Translate this page into:
Optimization of culture conditions by response surface methodology for production of extracellular esterase from Serratia sp. EST-4
⁎Corresponding author at: Faculty of Life Sciences, Department of Biotechnology, Himachal Pradesh University, Summer Hill, Shimla 171 005, India. reenagupta_2001@yahoo.com (Reena Gupta)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
The aim of present study was to determine the effect of interaction among different production variables on esterase production from Serratia sp. EST-4.
Method
The optimum and combined effect of independent parameters were determined using Plackett-Burman and Central Composite Design (CCD) (Design Expert 11).
Results
The effect of six parameters-cotton seed oil, peptone and maltose concentrations, pH, temperature and inoculum volume for production of esterase was determined by Plackett-Burman design. Four factors i.e. inoculum size, pH, peptone concentration and cotton seed oil concentration had significant effect on yield of esterase as suggested by the Pareto chart. Under optimized conditions, (inoculum size (1.0%, v/v), pH (8.0), peptone concentration (1.5%, w/v) and cotton seed oil concentration (4.0%, v/v)), the maximum esterase production was 9.77 U/ml. Analysis of variance (ANOVA) of the Central Composite Design (CCD)-based experiment exhibited the model F-value of 44.24 and P-value of <0.0001 which implies that model was significant and adequate to represent the system.
Conclusion
Inoculum size 1.0%, v/v, pH 8.0, concentration of peptone 1.5%, w/v and concentration of cotton seed oil 4.0%, v/v had positive effect on esterase production and provided 1.52-fold increase in esterase production than that obtained by optimizing one factor at one time.
Keywords
Response surface methodology
Esterase
Serratia sp.
Central composite design
1 Introduction
Microorganisms are main source of enzymes, because they can be manipulated genetically to increase the production of enzyme and are cultured on large scale in short time (Anbu et al., 2017; Sarkar et al., 2020). Phospholipases, lipases and esterases are lipolytic enzymes and belong to hydrolase class of enzymes that hydrolyze various substrates such as triacylglycerides, esters or peptides (Miranda et al., 2015,; Bhardwaj et al., 2017; Wang et al., 2018). Esterases are important group of hydrolases which catalyze different reactions such as esterification, inter-esterification and trans-esterification in water free or water-restricted mediumwithout using metal ions (Godinhoet al.,2011; Duarte et al., 2016). Esterases are widely used in the remediation of pyrethroid, ester-containing carbamate and organophosphate pesticides and are environment-friendly choice for the removal of recalcitrant compounds (Bhatt et al., 2020a; Bhatt et al., 2021). In general, all esterases contain the consensus sequence Glycine-X1-Serine-X2-Glycine as the catalytic moiety. (López-López et al., 2014; Ding et al., 2018; Bhardwaj et al., 2020).
The production of new esterases from different microbial sources is great compulsion for various biotechnological applications which requires cost effective production and stability of biocatalyst under extreme conditions (Maesteret al., 2020; Adiguzel, 2020). However, suitable production strategies must be developed to meet the growing demand of esterases. The optimization of enzyme production by varying one factor at a time does not consider the combined effect of all parameters, is laborious and cannot gave assurance of the optimum level of each parameter conditions (Lotfy et al., 2007; Hye et al., 2008; Kammoun et al., 2008; Gadhe et al., 2011; Tijani et al., 2011). On the other hand, Response Surface Methodology (RSM) can provide reliable optimization results as it helps in studying the effect of several variables at a time at different degrees to evaluate the combined effect of more than one parameters (Benzina et al., 2012; Yasmeen et al., 2013).
RSM is an experimental strategy commonly used to analyze effect of different parameters and to optimize several biological processes (Chen et al., 2002). The interaction outcome among various parameters on enzyme yield can be analyzed by different statistical models and desired variable response can be determined (Mohandas et al., 2010). This methodology can analyze the interaction of different parameters at a time and effect of several process variables on yield of enzyme (Box and Wilson, 1951). Different experimental combinations are accomplished in order to minimize impact of inexplicable variance in the actual enzyme yield due to irrelevant factors (Montgomery, 2005). Response Surface Methodology is less tedious or less extensive process and therefore used in various industrial applications to decrease the experimental runs (Quintavalla and Paralari, 1993).
2 Materials and methods
2.1 Chemicals
Tris buffer (99.8% purity), p-nitrophenyl acetate (p-NPA) (98% purity), peptone (99.5% purity), p-nitrophenol (p-NP) (98% purity), sodium chloride (99.8% purity), maltose (99.5% purity) used were of high analytical grade, obtained from Sigma Aldrich (U.S.A).
