Translate this page into:
Oxidative stress and hepatocellular mitochondrial dysfunction attenuated by asiatic acid in streptozotocin-induced diabetic rats
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Oxidative stress produced by mitochondria is one of the main causes for diabetes associated complications. This study was planned to assess the efficacy of asiatic acid (AA) on oxidative stress mediated hepatocellular mitochondrial dysfunction in streptozotocin (STZ)-induced type-2 diabetic rats. Diabetic rats were generated by intraperitoneal injection of STZ administered as single dose at 40 mg/kg body weight (b. wt.). Then the rats were treated with 20 mg of AA or 600 μg of glibenclamide (Glib)/kg b. wt, once in a day for 45 days. The results of this study illustrated that diabetic rat liver mitochondrial malondialdehyde (MDA), reactive oxygen species (ROS) and protein carbonyl (PCO) levels were significantly increased at the same time antioxidant enzymes and non-enzymes status was markedly diminished when compared with the liver mitochondria of normal control rats. A marked reduction in ROS, MDA, PCO and significant amelioration in non-enzymatic and enzymatic antioxidants were observed in diabetic rats treated with AA or Glib as compared with untreated diabetic control rats. Tricarboxylic acid (TCA) cycle enzymes like isocitrate dehydrogenase, alpha-ketoglutarate dehydrogenase, succinate dehydrogenase, malate dehydrogenase and electron transport chain (ETC) complexes activities were significantly diminished in diabetic rats. Besides, mitochondrial uncoupling protein-2 (UCP2) expression was significantly upregulated at the same time adenosine triphosphate (ATP) level and mitochondrial membrane potential (MMP) was markedly lowered in the mitochondria of diabetic rats. AA or Glib treatment with diabetic rats restored the TCA cycle enzymes activities, ETC complexes, UCP2, MMP and ATP level to near normal control rats as compared with untreated diabetic control rats. Hence, the results of the current study indicated that AA ameliorated mitochondrial function by attenuating oxidative stress in STZ-induced type-2 diabetic rats.
Keywords
Mitochondrial dysfunction
Oxidative stress
Lipid peroxidation
Antioxidants
Asiatic acid
Streptozotocin-induced diabetes
1 Introduction
Mitochondria are powerful and very important intracellular organelles in eukaryotic cells. They are a key player in the process of energy production and also implicate in numerous functions of eukaryotic cells. During normal cellular function mitochondria generates enormous amount of reactive oxygen species (ROS), at exceeded levels it may cause various diseases. Mitochondrial dysfunction has occurred due to most of the metabolic disorders including diabetes mellitus (Zavodnik et al., 2011). The main cause for the pathogenesis of diabetes is elevated oxidative stress and mitochondrial dysfunction. Diabetes mellitus is characterized by hyperglycemia associated with increased formation of ROS, reactive nitrogen species, advanced glycation end products, elevated lipolysis, ketogenesis and diminished level of antioxidants (Kumar et al., 2020). Hepatocytes play a major role in blood glucose homeostasis by regulation of glycolysis, glycogenolysis, glycogenesis and gluconeogenesis, and disruption of this regulation mechanism is affected during diabetes.
In general, oxidative stress has been noticed to be at increased level in type-2 diabetic patients. Elevated oxidant production in hepatic mitochondria is a basic mechanism that stimulates oxidative stress during diabetes. Uncoupling protein-2 (UCP2) is found in the mitochondrial inner membrane of various tissues, including liver. It can reduce the metabolic efficacy by dispersing the proton-motive force in the intermembrane space of the mitochondria that may affect ATP synthesis. Previous studies described that UCP2 can diminish ROS formation and regulate several cellular metabolisms (Arsenijevic et al., 2000). Overexpression of UCP2 will decrease the secretion of insulin by islets of Langerhans stimulated by blood glucose.
Hepatic mitochondria endure a forceful oxidative damage with a concurrent acceleration in ROS generation during hyperglycemia (Ji et al., 2020). Enhanced ROS production in mitochondria may also affect the mitochondrial energy metabolism by altering the flow of electrons via the electron transport chain (ETC) complexes. Besides, elevated ROS formation may decrease tricarboxylic acid (TCA) cycle enzymes activities. Diabetes related complications were increased by high amount of ROS formation, lipid peroxidation, protein oxidation and reduction of antioxidants in mitochondria. Mitochondrial transmembrane potential was altered by increased Ca2+ uptake that may affect the mitochondrial energy production during diabetes.
Asiatic acid (AA), a pentacyclic triterpene present in many plant species, and Centella asiatica (L) is considered as a main source of AA. It possesses a number of pharmacological properties like anti-inflammatory, antioxidant and hepatoprotective properties (Huang et al., 2011). Ramachandran et al. (2014) was illustrated the antidiabetic properties of AA by stimulating insulin levels, diminishing glucose levels and restoring the enzymes which are involved in carbohydrate and lipid metabolism. Further, they reported that AA reduced lipid peroxidation process and enhanced antioxidant levels in diabetic rats (Ramachandran and Saravanan, 2013). Nonetheless, according to literatures there were no studies published about the efficacy of AA on oxidative stress and hepatocellular mitochondrial dysfunction in STZ-induced diabetic rats. Thus, the current study was designed to assess the efficacy of AA on hyperglycemia prompted by oxidative stress and hepatocellular mitochondrial dysfunction in STZ-induced type-2 diabetic rats. In addition, the current study has compared the effects of AA with glibenclamide (Glib), an oral anti-hyperglycemic agent.
2 Materials and methods
2.1 Chemicals
The chemicals used for this study like streptozotocin (STZ), asiatic acid (AA), etc., were procured from Sigma-Aldrich (USA). Primary antibodies and secondary antibodies for UCP2 assay were obtained from Santa Cruz Biotechnology (USA). Enhanced chemiluminescence (ECL) reagent used for this study got from Bio-Rad Laboratories (USA). Most of the reagents and chemicals with highest purity used for this study were purchased from Sigma-Aldrich.
