Translate this page into:
Ovipositional response of Antilochus coquebertii and Dysdercus koenigii in different media under controled conditions
⁎Corresponding authors. sias337@yahoo.com (Syed Ishfaq Ali Shah), waqar4me@yahoo.com (Waqar Jaleel)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Cotton stainer, red cotton bug (Dysdercus spp., Heteroptera: Pyrrhocoridae) is one of the economical pest of cotton crop. Pyrrhocorid bugs have multiple hosts and are highly mobile, rendering their insecticidal control less effective. Among pyrrhocorid bugs, Antilochus coquebertii (Heteroptera: Pyrrhocoridae) is considered highly effective predator of cotton stainers. However, predator and prey proportion and their ecological behavior are very important and there is no previous study regarding oviposition behaviour both of predator and prey. In this study, effects of different media on the fecundity both of predator (A. coquebertii) and prey (D. koenigii) were explored for the first time under controlled conditions. Eight different media consisted of Dry Soil + Dry Leaves (DSDL), Dry Soil + Wet Leaves (DSWL), Wet Soil + Dry Leaves (WSDL), Wet Soil + Wet Leaves (WSWL), Dry Soil (DS), Wet Soil (WS), Dry Soil + Cylindrical Perforated Plastic Bottle (DS-CPPB), and Wet Soil + Cylindrical Perforated Plastic Bottle (WS-CPPB). The results revealed significantly higher number of egg batches both of predator and prey on WSWL followed by WSDL. It is, therefore concluded that both the predator and prey preferred WSWL and WSDL media for egg deposition.
Keywords
Antilochus coquebertii
Dysdercus koenigii
Egg-laying media
Wet Soil
Oviposition
1 Introduction
The red cotton bug or cotton stainer, Dysdercus koenigii Fabricius, (Hemiptera: Heteroptera: Pyrrhocoridae) causes economical damage to cotton crop in various cotton-growing countries including Pakistan since 2008 (Jaleel et al., 2013; Jaleel et al., 2014; Malinga and Laing, 2021; Naqqash et al., 2014). The red cotton bug was considered a minor pest in cotton crop till 2008 then after it became a major pest, due to the cultivation of transgenic cotton varieties containing Bacillus thuringiensis (Bt.): which reduce bollworm populations and use of foliar sprays in cotton growing areas (Greene and Turnipseed, 1996; Shah, 2014). Moreover, the early sowing of Bt. cotton varieties has also played a key role in completing multiple generations and turning their status to major pests of cotton. Dysdercus koenigii feeds on cotton seeds in developing cotton bolls resulting in warts (Shah, 2014) and also transmits fungus on lint and seeds (Bergé and Ricroch, 2010; Lacey et al., 2015), resulting in a stain on the lint (Jaleel et al., 2013; Shah et al., 2016). Feeding of D. koeinigii by puncturing the flower buds or young cotton bolls cause reduction in boll size or partial boll openings which later drop to the ground. Both nymphs and adults of the red cotton bug have a strong proboscis, a needle-like stylet meant for piercing and sucking plant sap (Malinga and Laing, 2021; Saeed et al., 2016).
Biological control of insect pests is the safest method of pest control since it is self-perpetuated, non-toxic, to humans, as well as is a key component of the Integrated Pest Management (IPM) (Heydari and Pessarakli, 2010). However, smooth mass production of natural enemies in the laboratory critically requires parallel mass production of their hosts. Among natural enemies, Antilochus coquebertii (Fabricius) (Heteroptera: Pyrrhocoridae) is the most effective biocontrol agent against cotton stainer and related species (Kohno, 2003; Sarmad et al., 2020). Though, A. coquebertii has a high fecundity rate (Sarmad et al., 2020), and its status as a highly voracious insect predator, just a few studies have been conducted on its ovipositional response. Therefore, this research was designed to investigate high fecundity rate both of A. coquebertii and D. koeinigii in different media under controlled conditions.
2 Materials and methods
2.1 Prey and predator
Adults of A. coquebertii were collected from cotton fields at Central Cotton Research Institute (CCRI), Multan, Punjab, Pakistan in 2020 and its culture was established in the bio-control laboratory on D. koenigii, which was reared from field collected adults in plastic cages (30×30×30cm), kept in the laboratory (26±2°C, 60–65% R.H., and 12L:12D photoperiod). Each Plasrtic cage contained sterilized soil as a natural medium for oviposition Water-soaked fuzzy cotton seeds were provided for food and replaced at 4-6 days intervals. Additionally, 3-6 Petri-dishes (5cm diameter) having moistened cotton wool covered with filter paper were placed in cages as red cotton bug likes moist environment.
