Translate this page into:
Ononin mitigates streptozotocin-induced diabetic nephropathy in rats via alleviating oxidative stress and inflammatory markers
⁎Corresponding author. talahmadi@ksu.edu.sa (Tahani Awad Alahmadi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
Diabetic nephropathy (DN) is a foremost complications among diabetic patients worldwide with growing prevalence and mortality rates annually. Nearly, 25–40% of diabetic patients suffer from DN.
Objective
The current research work was done to ascertain the therapeutic properties of ononin against the streptozotocin (STZ)-challenged DN in rats via alleviating oxidative stress and inflammatory responses.
Methodology
The DN was initiated to experimental rats via intraperitoneal injection of 60 mg/kg of STZ. Then DN animals were supplemented orally with three various doses of ononin at 10, 25, and 50 mg/kg once a day for 60 days. After the completion, the glucose level and bodyweight of the animals were recorded. The renal function markers like creatinine, BUN, uric acid, and microalbumin (U-mAlb) was assessed using hematological analyzer. The contents of IL-6, IL-1β, TNF-α, TGF-β1, and fibronectin was assessed using assay kits. The levels of ROS and antioxidant biomarkers were assessed using standard techniques and renal tissues were examined histologically.
Results
The ononin treatment substantially improved the bodyweight and depleted the FBG level, BUN, creatinine, uric acid, and U-mAlb levels in the STZ-challenged DN animals. The levels of inflammatory and fibrosis factors i.e. IL-6, IL-1β, TNF-α, TGF-β1, and fibronectin was remarkably depleted by the ononin treatment. The ononin also decreased the ROS level and improved the GSH content, SOD and GPx activities in the STZ-challenged DN animals. The findings of histological study also confirmed the therapeutic roles of ononin against STZ-induced changes in renal tissues.
Conclusion
Altogether, our results from this study evidences the beneficial roles of ononin treatment against the STZ-initiated DN in animals via its antioxidant and anti-inflammatory properties. Hence, it could be the potential candidate in the future for the management of DN.
Keywords
Diabetic nephropathy
Ononin
N-acetyl-β-D-glucosaminidase
ROS
Inflammation
1 Introduction
Diabetic nephropathy (DN) is a common problem among diabetic patients with increased morbidity and mortality rates worldwide. Almost 40% of diabetic patients develop DN followed by the initiation of hyperglycemia (Gheith et al., 2016). Hyperglycemia can result in the various grades of pathological renal damage, and alterations in kidney hemodynamics and glomerular permeability (Singh et al., 2011). The chronic diabetic condition augment the risks of DN characterized by kidney dysfunction, consequently, DN is considered as one of the most imperative long-term hyperglycaemic-associated disease (Velikova et al., 2021). The molecular mechanisms of DN development is highly complex and indefinable in its root cause. Though the complete underlying mechanisms participated in the initiation and progression of DN is not unveiled yet, but growing evidence suggests that oxidative stress and inflammation provoked by diabetic condition performs a imperative functions in the DN development (Bhattacharya et al., 2013). However, it is believed that the abnormalities in hemodynamic and metabolism may be the vital causes of DN, while it was suggested that excessive inflammatory and oxidative stress condition can directly injure the renal tissues (Barman et al., 2017).
During diabetes, hyperglycemia increases oxidative stress via affecting the redox equilibrium and/or over accumulation of free radicals that triggers cell membrane and DNA damage (Asmat et al., 2016). The oxidative stress is a essential player of DN along with the increased risks of macrovascular diseases, which may leads to death (Dabhi and Mistry, 2015). During hyperglycemia, the increased production of ROS by mitochondria the results in oxidative stress, triggers inflammation, and leads to severe micro- and macro-vascular diseases. Hence, it was suggested that the antioxidant therapy could be useful to treat this condition (Xu et al., 2016). Several earlier reports suggested that the hyperglycemic-associated complications is escorted by the depletion of antioxidants and increased oxidative stress (Kundu et al., 2020).
In addition, diabetic condition also stimulates the transcription of many inflammatory genes that in turn triggers the increased expression of several cytokines like TNF-α, IL-6, and IL-8 in the renal tissues. The initiation and development of DN is connected with augmented pro-inflammatory regulators in serum and apparent inflammatory cell penetrations into renal tissues like IL-6, IL-1β, and TNF-α (Rivero et al., 2009). Additionally, DN development is facilitated by the over production of inflammatory mediators like IL-6 and TNF-α that aggravates the systemic inflammatory condition and worsening of diabetes (Jung and Moon, 2021). TNF-α is a vital factor that is responsible for causing renal injury in diabetic patients (Fernandez-Real et al., 2012). Hence, it is highly necessary to ameliorate the pathophysiology of diabetes via multi-targeting approaches. Consequently, attenuating the oxidative stress and inflammation might potentially diminish kidney damages thereby avert the development of DN (Hsu et al., 2016).
