Translate this page into:
Pachypodol attenuates arsenic triggered cardiac damage via rectifying oxidative stress, inflammation, apoptosis and histopathological alterations
⁎Corresponding author at: Department of Zoology, Wildlife and Fisheries, University of Agriculture, Faisalabad, Pakistan. mehrabkhalil8@gmail.com (Mehrab Khalil)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Arsenic is a lethal toxicant found ubiquitously in the ecosystem which adversely affects the body organs including the heart. Pachypodol (PCHP) is a natural flavone which exhibits substantial pharmacotherapeutic potentials. The rats (n = 24) were distributed into 4 different groups i.e., control, arsenic-intoxicated group (50 mg/kg), arsenic + PCHP-supplemented group (50 mg/kg + 10 mg/kg) & PCHP-treated (10 mg/kg) group. It was assessed that arsenic administration subsided catalase (CAT), glutathione reductase (GSR), superoxide dismutase (SOD), glutathione peroxidase (GPx), & glutathione S-transferases (GST) activities while augmenting the levels of reactive oxygen species (ROS) and malondialdehyde (MDA). Furthermore, arsenic exposure increased the levels of cardiac injury markers such as creatine phosphokinase (CPK), creatine kinase-MB (CK-MB), troponin I & lactate dehydrogenase (LDH). Besides, the levels of inflammatory markers nuclear factor kappa B (NF-κB), interleukin-1β (IL-1β), Tumor necrosis factor α (TNF-α), Interleukin 6 (IL-6) levels and cyclooxygenase 2 (COX-2) activity were increased following the arsenic exposure. Similarly, Caspase-3, Bax and Caspase-9 levels were upsurged whereas Bcl-2 level was reduced after arsenic intoxication. In addition, the histopathological assessment revealed a substantial cardiac tissues impairment in the arsenic exposed group. Nonetheless, PCHP supplementation substantially (p < 0.05) recovered the abovementioned arsenic −induced impairments owing to its anti-inflammatory, antioxidative and anti-apoptotic abilities. Therefore, the current research revealed that PCHP might be a promising ameliorative agent to cure arsenic instigated cardiac damages.
Keywords
Pachypodol
Arsenic
Cardiac toxicity
Oxidative stress
Inflammation
1 Introduction
Arsenic is a well-known heavy metal which is ubiquitously present in our surroundings and considered as a major health concern across the globe. It is documented that arsenic leaches into drinking water through various sources such as bedrocks and earth crust (Vahter, 2008). Humans as well as other animals are exposed toarsenic via contaminated food and water, air as well as seafood (mussels, prawns, oyster, fish) (McCarty et al., 2011). It is reported that high concentrations of arsenic are present in urine, hoof, hair as well as nails (Roy et al., 2008). Furthermore, workers at chemical factories, mining sites and smelters are exposed to high concentration of arsenic. It is estimated that arsenic concentrations vary from one place to another such as the areas of low anthropogenic activities have 1–3 ng/m3 while industrial zones have 20–30 ng/m3 (ATSDR, 2000). Owing to high rate of absorption, slow elimination as well as small particle size, arsenic adversely affect various organs of body (Bergin et al., 2016).
It is revealed that arsenic exposure damages various body organs including skin, liver, lungs, heart, and urinary bladder (States et al., 2009; Moon et al., 2012). Various epidemiological investigations elucidated a strong relationship between arsenic contaminated water and cardiovascular disorders (Sanchez-Soria et al., 2012). Chowdhury and Daalen (2019) revealed that chronic exposure to arsenic instigates different cardiac impairments such as ischemia, arrhythmia as well as heart failure. It is reported that myocardial oxidative stress & apoptosis are primary factors underlying the arsenic instigated cardiac damages (Jiang et al., 2021). Furthermore, a long-term arsenic intoxication escalates the systolic blood pressure, total cholesterol, AST, bilirubin, creatinine, urea as well as reduces the levels of high-density lipoproteins (HDL) which ultimately instigate hypertension (Balarastaghi et al., 2022).
