Translate this page into:
Parasites on the wing; two new records of marine chewing lice (Phthiraptera) on Brown booby (Suliformes: Sulidae) from Egypt with notes on genus Pectinopygus/boobies phylogeny
⁎Corresponding author. mgnasser@sci.asu.edu.eg (Mohamed Nasser)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Little studies had been done on chewing lice of marine birds in the Middle East. Through this work, parasitic chewing lice of Brown Booby Sula leucogaster (Boddaert) in the Red Sea were recorded for the first time from Egypt.
Methods
One brown booby was examined for chewing lice in Giftun Island, Hurghada. A total of 27 specimens of lice were collected from this bird and phylogenetic analysis of species of genus Pectinopygus associated boobies has been done based on NCBI data.
Results and conclusions
Two species of marine chewing lice were identified: Eidmanniella albescens (Piaget, 1880) and Pectinopygus sulae (Rudow, 1869) ; diagnostic remarks for the two identified species, measurements, and material examined were provided through the manuscript. The final phylogenetic tree indicates the monophyletic origin of Pectinopygus spp. associated with boobies and their paraphyletic relation to other species of Pectinopygus that associated with other Suliformes. Also, independence of boobies’ speciation from chewing louse of genus Pectinopygus speciation. The present work forms a small step in a long way of studying marine chewing lice of Egypt and a better understanding of marine birds/chewing lice interaction.
Keywords
Egypt
Red Sea
New records
Chewing lice
Brown booby
1 Introduction
Many years of neglection were faced studying chewing lice fauna of the Middle East (Adly et al., 2019, 2020, Nasser et al., 2015a, 2020) and consequently, there was a lack of information concerning ectoparasites of marine bird through the area (Negm et al., 2013; Hafez and Madbouly, 1968). The chewing lice of marine birds are forming a very interesting group of insects as they form the largest hexapod fauna on the marine ecosystem (Nasser, 2015). Also, they are considered one of the most significant groups of lice which reflects a clear image about the ecology and evolution of their host birds (Nasser et al., 2019). Especially for true marine birds like Boobies which spend most of the year on the open water (Welty and Bapista, 1990).
Boobies are large sea birds with heavily built bodies, pointed long wings, and webbed large feet. They are perfect divers whose usually get their food of fishes and squids from deep water that reaches about 15 m. They are breeding in large colonies where nests are found on the ground with few nesting materials, female usually lays 1–3 eggs. The species of boobies are distributed through tropical and subtropical open seas around the globe (Scott, 2014). There are six species of boobies, most of them are non-migrant species while masked boobies are known by their long migration (Frances, 2009). The Brown Booby is a widely distributed sea bird of order Suliformes, the order which divided from Pelecaniformes recently by several phylogenetic analyses (Boles and Christidis, 2008; Gill and Donsker, 2014). These birds are clever diver as most of them feed mainly on squids (Alderton, 2008) and they had been very known in Pantropical open oceans and warm seas and the Red Sea form a part of its range (Anonymous, 2012).
There are two genera of chewing lice that have been listed as ectoparasites of Boobies through many parts of the world (Table 1): the genus Eidmanniella of suborder Amblycera and the genus Pectinopygus of suborder Ischnocera (Price, et al., 2003). The data about the two genera are rarely known from the Middle East. Recently, four species of chewing lice were recorded from the Gulf area infesting Suliformes: Eidmanniella albescens (Piaget, 1880), Eidmanniella nancyae Ryan & Price 1969, Pectinopygus socotranus Timmermann, 1964 and Pectinopygus sulae (Rudow, 1869) (Nasser et al., 2015b). Although the ornithological fauna of Egypt included only two recorded species of Suliformes (Brown booby, Sula leucogaster, and Great cormorant, Phalacrocorax carbo), there is no data available for their associated chewing lice.
