Translate this page into:
Optimization of medium components for the production of antimicrobial and anticancer secondary metabolites from Streptomyces sp. AS11 isolated from the marine environment
⁎Corresponding authors. myalansari@ksu.edu.sa (Mysoon Al-Ansari), venzymes@gmail.com (Ponnuswamy Vijayaraghavan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this study, 102 actinomycetes were isolated from the marine environment from three different locations at Saudi Arabia. Among the actinomycetes isolates, Streptomyces sp. AS11 showed significant activity against methicillin-resistant Staphylococcus aureus (MRSA) was selected for further studies. It has grown well at pH 7.3, 29 °C and with 34% seawater. Based on colony morphology, biochemical, and 16S rDNA sequencing the selected organism was identified as Streptomyces sp. AS11. Among the selected culture medium, ISP4 showed more activity against S. aureus and the zone of inhibition was 29 ± 2 mm. The factors such as, pH and temperature of the culture medium significantly affected secondary metabolite production. Secondary metabolite production was found to be maximum at pH 7.5 (31 ± 1 mm) and marginal decrease in antibiotics production was observed at pH 8.0 (27 ± 1 mm). Antibiotics production was found to be high at 38 °C and the zone of inhibition was 32 ± 2 mm. Among the carbon sources tested, supplementation of glucose and starch enhanced antibiotics production and the zone of inhibition was 31 ± 2 mm and 30 ± 3 mm, respectively. Among the nitrogen sources tested, sodium nitrate enhanced antibiotics production. The secondary metabolite concentration at 2× minimum inhibitory concentration (MIC) and 4× MIC was effectively reduced MRSA. 4× MIC concentration was found to be effective than 2× MIC values and reduced bacterial count considerably. In our study, the crude extract showed cytotoxic effect against cancer cell line and the IC50 value was 0.250 mg/ml. The crude extract acts on HeLa and abnormal cell morphology was observed due to cytotoxic effect.
Keywords
Streptomyces sp. AS11
Secondary metabolites
Anti-MRSA
Optimization
Cytotoxicity
1 Introduction
Antibacterial resistance among bacterial pathogens is an important public health problem (Al Dhabi et al., 2016, 2019a, 2020a). The bacterial pathogens such as, Enterococcus species and multiple drug resistant Staphylococcus aureus presently pose the serious threat (Al-Dhabi et al., 2020b). The pathogenic bacterium, methicillin-resistant Staphylococcus aureus has become an important source of community associated and nosocominal MRSA infections (Al-Dhabi et al., 2019; Boucher and Corey, 2008; Al-Dhabi et al., 2016, 2019a, 2019b, 2020a). The clinical strains of MRSA have very high mortality and morbidity than methicillin sensitive S. aureus (Chen et al., 2010; Al-Dhabi et al., 2018a). Like MRSA, Enterococcus faecium was also reported as multiple drug resistance against high-levels of aminoglycosides, ampicillin and vancomycin and associated with human infections (Al-Dhabi et al., 2018b). Vancomycin was frequently used as antibacterial drug to treat Enterococcus and MRSA (Al-Dhabi et al., 2019d). In Japan, the first case of MRSA was reported and the clinical isolate showed resistance against vancomycin (Al-Dhabi et al., 2014; Arasu et al., 2017). To cure this pathogen, antibacterial drugs such as, linezolid, synercid and daptomycin have been used; however report stated that these bacterial strains also have emerged resistance against these drugs (Sharma et al., 2008; Arasu et al., 2013a; Arasu et al., 2013b). Recently, MRSA cases have been reported outside of the hospitals, mainly affecting healthy individuals (Braun and Kahanov, 2018). Antibiotics such as tetracycline, aminoglycosides, clindamycin, macrolides and rifampin inhibit protein synthesis of bacteria. In recent years, aminoglycoside resistance, tetracycline resistance in bacteria and their mechanisms were reported by various research groups (Ong et al., 2017; Arasu et al., 2019). At present, vancomycin is the most useful drug to treat MRSA infections. Moreover, the emergence of MRSA with activity against vancomycin, daptomycin and resistance to linezolid were reported (Ghahremani et al., 2018). In recent years, many potent therapeutic lead molecules have been isolated and characterized from actinomycetes. Among actinomycetes, Streptomyces species have immense potential as they are well known producers of various lead molecules with novel biological properties (Kumar et al., 2012; Al-Dhabi et al., 2020d).