However, the management of arsenic induced toxicities is still complicated owing to lack of efficient treatment (Flora, 2020). Natural compounds are widely used as adjuvant therapy to treat various disorders (Men et al., 2022; Aboubakr et al., 2023). It is reported that plant-based flavonoids have potential to bind with heavy metals owing to their antioxidative abilities (Tay et al., 2013). PCHP is a plant derived flavonoid which demonstrated various pharmacological potentials including anti-microbial, cytoprotective, anti-apoptotic as well as anti-oxidative (Kim et al., 2019; Krithika et al., 2021; Zhang et al., 2021; Kim et al., 2019). Therefore, this investigation was executed to investigate the attenuative potential of PHCP to rectify arsenic provoked cardiac toxicity in rats.
2 Materials and methods
2.1 Chemicals
Arsenic (CAS No. 7440–38-2, Purity HPLC ≥ 98.0 %) & PCHP (CAS No. 33708–72-4, Purity HPLC ≥ 98.0 %) were bought from Sigma-Aldrich (Germany).
2.2 Experimental animals
Twenty-four albino rats, aged 6–7 weeks and weighing between 180–200 g, were housed in adequately ventilated enclosures under standard environmental conditions at the Animal Research Station of the University of Agriculture, Faisalabad (UAF). Standard laboratory conditions included a 12-hour light–dark photoperiod, a humidity level maintained at 60 ± 5 % and a temperature range of 20–26 °C. The rats were provided with a commercial diet (composition: Fibers, oils, proteins, carbohydrates, flour, minerals, and vitamins) and ad libitum access to tap water throughout the study period. Rats were handled according to the ethical guidelines provided by European Union of Animals Care and Experimentation (EU Directive 2010/63/EU).
2.3 Experimental protocol
Twenty-four rats were randomly distributed into 4 different groups, each comprising of six rats. The treatment regimens were as follows: the control group, the arsenic (50 mg/kg), PCHP + arsenic (50 mg/kg + 10 mg/kg), and the group (10 mg/kg). The experimental duration spanned a period of 30 days. On the 31st day of the experiment, the rats were decapitated, and cardiac blood samples were collected to assess the serum profile. Subsequently, the heart was surgically excised from the rats. The one part of heart tissue was placed in the zipper bags and preserved at −20 °C to facilitate the assessment of the biochemical biomarkers. Conversely, the 2nd part was taken in a formalin solution (10 %) to facilitate the subsequent examination of histopathological changes in cardiac tissues.
2.4 Cardiac injury markers estimation
The levels of CPK (Catalog no. E4608-100), LDH (CSB-E11324r), troponin I (CSB-E08594r) & CK-MB (Catalog No. MBS2515061) were estimated by employing ELISA kits.
2.5 Biochemical analysis
The assessment of CAT activity was carried out using the technique developed by Aebi, (1974). The activity of SOD was quantified following the methodology described by Kakkar et al. (1984). The measurement of GPx activity was performed by using the method established by Rotruck et al. (1973). The concentration of GSR was estimated following the protocol outlined by Carlberg and Mannervik, (1975). Additionally, the content of GSH was evaluated using the methodology demonstrated by Jollow et al. (1974).
2.6 Estimation of oxidative stress indices
The concentration of ROS was determined using the method established by Hayashi et al. (2007). The quantification of MDA levels was performed following the procedure outlined by Ohkawa et al. (1979).
2.7 Inflammatory markers assessment
The quantification of inflammatory biomarkers was carried out using ELISA kits followed by manufacture’s guidelines.
2.8 Apoptotic markers analysis
The ELISA kits were used to measure the level of apoptotic biomarkers according to the instruction of manufacturers.
2.9 Histopathological observation
A formaldehyde (10 %) solution was used to fix the cardiac tissues, after that dehydrated them with ascending grades of alcohol and trimmed into 4 μm pieces with the help of microtome (rotary). By using eosin as well as hematoxylin stains the staining was performed and the prepared slides were examined under light a microscope to study the histopathological disruption.
2.10 Statistical evaluation
All the data were illustrated as Mean ± SEM. The Tukey’s test & ANOVA (one way interaction) were used to statistically analyze the data using Graph pad Prism 5. The significance level was kept at p < 0.05.
