediately (400 μl hemolymph: 500 μl anticoagulant solution) (0.01 M EDTA, 0.1 M glucose, 0.062 M NaCl, and 0.026 M citric acid, pH 4.6) (Azambuja et al., 1991).
2.3 PO preparation
The diluted hemolymph was centrifuged at 12,000g for 5 min, and then the supernatant was removed and the pellet washed using phosphate buffer (K2HPO4 65 mM,KH2PO4 2.6 mM, NaCl 400 mM and NaN3 3 mM; pH = 6.5) (two times) (Leonard et al., 1985). The pellet was homogenized after adding 500 μl of cold phosphate buffer, and then centrifuged at 12,000g for 15 min. The hemocyte lysate supernatant (HLS) was transferred into new microcentrifuge tubes. Samples were preincubated with phosphate buffer at 30 °C for 30 min., then 50 μl of 10 mM l-dihydroxyphenylalanine (l-DOPA) as substrate was added and incubated for 5 min at 30 °C. PO activity was measured using a spectrophotometer (S 2100 Diode Array spectrophotometer) at 490 nm wavelength. One unit was described as 0.01 absorbance increase at 490 nm/min (Zibaee et al., 2011). Three biological replications were used to determine the average of PO activity in all experiments.
2.4 Kinetic parameters (Vmax and Km) of PO
PO kinetic parameters were measured by mixing different concentrations of l-DOPA (3, 3.5, 4, 5, 6, 7, 8, 9 and 10 mM) with 50 μl of enzyme solution and then the absorbance was read at 490 nm. The Michaelis constant (Km) and the maximal velocity (Vmax) were calculated by Sigma plot software version 12. The data of Km and Vmax were fixed as the means ± SE of three replicates (n = 3) for each concentration.
2.5 Assay of the optimal pH and stability of PO enzyme
Measurement of PO activity at different pHs was performed using 10 mM l-dihydroxyphenylalanine (l-DOPA) as substrate. The optimal pH was determined in the pH range of 4–10 at 30 °C using 25 mM Tris–HCl buffer. On the other hand, the stability of the PO enzyme at different pHs was determined by the keeping experimental solution in an incubator for 6, 12, 24, 48 and 72 h at optimized pHs (pH 6, 7 and 8; see results) (Liu et al., 2006). Three biological replications were used for every pH value.
2.6 Assay of the optimal temperature and stability of PO enzyme
Measurement of PO activity at different temperatures was performed using 10 mM solution of l-dihydroxyphenylalanine (l-DOPA) as substrate. The enzyme solution was preincubated at 25, 30, 35, 40, 45, 50, 55 and 60 °C for 30 min and then PO activity was determined for them. Finally, the highest activity of PO is considered as optimum temperature. Stability of the enzyme at optimized temperatures (25, 30 and 35 °C; see results) was determined by keeping the experimental solution in an incubator for 6, 12, 24, 48 and 72 h at the mentioned temperatures (Liu et al., 2006). Three biological replications were used for every temperature value.
2.7 Protein content determination
The total protein of PO preparation was measured according to the method of Bradford (1976), using bovine serum albumin (Bio-Rad) (Sigma) as the standard protein.
2.8 Statistical analysis
Data were compared by one-way analysis of variance (ANOVA) followed by Duncan’s studentized test at p < 0.05 (SAS, 1997). Differentially activities were shown (as different letters) in figures.
3 Results
3.1 Determination of the kinetic parameters of PO enzyme
The kinetic parameters of the PO enzyme on l-DOPA oxidation were determined as explained in section 2.4. Under the mentioned conditions, PO oxidizes l-DOPA as followed in the Lineweaver–Burk kinetics. These parameters contain Vmax (Maximal velocity) and Km (Michaelis constant) which were calculated as 0.35 μmol/min/mg protein and 1.86 mM, respectively (Fig. 1).![Double reciprocal plot to show the kinetic parameters of the PO from the hemocytes of H. armigera l-DOPA (10 mM) was used as substrate (1/Vmax = intercept on the 1/V0 ordinate, −1/Km = intercept on the negative side of the 1/[S] abscissa).](/content/185/2014/26/4/img/10.1016_j.jksus.2013.08.005-fig1.png)
Double reciprocal plot to show the kinetic parameters of the PO from the hemocytes of H. armigera l-DOPA (10 mM) was used as substrate (1/Vmax = intercept on the 1/V0 ordinate, −1/Km = intercept on the negative side of the 1/[S] abscissa).