DN also defined by the raised blood urea nitrogen (BUN) and creatinine levels, structural changes of kidney tissues, cellular epithelial thickening, glomerulosclerosis, mesangial extension, interstitial tubule fibrosis, kidney hypertrophy and dysfunction (Duan et al., 2021). The urea, uric acid, creatinine, BUN, and TGF are the crucial biomarkers regarded to be participated in the development of renal dysfunction (Rehman et al., 2013). In recent times, a strict blood sugar and blood pressure control are the mainstay approaches of DN management (Sanajou et al., 2018). Though, DN still appears in most number of diabetic victims. Hence, it is necessary to find a potential therapeutic options that particularly targets DN development.
Ononin is a natural isoflavone that broadly available in several herbal plants like broccoli, lupine, soybean, and astragalus (Dong et al., 2017). It was already reported that ononin exhibits several pharmacological properties like anti-inflammatory effect (Luo et al., 2019), pro-apoptotic effect in breast cancer cells (Zhang et al., 2018), and antiviral property (Yu et al., 2019). Hoo et al. (2010) reported that ononin ameliorated the obesity-provoked metabolic damage via its anti-inflammatory property. Meng et al. (2021) reported that the ononin demonstrated antiarthritic activity. Nonetheless, the beneficial properties of ononin against the amelioration of DN is not scientifically revealed yet. Accordingly, this research work targeted to ascertain the alleviative properties of ononin against the streptozotocin (STZ)-initiated DN in rats through averting the inflammation and oxidative stress.
2 Materials and methods
2.1 Chemicals
Ononin, STZ, buffered saline solution, and other reagents were procured from Sigma-Chemical, USA. The specific ELISA kits to assess the biochemical biomarkers were purchased from the Thermofisher, USA, R&D Systems, USA, and Biocompare, USA, respectively.
2.2 Experimental animals
The present research work was performed on the male wistar albino rats weighing about 240 ± 50 g and the animals were gained from institutional animal house. All animals were detained in infection free and sanitized polypropylene confines with highest care on the systematized laboratory conditions with 22–26 °C of temperature, 40–70% of air moisture, and 12-h of light/dark series. All rats were allowed freely to access the regular rodent chow and purified drinking water in throughout the experimental period. Before initiate the experiments, all the animals were accustomed for a week in an laboratory conditions.
2.3 Experimental design and treatment protocols
After the one week acclimatization, all animals were randomly distributed into five clusters with six rats in each cluster. Group I was reflected as control without any drug administrations and provided only with regular rodent diet in throughout the study. Control animals administered only buffered saline without any drugs. Group II were DN-initiated rats via injecting 60 mg/kg of STZ (newly prepared in citrate buffer, pH-4.5) by intraperitoneal route to initiate the DN. After 72 h of STZ-challenge, the fasting blood glucose (FBG) level was investigated and animals with more than 200 mg/dl were reflected as diabetic and utilized to additional treatments. Group III-V were DN-initiated animals and supplemented with ononin at the dose of 10, 25, and 50 mg/kg, respectively once a day via oral gavage route for 60 days. After the experiments completion, animals were sacrificed after anesthesia by cervical displacement. Then blood and renal tissues samples were gathered and utilized for additional assays.
2.4 Assessment of changes in FBG and bodyweight of experimental animals
The changes in the bodyweight of the experimental animals were noted carefully and data were tabulated. The alterations in the FBG status of control and experimental animals were investigated by using commercially procured glucometer (Roche, USA).
2.5 Measurement of renal biochemical markers level in the experimental animals
After the completion of experiments, the urine and blood samples were gathered from the sacrificed experimental animals used for the renal biochemical markers assessments. The contents of micro-albumin (u-mAlb), BUN, N-acetyl-β-D-glucosaminidase (NAG), uric acid, and serum creatinine were investigated with the aid of Automated Biochemical Analyzer (Hitachi 7100, Hitachi, Japan).
2.6 Quantification of pro-inflammatory marker levels in the renal tissues of experimental animals
The renal tissues were gathered from the experimental animals and homogenized using saline solution. Then the homogenate was centrifuged at 5000 rpm for 5 min and supernatant were employed for assessments. The contents of inflammatory markers i.e., inerleukin-1β (IL-1β), IL-6, TNF-α, and kidney fibrotic markers i.e., TGF-β1, and fibronectin were assessed renal tissue homogenate with the help of specific assay kits according to the guidelines described by manufacturer (R&D Systems, Minneapolis, USA).