2.2 Microorganism and esterase production
Oil contaminated soil, dairy soil, sweet shop waste, plastic wastes was collected from Hamirpur, Solan and Shimla districts of H.P., India. Production medium containing maltose (1.0%, w/v), cottonseed oil (4.0%, v/v), peptone (2.0%, w/v) and NaCl (0.5%, w/v) was autoclaved at temperature of 121 °C and 15 Ibs/inch2 for 20 min. The production medium was inoculated with seed culture (1.0%) and incubated for 2 days at 40 °C (Bhardwaj and Gupta, 2020).
2.3 Esterase activity
The esterase activity was measured by incubating 2.9 ml of Tris buffer (0.1 M, pH 8.0) with 60 μl of p-nitrophenyl acetate (10 mM). The reaction mixture was incubated at 35 °C for 10 min. 40 μl of enzyme was added in the reaction mixture and again incubated at 35 °C for 10 min. The reaction was stopped by chilling the reaction mixture at −20 °C for 2 min. The concentration of p-nitrophenol released was measured at A410 nm (Lab India 3000 + UV/VIS Spectrophotometer). By recording A410 of test sample, the corresponding p-NP concentration was analyzed from standard curve of p-NP (Immanuel et al., 2010).
The esterase activity was defined as amount of enzyme required to release one micromole of p-NP from p-NPA per minute under standard assay conditions.
2.4 Identification of microorganism
Maximum esterase producing bacterial isolate was previously characterized by 16S rRNA sequencing (Bhardwaj and Gupta, 2020).
2.5 Esterase production parameters optimization from Serratia sp. using response surface methodology (RSM)
2.5.1 Screening of important parameters using Plackett-Burman design
This testing methodology is mostly used for the screening of important variables that affect process output measures. Different experimental combinations are executed in order to minimize the effects of inexplicable variance in the actual esterase activity due to unrelated variables (Montgomery, 2005; Hajji et al., 2008). The design was used for the screening of different production parameters for esterase activity at two degrees (minimum (−1) and maximum (+1)). Six different variables examined in the study were concentration of cotton seed oil, peptone concentration, maltose concentration, pH, temperature and inoculum size. Design expert (11) was used for Plackett-Burman analyses and regression analyses. These different variables were previously optimized for esterase production from Serratia sp. by one factor at a time (Bhardwaj and Gupta, 2020). Effect of each parameter was determined using the equation given below:
E is the impact of parameter and M+ and M- are esterase activities of trials at which the variable was at its higher and lower values respectively and N is the experimental runs (Bhatia et al., 2013; Mehta et al., 2019). The error was determined by calculating the variability between two independent parameters by the following equation:
Veff is the variance effect, E is the effect of parameter and n is number of parameters used in experiment.
2.5.2 Central composite design (CCD)
The optimum level of significant parameters was determined for enhancement of esterase yield from Serratia sp. The independent parameters which showed significant effect on yield of esterase in the Plackett-Burman analysis were again optimized using Central Composite Design to evaluate optimum level and combined effect of parameters (Xiao et al., 2007). The combined effect of different variables on esterase yield was determined by plotting 3-D response curves against any two independent parameters while keeping other independent parameters at their ‘0′ levels. Analysis of variance analysis (ANOVA) was performed to analyze the significance of model and regression coefficients. The quality of polynomial equation was judged by determination coefficient (R2). The optimum values of selected parameters were obtained and 3D plots were plotted to examine the combined effects of different independent parameters on esterase yield from Serratia sp. (Kumar and Kanwar, 2019)
2.5.3 Validation of statistical model
The statistical model was validated for maximum yield of esterase from Serratia sp. EST-4 by performing at shake flask under predicted set of conditions. The lack of fit test was applied to determine the appropriacy and efficiency of the model. Statistical significance of the model was checked by Fisher’s F-test.
3 Results
3.1 Identification of microorganism
The bacterial strain was previously characterized by 16S rRNA sequencing and was identified as Serratia sp. EST-4 (NCBI Accession no.: MH538970) (Bhardwaj and Gupta, 2020).