2.2 Egg-laying media
The egg-laying media were Dry Soil + Dry Leaves (DSDL), Dry Soil + Wet Leaves (DSWL), Wet Soil + Dry Leaves (WSDL), Wet Soil + Wet Leaves (WSWL), Dry Soil (DS), Wet Soil (WS), Dry Soil + Cylindrical Perforated Plastic Bottle (DS-CPPB) and Wet Soil +Cylindrical Perforated Plastic Bottle (WS-CPPB). Dry and wet cotton leaves were provided as plant debris to observe the fecundity response both of the predator and its prey. Following is a detailed explanation of these eight different media inside every experimental cage:
2.3 DSDL, DSWL, WSDL, and WSWL media
Each of the four media contained 10g of dried cotton leaves. About 6ml water was sprinkled over the leaves of DSWL medium in a way that leaves were taken out carefully from the cage on a small plastic plate, water was sprinkled over the leaves with the help of a syringe and placed back in the cage. The same procedure was adopted with the WSDL medium (leaves were taken out and about 6ml water sprinkled over the soil and leaves placed back in the cage), whereas in the WSWL medium soil and leaves were simultaneously moistened with 6ml water inside the cages and DSDL medium was kept without water application.
2.4 DS and WS
Dry Soil medium was kept water-free whereas, WS medium was having only the soil sprinkled directly with 6ml water inside the cage.
2.5 DS-CPPB and WS-CPPB
Similarly, a plastic bottle measuring 7.62cm in length and 4.83cm in diameter having 24 holes drilled all around was filled with wet cotton wool. Delinted cotton seeds were inserted randomly in 12 holes, while the rest of the holes were left empty for sucking water both by predator and prey. Each perforated bottle was placed inverted in plastic Petri dishes of 5cm diameter (Fig. 5). Two CPPBs were kept i.e. (DS-CPPB and WS-CPPB) in each A. coqueberti and D. koenigii cages. Daily, 3ml water was applied with help of a syringe over the open-top perforated bottles, while additional 3ml water was applied in the soil around the WS-CPPBs, and the soil around DS-CPPBs was kept dry. The perforated bottles with cotton wool and seeds were replaced at 4-6 days intervals (Shah et al., 2016).
2.6 Predator and prey releases
In each replication, four newly formed pairs of A. coquebertii and four pairs of D. koenigii adults along with eight nymphs each of third, fourth, and fifth instars were collectively released over the above-mentioned eight different media. In each replication of the cotton stainer, ten newly formed adults were released over these eight different media.
2.7 Incubation
Egg batches of predators and its prey were collected from different media and were kept in glass Petri dishes of 5cm diameter conatining a layer of wet soil compact with thumb pressure. Eggs were placed on compact soil in the Petri-dishes andwere then kept in an incubator at 30°C and 50±5 % R.H.
2.8 Data recording
Observations regarding the egg batches, incubation period, egg hatching percentage, and predation were recorded at 24h intervals.
2.9 Data analyses
Data regarding fecundity/egg laying, incubation period, egg hatching percentage (both predator and prey), and predation efficacy (predator) were analyzed using one-way ANOVA with LSD test (Jaleel et al., 2013).
3 Results
3.1 Egg batches in different media
The highest number of egg batches (12.00±1.20) of A. coquebertii were observed on WSWL medium as compared to (DSWL, WSDL and WS-CPPB) media, whereas lowest number of egg batches (1.00±0.50) on DSWL (Fig. 1, P<0.05), however, no egg batches were recorded in whole course of the experiment on (DSDL, DS, WS and DS-CPPB). Similarly, highest number egg batches of D. koenigii were also recorded on WSWL (32.80±1.45) compared with (DSWL, WSDL and WS-CPPB) media, whereas, lowest number of egg batches (1.00±0.20) were collectively reported on DSDL and DS-CPPB (Fig. 1, P<0.001), however, no egg batch of D. koenigii was recorded throughout the experiment on DS and WS media (Fig. 1).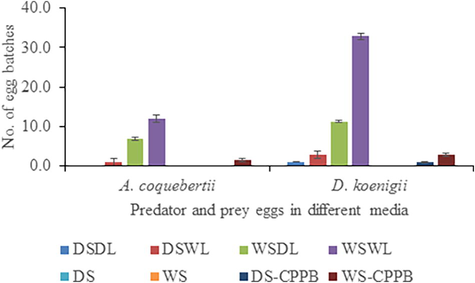
Number of egg batches laid by A. coquebertii and D. koenigii on different media. Dry Soil + Dry Leaves (DSDL), Dry Soil + Wet Leaves (DSWL), Wet Soil + Dry Leaves (WSDL), Wet Soil + Wet Leaves (WSWL), Dry Soil (DS), Wet Soil (WS), Dry Soil + Cylindrical Perforated Plastic Bottle (DS-CPPB), and Wet Soil + Cylindrical Perforated Plastic Bottle (WS-CPPB). Error bar within the column bar showing the standard error.