2.7 Assay of oxidative stress and antioxidant biomarkers
The renal tissue homogenate from the control and experimental animals were utilized to assess the oxidative stress and antioxidant biomarkers. The content of glutathione (GSH) was assessed by the Carlberg and Mannervik (1975) procedure and the outcomes were depicted as µM/mg protein. The SOD activity was investigated as per the technique of Marklund and Marklund (1974) and outcome was deliberated as U/mg protein. The activity of glutathione peroxidase (GPx) were assessed by the technique of Flohe and Günzler (1984) and the result was displayed as nmol/min/mg protein. The ROS production and AGEs content was assessed with the help of kits by the guidelines indicated by the manufacturer (Mybiosource, USA).
2.8 Histopathological analysis
For the analysis of histological changes, the renal tissues were gathered from the untreated control and experimental animals and processed with 10% of neutral formalin. Then the tissues were embedded in paraffin. The parafinized renal tissues were cut into 5 mm diameter and then stained with hematoxylin-eosin (H&E) solution. At last, the changes in the histopathology of kidney tissues were assessed under the microscope at 40× magnification.
2.9 Statistical analysis
The obtained data were scrutinized by using GraphPad prism software and final results were depicted as mean ± SD of triplicate assays. The outcomes were assessed using the one-way ANOVA and Tukey’s post hoc assay and the significant level was fixed at p < 0.05.
3 Results
3.1 Effect of ononin on the FBG level in the STZ-induced DN rats
Fig. 1 illustrates the effects of ononin on the FBG status of STZ-challenged DN animals. The drastic elevation in FBG level was found in the blood sample of STZ-provoked DN animals when related with control, which confirms the diabetic condition. Considerably, these elevation was alleviated by the ononin treatment. When compared with low dose (10 mg/kg) and middle dose (25 mg/kg) of ononin, the high dose (50 mg/kg) of ononin was demonstrated the appreciable reduction in the FBG level (Fig. 1). This finding confirmed the beneficial actions of ononin against the STZ-initiated diabetes in rats.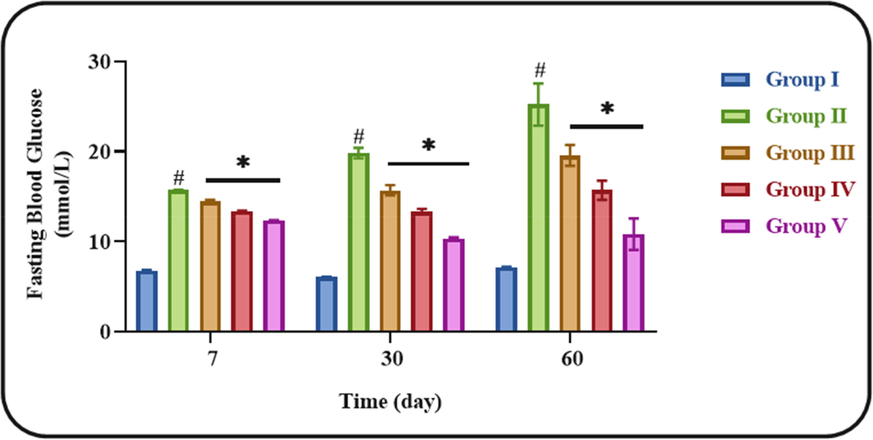
Effect of ononin on the FBG level in the experimental rats. Data were given as mean ± SD of three individual values. All results were statistically assessed by one-way ANOVA and Tukey’s post hoc test. ‘*’ indicates p < 0.05 compared with control ‘#’ indicates p < 0.01 compared with DN-initiated rats. Group I: Control group, Group II: STZ-induced DN group, Group III: Low dose (10 mg/kg) of ononin treated DN group, Group IV: Middle dose (25 mg/kg) of ononin treated DN group, and Group V: High dose (50 mg/kg) of ononin treated DN group.