3.2 Esterase production parameters optimization from bacterial isolate Serratia sp. using response surface methodology (RSM)
3.2.1 Screening of important parameters using Plackett-Burman design
Influence of 6 variables (concentrations of cotton seed oil, peptone, maltose, pH, temperature and inoculum size) for the study was evaluated by conducting 14 runs of experiments using Plackett-Burman design that involved different combinations of the variables. The Plackett-Burman experiments showed a wide variation in esterase production from 0.331 to 6.02 U/ml. The combination of parameters in run 3 was found to be significant and gave optimum response (enzyme activity 6.02 U/ml) (Table 1). This variation in enzyme activity showed the importance of optimizing these parameters for improving esterase production by the novel isolate. The experimental result made by using statistical Plackett-Burman design for esterase production from Serratia sp. revealed that the suitable media components were cotton seed oil 3.0% (v/v), maltose 1.5% (w/v), peptone 2.0% (w/v), pH 9.0, temperature 30 °C and inoculum size 1.5% (v/v). Results got from Plackett-Burman analysis with six parameters were used to create a Pareto chart (Fig. 1) to find out the order of significance of parameters on esterase yield from Serratia sp. Recently, the influence of starch, pH, NaCl, tryptone K2HPO4, inoculum volume, Tween 80 and inoculum age on esterase yield was evaluated by Plackett-Burman design and three variables (pH, Tween 80 and K2HPO4) were found to be significant for esterase production from Acinetobacter sp. B1 (Ma et al., 2019). In a different study, artificial neural networks were found to be advantageous than RSM for culture parameters optimization for maximum lipase yield from Geobacillus sp. (Ebrahimpour et al., 2008).
Run
Cotton seed oil concentration (%, v/v)
Peptone concentration (%, w/v)
Maltose concentration (%, w/v)
pH
Temperature (°C)
Inoculum size (%, v/v)
Responses (U/ml)
1
3
2
0.5
7
30
0.5
2.808
2
3
1
1.5
7
50
0.5
4.34
3
3
2
1.5
9
30
1.5
6.021
4
5
2
1.5
9
50
0.5
5.457
5
3
1
0.5
9
30
0.5
2.372
6
5
2
0.5
9
30
1.5
4.578
7
5
2
1.5
7
50
1.5
3.4
8
3
1
0.5
7
30
1.5
2.073
9
5
1
1.5
9
30
0.5
1.161
10
5
1
0.5
7
50
0.5
0.381
11
3
2
0.5
9
50
0.5
0.331
12
5
1
1.5
7
30
1.5
0.829
13
3
2
0.5
7
50
1.5
2.886
14
5
1
1.5
9
50
1.5
2.339
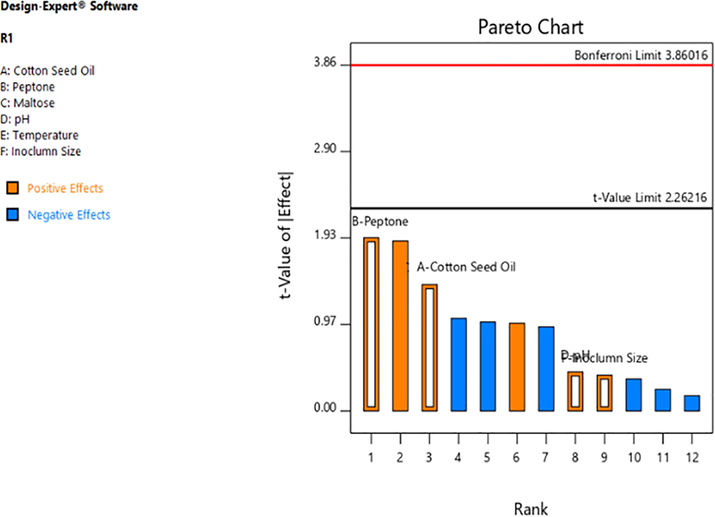
Effect of different parameters on esterase yield from Serratia sp. as shown by Plackett-Burman design.
3.2.2 Central composite design
The interaction of different parameters and their optimum amount (inoculum size, pH, peptone concentration and cotton seed oil concentration) having significant effect (Pareto chart) was evaluated by CCD (Table 2). 27 different experimental combinations were generated for four positive parameters by CCD, the results of different experimental responses (esterase yield) are shown in Table 2. Analysis of variance (ANOVA) showed a quite suitable model to optimize esterase production and four process order was suggested by Design expert 11. Based on the results of ANOVA of the CCD-based experiment, the model F-value of 44.24 and the P-value of <0.0001 suggest that model was appropriate and acceptable to represent the system (Table 3). In a previous study, ANOVA revealed that Tween 80, yeast extract and agitation were the variables that significantly affected the production of biomass and hence of lipolytic enzymes from A. facilis strain USBA-GBX-505. The Fisher F value at 3.17 and the P-value (0.0433, p < 0.05) conclude that the model was appropriate (Bernal et al., 2017). Cubic and quadratic process order gave similar results and quadratic process order was suggested due to R2 value of 0.9810 and low standard deviation (0.4685) and for further analysis. Coefficient of determination “R2,” value (0.9810) indicated that this model could explain 98% of the variability in the response and pointed to the accuracy of model (Table 4). In a recent study, inoculum volume 0.2% (v/v), lactose concentration 6 g/L (w/v), pH 5.5, temperature 23 °C and a pre and post-induced period of 5 h and 32 h respectively in a Luria–Bertani culture were found to be the optimal parameters for feruloyl esterase production from Burkholderia pyrrocinia B1213 (Fan et al., 2020).