2.2 Animals
The experimental study is conducted on male albino rats (180–210 g) that were kept in polypropylene cages and maintained at vivarium conditions (50% humidity, 12-hours light and 12-hours dark cycle at 25 ± 2 °C). The rats were freely allowed to have standard rat diet and drinking water. This study was conducted with strictly adhering to the ethical guidelines for experimental animals recommended by Prince Sattam bin Abdulaziz University, Kingdom of Saudi Arabia.
2.3 Induction of diabetic rats
Type-2 diabetic rats were produced by a single dose of STZ (40 mg/kg b. wt.) administrated as intraperitoneal injection after overnight fasting. STZ was prepared freshly by using citrate buffer (0.1 M, pH 4.5). To prevent STZ-induced sudden hypoglycemic mortality, the rats were allowed to have drinking water with 20% glucose for 24 h. STZ-induced diabetic rats were identified after three days by assessing fasting blood glucose level, estimated by a commercially available glucose assay kit (Cayman Chemical, USA). This entire study was conducted on selected diabetic rats based on the fasting blood glucose level that were higher than 230 mg/dL.
2.4 Experimental design
The study was designed to have five groups of six experimental rats in each group. AA and Glib were dissolved thoroughly in 5% dimethyl sulfoxide (DMSO, prepared in double distilled water) and then administered orally to experimental rats through intragastric tube for a period of 45 days at an intervals of single dose/day.
Group 1: Normal control (NC) rats were treated only 5% DMSO
Group 2: Normal rats + AA (N + AA) were treated (20 mg/kg b.wt.)
Group 3: Diabetic control (DC) rats were treated only 5% DMSO
Group 4: Diabetic rats + AA (D + AA) were treated (20 mg/kg b.wt.)
Group 5: Diabetic rats + Glib (D + Glib) were treated (600 µg/kg b.wt.)
At the end of experimental period, the rats were fasted for 12 h and anesthetized by 24 mg/kg body weight of ketamine, administered as intramuscular injection. The anesthetized rats were sacrificed by cervical dislocation, liver was dissected, washed thoroughly with ice-cold isotonic saline and removed the connective tissues and blood vessels. The dissected liver was weighed and used for mitochondria isolation.
2.5 Mitochondrial preparation
According to the protocol of Johnson and Lardy (1967) liver mitochondria were isolated with slight modification. Briefly, the cleaned liver tissue was weighed, minced thoroughly and homogenized with known volume of homogenization buffer (prepared by Tris-HCl (10 mM), sucrose (0.25 M) and EDTA (1 mM), pH 7.4). The liver homogenate was centrifuged at 600 × g for 10 min then the supernatant obtained was again centrifuged at 15,000 × g for 5 min. The resultant mitochondrial fraction was washed and resuspended with the homogenizing buffer to a known volume. The amount of mitochondrial protein was assessed by Lowry et al. (1951) method. Freshly isolated mitochondrial fraction was used to assess mitochondrial function.
2.6 Biochemical analysis
In the liver mitochondrial fraction, ROS was estimated as illustrated before (Shinomol and Muralidhara, 2007). The concentration of malondialdehyde (MDA), a lipid peroxidation product was assessed by the procedure of Ohkawa et al. (1979). Protein carbonyl (PCO) was measured followed by the protocol of Reznick and Packer (1994). Reduced glutathione (GSH) was estimated according to the instruction of Moron et al. (1979). Oxidized glutathione (GSSG) was quantified based on the protocol of Griffith (1980). Vitamin C was measured followed by the procedure of Omaye et al. (1979). Vitamin E was estimated followed by the instructions of Desai (1984). Superoxide dismutase (SOD) activity was assessed according to the procedure of Kakkar et al. (1998). Catalase (CAT) activity was measured followed by the protocol of Sinha (1972). Glutathione peroxidase (GPx) activity was investigated based on the procedure of Rotruck et al. (1973). Glutathione reductase (GR) activity was determined followed by the instruction of Horn and Burns (1978). Glutathione-S-transferase (GST) activity was assessed according to the protocol of Habig et al. (1974). Isocitrate dehydrogenase (ICDH) activity was measured followed by the procedure of Bell and Baron (1960). α-ketoglutarate dehydrogenase (α-KGDH) activity was quantified based on the instruction of Reed and Mukherjee (1969). Succinate dehydrogenase (SDH) activity was investigated followed by the protocol of Slater and Bonner (1952). Malate dehydrogenase (MDH) activity was assessed followed by the procedure of Mehler et al. (1948). Electron transport chain Complex-I, Complex-II, Complex-III and Complex-IV activities were determined based on the protocols of Brich-Machin et al. (1994), Krahenbuhl et al. (1994), and Smith (1955) respectively. ATP level was estimated according to procedure of Williams and Coorkey (1967). Mitochondrial membrane potential was determined as demonstrated previously (Apaijai et al., 2013).
2.7 Determination of UCP2 protein expression by western blot
UCP2 protein expression was assessed by western blot technique. 80 µg of protein sample from mitochondrial fraction was separated by using 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto polyvinylidene fluoride (PVDF) membrane (Bio-Rad Laboratories, USA). The membrane was blocked with 5% skimmed milk for 2 h and then incubated with primary antibodies against UCP2 (diluted 1:1000) and β-actin (diluted 1:2000) overnight at 4 °C. After washing the membrane in phosphate buffered saline with tween-20 (PBST), incubated with the secondary antibody (horseradish peroxidase-conjugated goat anti-rabbit IgG) at a dilution of 1:1000 for 2 h at room temperature. Then the membrane was washed after one hour and visualized with ECL reagent (Bio-Rad Laboratories, USA). The protein band density was assessed by Image J (Version 1.41) software (NIH, USA).
2.8 Statistical analyses
All the data obtained in this study were expressed as mean ± standard deviation (SD, n = 6). Significant differences were assessed by one-way analysis of variance with Tukey’s multiple comparison as a post-hoc test using a Graphpad prism software (Version 5.0). The values are considered as significant if the P < 0.05.