3.2 Effect of ononin on the bodyweight of STZ-induced DN rats
Fig. 2 indicates the variations in bodyweight of control and ononin treated DN animals. The STZ-initiated DN rats demonstrated the marked reduction in bodyweight when compared with control. However, these reduction in DN animals were inhibited by the ononin treatment. The ononin at three different doses substantially improved the bodyweight, however the high dose (50 mg/kg) of ononin demonstrated increased activity than other two doses (10 and 25 mg/kg). This finding proved that ononin treatment modulated the diabetes-induced changes in bodyweight of DN animals (Fig. 2).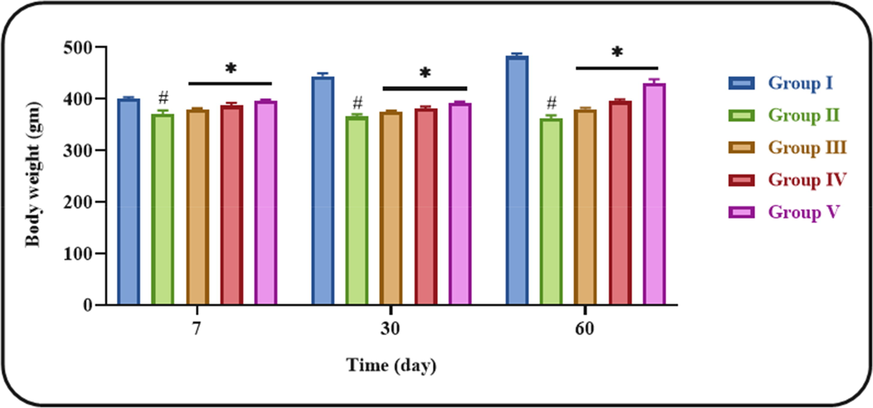
Effect of ononin on the bodyweight of experimental rats. Data were given as mean ± SD of three individual values. All results were statistically assessed by one-way ANOVA and Tukey’s post hoc test. ‘*’ indicates p < 0.05 compared with control ‘#’ indicates p < 0.01 compared with DN-initiated rats. Group I: Control group, Group II: STZ-induced DN group, Group III: Low dose (10 mg/kg) of ononin treated DN group, Group IV: Middle dose (25 mg/kg) of ononin treated DN group, and Group V: High dose (50 mg/kg) of ononin treated DN group.
3.3 Effect of ononin on the renal biochemical markers level in the STZ-induced DN rats
Fig. 3 shows the inhibitory effects of ononin on the renal biochemical markers in the STZ-challenged DN animals. The creatinine, BUN, uric acid, NAG, and U-mAlb contents were drastically elevated in the DN animals when compared with untreated normal control. These changes in creatinine, BUN, uric acid, NAG, and U-mAlb levels were effectively restrained by ononin. The supplementation of 50 mg/kg of ononin appreciably decreased the creatinine, BUN, uric acid, NAG, and U-mAlb contents in the STZ-provoked DN animals than the 10 and 25 mg/kg of ononin treatment (Fig. 3). This findings confirms the beneficial actions of ononin against STZ-initiated DN in rats.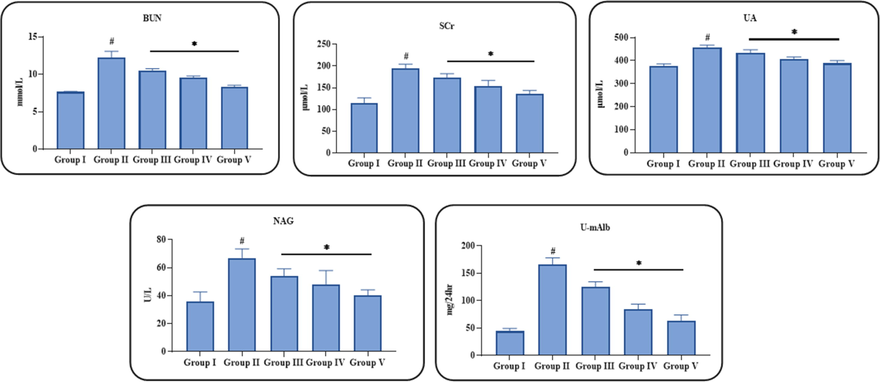
Effect of ononin on the renal biochemical markers level in the experimental rats. Data were given as mean ± SD of three individual values. All results were statistically assessed by one-way ANOVA and Tukey’s post hoc test. ‘*’ indicates p < 0.05 compared with control ‘#’ indicates p < 0.01 compared with DN-initiated rats. Group I: Control group, Group II: STZ-induced DN group, Group III: Low dose (10 mg/kg) of ononin treated DN group, Group IV: Middle dose (25 mg/kg) of ononin treated DN group, and Group V: High dose (50 mg/kg) of ononin treated DN group.