Run
Inoculum size(%, v/v)
pH
Peptone concentration(%, w/v)
Cotton seed oilconcentration(%, v/v)
Response (U/ml)
1
1.5
7
2
3
4.462
2
2
8
1.5
4
1.808
3
1
8
1.5
4
9.455
4
1
8
1.5
4
9.77
5
1.5
7
1
3
4.396
6
1.5
9
2
3
4.362
7
1.5
9
1
3
3.914
8
1
8
1.5
2
4.711
9
0.5
7
2
5
5.291
10
1.5
9
2
5
3.798
11
1.5
9
1
5
4.445
12
1
8
1.5
4
9.63
13
1.5
7
2
5
5.258
14
0.5
9
2
3
2.587
15
0.5
7
1
5
3.5
16
0.5
9
2
5
2.521
17
1
8
1.5
6
5.242
18
1.5
7
1
5
3.582
19
1
8
2.5
4
5.424
20
0.5
9
1
5
3.666
21
0.5
9
1
3
3.516
22
1
8
1.5
4
9.32
23
0.5
7
2
3
4.445
24
0.5
7
1
3
4.362
25
1
10
1.5
4
2.803
26
1
6
1.5
4
5.341
27
1
8
1.5
4
9.49
Source
Sum of Squares
df
Mean Square
F-value
p-value
Remark
Model
135.94
14
9.71
44.24
< 0.0001
significant
A-Inoculum size
1.13
1
1.13
5.14
0.0427
B-pH
5.57
1
5.57
25.38
0.0003
C-Peptone
0.0861
1
0.0861
0.3925
0.5427
D-Cotton seed oil
0.0485
1
0.0485
0.2210
0.6467
AB
1.07
1
1.07
4.86
0.0478
AC
0.1899
1
0.1899
0.8652
0.3706
AD
0.0009
1
0.0009
0.0040
0.9504
BC
2.17
1
2.17
9.88
0.0085
BD
0.0005
1
0.0005
0.0021
0.9646
CD
0.2518
1
0.2518
1.15
0.3052
A2
58.40
1
58.40
266.11
< 0.0001
B2
43.52
1
43.52
198.30
< 0.0001
C2
14.77
1
14.77
67.30
< 0.0001
D2
29.92
1
29.92
136.35
< 0.0001
Residual
2.63
12
0.2195
Lack of Fit
2.11
8
0.2635
2.00
0.2619
not significant
Pure Error
0.5257
4
0.1314
Cor Total
138.57
26
Source
Std. Dev.