Streptomyces species account for about 70% of the total production of antibiotics and the genus such as, Micromonospora showed less amount of antibiotics production. In a study, Kumar et al. (2010) isolated 117 secondary metabolite synthesizing actinomycetes from compost rich garden soil and wasteland alkaline soil. Cwala et al. (2011) isolated four new actinomycetes from the aquatic environment for antibacterial activity. In a study, Valli et al. (2012) isolated various actinomycetes from extreme environment and the screened actinomycete showed actibacterial activity against the selected bacteria. Kalyani et al. (2012) isolated more than 20 actinomycete species from the soil sample and among the 20 actinomycetes, three showed potent antibacterial activity against Escherichia coli and Staphylococcus aureus. Although, about two third of the commercially available antibiotics are produced from Streptomyces sp. however, only little have been explored. Hence, to fight with multiple drug resistance and to find novel lead molecules, it is essential to screen various Streptomyces sp. from various sources. Considering this view point, we isolated actinomycetes from soil sample showing activity against multidrug resistant bacteria.
2 Materials and methods
2.1 Samples and isolation of actinomycetes
Actinomycetes were isolated from the marine environment from three different locations at Saudi Arabia. Soil was collected and air dried for a day then heated at 40 ± 2 °C for five days. This pre-treated soil was used for the isolation of actinomycetes. For the isolation of actinomycetes, Bennet’s agar (g/l) (yeast extract-1, beef extract-1, casein enzymic hydrolysate-2, dextrose-10 and agar-15) and Starch casein agar (g/l) soluble starc-10, casein-0.3, potassium nitrate-2, magnesium sulphate-0.05, dipotassium hydrogen phosphate-2, sodium chloride-2, calcium carbonate-0.02, ferrous sulphate-0.01 and agar-18) was used. The sample was extracted with sea water and used for the isolation of actinomycetes. To the culture medium antibiotics such as, nalidixic acid (25 mg/ml) and cycloheximide (25 mg/ml) were supplemented.
2.2 Screening of actinomycetes for the secretion of antibiotics
About 20 µl cell free supernatant was loaded on filter disks. The plate was incubated for 24 h at 37 °C. All actinomyces isolates were screened against the selected bacterial isolates and the zone of inhibition was assayed (CLSI, 2015).
2.3 Characterization of actinomycetes
The potent actinomycete isolate was subjected for characterization studies. It was subjected to cultural, morphological, biochemical and physiological characteristics (Prauser, 1964). Spore chain morphology, spore bearing hyphae with aerial and substrate mycelium of actinomycete was evaluated by light microscope (Pridham and Gottlieb, 1948). Growth of mycelium and colour of colonies were observed. Growth of actinomycetes towards carbon sources were performed by supplementing carbon sources at 1% level. DNA extraction from the selected actinomycete isolate was performed as suggested by Marmur (1961). The purity of the extracted DNA was performed using a nanodrop spectrophotometer. The primers used were: 5′AGAGTTTGATCATGGC TCAG 3′ (forward) and 5′ TACGGCTACCTTGTTACGACTT-3′ (reverse) for 16S rDNA amplification (Lane, 1991).
2.4 Optimization of secondary metabolite production
Initially, the selected bacterial strain was grown in various culture media (starch casein agar medium, nutrient agar (g/l) (peptone-5, sodium chloride-5, HM peptone B-1.5, yeast extract-1.5 and agar-15), Bennett’s agar, ISP Medium 1 (g/l) (pancreatic digest of casein-5 and yeast extract − 3), ISP medium 2 (g/l) (yeast extract-4, malt extract-10, dextrose-4 and agar – 20) and ISP medium 4 (g/l) (soluble starch-10, dipotassium phosphate-1, magnesium sulphate-1, magnesium sulphate-1, sodium chorode-1, ammonium sulphate-2, calcium carbonate-2, ferrous sulphate-1, manganous chloride-1, zinc sulphate-1 and agar-20) and optimum medium was screened. The effect of sodium chloride on culture medium (2–6%), pH (5.0–8.0), and temperature (20–50 °C). The required concentration of carbon and nitrogen sources was performed as suggested by Fourati-Ben Fguira et al. (2005).
2.5 Submerged fermentation and extraction of secondary metabolites
The selected Streptomyces sp. AS11 was inoculated in to 500 ml culture flask containing 150 ml medium (ISP 4) supplemented with optimum carbon and nitrogen source. Then the culture was incubated for seven days and cell free extract was used for analysis. The cell free extract was obtained after extraction with ethyl acetate and bioactive compound was obtained by evaporating organic layer.