3.2 Incubation period in different media
The maximum incubation period of A. coquebertii was noticed on WSWL (8.50±1.23d) as compared with DSWL, WSDL and WS-CPPB (Fig. 2, P<0.05). But in case of D. koenigii, maximum incubation period was reported on WSDL (7.30±0.10d) compared with DSDL, DSWL, WSWL, DS-CPPB and WS-CPPB media (Fig. 2, P<0.001).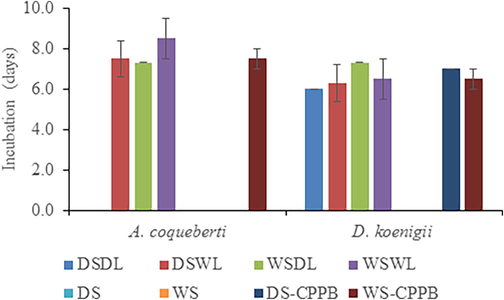
Effect of different media on the incubation period of eggs laid by A. coquebertii and D. koenigii. Dry Soil + Dry Leaves (DSDL), Dry Soil + Wet Leaves (DSWL), Wet Soil + Dry Leaves (WSDL), Wet Soil + Wet Leaves (WSWL), Dry Soil (DS), Wet Soil (WS), Dry Soil + Cylindrical Perforated Plastic Bottle (DS-CPPB), and Wet Soil + Cylindrical Perforated Plastic Bottle (WS-CPPB). Error bar within the column bar showing the standard error.
3.3 Number of eggs per batch and their hatchability
The maximum number of eggs per batch (78.80) and hatching percentage (90.00%) of A. coquebertii were collectively recorded on WSWL compared with other DSWL, WSDL and WS-CPPB media (Fig. 3). Likewise, the predator, D. koenigii also laid maximum number of eggs per batch (174.30) and highest hatching percentage (93.30%) on WSWL medium as compared with DSWL, WSDL, DS-CPPB and WS-CPPB media (Fig. 3).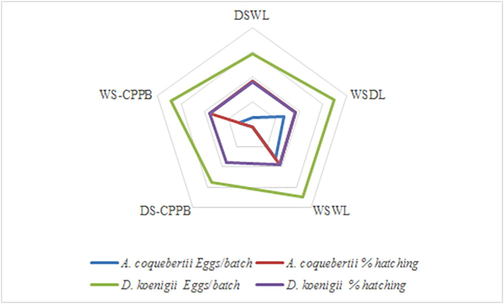
Number of eggs per batch and their hatching (%) of A. coquebertii and D. koenigii on different media. Dry Soil + Wet Leaves (DSWL), Wet Soil + Dry Leaves (WSDL), Wet Soil + Wet Leaves (WSWL), Dry Soil + Cylindrical Perforated Plastic Bottle (DS-CPPB), and Wet Soil + Cylindrical Perforated Plastic Bottle (WS-CPPB). Note: Dry Soil + Dry Leaves (DSDL), Dry Soil (DS), and Wet Soil (WS) were not suitable for the fecundity.
3.4 Predation
Maximum predation percentage (64.3) was recorded on third instar of D. koenigii as compared with fourth instar to adult stage, however, predation percentage of fourth instar of the prey was reported significantly different from fifth instar (Fig. 4, P<0.05), whereas predation percentage on fifth instar and adult stage were recorded statistically similar.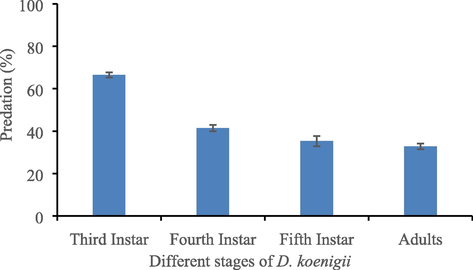
Predation (%) of A. coquebertii on choice of immature and mature stages of D. koenigii. Error bar within the column bar showing the standard error.