R2
Adjusted R2
Predicted R2
Press
Linear
2.45
0.0438
−0.1300
−0.1865
164.41
2FI
2.84
0.0703
−0.5107
−0.8679
258.84
Quadratic
0.4685
0.9810
0.9588
0.8865
15.72
Suggested
Cubic
0.3358
0.9959
0.9788
*
Aliased
Model was found to be significant and three-dimensional graphs were generated for regression analysis of Central Composite Design which represents the response surfaces for the interaction effects of inoculum size, pH, peptone concentration and cotton seed oil concentration respectively. These plots described the effect of different parameters and interaction effect of each parameter upon the response (Fig. 2a-2f). In a different study, effect of independent variables (metals, mineral salt and corn steep liquor) on production of esterase from E. coli was evaluated by a five-level three-factor Central Composite Design (Ren et al., 2006). Previously, fractional factorial central composite design was used for lipolytic enzyme production from Enterobacter aerogenes and optimized parameters were oil concentration 3.0% (v/v), temperature 34 °C, pH 7.0, inoculum volume 7.0% (v/v) and 60 h of incubation time (Kumari et al., 2009). In a recent study, response surface methodology was also used for the degradation of allethrin (pyrethroid) by esterase from Fusarium proliferatum CF2 and optimum conditions were temperature 26 °C and pH 6.0 (Bhatt et al., 2020b).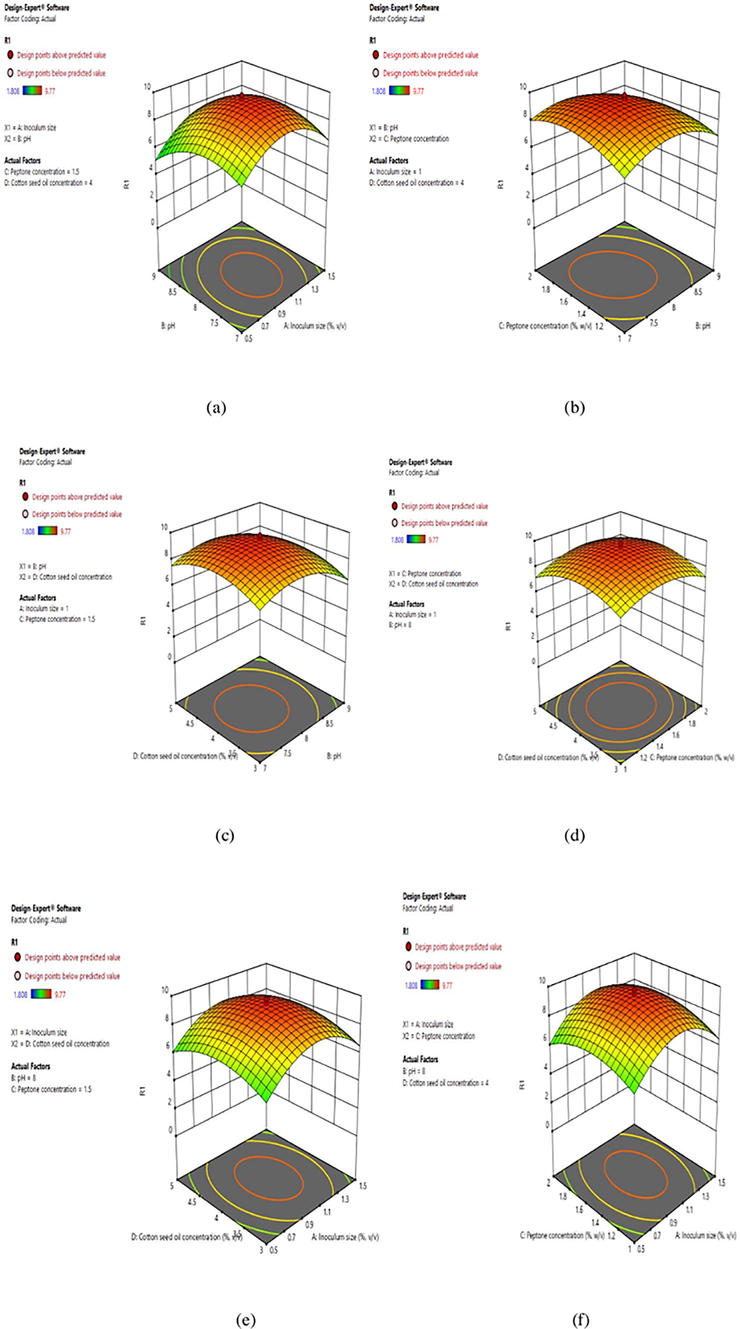
3-D response surface plots for influence of (a) inoculum size and pH (b) pH and peptone concentration (c) pH and cotton seed oil concentration (d) cotton seed oil concentration and peptone concentration (e) inoculum size and cotton seed oil concentration (f) peptone concentration and inoculum size on esterase production by Serratia sp.