3.2 Effect of pH on PO activity
The effect of pH on PO activity in H. armigera hemocytes was determined using l-DOPA (10 mM) as substrate at different pHs at 30 °C. Results showed that the activity of PO at pH 6, 7 and 8 was more than that in comparison to the other pHs. However, the highest and lowest of PO activities were observed at pH 6 (0.314 μmol/min/mg protein) and 4 (0.132 μmol/min/mg protein), respectively (Fig. 2). After that, the stability of PO was determined at pHs of 6, 7 and 8 for 72 h. Results showed that the enzyme activity was stable for 24 h and then differential reduction occurred at all pHs (Fig. 2).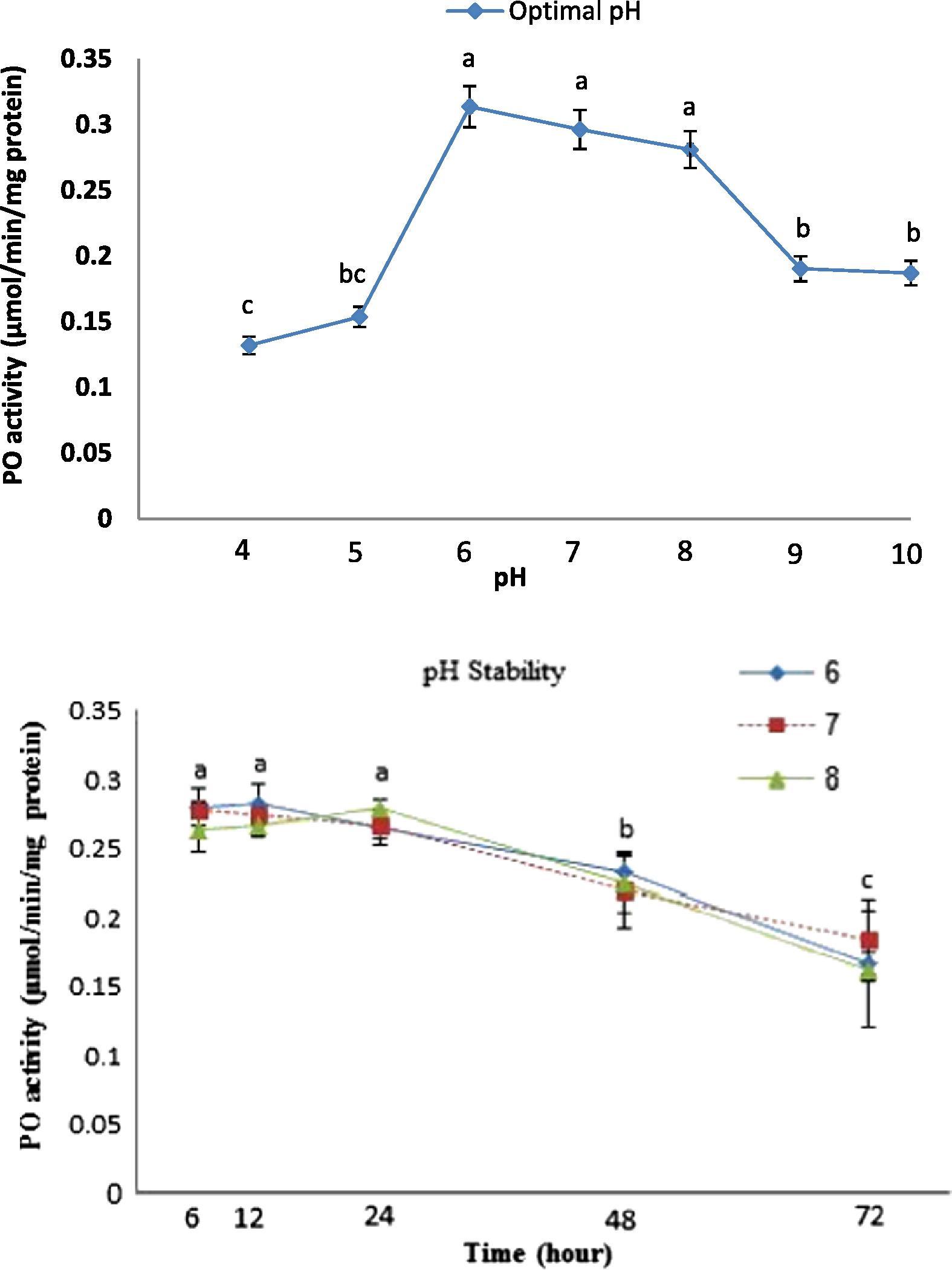
Effect of pH on the activity and stability of the hemocyte-derived phenoloxidase in H. armigera. Different letters show significant differences among means (Duncan’s test, p < 0.05).