Booby common name
Booby scientific name
Distribution
Chewing lice recorded
Blue-footed booby
Sula nebouxii
Milne-Edwards, 1882East Pacific especially south coast of North America and North coast of south America
E. albescens
P. minor
Brown booby
Sula leucogaster
(Boddaert, 1783)All tropical open seas
E. albescens
P. annulatus
P. garbei
P. sulae
Masked booby
Sula dactylatra
Lesson, 1831Tropical pat of pacific and Atlantic ocean and some regions of Indian oceans
E. albescens
P. annulatus
P. sulae
Nazca booby
Sula granti
Rothschild, 1902Eastern pacific and Galápagos Islands
-----
Peruvian booby
Sula variegata
(Tschudi, 1843)Chilean and Peruvian coast
E. albescens
P. annulatus
Red-footed booby
Sula sula
(Linnaeus, 1766)Through tropical open seas
E. albescens
P. annulatus
P. garbei
P. sulae
One of the interesting characters of Suliformes/chewing lice interaction is the special parasite/host distribution pattern. Chewing lice of cormorants show a high degree of host specificity in contrast to that infesting the boobies. Such relations need to be analyzed through the molecular level to clarifying the bases of speciation of the two groups and evaluate the impact of distribution to the picture that appears today. So, the objective of this work is to record chewing lice of Brown booby from Egypt for the first time and initially discussed the Pectinopygus /boobies interaction based on bioinformatics data.
2 Materials and methods
The brown booby was caught with broken wing in Giftun Island (27°13′N 33°56′E), Hurghada, Egypt. Live caught Brown Booby was examined visually and chewing lice were collected by fine forceps and placed in 95% alcohol. Then the examined booby was released at the capturing place. The collected lice were proceeded for mounting on microscopic slides for identification using the method described by Smart et al. (1965). Chewing lice were identified according to Kellogg and Kuwana (1902), Tenderio (1958), Bienko (1964), Price et al. (2003) and Nasser et al. (2015b). All specimens examined under BOECO BM-120 microscope and photographed by using S-EYE YW500 camera5mp. Measurements of specimen were taken using the same camera software in millimetres scale. Measurement abbreviations as follows: HL = Head Length; HW = Head Width; HI = Head Index; TL = Thorax Length; AL = Abdomen Length; TOL = Total Length; GL = Genitalia Length; GW = Genitalia Width. The specimens were preserved in Ain Shams University Collection (ASUC).
The database of the National Center for Biotechnology Information (NCBI) was used to study the phylogenetic relationships among some species of Pectinopygus. A conservative gene (elongation factor 1-alpha (EF1alpha)) was chosen to study such relationships, based on the availability of sequences of this gene from several Pectinopygus species. Sequences of five species of Pectinopygus were extracted in Fasta format from the GenBank database: three species which known to infest boobies, one species infest Cape gannet Morus capensis and one species infest Pygmy cormorant Microcarbo pygmaeus (Pectinopygus annulatus “DQ482984.1”, Pectinopygus bassani “DQ482978.1”, Pectinopygus excornis “DQ482976.1”, Pectinopygus minor “DQ482981.1” and Pectinopygus sulae “AF320444.1”). The evolutionary history was inferred using the Neighbor-Joining method based on Multiple Sequence Alignment with Cluster W (Saitou and Nei, 1987). The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Maximum Composite Likelihood method (Tamura et al., 2004). Codon positions included were 1st + 2nd + 3rd + Noncoding. All ambiguous positions were removed for each sequence pair (pairwise deletion option). There was a total of 347 positions in the final dataset. Evolutionary analyses were conducted in MEGA X (Kumar et al., 2018). For comparing the chewing lice phylogeny toward birds’ phylogeny, the phylogenetic tree of host birds was modified after Patterson et al., 2011.
3 Results
3.1 Species recorded
A total of 27 chewing lice specimens representing two new records of lice species to Egyptian fauna were collected from a Brown Booby, Eidmanniella albescens (Piaget, 1880), and Pectinopygus sulae (Rudow, 1869).