The combined effect and optimum conditions of different parameters optimized for enhanced esterase production were presented by three-dimensional response surface plots shown in Fig. 2a-2f. The influence of inoculum size and pH on esterase production is shown in Fig. 2a. 3-D plot depicts that slightly basic pH (8.0) caused maximum esterase production with inoculum size of 1.0%. The interaction effect of inoculum volume and pH was presented in 3-D plot and their optimized condition were 2.8%, v/v and 7.6 respectively for lipolytic enzyme production from Bacillus aryabhattai SE3-PB (Adetunji and Olaniran, 2018). A similar profile was observed in Fig. 2b (pH and peptone concentration) and Fig. 2c (pH and cotton seed oil concentration) where esterase activity increased when pH was 8.0 and peptone concentration increased to 1.5%, w/v and cotton seed oil concentration to 4.0%, v/v in relation to pH 8.0.Fig. 2d showed that simultaneous increase in cotton seed oil concentration and peptone concentration resulted in increase in esterase yield but further rise in cotton seed oil concentration and peptone concentration led to low esterase activity. Fig. 2e showed that an increase in the cotton seed oil concentration (4.0%, v/v) improved the esterase activity at low inoculum size (1.0%, v/v); with the optimum activity of esterase being 9.77 U/ml. Esterase production from Acinetobacter sp. B1 showed optimum enzyme yield in the presence of Tween 80 (0.8%) and pH (8.0) (Ma et al., 2019). Fig. 2f showed a curvature in the response surface stipulate that lower and higher concentration of both, peptone concentration and inoculum volume did not result in higher esterase yield. The increase of peptone concentration from 1.0 to 1.5% and inoculum size from 0.5 to 1.0% increased the esterase yield, but further increase in both the components decreased the enzyme yield (Fig. 2f). The shape of three- dimensional curve showed a significant interaction between these two parameters. In a different study, artificial neural networks was found to be advantageous than response surface methodology for culture parameters optimization for lipase production from Geobacillus sp. (Ebrahimpour et al., 2008).
3.2.3 Validation of model
The highest predicted value given by the software was 9.32 U/ml which was closer to actual obtained value 9.77 U/ml (Fig. 3). High resemblance between experimental and predicted responses reflects the applicability and accuracy of the RSM to optimize the process for enzyme production (Kar et al., 2010). Perturbation graph (Fig. 4) showed the optimum value for variables inoculum size 1.0 (%, v/v), pH 8.0, concentration of peptone 1.5 (% w/v), concentration of cotton seed oil 4.0 (%, v/v). There was 1.52-fold increase in esterase yield by using factorial design. In a different study, optimum parameters were found to be concentration of peptone 1.8 (%, w/v), pH 10.0, concentration of galactose 1.5 (%, w/v), incubation time 72 h and 45 °C temperature which resulted in 1.4-fold increase in lipase yield from Aspergillus fumigates (Mehta et al., 2019). In another study, the optimum parameters wereoil concentration 3.0 (%, v/v), pH 7.0, inoculum size 7.0 (%, v/v), temperature 34 °C and 60 h of incubation time for optimum yield of lipase (27.25 U/ml) from Enterobacter aerogenes. Optimization of various factors resulted in 1.4-fold increase in lipase yield from Enterobacter aerogenes (Kumari et al., 2009). Previously, there was 2.37-fold increase in yield of esterase from Lysinibacillus fusiformis and the optimum parameters predicted were found to be trisodium citrate 0.25 (%, w/v) , yeast extract 3.6 (%, w/v), pH of medium 7.9 and inoculum size 4.6 (%, v/v) (Prabha et al.,2015).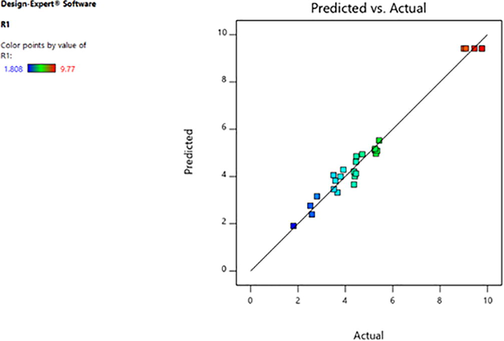
Comparison between predicted and actual enzyme yield from Serratia sp. in Central Composite Design.
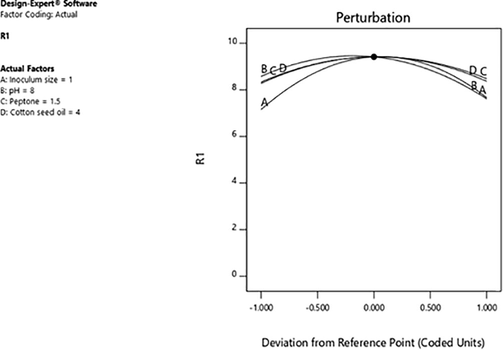
Perturbation graph representing comparison of influence of all factors considered in Central Composite Design showing impact on esterase production from Serratia sp.
4 Conclusion
The statistical optimization using RSM appears to be a successive and investigative tool for establishing interactions of independent parameters and to determine their optimum condition for maximum enzyme yield. Plackett-Burman analyses and CCD were used for optimizing production condition of enzyme. Inoculum size 1.0%, v/v, pH 8.0, peptone concentration 1.5%, w/v and cotton seed oil concentration 4.0%, v/v had positive effect on esterase production and provided 1.52-fold increase in esterase production than when produced by optimizing one factor at a time. It is concluded that Serratia sp. is a good source of esterase enzyme and it can be used in different industrial sectors.