3 Results
3.1 Effect of asiatic acid on ROS, lipid peroxidation and protein oxidation
The liver mitochondrial ROS, MDA and PCO levels of experimental and normal control rats were demonstrated in Fig. 1. The levels of ROS, MDA and PCO were markedly elevated (P < 0.001) in the liver mitochondria of STZ-induced diabetic rats as compared with the liver mitochondria of normal control rats. When STZ-induced diabetic rats treated with AA and Glib were reduced the levels of ROS, MDA and PCO as compared with untreated diabetic control rats. AA treated with normal control rats did not depict any significant changes in ROS, MDA and PCO levels.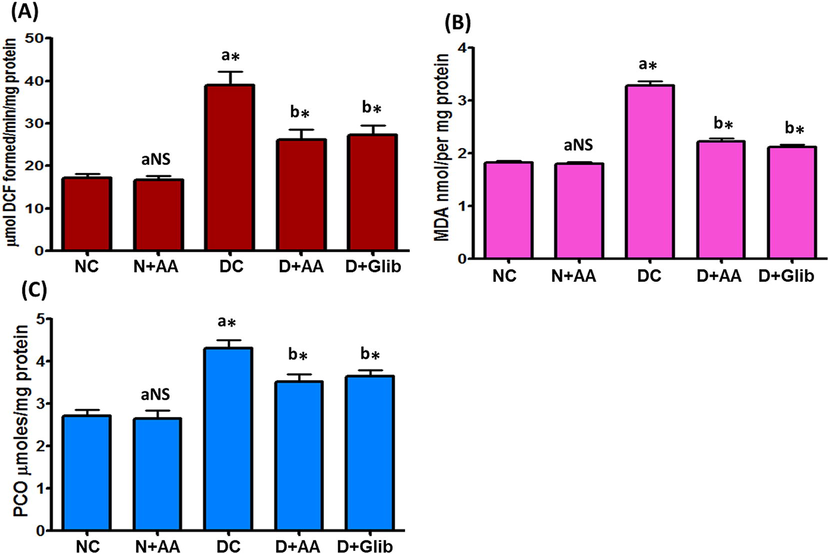
Change of ROS (A), MDA (B) and PCO (C) levels in the liver mitochondria of each group. Data were illustrated as mean ± SD (n = 6). Statistical comparisons were made as “a” diabetic control (DC) rats and normal rats treated with asiatic acid (N + AA) compared with normal control (NC) rats, “b” diabetic rats treated with AA (D + AA) and glibenclamide (D + Glib) compared with diabetic control (DC) rats. NS represent as non-significant, *represents significant difference at p < 0.001.
3.2 Effect of asiatic acid on non-enzymatic antioxidants
Liver mitochondrial GSH, GSSG, vitamin C and vitamin E levels of experimental and normal control rats were illustrated in Fig. 2. GSH, vitamin C, and vitamin E levels were significantly diminished (P < 0.001) at the same time GSSG level was significantly elevated (P < 0.001) in the liver mitochondria of STZ-induced diabetic rats when compared with the liver mitochondria of normal control rats. AA and Glib treatment with STZ-induced diabetic rats were reverted these values near to normal control rats as compared with untreated diabetic control rats. These levels were not altered in the liver mitochondria of normal control rats treated with AA.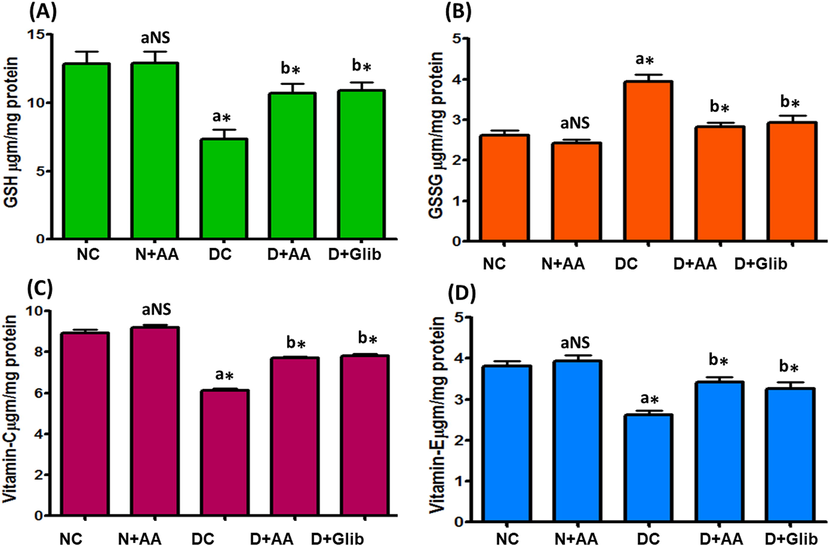
Changes of GSH (A), GSSG (B), vitamin C (C) and vitamin E (D) levels in the liver mitochondria of each group. Data were illustrated as mean ± SD (n = 6). Statistical comparisons were made as “a” diabetic control (DC) rats and normal rats treated with asiatic acid (N + AA) compared with normal control (NC) rats, “b” diabetic rats treated with AA (D + AA) and glibenclamide (D + Glib) compared with diabetic control (DC) rats. NS represent as non-significant, *represents significant difference at p < 0.001.
3.3 Effect of asiatic acid on enzymatic antioxidants
Liver mitochondrial antioxidant enzymes such as SOD, CAT, GPx, GR and GST activities of experimental and normal control rats were illustrated in Figs. 3 and 4. These antioxidant enzymes activities were markedly decreased (P < 0.001) in the liver mitochondria of STZ-induced diabetic rats as compared with normal control rats. AA and Glib treatment were ameliorated these antioxidant enzymes activities to near normal in the liver mitochondria of STZ-induced diabetic rats when compared with untreated diabetic control rats. AA treated normal control rats were not expressed any marked changes in the liver mitochondrial antioxidant enzymes activities.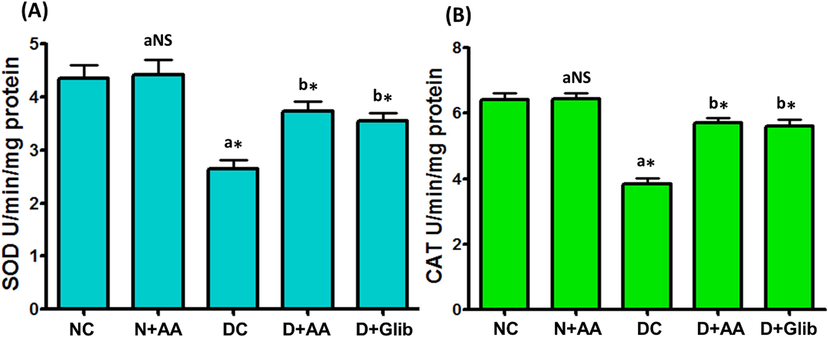
Changes of SOD (A) and CAT (B) activities in the liver mitochondria of each group. Data were illustrated as mean ± SD (n = 6). Statistical comparisons were made as “a” diabetic control (DC) rats and normal rats treated with asiatic acid (N + AA) compared with normal control (NC) rats, “b” diabetic rats treated with AA (D + AA) and glibenclamide (D + Glib) compared with diabetic control (DC) rats. NS represent as non-significant, *represents significant difference at p < 0.001.