2.6 Time kill assay
To determine time kill assay the anticancer secondary metabolite was prepared as 2× MIC and 4× MIC concentrations. It was added to the drug resistant culture broth and the growth was monitored for every 2 h up to 24 h. The broth was diluted appropriately and plated on MHA and incubated for 24 h at 37 ± 2 °C. The reduction in viable bacteria was counted and killing rate was analyzed (log10 CFU/mL) Pucci et al. (2011) and NCCLS (1999). Experiments were performed in triplicates and killing curve was plotted.
2.7 Cytotoxicity analysis
In this study, anticancer properties of the secondary metabolites were determined using HeLa (cervical carcinoma) cell line according to the method suggested by Roch et al. (2017) with minor modifications.
3 Results
3.1 Recovering and screening of actinomycetes for secondary metabolite production
In this study 102 actinomycetes were initially isolated from the marine environment collected three different locations. Among the actinomycetes isolates, the strain named as, Streptomyces sp. AS11 showed significant activity against MRSA was selected for further studies. The cell free extract showed potent antibacterial activity against MRSA. The selected actinomycetes isolate showed grey colour, aerial mycelium and observed spore chain under microscope (1000 ×). Also, spore surface was very smooth. The selected actinomycete grown well in the ISP 4 medium supplemented with sea water. The growth of this organism was found to be high than that of Actinomycetes isolation agar. It was grown well at pH 7.3, 29 °C and with 34% seawater. The selected actinomycete utilized sugars such as, arabinose, xylose, rhammose, raffinose, sucrose and mannitol (Table 1). The selected organism utilized various inorganic nitrogen sources such as, L-phenylalanine, L-asparagine and L-hydroxyprolone at 0.2% level. It was tolerated at various concentrations of NaCl (upto 15%). Based on colony morphology, biochemical, physiochemical factors and 16S rDNA sequence, the selected organism was identified as Streptomyces sp. SA 11. Fig. 1 shows antagonistic activity of extract of Streptomyces sp. SA 11 against selected bacteria.
Streptomyces sp. SA 11
Characteristics
Gram's staining
Positive
Shape
Filamentous
Anaerobic growth
Negative
Motility test
Non-motile
Growth range (pH)
6.0–8.5
Optimum pH
7.3
Growth range (Temperature)
25–40 °C
Optimum temperature
29 °C
NaCl tolerance
Survives up to 15%
Melanoid pigments
Negative
Aerial mass colour
White
Soluble pigments
Negative
Reverse side pigments
Negative
Xylose
positive
Arabinose
Positive
Mannitol
Positive
Rhammose
Negative
Raffinose
Positive
Sucrose
Positive
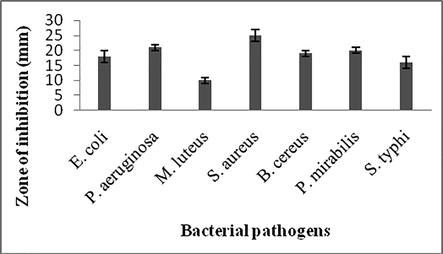
Antimicrobial property of secondary metabolites against various bacterial pathogens. Values are mean ± SD of duplicate experiments.
The crude extract was tested against various pathogenic bacteria and showed potential activity against most of the organisms. Zone of inhibition varied widely among the bacteria. Zone of inhibition was 18 ± 2 mm, 21 ± 1 mm, 10 ± 1 mm against E. coli, P. aeruginosa and M. luteus, respectively. Among the selected bacterial isolates, S. aureus was highly sensitive to the crude extracts. The tested bacteria such as, P. mirabilis and S. typhi showed 20 ± 2 mm, 16 ± 2 mm zone, respectively (Fig. 1).
3.2 Effect of various culture media on antibiotics production
The selected culture medium influenced on secondary metabolites production. The actinomycete isolate was inoculated at 5% level and incubated at 28 °C for 7 days and the zone of inhibition was assayed. Among the selected culture medium, ISP4 showed more activity against S. aureus and the zone of inhibition was 29 ± 2 mm. For further studies, ISP4 was selected until otherwise stated (Fig. 2).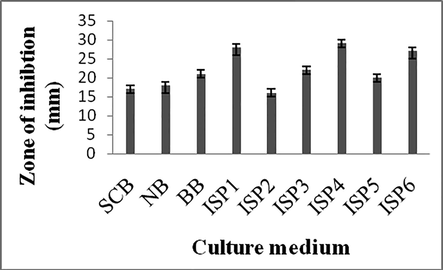
Influence of various culture media on secondary metabolites production by Streptomyces sp. SA 11.