3 Results
3.1 Impact of PCHP on biochemical profile
Arsenic intoxication substantially (p < 0.05) downregulated the GSH, GSR, GST, GPx, SOD as well as CAT activity. Furthermore, co– treatment of arsenic + PCHP considerably (p < 0.05) augmented these enzyme’s activities in comparison to arsenic exposed rats. Moreover, insignificant differences were observed among PCHP alone and the control group (Table 1). Distinct superscripts on various values demonstrated discrepancies among other groups.
Parameters
Groups
Control
Arsenic
Arsenic + PCHP
PCHP
CAT (U/mg protein)
13.99 ± 1.58a
6.74 ± 0.50b
11.53 ± 1.32a
14.13 ± 1.94a
SOD (U/mg protein)
9.37 ± 1.13a
3.81 ± 0.22c
6.79 ± 0.40b
9.42 ± 1.10a
GSR (nM NADPH oxidized/min/mg tissue
7.28 ± 0.25a
2.29 ± 0.20c
5.54 ± 0.33b
7.32 ± 0.29a
GPx (U/mg protein)
25.12 ± 1.93a
9.09 ± 1.18c
16.93 ± 1.61b
25.83 ± 2.43a
GSH (U/mg protein)
15.34 ± 2.07a
4.01 ± 0.27b
13.62 ± 1.11a
15.79 ± 2.54a
GST (U/mg protein)
25.12 ± 1.93a
9.09 ± 1.18c
16.93 ± 1.61b
25.83 ± 2.43a
MDA (nmol/g)
0.75 ± 0.12c
7.22 ± 0.33a
3.19 ± 0.20b
0.71 ± 0.10c
ROS (nmol/g)
0.66 ± 0.12c
8.66 ± 0.50a
2.51 ± 0.28b
0.63 ± 0.11c
3.2 Impact of PCHP on oxidative stress biomarkers
Arsenic treatment remarkably (p < 0.05) elevated MDA along with ROS concentration in arsenic group in contradiction to the control rats. PCHP + Arsenic group substantially (p < 0.05) reduced their levels when matched with arsenic rats. No substantial changes were detected in the mean values of these markers in the control & PCHP rats (Table 1).
3.3 Impact of PCHP on cardiac injury markers
Arsenic supplementation substantially (p < 0.05) elevated LDH, CPK & CK-MB in comparison to the control rats. C-administration of PCHP notably (p < 0.05) restored their levels to normal state arsenic as compared to arsenic group. Furthermore, there was no substantial changes noticed in the mean values of only PCHP and the control groups (Table 2). Distinct superscripts on various values demonstrated discrepancies among other groups.
Parameters
Groups
Control
Arsenic
Arsenic + PCHP
PCHP
LDH (mg/dl)
15.81 ± 1.51c
56.85 ± 2.52a
25.70 ± 2.03b
15.69 ± 1.51c
CPK (mcg/L)
146.16 ± 9.99c
429.7 ± 24.1a
237.38 ± 12.69b
141.99 ± 10.24c
CK-MB (ng/mL)
32.83 ± 1.50a
92.37 ± 2.11a
222 ± 286a
32.79 ± 1.51a
Troponin (pg/ml)
0.66 ± 0.20c
4.30 ± 0.23a
1.72 ± 0.16b
0.64 ± 0.20c
3.4 Impact of PCHP on inflammatory biomarkers
Exposure to arsenic significantly (p < 0.05) augmented the TNF-α, IL- 6, IL-1β, NF-κB along with the COX-2 activity in the arsenic rats in contrast to control rats. PCHP administration along with arsenic markedly (p < 0.05) decreased the activity and levels of the aforesaid inflammatory biomarkers as compared to arsenic rats. Moreover, no substantial alterations were observed among the control groups and PCHP (Table 3). Distinct superscripts on various values demonstrated discrepancies among other groups.