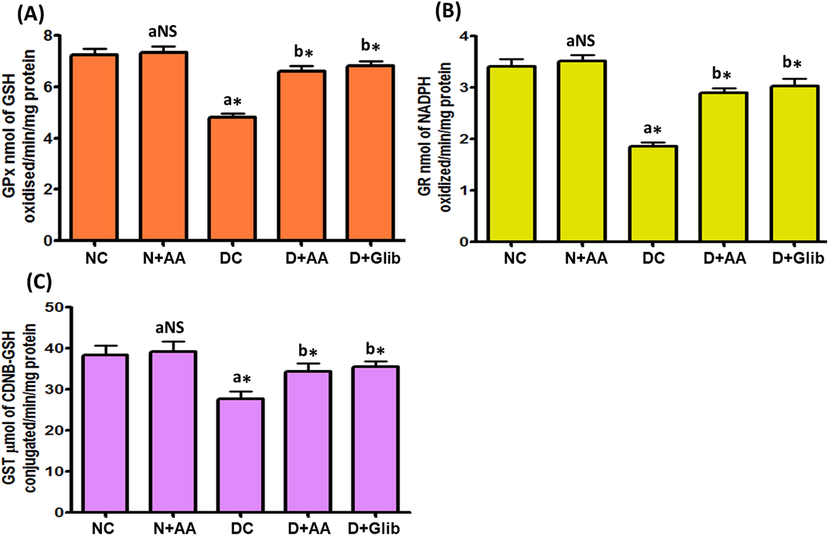
Changes of GPx (A), GR (B) and GST (C) activities in the liver mitochondria of each group. Data were illustrated as mean ± SD (n = 6). Statistical comparisons were made as “a” diabetic control (DC) rats and normal rats treated with asiatic acid (N + AA) compared with normal control (NC) rats, “b” diabetic rats treated with AA (D + AA) and glibenclamide (D + Glib) compared with diabetic control (DC) rats. NS represent as non-significant, *represents significant difference at p < 0.001.
3.4 Effect of asiatic acid on TCA cycle enzymes
The TCA cycle enzymes mainly ICDH, α-KGDH, SDH and MDH activities in the liver mitochondria of experimental and normal control rats were given in Fig. 5. STZ-induced diabetic rats illustrated a marked (P < 0.001) reduction in the activities of ICDH, α-KGDH, SDH and MDH as compared with normal control rats. After AA and Glib treatment, STZ-induced diabetic rats were shown a marked (P < 0.001) elevation in these enzymes activities as compared with untreated diabetic control rats. Besides, these TCA cycle enzymes activities were not changed in the liver mitochondria of normal control rats treated with AA.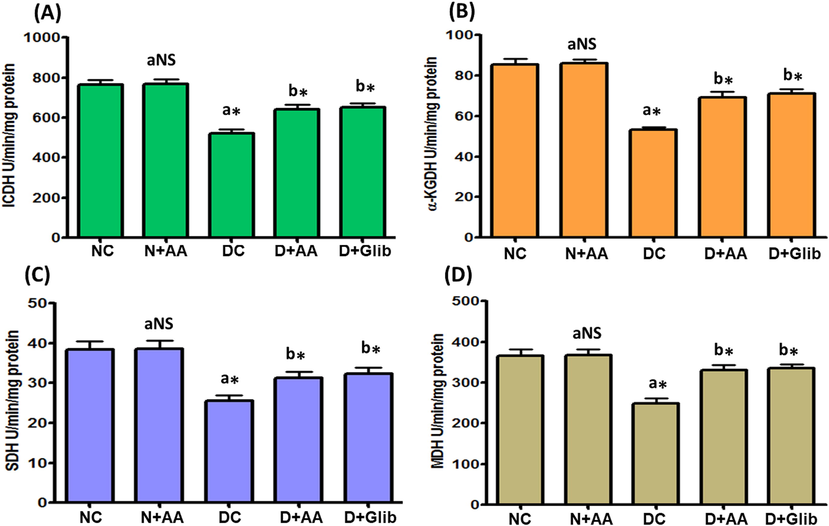
Changes of ICDH (A), α-KGDH (B), SDH (C) and MDH (D) activities in the liver mitochondria of each group. Data were illustrated as mean ± SD (n = 6). Statistical comparisons were made as “a” diabetic control (DC) rats and normal rats treated with asiatic acid (N + AA) compared with normal control (NC) rats, “b” diabetic rats treated with AA (D + AA) and glibenclamide (D + Glib) compared with diabetic control (DC) rats. NS represent as non-significant, *represents significant difference at p < 0.001.