3.3 Optimization of physical factors to enhance secondary metabolites production
In this study, pH and temperature of the culture medium significantly affected secondary metabolite production. The activity was noted maximum at pH 7.5 (31 ± 1 mm) and marginal decrease in antibiotic production was observed at pH 8.0 (27 ± 1 mm) (Fig. 3a). Temperature is one of the important factors affect on antibiotics production. Antibiotics production was found to be high at 38 °C and the zone of inhibition was 32 ± 2 mm (Fig. 3b).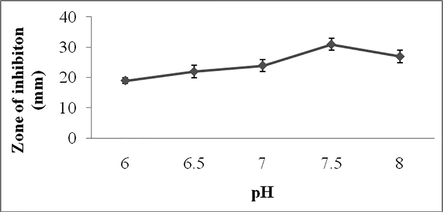
Effect of pH on secondary metabolite production. Values are mean ± SD of duplicate experiments.
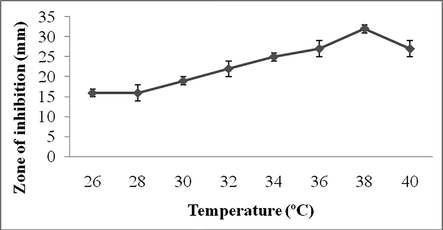
Effect of temperature on secondary metabolite production. Values are mean ± SD of duplicate experiments.
3.4 Effect of nutritional components on antibiotics production
In the present study, carbon sources such as, glucose, sucrose, lactose, fructose, maltose and starch were supplemented with the culture medium. To the control, carbon sources were not included. Among the carbon sources tested, supplementation of glucose and starch enhanced antibiotics production and the zone of inhibition was 31 ± 2 mm and 30 ± 3 mm, respectively (Fig. 4a). Among the nitrogen sources tested sodium nitrate enhanced antibiotics production. The control experiment showed 32 ± 2 mm zone of inhibition and the organic nitrogen sources such as, tryptone, beef extract and yeast extract affected antibiotics production. However, supplemented peptone showed positive impact on secondary metabolites production (Fig. 4b).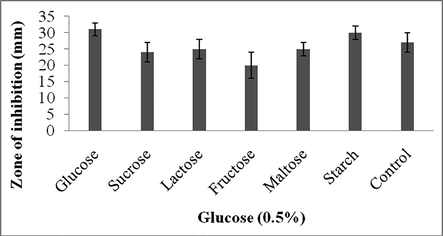
Effect of carbon sources on secondary metabolite production. Values are mean ± SD of duplicate experiments.
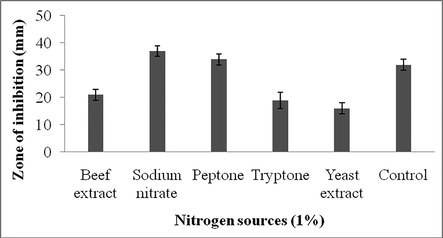
Effect of nitrogen sources on secondary metabolite production. Values are mean ± SD of duplicate experiments.
3.5 Time kill assay
The secondary metabolite was incubated with S. aureus at 37 °C for 24 h. The antibiotics concentration at 2× MIC and 4× MIC were effectively reduced the bacterial pathogen. 4× MIC concentration was found to be effective than 2× MIC values and reduced bacterial count considerably. At 2× MIC concentration, it reduced inoculum level with 18 h incubation, however within 14 h incubation 4× concentration eliminated all pathogens. The present finding revealed bactericidal activity of secondary metabolites synthesized by Streptomyces sp. AS11 (Fig. 5).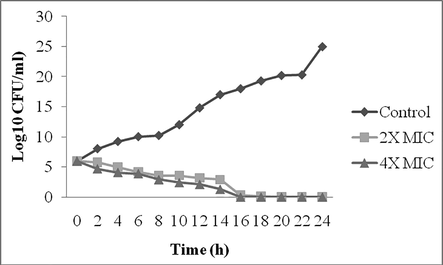
Time kill assay of compounds at 2× MIC and 4× MIC concentrations against S. aureus. The culture was incubated for 24 h at 37 °C in an optimized medium and antibiotics were supplemented at two different doses (2× MIC and 4× MIC).