Suborder Amblycera Kellogg, 1896
Family Menoponidae Mjöberg, 1910
Genus: Eidmanniella Kéler, 1938
Eidmanniella albescens (Piaget, 1880:491) (Fig. 1a)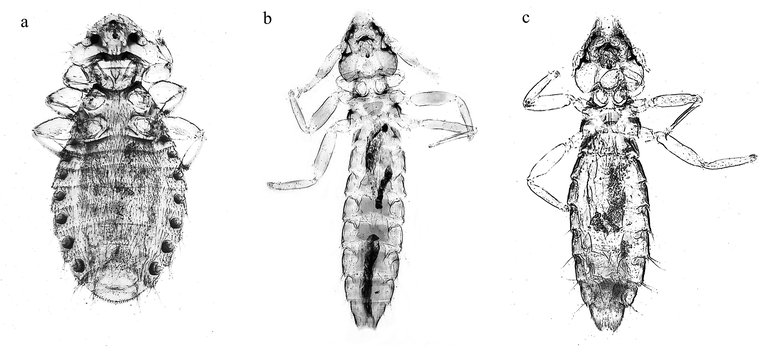
a. Female Eidmanniella albescens; b. Male Pectinopygus sulae & c. Female Pectinopygus sulae.
Menopon albescens Piaget, 1880:491
Menopon singulans Kellogg & Kuwana, 1902:485
Eidmanniella sula Tendeiro, 1958f:443
Type host: Morus serrator Gray, 1843, Australasian gannet.
Other hosts: Papasula abbotti (Ridgway, 1893), Abbott's booby; Sula nebouxii Milne-Edwards, 1882, Bluw-footed booby; Sula variegata (Tschudi, 1843), Peruvian booby; Sula dactylatra Lesson, 1831, Masked booby; Sula sula (L., 1766), Red-footed booby; Sula leucogaster (Boddaert, 1783), Brown booby (Suliformes: Sulidae).
Local host: Sula leucogaster (Boddaert, 1783), Brown booby.
Remarks: This is the first record of Eidmanniella albescens from Egypt. The Eidmanniella albescens dislocated from downside of wing feathers and abdomen feathers of brown booby.
Description: Body medium; head semicircular, broad with preocular slit, well developed hypopharyngeal sclerite, fourth segmented antennae with terminally distinctive club shape, temple with four medium setae; thorax with characteristic hexagon prothorax shape with numerous short setae on margins; meso and metathorax normally flattened; length of legs nearly equal; abdomen well-distinguish with stretched oval shape; The key characters that distinguish Eidmanniella albescens from other Eidmanniella spp.; the hypopharyngeal well-developed sitophore sclerite, the posterior margin of last tergite with short blunt spiniform setae.
Material examined: 3 females and 1 nymph of chewing lice from Sula leucogaster at Giftun Island, Egypt June 2019.
Measurements: Female HL: 0.44 (0.40–0.47); HW: 0.77 (0.72–0.83); HI: 0.57 (0.56–0.67); TL: 0.73 (0.70–0.75); AL: 1.46 (1.43–1.50); TOL: 2.63 (2.53–2.72).
Suborder Ischnocera Kellogg, 1896
Family Philopteridae Burmeister, 1838
Genus: Pectinopygus Mjöberg, 1910
Pectinopygus sulae (Rudow, 1869) (Fig. 1b, c)
Lipeurus sulae Rudow, 1869
Lipeurus helleri Kellogg & Kuwana, 1902:479
Lipeurus tuberculatus Piaget, 1885:61
Type host: Sula leucogaster (Boddaert, 1783), Brown booby.
Other hosts: Sula dactylatra Lesson, 1831, Masked booby; Sula sula (L., 1766), Red-footed booby (Suliformes: Sulidae).