3.5 Effect of asiatic acid on electron transport chain complexes
The ETC complexes such as Complex I, Complex II, Complex III and Complex IV activities in the liver mitochondria of experimental and normal control rats were exhibited in Fig. 6. In STZ-induced diabetic rats, the ETC complexes activities were markedly diminished (P < 0.001) as compared with normal control rats. AA and Glib treatment were reverted the ETC complexes activities to near normal in the liver mitochondria of STZ-induced diabetic rats as compared with untreated diabetic control rats. AA treatment with normal control rats did not depict any significant changes in the ETC complexes activities.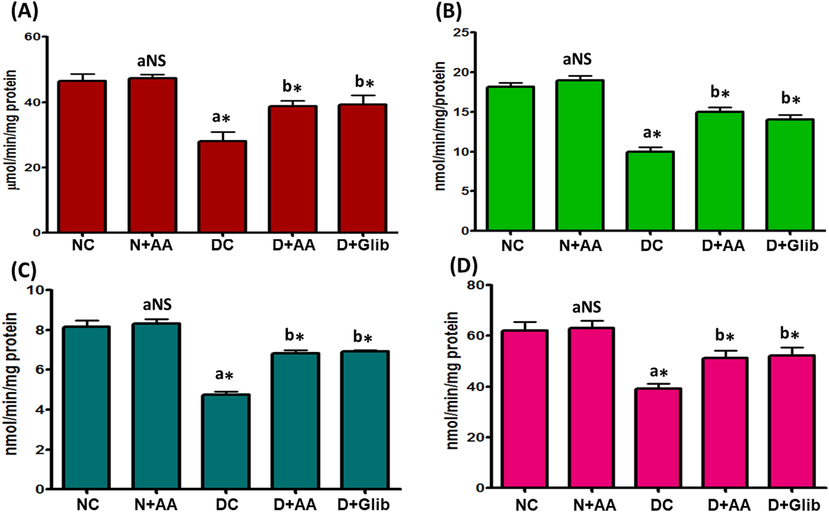
Changes of ETC Complex-I (A), Complex-II (B), Complex-III (C) and Complex-IV (D) activities in the liver mitochondria of each group. Data were illustrated as mean ± SD (n = 6). Statistical comparisons were made as “a” diabetic control (DC) rats and normal rats treated with asiatic acid (N + AA) compared with normal control (NC) rats, “b” diabetic rats treated with AA (D + AA) and glibenclamide (D + Glib) compared with diabetic control (DC) rats. NS represent as non-significant, *represents significant difference at p < 0.001.
3.6 Effect of asiatic acid on UCP2 protein expression
UCP2 protein expression in the liver mitochondria of experimental and normal control rats were revealed in Fig. 7. A marked (P < 0.001) upregulation of UCP2 protein expression was observed in the liver mitochondria of STZ-induced diabetic rats as compared with normal control rats. AA and Glib treatment were ameliorated (P < 0.001) the UCP2 protein expression in the liver mitochondria of STZ-induced diabetic rats as compared with untreated diabetic control rats. The expression of UCP2 was not changed in the liver mitochondria of normal control rats treated with AA.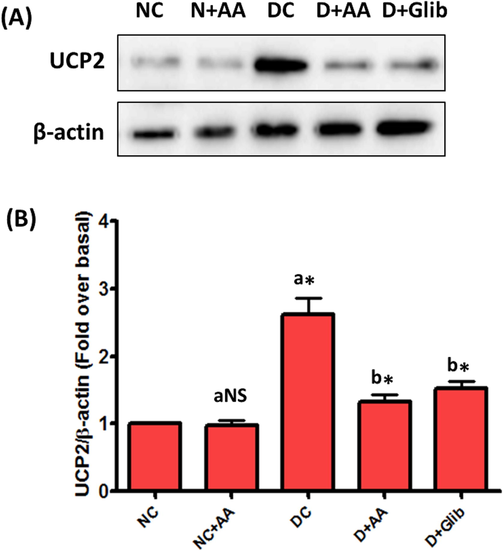
Changes of UCP2 protein expression in the liver mitochondria of each group. Data were illustrated as mean ± SD (n = 6). Statistical comparisons were made as “a” diabetic control (DC) rats and normal rats treated with asiatic acid (N + AA) compared with normal control (NC) rats, “b” diabetic rats treated with AA (D + AA) and glibenclamide (D + Glib) compared with diabetic control (DC) rats. NS represent as non-significant, *represents significant difference at p < 0.001.
3.7 Effect of asiatic acid on mitochondrial membrane potential and ATP level
MMP and ATP level in the liver mitochondria of experimental and normal control rats were shown in Fig. 8. MMP and ATP level was markedly (P < 0.001) diminished in the liver mitochondria of STZ-induced diabetic rats as compared with normal control rats. AA and Glib treatment was brought (P < 0.001) MMP and ATP level to near normal in STZ-induced diabetic rats as compared with untreated diabetic control rats. AA treatment with normal control rats did not show any significant changes in MMP and ATP level.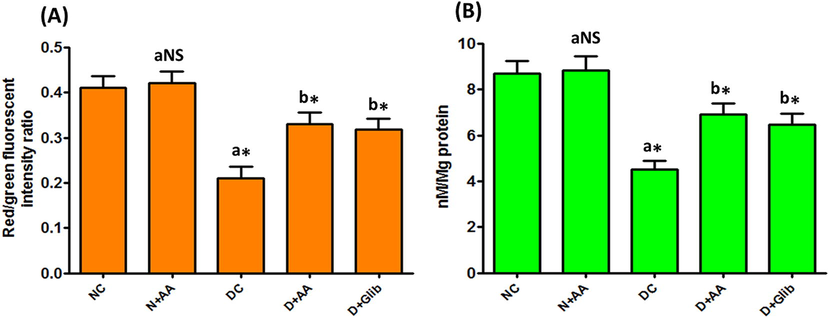
Changes of MMP (A) and ATP (B) levels in the liver mitochondria of each group. Data were illustrated as mean ± SD (n = 6). Statistical comparisons were made as “a” diabetic control (DC) rats and normal rats treated with asiatic acid (N + AA) compared with normal control (NC) rats, “b” diabetic rats treated with AA (D + AA) and glibenclamide (D + Glib) compared with diabetic control (DC) rats. NS represent as non-significant, *represents significant difference at p < 0.001.