3.6 Crude Streptomyces sp. AS11 extract and cytotoxicity against cancer cell line
In our study, the crude extract showed cytotoxic effect against cancer cell line and the IC50 value was 0.250 mg/ml. The crude extract from Streptomyces sp. extract was highly active against HeLa cell line. The crude extract acts on HeLa and abnormal cell morphology was observed due to cytotoxic effect.
4 Discussion
In these days, the emergence of multiple drug resistant pathogens poses serious risk throughout the world. Among these drug resistance organisms, MRSA is important one; it is a common nosocominal pathogen in human population (Cardozo et al., 2013; Arasu et al., 2015; Arokiyaraj et al., 2015; Balachandran et al., 2015; Valsalam et al., 2019a; Valsalam et al., 2019b). The cell wall of MRSA is very thick and showed high resistance against almost all β-lactam antibiotics such as, penicillin, methicillin, amoxicillin and oxacillin. Earlier, many marine (Al-Dhabi et al., 2020c) Pseudomonas species were subjected for the isolation of various types of anti-MRSA molecules. In a study, Darabpour et al. (2012) isolated Pseudoalteromonas piscicida PG-01 from the soil sediment from Persian Gulf showing novel anti-MRSA properties. Also, Isnansetyo and Kamei (2009) isolated potent anti MRSA molecule from Pseudoalteromonas phenolica O-BC30 isolated from the marine environment. Likewise, Lee et al. (2013) isolated and characterized anti MRSA molecule from the marine bacterium, Pseudomonas sp. Also, anti-MRSA molecules such as, Bogorol A, Abyssomicin C, 2,4-Diacetylphloroglucinol, Loloatins A–D, Fijimycin A, Marinopyrroles A, Lipoxazolidinone A–C were isolated from marine microorganisms (Eom et al., 2013; Rajkumaria et al., 2019). However, Streptomyces species has the potential to produce various bioactive molecules. In our study, the anti-MRSA producing Streptomyces sp. was isolated from the marine environment and lead molecule production was found to be maximum in the optimized culture medium. Previously, it was reported the influence of process parameters such as, pH, temperature, incubation period, nitrogen and carbon sources on secondary metabolites production (Wang et al., 2011). In this study, the environmental and nutritional factors were optimized to enhance antibiotics production in ISP4 medium. The optimum temperature was 31 °C, 4.5% sodium chloride and pH 7.3 in ISP4 medium enhanced the production of anti-MRSA molecules. Previously various anti-MRSA molecules, mainly phenolic compounds have been synthesized for antagonistic activity against drug resistance pathogens.
In a study, Mohammad et al. (2015) synthesized anti-MRSA molecules, phenylthiazole compounds against MRSA pathogens and MIC was determined. In another study, Patel et al. (2013) synthesized anti-MRSA molecules and it was found to be effective against various ATTC strains. Many synthesized compounds showed activity against MRSA and these compounds showed optimum MIC values. These synthesized compounds have various hazards to biological system and also to the natural environment. Hence, naturally occurring antimicrobial compounds are beneficial. In recent years many novel Streptomyces species were isolated from the marine environment and it covers about 70% of earth surface (Beygmoradi and Homaei, 2017), however most of the resources were unexplored (Mora et al., 2011). More than 42 Streptomyces strains were isolated from marine environment and have the ability to produce potent anti-MRSA activity (Kemung et al., 2018). These findings critically suggest an increasing trend in the discovery of various anti-MRSA molecules from the marine environment.
In our study, the Streptomyces AS11 showed cytotoxicity against cancer cells. Actinomycete such as, Streptomyces sp. VITSDK1 and Streptomyces sp. LCJ85 showed potent anticancer activity and it effectively inhibit angiogenesis. These investigations showed that actinomyctes from marine environment are a potent source for the synthesis of anticancer cytotoxic alkaloids. Also, various research groups have also observed anticancer secondary metabolites from non-alkaloid origin from Streptomyces sp. VITSDK1 (Suthindhiran and Kannabiran, 2013). Streptomyces purpurascens produced rhodomycin-B and this molecule showed cytotoxic activity against HeLa cancer cell line (Holkar et al., 2013). Kadiri et al. (2013) screened a cytotoxic compound, aporphine alkaloid SSV from Streptomyces sp. KS1908 against HL-60, MCF7cells, Hep2 and HeLa and cytotoxic effect was reported. Mohanraj and Sekar (2013) isolated a novel cytotoxic compound 1-(3-bromo-5-methylphenyl)-1H-indole from Streptomyces sp. LCJ85 which was found to be highly effective against HePG2 cell line.