3.4 Effect of ononin on the inflammatory and renal fibrotic markers level in the STZ-induced DN rats
Fig. 4 illustrates the inhibitory role of ononin on the contents of pro-inflammatory (IL-6, IL-1β, and TNF-α) and kidney fibrotic markers (fibronectin and TGF-β1) in the STZ-challenged DN animals. The remarkably increased status of IL-6, IL-1β, TNF-α, fibronectin and TGF-β1 were noted in the STZ-provoked DN animals, when compared with control. Interestingly, these increments were effectively ameliorated by the ononin treatment. The contents of IL-6, IL-1β, TNF-α, fibronectin, and TGF-β1 were substantially decreased in DN animals by the 50 mg/kg of ononin treatment (Fig. 4). The other two (10 and 25 mg/kg) doses of ononin treatments also decreased the IL-6, IL-1β, TNF-α, fibronectin and TGF-β1 levels but comparatively less effective when compared with 50 mg/kg of ononin treatment.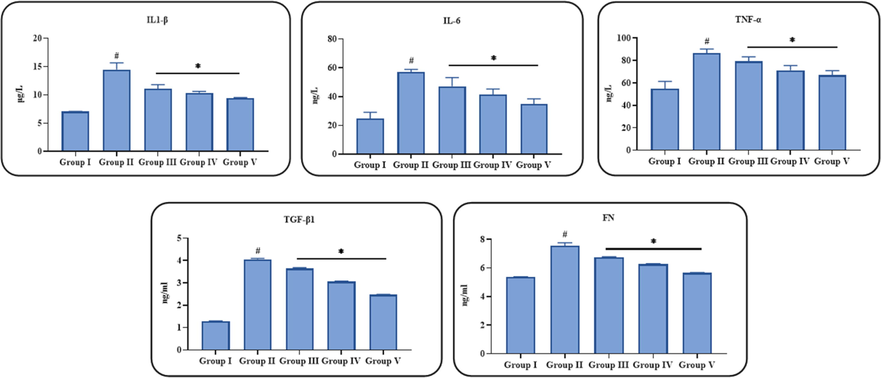
Effect of ononin on the inflammatory and renal fibrotic markers in the experimental rats. Data were given as mean ± SD of three individual values. All results were statistically assessed by one-way ANOVA and Tukey’s post hoc test. ‘*’ indicates p < 0.05 compared with control ‘#’ indicates p < 0.01 compared with DN-initiated rats. Group I: Control group, Group II: STZ-induced DN group, Group III: Low dose (10 mg/kg) of ononin treated DN group, Group IV: Middle dose (25 mg/kg) of ononin treated DN group, and Group V: High dose (50 mg/kg) of ononin treated DN group.
3.5 Effect of ononin on the oxidative stress and antioxidant biomarkers in the STZ-induced DN rats
Fig. 5 exhibits the influence of ononin treatment on the oxidative stress and antioxidant biomarkers in the STZ-initiated DN animals. The present finding demonstrated that the levels of ROS and AGEs were found raised and the GSH content, SOD and GPx activities were found decreased in the STZ-provoked DN animals when compared with control. The ononin treatment effectively attenuated these changes in ROS and antioxidant biomarkers. The supplementation of 50 mg/kg of ononin to STZ-challenged DN rats displayed the remarkable reduction in ROS and AGE contents and also effectively improved the GSH content, SOD, and GPx activities, which evidenced the antioxidant action of ononin (Fig. 5). The low and middle (10 and 25 mg/kg) doses of ononin treated DN animals also displayed the reduced ROS and AGE contents and improved GSH content, SOD, and GPx activities in the STZ-challenged DN animals.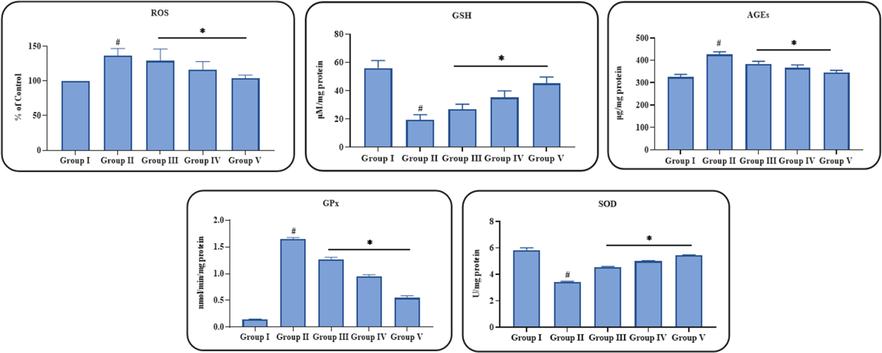
Effect of ononin on the oxidative stress and antioxidant biomarkers in the experimental rats. Data were given as mean ± SD of three individual values. All results were statistically assessed by one-way ANOVA and Tukey’s post hoc test. ‘*’ indicates p < 0.05 compared with control ‘#’ indicates p < 0.01 compared with DN-initiated rats. Group I: Control group, Group II: STZ-induced DN group, Group III: Low dose (10 mg/kg) of ononin treated DN group, Group IV: Middle dose (25 mg/kg) of ononin treated DN group, and Group V: High dose (50 mg/kg) of ononin treated DN group.