Parameters
Groups
Control
Arsenic
Arsenic + PCHP
PCHP
NF-kB (ng/g tissue)
19.89 ± 1.74c
85.82 ± 1.34a
44.29 ± 2.50b
19.67 ± 1.52c
TNFα (ng/g tissue)
9.33 ± 1.44c
34.65 ± 3.00a
15.81 ± 1.33b
9.26 ± 1.33c
IL-1ß (ng/g tissue)
19.94 ± 2.95c
78.30 ± 2.96a
43.87 ± 1.57b
19.79 ± 2.78c
IL-6 (ng/g tissue)
9.24 ± 0.11c
54.91 ± 2.58a
24.24 ± 2.01b
9.21 ± 0.08c
COX-2 (ng/g tissue)
22.82 ± 1.70c
65.18 ± 2.23a
34.62 ± 2.10b
22.71 ± 1.75c
3.5 Impact of PCHP on apoptotic biomarkers
Arsenic administration considerably (p < 0.05) esclated caspase-9, Bax and caspase-3, while reducing the concentration of Bcl-2 in arsenic rat in contrast to control. PCHP supplementation recovered the altered levels of these contrary to arsenic treated group. However, insignificant difference was observed in apoptotic markers level among PCHP and the control group (Table 4). Distinct superscripts on various values demonstrated discrepancies among other groups.
Parameters
Groups
Control
Arsenic
Arsenic + PCHP
PCHP
Bax (pg/mL)
1.38 ± 0.15c
9.50 ± 1.04a
3.32 ± 0.20b
1.36 ± 0.16c
Caspase-3 (ng/mL)
3.32 ± 0.20b
19.33 ± 2.05a
5.23 ± 0.11b
3.29 ± 0.19b
Caspase-9 (pg/mL)
3.32 ± 0.20b
19.33 ± 2.05a
5.23 ± 0.11b
3.29 ± 0.19b
Bcl-2 (pg/mL)
14.52 ± 1.04a
3.85 ± 0.25c
11.03 ± 0.62b
14.76 ± 1.12a
3.6 Impact of PCHP on histology
Arsenic intoxication instigated inflammation, degeneration of endocardium, cardiac edema, as well as abnormal shape of cardiomyocytes in contrast to control group. However, PCHP + arsenic treatment remarkably (p < 0. 05) restored aforementioned histopathological impairments. Nonetheless, control and PCHP alone treated group showed normal histology of cardiac tissues (Fig. 1).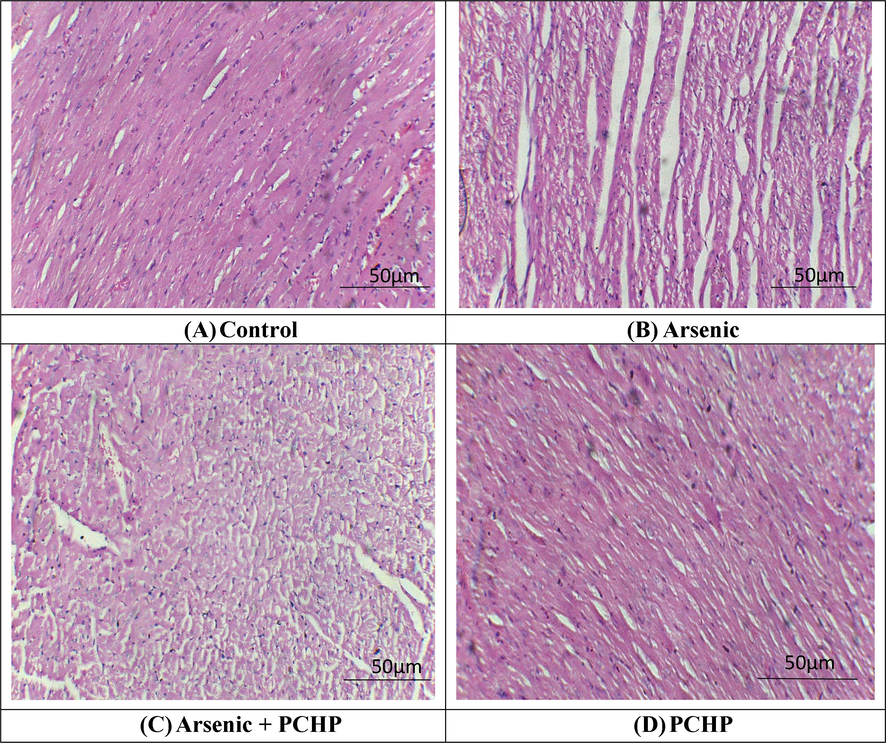
Histopathological analysis of heart tissues. H&E stain; 40X (A) Control group exhibited normal architecture of heart tissues (B) Arsenic exposed group demonstrated fibrosis, inflammation as well as large interstitial spaces (edema) (C) Arsenic + PCHP group showed a remarkable recovery in contrast to Arsenic exposed group (D) Only PCHP supplemented group showed normal morphology of cardiac tissues as in control group.