5 Conclusion
The extensive screening of actinomyces conducted in this study indicates Streptomyces sp. AS11 is a novel source of antibiotic and anticancer molecules. The crude extract was highly effective against S. aureus (25 ± 2 mm), followed by P. mirabilis (20 ± 2 mm) and S. typhi (20 ± 2 mm). The crude extract from Streptomyces sp. AS11 extract was highly active against HeLa cell line. The antimicrobial and anticancer activity of the compound clearly demonstrated its application as pharmaceutical products.
Acknowledgement
This research Project was funded by a grant from the Research Center of the Center for Female Scientific and Medical Colleges at King Saud University.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Isolation, identification and screening of antimicrobial thermophilic Streptomyces sp. Al-Dhabi-1 isolated from Tharban hot spring, Saudi Arabia. Extremophiles. 2016;20:79-90.
- [Google Scholar]
- Chemical profiling of Streptomyces sp. Al-Dhabi-2 recovered from an extreme environment in Saudi Arabia as a novel drug source for medical and industrial applications. Saudi J. Biol. Sci.. 2019;26:758-766.
- [Google Scholar]
- Environmental friendly synthesis of silver nanomaterials from the promising Streptomyces parvus strain Al-Dhabi-91 recovered from the Saudi Arabian marine regions for antimicrobial and antioxidant properties. J. Photochem. Photobiol., B. 2019;197:111529.
- [Google Scholar]
- Metabolite profiling of Streptomyces sp. Al-Dhabi-100 isolated from the marine environment in Saudi Arabia with anti-bacterial, anti-tubercular and anti-oxidant potentials. J. King Saud Univ.-Sci. 2019
- [Google Scholar]
- Isolation and screening of Streptomyces sp. Al-Dhabi-49 from the environment of Saudi Arabia with concomitant production of lipase and protease in submerged fermentation. SaudiJ. Biol. Sci. 2020
- [CrossRef] [Google Scholar]
- Characterization and fermentation optimization of novel thermo stable alkaline protease from Streptomyces sp. Al-Dhabi-82 from the Saudi Arabian environment for eco-friendly and industrial applications. J. King Saud Univ. – Sci.. 2020;32(1):1258-1264.
- [Google Scholar]
- Isolation and purification of starch hydrolysing amylase from Streptomyces sp. Al-Dhabi-46 obtained from the Jazan region of Saudi Arabia with industrial applications. J. King Saud Univ. – Sci.. 2020;32:1226-1232.
- [Google Scholar]
- Isolation and screening of Streptomyces sp. Al-Dhabi-49 from the environment of Saudi Arabia with concomitant production of lipase and protease in submerged fermentation. Saudi J. Biol. Sci.. 2020;27(1):474-479.
- [Google Scholar]
- Green biosynthesis of silver nanoparticles produced from marine Streptomyces sp. Al-Dhabi-89 and their potential applications against wound infection and drug resistant clinical pathogens. J. Photochem. Photobiol., B. 2018;189:176-184.
- [Google Scholar]
- Characterization of silver nanomaterials derived from marine Streptomyces sp. Al-Dhabi-87 and its in vitro application against multidrug resistant and extended-spectrum beta-lactamase. Clinical Pathogens. Nanomater.. 2018;8:5.
- [Google Scholar]
- Bioactivity assessment of the Saudi Arabian Marine Streptomyces sp. Al-Dhabi-90, metabolic profiling and its in vitro inhibitory property against multidrug resistant and extended-spectrum beta-lactamase clinical bacterial pathogens. J. Infect. Public Health 2019:549-556.
- [Google Scholar]
- Antifungal metabolites from sponge associated marine Streptomyces sp. strain (ERIMA-01) J. Pure Appl. Microbiol.. 2014;8(2):115-128.
- [Google Scholar]
- Green chemical approach towards the synthesis of CeO2 doped with seashell and its bacterial applications intermediated with fruit extracts. J. Photochem. Photobiol., B. 2017;172:50-60.