Local host: Sula leucogaster (Boddaert, 1783), Brown booby.
Remarks: This report constitutes a new geographical record of Pectinopygus sulae from Egypt. All specimens of Pectinopygus sulae were located on head and neck feathers of host. & feathers of wing and tail.
Description: Body elongated, female wider and shorter than male, head conic shape with numerous setae around margin of hyaline, antennae sexually dimorphic with male scape enlarged; thorax slightly narrower than head, trapezoidal in shape, fore legs very shorter than other two pairs of legs; abdomen elongated, tergites with characteristic chitinized margin and with two centrals short setae, four short setae in each sternum. The open of anus of female angularly with many setae, asymmetric curved genitalia of male with granulated club shaped head and pointed end.
Material examined: 11 males, 6 females and 6 nymphs of chewing lice from Sula leucogaster Giftun Island, Egypt June 2019.
Measurements: Male HL: 0.78 (0.75–0.79); HW: 0.66 (0.64–0.67); HI: 2.38 (2.35–2.41); TL: 0.50 (0.48–0.53); AL: 2.30 (2.28–2.32); TOL: 3.58 (2.58–2.64), female HL: 0.75 (0.73–0.78); HW: 0.66 (0.62–0.69); HI: 2.22 (2.19–2.25); TL: 0.44 (0.41–0.47); AL: 2.13 (2.11–2.16); TOL: 3.32 (3.30–3.34); GL: 1.03 (1.02–1.04); GW: 0.21 (0.20–0.22).
3.2 Phylogeny of Boobies’ chewing lice of genus Pectinopygus
The sequence data of elongation factor 1-alpha (EF1alpha) gene of Boobies’ chewing lice of Genus Pectinopygus were used to construct an evolutionary tree (Fig. 2). The produced tree indicated the monophyletic relation of the three recorded chewing lice species of Genus Pectinopygus which infected boobies and their paraphyletic relation toward other Pectinopygus spp. which infest other Suliformes. Pectinopygus sulae appears to the last recently developed species of the group while Pectinopygus bassani of Cape gannet Morus capensis was the oldest.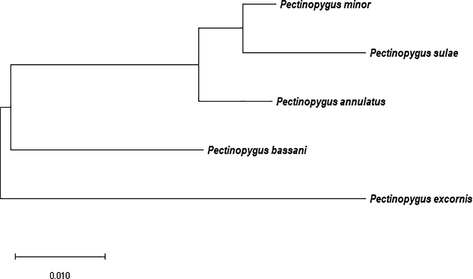
Annotated tree illustrates phylogenetic relations among Pectinopygus spp. based on Neighbor-Joining method of elongation factor 1-alpha (EF1alpha) sequence.
Comparing the produced tree to host bird phylogeny (Fig. 3) indicated independence of boobies’ evolution from the Pectinopygus chewing lice evolution. Only the Pectinopygus minor has a specific relation with blue-footed booby (Sula nebouxii) of the Pacific Ocean, while Pectinopygus annulatus and Pectinopygus sulae recorded from four and three species of boobies respectively. Such results indicated sympatric speciation of Genus Pectinopygus through boobies’ population around the globe.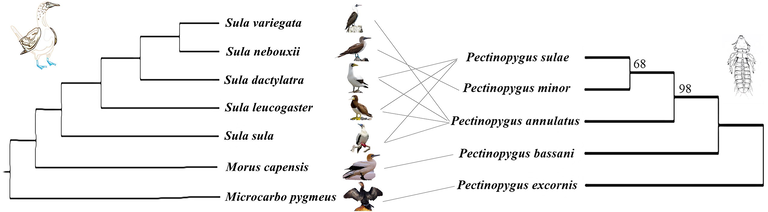
Comparing the phylogenetic relations among Pectinopygus spp. toward their host birds’ phylogeny “the phylogenetic tree of birds was modified after Patterson et al., 2011″
4 Discussion
The diversity of Marien birds’ ectoparasites is not completely understood through different parts of the world, as many new species and new records are discovered every year (Shobrak et al., 2015; Nasser, 2015). This is not far from the data about chewing lice of Egypt that are very old and not compatible with the great diversity of avifauna of the country (Adly et al., 2019). So, the present work forms a small step to better understanding chewing lice/ marine birds’ association to the region. Eidmanniella Kéler, 1938 of the suborder Amblycera, and Pectinopygus Mjöberg, 1910 of the suborder Ischnocera are two newly recorded genera to Egyptian fauna with the two species Eidmanniella albescens and Pectinopygus sulae from brown booby. Several previous studies have revised the information on chewing lice of these two species and recorded them on brown booby from different parts of the world (Kellogg and Kuwana, 1902; Timmermann, 1967; Ryan and Price, 1969; Nasser, et al., 2015b).