4 Discussion
Mitochondria are essential subcellular organelles that plays major role in ATP synthesis and are highly vulnerable to oxidative stress. Dysfunction of mitochondria has been projected as a perilous modulator of ROS formation and inception of apoptosis in diabetic conditions. The potency of the mitochondrial membrane might be lost due to increased formation of ROS in the mitochondria. In agreement with these points, the current study illustrated increased level of ROS in STZ-induced diabetic rat liver mitochondria (Fig. 1A). The increased ROS level may affect the mitochondrial inner membrane efficiency and intends the loss of mitochondrial membrane integrity in diabetic rats. The inner mitochondrial membrane and its physiological functions were disturbed by oxidative stress; this might be attributed to polyunsaturated fatty acids (PUFA) abundantly present in these membrane. During diabetic conditions, the increased ROS reacts with PUFA that stimulate oxidation of lipids present in the mitochondrial membrane which causes mitochondrial membrane structural changes, swelling and interruptions of mitochondrial functions. In addition, increased ROS production may diminish antioxidant defensive system that leads to increased susceptibility of membrane lipid peroxidation, oxidative damage and distraction of cellular functions. In agreement with earlier studies, the present study also observed increased MDA level in the liver mitochondria of STZ-induced diabetic rats (Fig. 1B). Elevated MDA level in the liver mitochondria directs increased lipid peroxidation; this may modify the mitochondrial membrane integrity resulting in mitochondrial damage. In this study, AA treatment decreased the level of ROS and MDA in the liver mitochondria of STZ-induced diabetic rats. This result is concordant with previous reports; AA decreased lipid peroxidation level by increased antioxidant status (Ramachandran and Saravanan, 2013, 2015).
Oxidative damage of proteins is evaluated by generation of PCO, it is also a highly damaging event, and this may arise in the absence of lipid peroxidation. The alteration of proteins by ROS is main cause for several pathophysiological disorders and diseases. Free radicals produced during oxidative stress may activate protein modification and oxidative damage in the mitochondria. Oxidative protein damage has identified as a crucial point in diabetes associated complications. Diabetes-associated alteration of proteins might be attributed to an elevated free radical induced oxidative damage. In this study PCO level was markedly elevated in the liver mitochondria of STZ-induced diabetic rats (Fig. 1C). This result is concomitant with earlier reports (Raza et al., 2011; Alejandra Sánchez-Muñoz et al., 2018). AA treatment was decreased PCO level in the liver mitochondria of diabetic rats. AA might protect the proteins from carbonylation and preserves many enzymes activities which are important for mitochondrial function through its free radical scavenging activity (Ramachandran and Saravanan, 2013, 2015).
All the cells and tissues possess defensive mechanism to protect from oxidative damage. These mechanisms are including non-enzymatic and enzymatic antioxidant system. In the present investigation, a marked reduction of non-enzymatic antioxidants mainly GSH, vitamin C and vitamin E levels were observed at the same time GSSG level was markedly elevated in the liver mitochondria of STZ-induced diabetic rats (Fig. 2). These results are concordant with earlier reports (Raza et al., 2011; Alejandra Sánchez-Muñoz et al., 2018; Aloud et al., 2018). This might be attributed to diabetes-dependent elevation of free radical production in the mitochondria which causes oxidative damage. Besides, decreased NADPH levels will inhibit the conversion of GSSG to GSH by GR; which may accelerate the process of lipid peroxidation and decrease the antioxidant defensive system (Alejandra Sánchez-Muñoz et al., 2018). In this study, administration of AA was enhanced GSH, vitamin C and vitamin E levels likewise reduced GSSG level in the liver mitochondria of STZ-induced diabetic rats as compared with untreated diabetic control rats. These observations are consistent with earlier studies which demonstrate the antioxidant property of AA (Ramachandran and Saravanan, 2013, 2015). AA effectively removes superoxide, peroxide and hydroxyl radicals by enhancing antioxidant status. Therefore, AA supplementation may develop antioxidant defensive system which will decrease the diabetic complications.
Enzymatic antioxidants provide a constant support to defend against free radicals. SOD is the first line enzyme in antioxidant defensive system which will decrease superoxide radicals. In uncontrolled diabetic condition, huge amount superoxide radicals formed by autooxidation of increased glucose that cause lipid peroxidation. SOD produce H2O2 that will be converted in to water and molecular oxygen by CAT, through this mechanism CAT might protect the cells from H2O2 induced oxidative stress. In this study, SOD and CAT activities were markedly lowered in the liver mitochondria of STZ-induced diabetic rats (Fig. 3). Earlier report of SOD and CAT activities (Aloud et al., 2018) are in agreement with this study. Decreased SOD and CAT activities might be attributed to more utilization for scavenging superoxide radicals and these enzymes activities were inactivated by increased production of ROS. AA treatment with STZ-induced diabetic rats brought SOD and CAT activities to near normal. This result depicts that AA might be ameliorated SOD and CAT activities by scavenging superoxide radicals and reducing formation of H2O2 (Ramachandran and Saravanan, 2013, 2015). In addition to these antioxidant enzymes activities, this study also observed diminished GPx, GR and GST activities in the liver mitochondria of STZ-induced diabetic rats (Fig. 4). These GSH dependent antioxidant enzymes plays major role in detoxification of toxic electrophiles, maintaining intracellular GSH level and reduce the lipid peroxidation process. These enzymes activities were markedly depleted in the whole liver of STZ-induced diabetic rats. The lowered activities of these GSH dependent antioxidant enzymes might be linked with decreased level of GSH and an elevated level of lipid peroxidation (Alejandra Sánchez-Muñoz et al., 2018; Aloud et al., 2018). In this study, increased lipid peroxidation and diminished GSH level might be reduced the GPx, GR and GST activities in the liver mitochondria of STZ-induced diabetic rats. Increased activities of GPx, GR and GST were attained in the liver mitochondria of AA treated STZ-induced diabetic rats; this result show the protective effect of AA against diabetic complications. Amelioration of GSH and reduction of lipid peroxidation might be the reason for the elevation of GPx, GR and GST activities in STZ-induced diabetic rats treated with AA (Ramachandran and Saravanan, 2013, 2015).