- [Google Scholar]
- Antifeedant, larvicidal and growth inhibitory bioactivities of novel polyketide metabolite isolated from Streptomyces sp. AP-123 against Helicoverpa armigera and Spodoptera litura. BMC Microbiol.. 2013;13(1):105.
- [Google Scholar]
- One step green synthesis of larvicidal, and azo dye degrading antibacterial nanoparticles by response surface methodology. J. Photochem. Photobiol., B. 2019;190:154-162.
- [Google Scholar]
- Antibacterial and antifungal activities of polyketide metabolite from marine Streptomyces sp. AP-123 and its cytotoxic effect. Chemosphere. 2013;90(2):479-487.
- [Google Scholar]
- Identification of novel quinine metabolite from marine actinomycetes with antifungal and anticancer bio-prospective. Fresenius Environ. Bull.. 2015;24(10a):3281-3287.
- [Google Scholar]
- Green synthesis of Silver nanoparticles using aqueous extract of Taraxacum officinale and its antimicrobial activity. South Indian J. Biol. Sci.. 2015;2:115-118.
- [Google Scholar]
- Antimicrobial and cytotoxic properties of Streptomyces sp. (ERINLG-51) isolated from Southern Western Ghats. South Indian J. Biol. Sci.. 2015;1:7-14.
- [Google Scholar]
- Marine microbes as a valuable resource for brand new industrial biocatalysts. Biocat. Agricul. Biotechnol.. 2017;11:131-152.
- [Google Scholar]
- Epidemiology of methicillin-resistant Staphylococcus aureus. Clin. Infect. Diseas.. 2008;1(46(Supplement_5)):S344-S349.
- [Google Scholar]
- CA-MRSA infection rates and management among student-athletes. Med. Sci. Sports Exerc.. 2018;50:1802-1809.
- [Google Scholar]
- Antibacterial activity of extracellular compounds produced by a Pseudomonas strain against methicillin-resistant Staphylococcus aureus (MRSA) strains. Ann. Clin. Microbiol. Antimicrob.. 2013;12:12.
- [Google Scholar]
- Differences between methicillin-resistant Staphylococcus aureus bacteremic isolates harboring type IV and type V staphylococcal cassette chromosome mec genes based on prior patient healthcare exposure. Eur. J. Clin. Microbiol. Infect. Dis.. 2010;29(12):1539-1546.
- [Google Scholar]
- Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Tenth Edition: Approved Standard M07–A10. Wayne, PA: CLSI; 2015. Available online at:
- Assessment of antibiotics production potential in four actinomycetes isolated from aquatic environments of the Eastern Cape Province of South Africa. Afr. J. Pharm. Pharmacol.. 2011;5(2):118-124.
- [Google Scholar]
- Isolation of a potent antibiotic producer bacterium, especially against MRSA, from northern region of the Persian Gulf. Bosn. J. Basic Med. Sci.. 2012;12:108-121.
- [Google Scholar]
- Marine bacteria: potential sources for compounds to overcome antibiotic resistance. Appl. Microbiol. Biotechnol.. 2013;97:4763-4773.
- [Google Scholar]
- Purification and structure elucidation of antifungal and antibacterial activities of newly isolated Streptomyces sp. strain US80. Res. Microbiol.. 2005;156:341-347.
- [Google Scholar]
- Emergence of vancomycin-intermediate and-resistant Staphylococcus aureus among methicillin-resistant S. aureus isolated from clinical specimens in the northwest of Iran. J. Glob. Antimicrob. Resist.. 2018;14:4-9.
- [Google Scholar]
- Rhodomycin analogues from Streptomyces purpurascens: isolation, characterization and biological activities. Springerplus. 2013;2:93.
- [Google Scholar]
- Anti-methicillin-resistant Staphylococcus aureus (MRSA) activity of MC21-B, an antibacterial compound produced by the marine bacterium Pseudoalteromonas phenolica O-BC30. Int. J. Antimicrob. Agents. 2009;34:131-135.
- [Google Scholar]
- Isolation and identification of a novel aporphine alkaloid SSV, an antitumor antibiotic from fermented broth of marine associated Streptomyces sp. KS1908. J. Mar. Sci. Res. Dev.. 2013;3:137.
- [Google Scholar]
- Isolation and characterization of antibiotic producing actinomycetes from marine soils samples. Int. J. Curr. Pharmaceut. Res.. 2012;4(2):109-112.
- [Google Scholar]
- Streptomyces as a prominent resource of future anti-MRSA drugs. Front. Microbiol.. 2018;9:2221.