3.6 Effect of ononin on the renal histopathology of the STZ-induced DN rats
Fig. 6 demonstrates the histopathological findings of renal tissues of experimental rats. The kidney tissues of control animals demonstrated the healthy appearance with typical renal histo-architectures. However, the renal tissues of STZ-challenged DN animals demonstrated the glomerular shrinkages and necrosis, renal tubular epithelial cell damages and necrosis, degeneration, edema, and increased neutrophils infiltration. Interestingly, these histological changes were effectively attenuated by the ononin treatment. The ononin administration at all three diverse doses demonstrated the therapeutic properties on renal histopathology (Fig. 6). On the other hand, the 50 mg/kg of ononin effectively attenuated these histological alterations in the renal tissues of DN animals than other two (10 and 25 mg/kg) doses.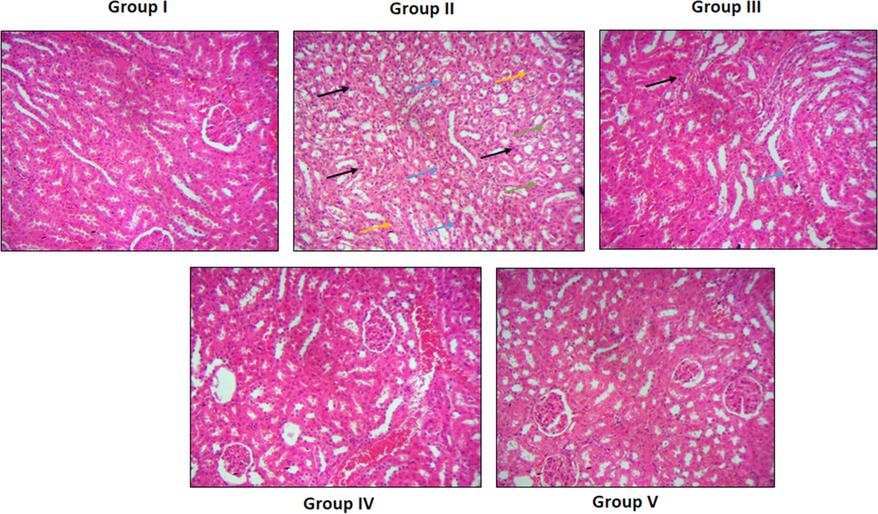
Effect of ononin on the renal histopathology of the experimental rats. Control rats exhibits the healthy tissue appearance with normal histo-architectures (Group I). The STZ-provoked DN animals showed the shrinkages and necrosis of glomeruli (black arrows), renal tubular epithelial cell damages (blue arrows), edema (green arrows), and neutrophils infiltration (yellow arrows) (Group II). The ononin at three different doses i.e. 10, 25, and 50 mg/kg treated DN animals demonstrated the reduced histological changes such as reduced edema, glomerular and tubular damage, and neutrophil penetrations in the renal tissues (Group III-V, respectively).
4 Discussion
The uncontrolled diabetic condition in patients generally develops several complications like nephropathy, retinopathy, and neuropathy. DN is the most serious one among them, which results in the end-stage renal disease. Almost 25% of type-1 diabetes and 40% of type-2 diabetic patients suffer from DN (Piscitelli et al., 2017). The possible mechanisms of development of DN was well established in the STZ-triggered animal diabetic model, which is broadly utilized in the confirmative experiments of antidiabetic effects of sample drugs. This type of induction can result in increased FBG, insulin resistance, and kidney injury (Sun et al., 2019). The augmentation in the FBG, and depletion in bodyweight is a general clinical signs of diabetes. Similarly, here we evidenced that the STZ-challenged animals demonstrated increased FBG and depleted bodyweight, which is effectively attenuated by the ononin treatment. Ononin decreased the FBG and improved the bodyweight in the STZ-challenged animals (Fig. 1). The current findings were supported by the previous report done by Ma et al. (2020).
DN is developed due to the structural and functional remodelling of the kidneys that are provoked by the uncontrolled diabetes (Arora and Singh, 2013). These pathological developments are accredited to persistent hyperglycemia and elevated creatinine, uric acid, urea, and BUN levels, which is witnessed in this current work. The raise in the blood urea status concurrently with augmented blood sugar denotes the injury to the renal tissues of diabetic victims. This statement were in agreement with preceding report that exhibited augmented creatinine and urea status in diabetic animals with kidney injury (Anjaneyulu and Chopra, 2004). The reduced excretory ability of the kidneys leads to the accretion of uric acid, urea, and creatinine in the blood stream (Al-Rasheed et al., 2018). Wali et al. (2019) reported that the BUN, creatinine, uric acid, NAG, and U-mAlb contents were elevated drastically during the progression of nephropathy, which is the biomarkers of DN. In similar manner, our findings from this study showed the increased contents of creatinine, BUN, uric acid, NAG, and U-mAlb levels in the STZ-challenged DN animals. Interestingly, the treatment with the ononin appreciably decreased the levels of creatinine, BUN, uric acid, NAG, and U-mAlb DN animals, which confirmed the therapeutic roles of ononin (Fig. 3). A previous report done by the Jiang et al. (2020) were supported the findings of this study.