The mitochondrial enzymes mainly ICDH, α-KGDH, SDH and MDH plays a key role in oxidation of many substrates via TCA cycle or citric acid cycle, produced reducing equivalents, which are transferred to electron transport chain for ATP synthesis through oxidative phosphorylation. ICDH is known as NADP+-dependent enzyme, found in many tissues and localized in the mitochondria as well as in the cytoplasm. α-KGDH enzyme is a main regulation point in the TCA cycle; controlled by its products succinyl CoA and NADH. SDH is one of the key enzymes in the TCA cycle; which in turn will be regulated by succinate, phosphate and ATP. MDH is another important enzyme in the TCA cycle used to synthesis oxaloacetate from malate. In this study ICDH, α-KGDH, SDH and MDH activities were markedly diminished in the liver mitochondria of STZ-induced diabetic rats (Fig. 5). These observations are concomitant with earlier report (Aloud et al., 2018). Decreased activities of these mitochondrial enzymes might be attributed to elevated level of ROS which could be inactivated mitochondrial enzymes by oxidative protein damage. The diminished mitochondrial enzymes activities may decrease mitochondrial function during diabetes. Besides, increased ROS may interfere with substrate oxidation process in the mitochondria; this may lead low level of substrate oxidation result in less amount of reducing equivalents transfer to molecular oxygen and decreased synthesis of ATP (Raza et al., 2011). The TCA cycle enzymes activities were ameliorated in STZ-induced diabetic rats treated with AA as compared with untreated diabetic rats. This result indicate that AA might have restored the mitochondrial function by enhancing antioxidant defensive mechanism (Ramachandran and Saravanan, 2013, 2015), and reducing the mitochondrial damage linked with diabetic complications.
Alterations in the ETC complex activities and oxygen consumption are crucial point for ROS formation in normal physiological conditions. In several pathological conditions, ETC complex activities were altered due to increased production of ROS. Numerous evidences suggested that there may be a close relation between oxidative stress, hyperglycemia and diabetes associated complications (Rolo and Palmeira, 2006). Hyperglycemia increases the electron carriers which may reduce ETC complex activities (Rolo and Palmeira, 2006). This reduction may increase electron leakage, causing a massive formation of ROS, that causes intracellular organ damage. Complex I is an important membrane protein complex of the ETC which stimulates the transfer of electrons from NADH to ubiquinone. It plays a major role in transfer of electrons; protons to the inner mitochondrial membrane that creates an electrochemical gradient which will be used for ATP synthesis. This study observed a marked decrease in the activity of Complex I in the mitochondria of diabetic rats (Fig. 6A). This result is concordant with the reports of previous studies (Al-Nahdi et al., 2017; Aloud et al., 2018). The decreased Complex I activity might be due to less availability of its substrate, NADH produced by TCA cycle. Complex I activity was markedly elevated in the liver mitochondria of AA treated diabetic rats as compared with untreated diabetic rats. This might be attributed to the enhancement of TCA cycle enzymes activities by AA, which supply the substrates for the ETC. Complex III is very important for the whole ETC activities and it is coupled well with Complex II. In this study, Complexes II and Complex III activities were markedly decreased in the mitochondria of diabetic rats (Fig. 6B and C). These observations are concordant with previous studies (Al-Nahdi et al., 2017; Agil et al., 2015; Raza et al., 2015). AA treatment enhanced Complexes II and III activities in the diabetic rat liver mitochondria. The terminal enzyme of ETC is Complex IV; it stimulates the reduction of molecular oxygen with electrons from reduced cytochrome c and synchronously maintaining the reaction potency by pumping protons from mitochondrial matrix to intermembrane space for generation of electrochemical gradient which is essential for ATP synthesis in mitochondria. In this study, Complex IV activity was markedly decreased in the liver mitochondria of diabetic rats (Fig. 6D). Similar result was observed in the earlier studies, which depict a diminution of Complex IV activity in diabetes (Aloud et al., 2018; Al-Nahdi et al., 2017; Agil et al., 2015; Raza et al., 2015). In this study, AA treatment has stimulated Complex IV activity in the liver mitochondria of diabetic rats. Activation of ETC complexes by AA might be an alternate way to decrease extreme ROS generation and reduce the possible impairment of vital components of the cells.
UCP2 present in the mitochondrial inner membrane, is involve in the uncoupling of oxidative phosphorylation from ATP synthesis and also make protons to leak across the inner mitochondrial membrane. UCP2 is identified as a key player for the predisposition of many diseases including diabetes. UCP2 upregulation was observed during excessive ROS formation (Chan et al., 2004). UCP2 also diminish the MMP and reduce ATP synthesis by enhancing proton leakage. Decreased ATP synthesis is a main cause for cellular dysfunction. Earlier study described that ATP synthesis was negatively regulated by UCP2 (Chan et al., 2004). Actually, maximum ATP production is essential for active cellular functions. In this study UCP2 protein expression was markedly increased at the same time MMP and ATP synthesis was significantly lowered in the mitochondria of SZT-induced diabetic rat (Figs. 7 and 8). The findings of this study depicted that increased UCP2 protein expression was activated by excessive generation of ROS. The increased UCP2 may create more proton leakage across the inner mitochondrial membrane and diminished ATP synthesis, which may lead hepatocellular dysfunction. AA treatment was decreased UCP2 protein expression at the same time enhanced MMP and ATP production in the mitochondria of STZ-induced diabetic rats.
5 Conclusion
The data presented here illustrates the effect of AA on hepatic mitochondrial dysfunction through decreased production of ROS, lipid peroxidation, protein oxidation and increased level of antioxidants, TCA cycle enzymes, ETC complexes activities, MMP, ATP production and downregulation of UCP2 protein expression in STZ-induced diabetic rats. The possible protective mechanism of AA might be due to enhancing antioxidants by scavenging free radicals. This study suggest that AA could be a potential compound to protect hepatic mitochondria from oxidative damage associated with diabetic complications. Hence, further studies are necessary to elucidate thorough molecular mechanism of AA and to develop the therapeutic efficacy for the treatment of diabetes associated complications.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Melatonin reduces hepatic mitochondrial dysfunction in diabetic obese rats. J. Pineal Res.. 2015;59:70-79.
- [Google Scholar]
- Alejandra Sánchez-Muñoz, M., Valdez-Solana, M.A., Campos-Almazán, M.I., Flores-Herrera, Ó., Esparza-Perusquía, M., Olvera-Sánchez, S., García-Arenas, G., Avitia-Domínguez, C., Téllez-Valencia, A., Sierra-Campos, E., 2018. Streptozotocin-induced adaptive modification of mitochondrial supercomplexes in liver of wistar rats and the protective effect of Moringa oleifera Lam. Biochem. Res. Int. 5681081.