- [Google Scholar]
- Isolation and screening of soil actinomycetes as source of antibiotics active against bacteria. Int. J. Microbiol. Res.. 2010;2(2):12-16.
- [Google Scholar]
- Antibacterial activity of some actinomycetes from Tamil Nadu. India. Asian Pac. J. Trop. Biomed.. 2012;2:936-943.
- [Google Scholar]
- 16S/23S rRNA sequencing. In: Stackebrandt E., Goodfellow M., eds. Nucleic Acid Techniques in Bacterial Systematics. New York: John Wiley and Sons; 1991. p. :115-175.
- [Google Scholar]
- Anti-methicillin-resistant Staphylococcus aureus (MRSA) substance from the marine bacterium Pseudomonas sp. UJ-6. Environ. Toxicol. Pharmacol.. 2013;35:171-177.
- [Google Scholar]
- A procedure for the isolation of deoxyribonucleic acid from microorganisms. J. Mol. Biol.. 1961;3:208-218.
- [Google Scholar]
- Synthesis and antibacterial evaluation of a novel series of synthetic phenylthiazole compounds against methicillin-resistant Staphylococcus aureus (MRSA) Eur. J. Med. Chem.. 2015;94:306-316.
- [Google Scholar]
- Isolation and screening of actinomycete from marine sediments for their potential to produce antimicrobials. Int. J. Life Sci. Pharma. Res.. 2013;2(12):115-126.
- [Google Scholar]
- How many species are there on Earth and in the ocean? PLoS Biol.. 2011;9(8):e1001127.
- [Google Scholar]
- NCCLS—National Committee for Clinical Laboratory Standards, 1999. Methods for determining bactericidal activity of antimicrobial agents. Approved guideline, NCCLS document M26-A. National Committee for Clinical Laboratory Standards, Wayne PA, USA.
- High prevalence of tetM as compared to tetK amongst methicillin-resistant Staphylococcus aureus (MRSA) isolates from hospitals in Perak, Malaysia. Jundishapur J. Microbiol.. 2017;10:e13935.
- [Google Scholar]
- The synthesis and SAR study of phenylalanine-derived (Z)-5-arylmethylidene rhodanines as anti-methicillin-resistant Staphylococcus aureus (MRSA) compounds. Bioorg. Med. Chem. Lett.. 2013;23:5523-5527.
- [Google Scholar]
- Aptness and application of colour for exact description of colours of Streptomyces. J. Basic Microbiol.. 1964;4:95-98.
- [Google Scholar]
- The utilization of carbon compounds by some actinomycetales as an aid for species determination. J. Bacteriol.. 1948;56:107-114.
- [Google Scholar]
- In vitro and in vivo profiles of ACH-702, an isothiazoloquinolone, against bacterial pathogens. Antimicrob. Agents Chemother.. 2011;55:2860.
- [Google Scholar]
- Synthesis of titanium oxide nanoparticles using Aloe barbadensis mill and evaluation of its antibiofilm potential against Pseudomonas aeruginosa PAO1. J. Photochem. Photobiol. B: Biol. 2019:111667.
- [Google Scholar]
- Daptomycin resistance in clinical MRSA strains is associated with a high biological fitness cost. Front. Microbiol.. 2017;8:2303.
- [Google Scholar]
- High rate of decreasing daptomycin susceptibility during the treatment of persistent Staphylococcus aureus bacteremia. Eur. J. Clin. Microbiol. Infect. Dis.. 2008;27(6):433-437.
- [Google Scholar]
- Probin the mechanism of cytotoxic furan 2-YL acetate using in vitro and in silicoanalysis-pharmacological study. J. Pharmacol. Toxicol.. 2013;8:1-18.
- [Google Scholar]
- Antimicrobial potential of actinomycetes species isolated from marine environment. Asian Pacific J. Trop. Biomed.. 2012;9:416-473.
- [Google Scholar]
- Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol., B. 2019;191:65-74.
- [Google Scholar]
- Biosynthesis of silver and gold nanoparticles using Musa acuminata colla flower and its pharmaceutical activity against bacteria and anticancer efficacy. J. Photochem. Photobiol., B 2019:111670.
- [Google Scholar]
- Improvement of antibiotic activity of Xenorhabdus bovienii by medium optimization using response surface methodology. Microb. Cell Fact.. 2011;10:98.
- [Google Scholar]