3.3 Effect of temperature on PO activity
The effect of temperature on PO activity in H. armigera hemocytes was studied at various temperatures from 25 to 60 °C (Fig. 3). However, the highest level of enzyme activity differentially occurred from 25 to 35 °C which was more than that in the other temperatures. The highest and lowest of PO activities were observed at the 30 (0.308 μmol/min/mg protein) and 55 °C (0.202 μmol/min/mg protein), respectively. Stability of the enzyme was studied at temperatures ranging from 25–35 °C for 72 h. Results showed that the enzyme activity was stable for 12 h and then a sharp decrease occurred at all temperatures (Fig. 3).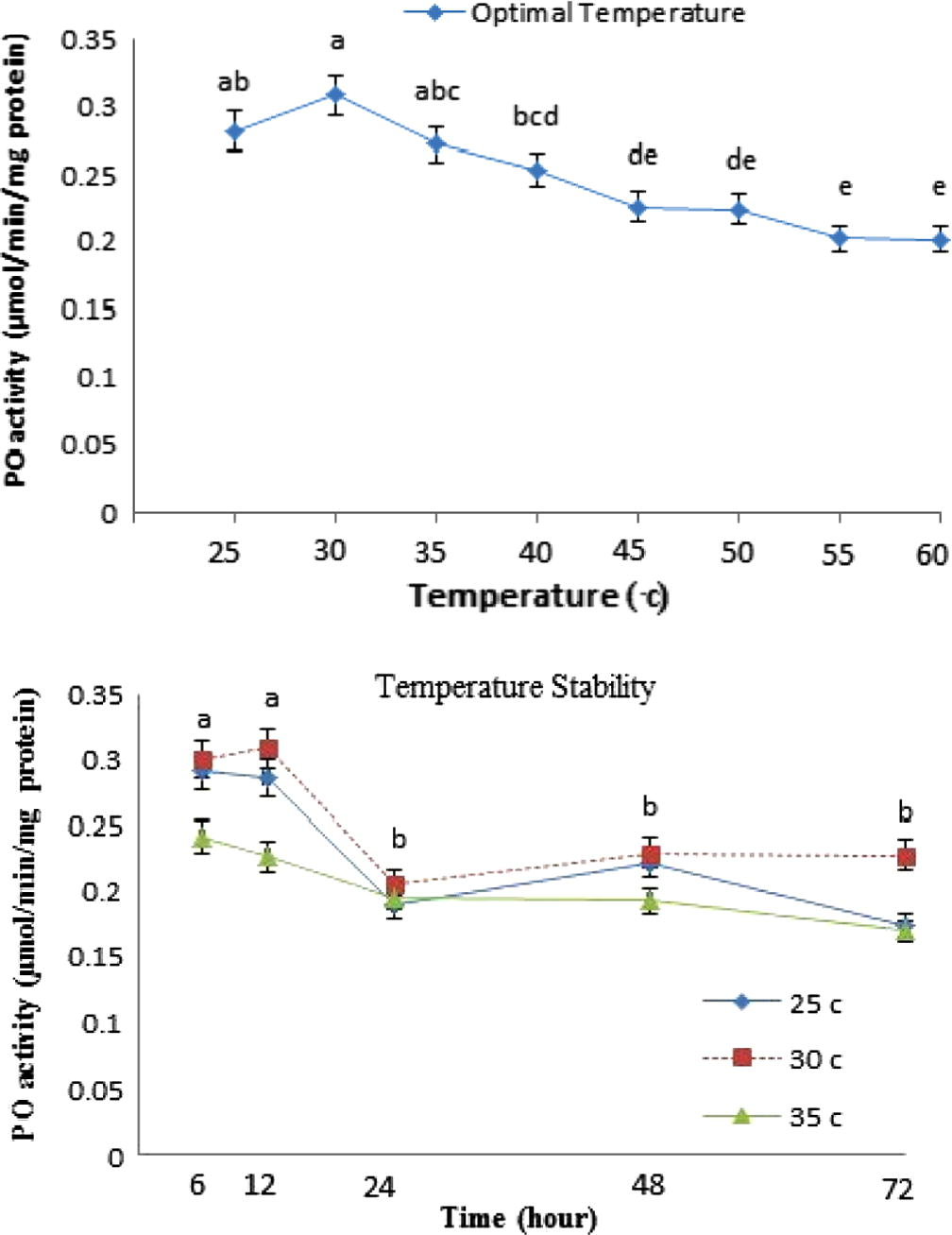
Effect of temperature (°C) on the activity and stability of the hemocyte lysate extracted phenoloxidase in H. armigera. The optimum temperature was determined by assaying enzyme activity at various temperature values using phosphate buffer pH = 6.5. Different letters show significant differences among means (Duncan’s test, p □ 0.05).
4 Discussion
In insects, phenoloxidases have important roles in normal developmental and physiological processes, such as cuticular tanning, sclerotization, wound healing, encapsulation and nodule formation (Lokstan and Li, 1988). In this study the kinetic parameters of the enzyme were determined by analyzing Lineweaver–Burk plots in which Vmax (Maximal velocity) and Km (Michaelis constant) were calculated as 0.35 U/mg proteins and 1.86 mM, respectively. These data showed that the Km value of H. armigera was higher than that of other insects, such as the Apis mellifera L. (Hymenoptera: Apidae) with 0.17 mM (Zufelato et al., 2004), Drosophila