4 Discussion
Our findings demonstrated that arsenic intoxication reduced the antioxidant enzyme’s concentration (CAT, GPx, SOD, GSR & GSH) while downregulating the levels of ROS & MDA. Antioxidant enzymes are primary barriers against oxidative damage (Berroukeche et al., 2022). Various studies showed a strong association between ROS and arsenic instigated cellular damages (Liu and Jan, 2000). The overproduction of ROS retards the levels of these enzymes and disrupts the normal architecture of cells which ultimately leads to the progression of various pathologies (Mukherjee et al., 2015). Moreover, excessive production of free radicals decreases the activities of antixodiant enzymes which ultimately impairs the endogenous cellular defense system (Ahmad et al., 2023). It is reported that arsenic reduces the activities of SOD along with CAT via decreasing the availability of NADH while escalating the levels of superoxide anions (Das et al., 2010). Our results matched with the investigations of Ijaz et al. (2023) who elucidated that administration of arsenic reduced these enzyme’s concentration while escalating the levels of lipid peroxidation. Plant-based flavonoid has potential to increase the activities of antioxidant enzymes (Ijaz et al., 2022). However, co-treatment of PCHP remarkably reduced antioxidant enzymes activities via regulating the levels of ROS owing to its ROS scavenging abilities.
Our investigation manifested that arsenic exposure augmented the levels of CK-MB, LHD, CPK as well as troponin-1 in cardiac tissues of rats. Abovementioned cardiac biomarkers are primary indicators of cardiac injury therefore, elevated levels of these biomarkers in the bloodstream indicate severe cardiac damage (Warings et al., 2000). Our findings are corroborated by the previous investigation which elucidated that arsenic administration triggers various alterations in cardiac tissues which are characterized by escalated levels of abovementioned cardiac injury markers (Jalaludeen et al., 2015). Nonetheless, the supplementation of PCHP reduced the levels of these biomarkers which demonstrates its cardioprotective potential.
Our findings demonstrated that the administration of arsenic culminated in an increase in the concentration of inflammatory biomarkers (IL-1β, TNF-α, IL-6, NF-κB & COX-2 activity). NF-κB is a principal biomarker contributing substantially to the augmentation of the aforementioned proinflammatory biomarkers, ultimately leading to the onset of acute inflammation and the subsequent damages associated with ROS (Zha et al., 2014). Furthermore, COX-2 serves as a substantial mediator that prompts the incipience of inflammatory cascade in the cardiac tissues (Gilroy and Colville-Nash, 2000). The outcomes of our study revealed that the arsenic caused a substantial escalation in the concentration of these inflammatory biomarkers owing to increased OS levels which reflects the state of inflammation in the heart tissues. Supplementation of PCHP effectively reduced the stimulation of NF-κB that serves as the primary catalyst for tissue inflammation. Therefore, it can be inferred that PCHP may have substantial therapeutic action in mitigating the raised proinflammatory biomarkers, owing to its noticeable anti-inflammatory nature.
Apoptosis can be instigated by dysregulation in the homeostasis between apoptotic and antiapoptotic enzymes which are mediated through mitochondrial-independent or dependent cascades (Selimovic et al., 2011). Downregulation of Bcl-2 and concurrent upregulation of Bax markedly disrupt the integrity of the mitochondrial membrane (Reed, 2006). Bcl-2 & Bax mediates the secretion of cytochrome c from the mitochondrial system which ultimately triggers the process of apoptosis (Shou et al., 2002). Caspase-3 is recognized as a pivotal mediator of apoptosis which activates other pro-apoptotic markers (D’Amelio et al., 2012). Treatment with arsenic resulted in a substantial upsurge in the Bax & Caspase-3 following a remarkable decline in the Bcl-2 concentration, However, PCHP supplementation alleviated arsenic caused cardiac apoptosis by reducing the Caspase-3 & Bax levels while upregulating the levels of Bcl-2.