Acknowledgements
We acknowledge Council of Scientific and Industrial Research (CSIR) for providing fellowship to Mr. Kamal Kumar Bhardwaj (CSIR Fellowship- 09/237(0156)/2016-EMR-I) in the form of SRF.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Optimization of culture conditions for enhanced lipase production by an indigenous Bacillusaryabhattai SE3-PB using response surface methodology. Biotechnol. Biotechnol. Equip.. 2018;32(6):1514-1526.
- [Google Scholar]
- Production and characterization of thermo-, halo- and solvent-stable esterase from Bacillus mojavensis TH309. Biocatal. Biotransfor.. 2020;38:210-226.
- [Google Scholar]
- Microbial enzymes and their applications in industries and medicine. BioMed. Res. Int.. 2017;2017:1-3.
- [Google Scholar]
- Enhanced decolorization of the azo dye Sirius rose BB by laccase-HBT system. 3. Biotech.. 2012;2:149-157.
- [Google Scholar]
- Response Surface Methodology (RSM) for analysing culture conditions of Acidocella facilis strain USBA-GBX-505 and partial purification and biochemical characterization of lipase 505 LIP. Univ. Sci.. 2017;22:45-70.
- [Google Scholar]
- Bhardwaj, K.K., Dogra, A., Kapoor, S., Mehta A., Gupta, R., 2020. Purification and properties of an esterase from Bacillus licheniformis and its application in synthesis of octyl acetate.14, 113-121.
- Screening and isolation of esterase producing microorganisms and optimization of enzyme production conditions from Serratia sp. Trends Carbohyd. Res.. 2020;12:34-44.
- [Google Scholar]
- Immobilization of lipase from Geobacillus sp. and its application in synthesis of methyl salicylate. J. Oleo Sci.. 2017;66:391-398.
- [Google Scholar]
- An isobutyronitrile-induced bienzymatic system of Alcaligenes sp. MTCC 10674 and its application in the synthesis of α-hydroxyisobutyric acid. Bioproc. Biosyst. Eng.. 2013;36(5):613-625.
- [Google Scholar]
- Esterase is a powerful tool for the biodegradation of pyrethroid insecticides. Chemosphere.. 2020;244:125507.
- [CrossRef] [Google Scholar]
- Biodegradation of allethrin by a novel fungus Fusarium proliferatum Strain CF2, isolated from contaminated soils. Microorganisms.. 2020;8:1-15.
- [Google Scholar]
- Characterization of the role of esterases in the biodegradation of organophosphate, carbamate, and pyrethroid pesticides. J. Hazard. Mater.. 2021;411:125026.
- [CrossRef] [Google Scholar]
- On the experimental attainment of optimum conditions. J. R. Stat. Soc. Series B.. 1951;13(1):1-38.
- [Google Scholar]
- Optimization of medium composition for the production of elastase by Bacillus sp. EL31410 with response surface methodology. Enzyme Microb. Technol.. 2002;30(5):667-672.
- [Google Scholar]
- A thermostable and alkaline GDSL-motif esterase from Bacillus sp. K91: crystallization and X-ray crystallographic analysis. Acta Cryst.. 2018;74(2):117-121.
- [Google Scholar]
- Rapid determination of the synthetic activity of lipases/esterases via transesterification and esterification zymography. Fuel.. 2016;177:123-129.
- [Google Scholar]
- A modeling study by response surface methodology and artificial neural network on culture parameters optimization for thermostable lipase production from a newly isolated thermophilic Geobacillus sp. strain ARM. BMC Biotechnol.. 2008;8:1-15.
- [Google Scholar]
- Fan, G., Zhu, Y., Fu, Z., Sun, B., Teng, C., Yang, R., Li, X., 2020. Optimization of fermentation conditions for the production of recombinant feruloyl esterase from Burkholderia pyrrocinia B1213. 3 Biotech. 10, 1-10.
- Statistical optimization of process parameters for the production of vanillic acid by solid state fermentation of groundnut shell waste using response surface methodology. J. Chem. Technol. Biotechnol.. 2011;86(12):1535-1541.
- [Google Scholar]
- Discovery of an Escherichia coli esterase with high activity and enantioselectivity towards 1, 2-O-Isopropylidene glycerol esters. Appl. Environ. Microbiol.. 2011;77:6094-6099.