melanogaster L. (Diptera: Drosophilidae) with 1.30 mM (Wang et al., 2004), Pieris rapae L. (Lepidoptera: Pieridae) larvae with 0.8 mM (Xue et al., 2006) and Ostrinia furnacalis G. (Lepidoptera: Pyralidae) larvae with 0.92 mM (Feng et al., 2008), on the other hand it was lower than that in Heliothis virescens F. (Lepidoptera: Noctuidae) with 2.25 mM (Lockey and Ourth, 1992), Musca domestica L. (Diptera: Muscidae) pupae with 3.93 mM (Wang et al., 2004) and Eurygaster integriceps P. (Hemiptera: Scutelleridae) with 10 mM (Zibaee et al., 2011). The differences in substrate-protein contact points or differences in the size of the substrate-binding pocket can affect substrate catalysis by PO in different insects (Feng et al., 2008). Our results suggest that the low Km of PO in H. armigera may be related to the higher affinity of the enzyme to l-DOPA in comparison to the other insects.
Our data showed that the optimal pH of the PO activity in cotton bollworm was 6, although there was no significant difference among pHs 6, 7 and 8. These results were similar to the optimal pH of PO in the Bombyx mori L. (Lepidoptera: Bombycidae) (pH 6, Ashida, 1971), P. rapae (L.) (pH 7, Lepidoptera: Pieridae) (Xue et al., 2006) and sunn pest, E. integriceps P. (Hemiptera: Scutelleridae) (pH 6, Zibaee et al., 2011). Also, it was different from Sarcophaga bullata P. (Diptera: Sarcophagidae) (pH 4, Barrett, 1986) and H. virescens F. (Lepidoptera: Noctuidae) (pH 9, Lockey and Ourth, 1992). The different species and survival conditions (for example, temperatures and invading pathogens) have been considered as main factors in changing of optimal pH and temperature (Liu et al., 2006).