Cylindrical Perforated Plastic Bottle (CPPB; Shah et al., 2016).
4 Discussion
Among the sucking insect pests of cotton, red cotton bug is the most noxious pests in Pakistan (Noman et al., 2016). Interestingly, A. coquebertii is reported to be a voracious predator on it (Vreysen et al., 2016), however, for its eco-friendly management, the predator needs to be further investigated (Shafiq et al., 2020). This study was mainly focused to evaluate factors that enhance the fecundity of A. coquebertii and D. koeinigii for their rapid mass production under laboratory conditions for augmentative field releases of A. coquebertii, which will help maintaining ecological balance.
Kohno (2003) experimented on the fecundity of A. coquebertii, but the collection sites of eggs were not mentioned as to where he actually collected the egg batches. We noticed maximum fecundities of A. coquebertii and D. koenigii on WSWL medium. However, no egg batch of A. coquebertii was recorded on DSDL, DS, WS, and DS-CPPB. It means, A. coquebertii females mostly preferred moist soil below the plant debris or residues for egg-laying.
Similarly, most of the studies reported that D. cingulatus deposited eggs under the bottom of Petri-dishes provided for moisture and food, while in the field it oviposited in shallow depressions made in the soil under debris or occasionally on undersides of cotton leaves below the plant canopy (Bissdorf, 2005). Likewise, the predator, no eggs batch of D. koenigii was found on DS and WS media throughout the experiment. Our findings are in accordance with (Verma et al., 2013), who reported egg batches mostly under plant debris in field conditions and Karar et al. (2020) noticed oviposition in moist patches below the plant residues.
Soil medium is also important for the egg period and development of red cotton bug. Jaleel et al. (2013) recorded incubation period of 6.70 days: Verma et al. (2013) recorded average incubation period of 4.97 days. However, the egg-laying media had a significant effect on the incubation period of bugs. In our study, the longest incubation period of A. coquebertii eggs was (8.50±1.23d) on WSWL medium, while in case of D. koenigii, it was observed to be (7.30±0.10) on WSDL.
Jaleel et al. (2013), Verma et al. (2013) and Bissdorf (2005) collectively reported 7 to 100 eggs per batch of red cotton bug, while in case of A. coquebertii, Shafiq et al. (2020) recorded 61 eggs per batch. It is evident from the results that fecundity of both the predator and its prey could be enhanced on moist soil patches covered with plant decaying material (Shafiq et al., 2020), as we reported (174.3 and 78.80) eggs per batch of D. koenigii and A. coquebertii respectively on WSWL medium.
The most interesting findings of this research indicated the common behavior both of predator and the prey regarding fecundity and oviposition sites which can lead to an efficient source of mechanical control (Muthupandi et al., 2014) and their convenient eggs collection.
Significantly maximum predation was recorded third instar of the prey as compared with fourth to adult stage which indicates that predation is inversely proportional to the prey size. Analogous findings were recorded by Kohno (2003), who reported that the adult stage of the A. coquebertii consumed fewer adults of D. koenigii and fifth instar nymphs, while it consumed higher number of rests of the younger stages of the prey, which suggested that prey body mass and size affect predation efficiency.
5 Conclusion
Generally, A. coquebertii preferred moist soil for egg-laying whereas, its prey, D. koenigii laid eggs on soil irrespective of its moisture content under the decaying plant residues. However, moist soil under the plant debris increases their eggs-laying potential. Such findings regarding the egg-laying behavior both of the predator and its prey suggest key factors for rapid mass production in the laboratory for safe management of red cotton bug including other pyrrhocorid pests. Rapid reproduction rate, high mobility and wide host range are major bottle-necks that make red cotton bug control rather difficult under field conditions, however, information about such habitats would provide a suitable platform for employing IPM tactics against the red cotton bug and colonies of its early nymphal instars could give a chance for mass collection of A. coquebertii.