A normal histo-architectural integrity of any organs is essential for its physiological functions (Oyeyipo et al., 2010). In the current study arsenic exposure remarkably induced histopathological disruptions in cardiac tissues i.e., myocardial damage, fibrosis, inflammatory responses, modification in cellular morphology as well as cellular necrosis. OS is recognized as central element underlying these morphological disturbances (Goyal et al., 2016). ROS disrupts normal activity of mitochondria, resulting in decreased ATP formation which leads towards the cell death in the cardiac tissues. Besides, OS triggered cascade of inflammatory events, instigating myocardial inflammation (Fabiani et al., 2011). Furthermore, these detrimental alterations influence contractility and cardiac perfusion, thus triggering the proliferation of wide range of cardiovascular pathologies (Fabiani et al., 2011). Our findings demonstrated that PCHP is an effective curative compound which recovered aforesaid cardiac histopathological damages provoked by arsenic.
5 Conclusion
Taken together, arsenic reduced the activities of antioxidant enzymes while upregulating the levels of oxidative stress. Furthermore, arsenic promoted the levels of cardiac injury as well as inflammatory markers. The levels of pro-apoptotic markers were escalated while the levels of anti-apoptotic markers were reduced following the arsenic treatment. Moreover, arsenic instigated various histopathological impairments. However, PCHP treatment protected the cardiac tissues via regulating abovementioned dysregulations.
CRediT authorship contribution statement
Syeda Sania Zahara: Writing – review & editing, Writing – original draft, Methodology, Investigation, Conceptualization. Mehrab Khalil: Writing – original draft, Validation, Methodology, Investigation. Moazama Batool: Visualization, Validation, Software, Formal analysis, Data curation. Muhammad Faisal Hayat: Writing – review & editing, Writing – original draft, Methodology, Investigation. Bader O. Almutairi: Writing – original draft, Resources, Funding acquisition. Mian Nadeem Riaz: Writing – original draft, Visualization, Validation.
Acknowledgement
This work was funded by Researchers Supporting Project number (RSP2024R414), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Protective effects of N acetylcysteine and vitamin E against acrylamide-induced neurotoxicity in rats. Pak. Vet. J.. 2023;43(2):262-268.
- [Google Scholar]
- Catalase. Methods. of Enzymatic. Analysis. Academic press; 1974. p. :673-684.
- ATSDR, T., 2000. ATSDR (Agency for toxic substances and disease registry). Prepared by clement international corp., under contract. 205, 88-0608.
- Ameliorative effects of rhamnetin against polystyrene microplastics-induced nephrotoxicity in rats. Pak. Vet. J.. 2023;43:623-627.
- [Google Scholar]
- Mechanisms of arsenic exposure-induced hypertension and atherosclerosis: an updated overview. Biol. Trace. Elem. Res.. 2023;201:98-113.
- [Google Scholar]
- Effects of particle size and coating on toxicologic parameters, fecal elimination kinetics and tissue distribution of acutely ingested silver nanoparticles in a mouse model. Nanotoxicology. 2016;10:352-360.
- [Google Scholar]
- Investigation of antioxidant and anti-hemolytic properties of Algerian Bunium incrassatum tubers and their effects as diet on histological and biochemical parameters of normal Wistar rats. Asian J. Agric. Biol. 2022202007391
- [Google Scholar]
- Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem.. 1975;250:5475-5480.
- [Google Scholar]
- Arsenic: a metal that might break your heart. Circ. Cardiovasc. Imaging.. 2019;12:009185
- [Google Scholar]
- Caspase-3 in the central nervous system: beyond apoptosis. Trends. Neurosci.. 2012;35:700-709.
- [Google Scholar]
- Protective effect of Corchorus olitorius leaves against arsenic-induced oxidative stress in rat brain. Environ. Toxicol. Pharmacol.. 2010;29:64-69.
- [Google Scholar]
- Oxidative stress and inflammation: determinants of anthracycline cardiotoxicity and possible therapeutic targets. Heart. Fail. Rev.. 2021;26:881-890.