Our results showed that the highest level of PO activity in H. armigera occurred at 30 °C, although, there are no significant differences with 25 and 35 °C. Our data about optimal temperature of PO activity were similar to those in other insects such as Locusta migratoria L. (Orthoptera: Acrididae) (30–35 °C, Cherqui et al., 1998) and E. integriceps P. (30–35 °C, Zibaee et al., 2011). Also, it was different with H. virescens F. (45 °C, Lockey and Ourth, 1992), Hyphantria cunea (D.) (Lepidoptera: Arctiidae) (35–40 °C, Ajamhassani et al., 2012) and S. bullata P. (40 °C, Wang et al., 2004). Zibaee et al. (2009) stated that the main effective agent on PO activity was the temperature in which enzyme activity increased according to increasing temperature, but in high temperatures the enzyme activity and the rate of the reaction decreased sharply, simultaneously. It is well known that the biochemical properties of the phenoloxidase enzyme were affected by different pHs and temperatures; different types of inhibitors and activators (Thomas et al., 1989).
One of the main goals in the study of enzyme characterization is finding new strategies for pest control using various inhibitors to disrupt enzymatic reactions in normal conditions (Saadati et al., 2008). In order to be of practical use for the production of enzyme inhibitors, Enzyme targeting and characterizing should have been appropriately performed (Saadati et al., 2007). These results could be appropriate to describe comparative biochemistry about PO properties; also it can improve our knowledge of the immune system of cotton bollworm and provide new opportunities for non chemical control of this important pest in future.
Acknowledgments
We thank Mr. Kahnamui and Mrs. Majidiani for their assistance in the technical process and insect collection.
References
- Purification and characterization of phenoloxidase from the hemolymph of Hyphantria cunea (Lepidoptera: Arctiidae) ISJ. 2012;9:64-71.
- [Google Scholar]
- Purification and characterization of pre-phenoloxidase from hemolymph of the silkworm Bombyx mori. Arch. Biochem. Biophys.. 1971;144:749-762.
- [Google Scholar]
- Recent advances in research on the insect phenoloxidase cascade. In: Brey P.T., Hultmark D., eds. Molecular Mechanisms of Immune Responses in Insects. London: Chapman and Hall; 1998. p. :135-172.
- [Google Scholar]
- Aspects of classification of Hemiptera hemocytes from six triatomine species. Mem. Inst. Oswaldo Cruz. 1991;86:1-10.
- [Google Scholar]
- Characterization of phenoloxidase from larval cuticle of Sarcophaga bullata and a comparison with cuticular enzymes from other species. Can. J. Zool.. 1986;65:1158-1166.
- [Google Scholar]
- Insect Immunology. San Diego: Academic press; 2008. p. 348
- A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein–dye binding. Anal. Biochem.. 1976;72:248-254.
- [Google Scholar]
- The prophenoloxidase-activating system in invertebrates. Immunol. Rev.. 2004;198:116-126.
- [Google Scholar]
- The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol.. 2008;29:263-271.
- [Google Scholar]
- Purification, characterization and molecular cloning of prophenoloxidase from Sarcophaga bullata. Insect Biochem. Mol. Biol.. 2000;30:953-967.
- [Google Scholar]
- Cooperation of dopachrome conversion factor with phenoloxidase in the eumelanin pathway in haemolymph of Locusta migratoria (Insecta) Insect Biochem. Mol. Biol.. 1998;28:839-848.
- [Google Scholar]
- Purification and characterization of hemolymph prophenoloxidase from Ostrinia furnacalis (Lepidoptera: Pyralidae) larvae. Comp. Biochem. Physiol.. 2008;151(B):139-146.
- [Google Scholar]
- The ecology of Heliothis species in relation to agroecosystems. Annu. Rev. Entomol.. 1989;34:17-52.
- [Google Scholar]
- Studies on prophenoloxidase and protease activity of Blaberua craniifer hemocytes. Insect Biochem.. 1985;15:803-810.
- [Google Scholar]
- Purification and characterization of phenoloxidase from crab Charybdis japonica. Fish. Shell fish Immunol.. 2006;20:47-57.