- Elucidation of molecular mechanisms of streptozotocin-induced oxidative stress, apoptosis, and mitochondrial dysfunction in rin-5F pancreatic β-cells. Oxid. Med. Cell Longev.. 2017;2017:7054272.
- [Google Scholar]
- Galangin, a natural flavonoid reduces mitochondrial oxidative damage in streptozotocin-induced diabetic rats. Redox Rep.. 2018;23:29-34.
- [Google Scholar]
- Effects of vildagliptin versus sitagliptin, on cardiac function, heart rate variability and mitochondrial function in obese insulin-resistant rats. British J. Pharmacol.. 2013;169:1048-1057.
- [Google Scholar]
- Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat. Genet.. 2000;26:435-439.
- [Google Scholar]
- A colorimetric method for determination of isocitric dehydrogenase. Clin. Chem. Acta. 1960;5:740-747.
- [Google Scholar]
- An evaluation of the measurement of the activities of complex I-IV in the respiratory chain of human skeletal muscle mitochondria. Biochem. Med. Metab. Biol.. 1994;51:35-42.
- [Google Scholar]
- Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem.. 1980;106:207-212.
- [Google Scholar]
- Glutathione S-transferase: the first enzymatic step in mercapturic acid formation. J. Biol. Chem.. 1974;249:7130-7139.
- [Google Scholar]
- Glutathione reductase. In: Bergmeyer H.V., ed. Methods of Enzymatic Analysis. NewYork: Academic Press; 1978. p. :875-879.
- [Google Scholar]
- Antinociceptive activities and the mechanisms of anti-inflammation of asiatic acid in mice. Evid. Based Complement. Alternat. Med.. 2011;2011:895857.
- [Google Scholar]
- Vannilic acid ameliorates hyperglycemia-induced oxidative stress and inflammation in streptozotocin-induced diabetic rats. J. King Saud Univ. Sci.. 2020;32:2905-2911.
- [Google Scholar]
- Johnson, D., Lardy, H., 1967. Isolation of liver or kidney mitochondria. In: Estabrook, R.W., Pullman, M.E. (Eds.), Methods in Enzymology, Vol. 10, New York. Academic Press, pp. 94–96.
- Increased oxidative stress in rat liver and pancreas during progression of streptozotocin-induced diabetes. Clin. Sci. (Lond.). 1998;94:623-632.
- [Google Scholar]
- Development and evaluation of a spectrophotometric assay for complex III in isolated mitochondria, tissue and fibroblast from rats and humans. Clin. Chim. Acta. 1994;230:177-187.
- [Google Scholar]
- Kumar, M.P., Poornima, Mamidala, E., Al-Ghanim, K., Al-Misned, F., Ahmed, Z., Mahboob, S., 2020. Effects of D-Limonene on aldose reductase and protein glycation in diabetic rats. J. King Saud Univ. Sci. 32, 1953–1958.
- Protein measurement with the Folin phenol reagent. J. Biol. Chem.. 1951;193:265-275.
- [Google Scholar]
- The enzymatic mechanism of oxidation-reductions between malate or isocitrate and pyruvate. J. Biol. Chem.. 1948;174:961-977.
- [Google Scholar]
- Moron, M.S., Depierre, J.W., Mannervik, B., 1979. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta 582, 67–78.
- Assay for lipid peroxides in animal tissues by thiobarbituric aced reaction. Anal. Biochem.. 1979;95:351-358.
- [Google Scholar]
- Selected methods for the determination of ascorbic acid in animal cells, tissues and fluids. Methods Enzymol.. 1979;62:3-11.
- [Google Scholar]
- Asiatic acid prevents lipid peroxidation and improves antioxidant status in rats with streptozotocin-induced diabetes. J. Funct. Foods. 2013;5:1077-1087.
- [Google Scholar]
- Glucose uptake through translocation and activation of GLUT4 in PI3K/Akt signaling pathway by asiatic acid in diabetic rats. Hum. Exp. Toxicol.. 2015;34:884-893.
- [Google Scholar]
- Antidiabetic and antihyperlipidemic activity of asiatic acid in diabetic rats, role of HMG CoA: in vivo and in silico approaches. Phytomedicine. 2014;21:225-232.
- [Google Scholar]
- Increased oxidative stress and mitochondrial dysfunction in zucker diabetic rat liver and brain. Cell Physiol. Biochem.. 2015;35:1241-1251.
- [Google Scholar]
- Impaired mitochondrial respiratory functions and oxidative stress in streptozotocin-induced diabetic rats. Int. J. Mol. Sci.. 2011;12:3133-3147.
- [Google Scholar]
- α-Ketoglutarate dehydrogenase complex from Escherichia coli. In: Colowick S.P., Kaplon N.O., eds. Methods in Enzymology. Vol vol. 13. New York: Academic Press; 1969. p. :53-61.
- [Google Scholar]
- Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol.. 1994;233:357-363.
- [Google Scholar]
- Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol. Appl. Pharm.. 2006;212:167-178.
- [Google Scholar]
- Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588-590.
- [Google Scholar]
- Shinomol, G.K., Muralidhara, 2007. Differential induction of oxidative impairments in brain regions of male mice following subchronic consumption of Khesari dhal (Lathyrus sativus) and detoxified Khesari dhal. Neurotoxicology 28, 798–806.
- Slater, E.C., Bonner, Jun, W.D., 1952. The effect of fluoride on succinic oxidase system. Biochem. J. 52, 185–196.
- Spectrophotometric assay of cytochrome c oxidase. Methods Biochem. Anal.. 1955;2:427-434.
- [Google Scholar]
- Williams, J.R., Coorkey, B.E., 1967. Assay of intermediates of the citric acid cycle and related compounds by flourimetric enzymatic methods. In: Lowenstein, J.M. (Ed.), Methods Enzymol, Academic Press: New York, NY, USA, pp. 488–492.
- Melatonin and succinate reduce rat liver mitochondrial dysfunction in diabetes. J. Physiol. Pharmacol.. 2011;62:421-427.
- [Google Scholar]