The chewing lice/boobies interaction indicates the absence of clear parasitic specificity except for Pectinopygus minor on blue-footed booby (Sula nebouxii) of the Pacific Ocean. This is maybe due to the niche overlapping of boobies’ species through pantropic seas and oceans. Such host distribution pattern could confirm parasite transfer through different species of boobies’ population and preventing chewing lice specificity and speciation. The phylogenetic analysis of Pectinopygus spp. support such hypothesis and indicated sympatric speciation of the three Pectinopygus spp. infesting boobies. On the other hand, each species of the genus Pectinopygus parasitizes a unique species of a cormorant as usually there is a clear geographical isolation pattern of cormorant species populations (Price et al. 2003). More studies are needed to get a complete understanding of interactions between Suliformes and their associated chewing lice, especially on the molecular level. Also, knowledge of the diversity of chewing lice from wild birds in Egypt is still very sparse and great efforts are needed to end their survey.
5 Conclusion
A significant lack of information about ectoparasites of marine birds around the world especially in the Middle East region. A total of 27 specimens of lice were collected from Brown Booby Sula leucogaster (Boddaert) in Giftun Island, Hurghada, Red Sea, Egypt. Two species of marine chewing lice (Eidmanniella albescens (Piaget, 1880) and Pectinopygus sulae (Rudow, 1869)) were identified for the first time in Egypt. Also, we did a bioinformatic analysis of genus Pectinopygus associated with boobies to produce a phylogenetic tree that indicated the independence of boobies’ speciation from chewing louse of genus Pectinopygus speciation. A lot of effort is needed for this important point of the study.
Acknowledgments
This project was supported by Researchers Supporting Project Number (RSP-2021/7) King Saud University, Riyadh, Saudi Arabia. Thanks also go to the staff of Entomology Departments, Faculty of Science, Ain Shams University, Cairo 11566, Egypt for their continuous support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Analysis of phoretic relation between chewing lice and hippoboscid flies of Columba livia. Veterinary Parasitology: Regional Studies and Reports. 2020;22:100496
- [CrossRef] [Google Scholar]
- New records of chewing lice (Phthiraptera: Amblycera, Ischnocera) from Egyptian pigeons and doves (Columbiformes), with description of one new species. Acta Trop.. 2019;190:22-27.
- [CrossRef] [Google Scholar]
- The World Encyclopedia of Birds and Bird Watching. London: Anness Publishing; 2008.
- Anonymous, 2012. BirdLife International Sula leucogaster. The IUCN Red List of Threatened Species. Version 2014.3. (http://www.iucnredlist.org; accessed 26/12/2014).
- Keys to the Insects of the European USSR: Apterygota, Palaeoptera, Hemimetabola. Israel Program for Scientific Translations. 1964;1:385-402.
- [Google Scholar]
- Systematics and Taxonomy of Australian Birds. Collingwood, Australia: CSIRO Publishing; 2008.