- [Google Scholar]
- Preventive and therapeutic strategies for acute and chronic human arsenic exposure. Arsenic in Drinking Water and Food. 2020:341-370.
- [Google Scholar]
- New insights into the role of COX 2 in inflammation. J. Mol. Med.. 2000;78:121-129.
- [Google Scholar]
- Protective effect of oleanolic acid on oxidative injury and cellular abnormalities in doxorubicin induced cardiac toxicity in rats. Am. J. Transl. Res.. 2016;8:60.
- [Google Scholar]
- High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat. Res. Genet. Toxicol. Environ. Mutagen.. 2007;631:55-61.
- [Google Scholar]
- Evaluation of the possible protective role of nobiletin against arsenic-induced liver damage in male albino rats. Toxics.. 2023;11:110.
- [Google Scholar]
- Hepatoprotective potential of Genkwanin against aflatoxin B1-induced biochemical, inflammatory and histopathological toxicity in rats. Pak. Vet. J.. 2022;42(4):499-504.
- [Google Scholar]
- Therapeutic efficacy of biochanin A against arsenic-induced renal and cardiac damage in rats. Environ. Toxicol. Pharmacol.. 2015;39:1221-1231.
- [Google Scholar]
- Arsenic (III) and/or Antimony (III) induced disruption of calcium homeostasis and endoplasmic reticulum stress resulting in apoptosis in mice heart. Ecotoxicol. Environ. Saf.. 2021;220:112394
- [Google Scholar]
- Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. J. Pharmacol.. 1974;11:151-169.
- [Google Scholar]
- Kakkar, P., Das, B. and Viswanathan, P.N., 1984. A modified spectrophotometric assay of superoxide dismutase.
- DNA damage in arsenite-and cadmium-treated bovine aortic endothelial cells. Free. Radic. Biol. Med.. 2000;28:55-63.
- [Google Scholar]
- McCarty, K.M., Hanh, H.T. and Kim, K.W., 2011. Arsenic geochemistry and human health in South East Asia.
- Phytochemical constituents and antioxidant activity of some medicinal plants collected from the Mekong Delta, Vietnam. Asian J. Agric. Biol. 2022
- [Google Scholar]
- Arsenic exposure and cardiovascular disease: an updated systematic review. Curr. Atheroscler. Rep.. 2012;14:542-555.
- [Google Scholar]
- Detection of oxidative stress-induced carbonylation in live mammalian cells. Free. Radic. Biol. Med.. 2015;84:11-21.
- [Google Scholar]
- Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;44:276-278.
- [Google Scholar]
- Effects of oral administration of nicotine on organ weight, serum testosterone level and testicular histology in adult male rats. Niger. J. Physiol. Sci.. 2010;25:81-86.
- [Google Scholar]
- Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell. Death. Differ.. 2006;13:1378-1386.
- [Google Scholar]
- Selenium: biochemical role as a component of glutathione peroxidase. J. Sci.. 1973;179:588-590.
- [Google Scholar]
- Arsenic induced haematobiochemical and histopathological alterations and its level in certain biological samples. Toxicol. Int.. 2008;25:57-62.
- [Google Scholar]
- Chronic low-level arsenite exposure through drinking water increases blood pressure and promotes concentric left ventricular hypertrophy in female mice. Toxicol. Pathol.. 2012;40:504-512.
- [Google Scholar]
- Apoptosis-related protein-2 triggers melanoma cell death by a mechanism including both endoplasmic reticulum stress and mitochondrial dysregulation. J. Carcinog.. 2011;32:1268-1278.
- [Google Scholar]
- NF-κB-mediated up-regulation of Bcl-XS and Bax contributes to cytochrome c release in cyanide-induced apoptosis. J. Neurochem.. 2002;81:842-852.
- [Google Scholar]
- Health effects of early life exposure to arsenic. Basic. Clin. Pharmacol. Toxicol.. 2008;102:204-211.
- [Google Scholar]
- Soyasaponins can blunt inflammation by inhibiting the reactive oxygen species-mediated activation of PI3K/Akt/NF-kB pathway. PLoS One. 2014;9:107655
- [Google Scholar]