Hyperglycemia generates oxidative stress in the organs and triggers over accumulation of ROS, which facilitates DN development (Hong et al., 2017; Sagoo and Gnudi, 2018). The imbalance between oxidant and antioxidants is believed to develop the kidney lesions (Shao et al., 2013). Chronic diabetic stress in the kidneys causes distortion of renal homeostasis and triggers severe morphological and biochemical alterations in the glomerular endothelium, tubular and mesangial epithelium (Sifuentes-Franco et al., 2018). Diabetes-provoked oxidative stress in renal tissues also aggravate the accumulation of free radicals that further causes nephropathy (Fernandes et al., 2016). ROS lessen the antioxidant mechanisms in the cells thereby making them more vulnerable to oxidative injury. ROS further damages protein, DNA, and lipid molecules that results in their oxidation and leads to structural and functional alterations like renal cell necrosis (Nita and Grzybowski, 2016). The considerably elevated ROS status and depleted antioxidant markers like SOD, GPx and GSH are considered as a hallmarks of diabetes initiated complications. SOD is a well known antioxidant, which is essential for dismutating free radicals and improving the antioxidant mechanisms that makes it important factor to examine the antioxidant ability in cells (Zhang et al., 2018). A previous investigation done by Olatunji et al. (2018) also reported that same kind of observations in the diabetic model. In similar manner, our findings also evidenced that the STZ-provoked DN animals demonstrated increased ROS production and depleted antioxidants mechanisms. But the treatment with ononin remarkably decreased the ROS and improved the GSH content, SOD and GPx activities in the DN animals (Fig. 5). The present findings were coincides with the previous research report done by the Qiao et al. (2020).
The systemic inflammatory cytokines is believed as a reliable biomarkers to investigate the nephropathy. Such cytokines can aggravate the risks of diabetic-related complications like DN (Miranda-Diaz et al., 2016). The increased levels of pro-inflammatory markers plays an essential function in DN development. For instance, TNF-α, IL-6, and IL-1β are believed as a vital players of DN progression (Suryavanshi and Kulkarni, 2017). During DN initiation, macrophages in the renal tissues generate IL-6, IL-1β, and TNF-α. The increased status of these cytokines enhances the expansion of kidney injury. The higher status of IL-6, TNF-α, and IL-1β in the renal tissues indicates the suppressed permeability of endothelium (Donate-Correa et al., 2015). Additionally, the release of these cytokines during DN can speed up the macrophages penetration that in turn damages the renal tissues leading to increased urinary protein level. Though, the clear mechanisms of participation of these inflammatory markers in DN has not been discovered yet, it was believed that the of IL-6 plays a major role. Moreover, IL-1β is actively participating in the DN development (Stefanidis et al., 2014). The increased expression of TNF-α can speed up the ROS production and trigger oxidative injury in renal tissues (Navarro-González et al., 2011). The contents of cytokines like IL-6, TNF-α, and IL-1β to be elevated during DN (Lim and Tesch, 2012). Our findings from this investigation proved that the ononin treatment appreciably depleted the IL-6, TNF-α, and IL-1β contents in the STZ-challenged DN animals (Fig. 4), which confirmed the anti-inflammatory potential of ononin. A earlier findings reported by the Ebaid et al. (2020) were supported the current findings of this research work.
The fibronectin and TGF-β1 are believed to be a vital fibrotic markers that enhance the kidney interstitial fibrosis. It is reported that fibronection and TGF-β1 can trigger mesangial cells, via an elevation in protein formation and reduction in degradation of matrix protein that leads to the chronic renal damage (Sorrentino et al., 2018). The depletion in fibronectin and TGF-β1 contents could ameliorate the tubule-interstitial and glomerulosclerotic fibrosis. Here, our findings of this exploration witnessed that the ononin treatment remarkable suppressed the contents of fibronectin and TGF-β1 in the STZ-initiated DN animals (Fig. 4). This finding proved that the ononin can ameliorate the tubule-interstitial and glomerulosclerosis fibrosis in DN animals.
Chronic diabetic condition generates pathological alterations in the tubules and glomeruli that results in lessened filtration of metabolites and electrolytes by the kidneys. The persistent diabetes also triggers glomerular endothelial injury that results in micro-proteinuria (Dabla, 2010). During DN development, the signs comprising the mesangial expansion in glomeruli, and basement membrane thickening with tubular hypertrophy occurs (Lim, 2014). Additionally, DN causes manifold metabolic changes like alteration in blood stream, production of excessive ROS and inflammatory regulators (Ebaid et al., 2014). The augmentation in such factors might act as a risk factors of developing diabetic-associated problems in the prolonged condition, especially in the development of DN (Tonelli et al., 2015; Papatheodorou et al., 2017). Diabetic condition deteriorates the glomerular filtration barrier, provokes glomerular injury, and results in leakage of albumin that aggravates DN (Ma et al., 2013). Our results of this work demonstrated that ononin treatment appreciably attenuated the histological alterations in the renal tissues of STZ-provoked DN rats (Fig. 6). These findings witnessed the therapeutic roles of ononin against STZ-initiated DN. The earlier studies done by the Chowdhury et al. (2019) and Ahmed et al. (2019) were supported the findings of our present study.