- [Google Scholar]
- Isolation and characterization of hemolymph phenoloxidase from Heliothis virescens larvae. Comp. Biochem. Physiol.. 1992;102(B):891-896.
- [Google Scholar]
- Lokstan, M. (Eds.), Li, S.W. (Trans.). 1988. Insect Biochemistry. Science Press, Beijing, pp. 151–153.
- Melanogenesis and associated cytotoxic reactions: applications to insect innate immunity. Insect Biochem. Mol. Biol.. 2005;35:443-459.
- [Google Scholar]
- Laboratory investigationson the potential of entomopathogenic for biocontrol of Helicoverpa armigera (Lepidoptera: Noctuidae) larvae and pupae. Biocontrol Sci. Techn.. 2007;17(8):853-864.
- [Google Scholar]
- Mapping the larval midgut lumen proteome of Helicoverpa armigera, a generalist herbivourous insect. J. Proteome Res.. 2008;7:1629-1639.
- [Google Scholar]
- IPM Research and Development in India: Progress and Priorities. In: Lal O.P., ed. Recent Advances in Indian Entomology. New Delhi: APC Publications Pvt. Ltd.; 1996. p. :115-126.
- [Google Scholar]
- Prophenoloxidase activation: nonself recognition and cell cooperation in insect immunity. Science. 1984;226:557-559.
- [Google Scholar]
- Effects of some mineral compounds on the salivary α-amylase activity of the sunn pest, Eurygaster integriceps (Put.) Turk. J. Entomol.. 2007;31:163-173.
- [Google Scholar]
- Some properties of alpha-amylase in the salivary gland of Eurygaster integriceps (Put) Mun. Ent. Zool.. 2008;3:733-743.
- [Google Scholar]
- Proteome analysis of gut and salivary gland proteins of fifth-instar nymph and adults of sunn pest, Eurygaster integriceps. Arch. Insect Biochem. Physiol.. 2012;81:105-119.
- [Google Scholar]
- SAS/STAT User’s Guide for Personal Computers. Cary, Nc: SAS Institute; 1997.
- Role of the prophenoloxidase-activating system in invertebrate immunity. Curr. Opin. Immunol.. 1998;10:8-23.
- [Google Scholar]
- Comparative biochemistry of eumelanogenesis and the protective roles of phenoloxidase and melanin in insects. Pigment Cell Res.. 2002;15:2-9.
- [Google Scholar]
- A trypsin-solubilized laccase from pharate pupal integument of the tobacco hornworm Manduca sexta. Insect. Biochem.. 1989;19:611-622.
- [Google Scholar]
- A novel lectin with a fibrinogen-like domain and its potential involvement in the innate immune response of Armigeres subalbatus against bacteria. Insect Mol. Biol.. 2004;13:273-282.
- [Google Scholar]
- Enzymatic properties of phenoloxidase from Pieris rapae (Lepidoptera) larvae. Insect Sci.. 2006;13:251-256.
- [Google Scholar]
- The biology and ecology of Heliothis armigera (Hübner) and H. punctigera Wallengren (Lepidoptera:Noctuidae) in Australia: what do we know? Austr. J. Zool.. 1986;34:779-814.
- [Google Scholar]
- Effects of Artemisia annua L. (Asteracea) on the digestive enzymatic profiles and the cellular immune reactions of the Sunn pest, Eurygaster integriceps (Heteroptera: Scutelleridae), against Beauveria bassiana. Bull. Entomol. Res.. 2010;100:185-196.
- [Google Scholar]
- Temperature and Ca2+ ion as modulators in cellular immunity of the Sunn pest, Eurygaster integriceps Puton (Heteroptera: Scutelleridae) Entomol. Res.. 2009;39:364-371.
- [Google Scholar]
- Purification and characterization of phenoloxidase from the hemocytes of Eurygaster integriceps (Hemiptera: Scutelleridae) Comp. Biochem. Physiol.. 2011;158(B):117-123.
- [Google Scholar]
- Phenoloxidase activity in Apis mellifera honey bee pupae, and ecdysteroid-dependent expression of the prophenoloxidase mRNA. Insect Biochem. Mol. Biol.. 2004;34:1257-1268.
- [Google Scholar]