Acknowledgements
The authors extend their appreciation to the support of the Research Center for Advanced Materials Science (RCAMS) at King Khalid University, Abha, Kingdom of Saudi Arabia through a project number RCAMS/KKU/G003-21. The principal authors cordially acknowledge the cooperation of Dr Zhaid Mahmood, Director, Central Cotton Research Institute, Multan and entire staff of the Entomology Section, especially, Muhammad Akbar, Nadeem Saquib, and Muhammad Ibrahim for their help in the collection of predators and its prey and maintenance of laboratory cultures. We are highly grateful to Mr. Iqbal Arif, Senior Scientific Officer (Rtd.), CCRI, Multan for the suggestions and critical review of the article.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Emergence of minor pests becoming major pests in GE cotton in China: what are the reasons? What are the alternatives practices to this change of status? GM Crops. 2010;1(4):214-219.
- [Google Scholar]
- Field guide to non-chemical pest management in cotton production–Pesticide Action Network (PAN). Germany: Hamburg; 2005.
- Greene J, Turnipseed S (1996): Stink bug thresholds in transgenic Bt cotton, 1996 Proceedings Beltwide Cotton Conferences, Nashville, TN, USA, January 9-12, 1996: Volume 2. National Cotton Council, pp. 936-938
- A review on biological control of fungal plant pathogens using microbial antagonists. J. Biol. Sci.. 2010;10(4):273-290.
- [Google Scholar]
- Biology and bionomics of Dysdercus koenigii F. (Hemiptera: Pyrrhocoridae) under laboratory conditions. Pakistan J. Agri. Sci.. 2013;50:373-378.
- [Google Scholar]
- Survey of Bt cotton in Punjab Pakistan related to the knowledge, perception and practices of farmers regarding insect pests. Int. J. Agric. Crop Sci.. 2014;7:10.
- [Google Scholar]
- The impact of adjacent habitats on population dynamics of red cotton bugs and lint quality. PLoS ONE. 2020;15(12) e0242787
- [Google Scholar]
- Development and reproduction of Antilochus coqueberti (Heteroptera: Pyrrhocoridae), the specific predator of Dysdercus spp. (Heteroptera: Pyrrhocoridae) App. Entomol. Zool.. 2003;38(1):103-108.
- [Google Scholar]
- Insect pathogens as biological control agents: back to the future. J. Invert. Pathol.. 2015;132:1-41.
- [Google Scholar]
- Efficacy of three biopesticides against cotton pests under field conditions in South Africa. Crop Prot.. 2021;145:105-578.
- [Google Scholar]
- Biology of pyrrhocorid predator, Antilochus conqueberti Fabr. (Hemiptera: Pyrrhocoridae) and its predatory potential on Dysdercus cingulatus Fabr. (Hemiptera: Pyrrhocoridae) J. Ent. Zool. Stud.. 2014;2:91-96.
- [Google Scholar]
- Effect of host plants on life history traits of Dysdercus koenigii (Hemiptera: Pyrrhocoridae) J. Biol. Environ. Sci.. 2014;4:187-194.
- [Google Scholar]
- Cotton stainer, Dysdercus koenigii (Heteroptera: Pyrrhocoridae) eggs laying preference and its ecto-parasite, Hemipteroseius spp levels of parasitism on it. Appl. Sci. Bus. Econ.. 2016;3:01-07.
- [Google Scholar]
- Toxicological studies on some important chemicals against Dysdercus koenigii Fabr (Hemiptera: Pyrrhocoridae) Pakistan J. Zool.. 2016;48:1249-1254.
- [Google Scholar]
- Fitness and Predating Potential of Antilochus coquebertii (Hemiptera: Pyrrhocoridae): A Predator of the Red Cotton Bug (Dysdercus koenigii) J. Kansas Entomol. Soc.. 2020;92:589-601.
- [Google Scholar]
- Survival and fecundity of Antilochus coquebertii (Hemiptera: Pyrrhocoridae) on different stages of Dysdercus koenigii (Hemiptera: Pyrrhocoridae) J. Kansas Entomol. Soc.. 2020;92:526-535.
- [Google Scholar]
- The cotton stainer (Dysdercus koenigii): An emerging serious threat for cotton crop in Pakistan. Pakistan J. Zool.. 2014;46:329-335.
- [Google Scholar]
- Comparison of the newly introduced rearing methods of cotton stainer, Dysdercus koenigii (Hemiptera: Pyrrhocoridae) with classical methods. Pakistan J. Zool.. 2016;48:781-787.
- [Google Scholar]
- Overview of technological advances toward greater efficiency and efficacy in sterile insect-inherited sterility programs against moth pests. Florida Entomol.. 2016;99(sp1):1-12.
- [Google Scholar]