- [Google Scholar]
- Optimization of alkaline protease production by Aspergillus clavatus ES1 in Mirabilis jalapa tuber powder using statistical experimental design. Appl Microbiol. Biotechnol.. 2008;79:915-923.
- [Google Scholar]
- Optimization of key process variables for enhanced hydrogen production by Enterobacter aerogenes using statistical methods. Bioresour. Technol.. 2008;99:2061-2066.
- [Google Scholar]
- Solid state production of esterase using groundnut oil cake by fish intestinal isolate Bacillus circulans. KKU Res. J.. 2010;15:459-474.
- [Google Scholar]
- Application of statistical design to the optimization of parameters and culture medium for α-amylase production by Aspergillus oryzae CBS 819.72 grown on gruel (wheat grinding by product) Bioresour. Technol.. 2008;99:5602-5609.
- [Google Scholar]
- Optimization of thermostable α-amylase production by Streptomyces erumpens MTCC7317 in solid state fermentation using cassava fibrous residue. Braz. Arch. Biol. Technol.. 2010;53:301-309.
- [Google Scholar]
- Five-factor-at-a-time (FFAT) approach for optimal production of an extracellular RNase from Bacillus safensis RB-5. Prep. Biochem. Biotechnol.. 2019;49:916-926.
- [Google Scholar]
- Statistical optimization of culture conditions by response surface methodology for synthesis of lipase with Enterobacter aerogenes. Braz. Arch. Biol. Technol.. 2009;52(6):1349-1356.
- [Google Scholar]
- López-López, O., Cerdán, M.E., González, S.M.I., 2014. New extremophilic lipases and esterases from metagenomics. Curr Protein Pept Sci.15, 445-55.
- Citric acid production by a novel Aspergillus niger isolate:II. Optimization of process parameters through statistical designs. Bioresour. Technol.. 2007;98:3470-3477.
- [Google Scholar]
- Production of Tween 80-inducing esterase by Acinetobacter sp. B1 using Response Surface Methodology. Microbiol. Biotechnol. Lett.. 2019;47(1):87-95.
- [Google Scholar]
- Maester, T.C., Pereira, M.R., Malaman, A.M.G., Borges, J.P., Pereira, P.A.M., Lemos, E.G.M. 2020. Exploring metagenomic enzymes: a novel esterase useful for short-chain ester synthesis. 10, 1-18.
- Statistical optimization by response surface methodology to enhance lipase production by Aspergillus fumigates. Open Microbiol J.. 2019;13:86-93.
- [Google Scholar]
- Lipases: valuable catalysts for dynamic kinetic resolutions. Biotechnol. Adv.. 2015;33(5):372-393.
- [Google Scholar]
- Statistical optimization and neural modeling of amylase production from banana peel using Bacillus subtilis MTCC 441. Int. J. Food Eng.. 2010;6:1-6.
- [Google Scholar]
- Montgomery, D.C., 2005. Response surface method and designs. In: R.A. Myers, D.C. Montgomery (Eds.). Design and Analysis of Experiments (6th edn.). John Wiley and Sons, New York, pp. 39-49.
- Statistical analysis of production of protease and esterase by a newly isolated Lysinibacillus fusiformis purification and application of protease in sub-culturing cell lines. Ann. Microbiol.. 2015;65:33-46.
- [Google Scholar]
- Effects of temperature, aw and pH on the growth of Bacillus cells and spores: a response surface methodology study. Int. J. Food Microbiol.. 1993;19(3):207-216.
- [Google Scholar]
- Optimization of recombinant hyperthermophilic esterase production from agricultural waste using response surface methodology. Bioresour. Technol.. 2006;97(18):2345-2349.
- [Google Scholar]
- Characterization of a novel family VIII esterase EstM2 from soil metagenome capable of hydrolyzing estrogenic phthalates. Microb. Cell Fact.. 2020;19:77-89.
- [Google Scholar]
- Lignin modifying enzyme activities by some Malaysian white rot fungi. ACT-Biotechnol. Res. Commun.. 2011;1:14-19.
- [Google Scholar]
- Identification, characterization, immobilization, and mutational analysis of a novel acetylesterase with industrial potential (LaAcE) from Lactobacillus acidophilus. Biochim. Biophys. Acta.. 2018;1862(1):197-210.
- [Google Scholar]
- Statistical optimization of medium components for enhanced acetoin production from molasses and soybean meal hydrolyzate. Appl. Microbiol. Biotechnol.. 2007;74:61-68.
- [Google Scholar]
- Optimization of lignolytic enzyme production through response surface methodology. BioResources. 2013;8:944-968.
- [Google Scholar]