- Gill, F., Donsker, D., eds. 2014. IOC World Bird List (v 4.4). doi: 10.14344/IOC.ML.4.4. (http://www.worldbirdnames.org; accessed 26/12/2014).
- Mallophaga infesting migratory birds in Egypt. Bulletin de la Societe de Entomologique d'Egypte. 1968;52:113-154.
- [Google Scholar]
- Papers from the Hopkins Stanford Galapagos expedition, 1898–1899. X. Entomological results (8). Mallophaga from birds. Proc. Washington Acad. Sci.. 1902;4:457-499.
- [Google Scholar]
- MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol.. 2018;35:1547-1549.
- [Google Scholar]
- Chewing Lice: Tiny Insects in Raging Seas. J. Marine Sci. Res. Dev.. 2015;5:e137
- [CrossRef] [Google Scholar]
- Identification key for chewing lice (Phthiraptera: Amblycera, Ishnocera) infesting the Indian Peafowl (Pavo cristatus) with one new country record and new host record for Saudi Arabia. Turk. J. Zool.. 2015;39:88-94.
- [CrossRef] [Google Scholar]
- Chewing lice (Phthiraptera) infesting breeding Suliformes (Aves: Aequornithes) of the Arabian Peninsula. African Invertebrates. 2015;56(3):709-717.
- [Google Scholar]
- Host habitat and position on host affecting the evolution of chewing lice (Phthiraptera): Phylogenetic analysis of Ischnocera in Saudi Arabia. J. Insect Biodiversity Systematics. 2020;6(1):101-112.
- [Google Scholar]
- An analysis of osprey/chewing lice interaction, with a new record for Saudi Arabia. African Entomol.. 2019;27(1):178-184.
- [CrossRef] [Google Scholar]
- Feather mites of the genus Zachvatkinia Dubinin, 1949 (Astigmata: Analgoidea: Avenzoariidae) from Saudi Arabia: a new species and two new records. Zootaxa. 2013;3710(1):61-71.
- [Google Scholar]
- A multilocus phylogeny of the Sulidae (Aves: Pelecaniformes) Mol. Phylogenet. Evol.. 2011;58(2):181-191.
- [CrossRef] [Google Scholar]
- The Chewing Lice: World Checklist and Biological Overview. Illinois Natural History Survey; 2003.
- A review of the genus Eidmanniella (Mallophaga: Menoponidae) from the Pelecaniformes. Ann. Entomol. Soc. Am.. 1969;62(4):815-823.
- [Google Scholar]
- The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol.. 1987;4:406-425.
- [Google Scholar]
- Scott, P., 2014. The world atlas of birds. Bounty books, a division of Octopus publishing group, UK, P 228.
- New records of species of Saemundssonia (Insecta: Phthiraptera: Philopteridae) infesting breeding terns in the Arabian Peninsula, with notes on their phylogeny and ecology. Parasitol. Res.. 2015;114:2587-2597.
- [Google Scholar]
- Smart, J., Jordan, K., Whittick, R.J., 1965. Insects of Medical Importance. 4th Ed. British Museum, Natural History. Oxford: Adien Press.
- Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Nat. .Acad. Sci. (USA). 2004;101:11030-11035.
- [Google Scholar]
- Études sur les Mallophages. Sur une nouvelle espèce du genre Eidmanniella von Kéler 1938 (Amblycera, Menoponidae), parasite de Sula leucogaster leucogaster (Boddaert) et Sula leucogaster plotus Forster. Garcia de Orta. 1958;6(3):443-449.
- [Google Scholar]
- Gruppen-Revisionen bei Mallophagen. VII. Die Pectinopygus-Arten der Großkormorane (Gen. Phalacrocorax Brisson, 1760 s.st.) Mitteilungen aus dem Hamburg Zoologischen Museum Institut. 1967;64:83-85.
- [Google Scholar]
- The Life of Birds (4th ed.). New York: Harcourt Brace Jovanovich College Publishers; 1990.