5 Conclusion
In conclusion, our results from this work suggests the possible therapeutic roles of ononin against the STZ-challenged DN animals via alleviating oxidative stress and inflammation. The ononin treatment substantially improved bodyweight and decreased the FBG and renal function markers in the DN animals. Ononin treatment also reduced the inflammatory, renal fibrosis, and oxidative stress markers and elevated the antioxidant biomarkers in the DN rats. Overall, these findings recommend that ononin could be a hopeful candidate in the future for the DN treatment. Also, additional works still needed in the future in order to discover the exact therapeutic mechanisms of the ononin against the DN.
Conflict of interest
The authors declare no conflict of interest
Acknowledgement
This project was supported by Researchers Supporting Project number (RSP-2021/230) King Saud University, Riyadh, Saudi Arabia.
References
- Effects of enalapril and paricalcitol treatment on diabetic nephropathy and renal expressions of TNF-α, p53, caspase-3 and Bcl-2 in STZ-induced diabetic rats. PLoS ONE. 2019;14(9)
- [Google Scholar]
- Simvastatin ameliorates diabetic nephropathy by attenuating oxidative stress and apoptosis in a rat model of streptozotocinm induced type 1 diabetes. Biomed. Pharmacother.. 2018;105:290-298.
- [Google Scholar]
- Quercetin, an anti-oxidant bioflavonoid, attenuates diabetic nephropathy in rats. Clin. Exp. Pharmacol. Physiol.. 2004;31(4):244-248.
- [Google Scholar]
- Molecular mechanisms in the pathogenesis of diabetic nephropathy: an update. Vasc. Pharmacol.. 2013;58(4):259-271.
- [Google Scholar]
- Diabetes mellitus and oxidative stress—a concise review. Saudi Pharm. J.. 2016;24(5):547-553.
- [Google Scholar]
- Zinc supplementation alleviates the progression of diabetic nephropathy by inhibiting the overexpression of oxidative stress-mediated molecular markers in streptozotocin-induced experimental rats. J. Nutr. Biochem.. 2017;54:113-129.
- [Google Scholar]
- d-Saccharic acid 1,4-lactone protects diabetic rat kidney by ameliorating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via NF-kB and PKC signaling. Toxicol. Appl. Pharmacol.. 2013;267:16-29.
- [Google Scholar]
- Ferulic acid protects hyperglycemia-induced kidney damage by regulating oxidative insult, inflammation and autophagy. Front. Pharmacol.. 2019;5(10):27.
- [Google Scholar]
- Oxidative stress and its association with TNF-α-308 G/C and IL-1α-889 C/T gene polymorphisms in patients with diabetes and diabetic nephropathy. Gene. 2015;562:197-202.
- [Google Scholar]
- Anti-inflammatory effects of ononin on lipopolysaccharide-stimulated RAW 264.7 cells. Mol. Immunol.. 2017;83:46-51.
- [Google Scholar]
- The role of inflammation in diabetic kidney disease. Korean J. Intern. Med.. 2021;36(4):753-766.
- [Google Scholar]
- Antioxidant bioactivity of Samsum ant (Pachycondyla sennaarensis) venom protects against CCL4-induced nephrotoxicity in mice. Oxidative Med. Cell Longev.. 2014;2014:1-8.
- [Google Scholar]
- Folic acid and melatonin mitigate diabetic nephropathy in rats via inhibition of oxidative stress. Nutr. Metab. (Lond). 2020;14(17):6.
- [Google Scholar]
- The role of oxidative stress in streptozotocin-induced diabetic nephropathy in rats. Arch. Endocrinol. Metabol.. 2016;60(5):443-449.
- [Google Scholar]
- Structural damage in diabetic nephropathy is associated with TNF-α system activity. Acta Diabetol.. 2012;49(4):301-305.
- [Google Scholar]
- Diabetic kidney disease: world wide difference of prevalence and risk factors. J. Nephropharmacol.. 2016;5(1):49-56.
- [Google Scholar]
- Targeting inflammatory cytokines to improve type 2 diabetes control. Biomed. Res. Int.. 2021;2021:1-12.
- [Google Scholar]
- Extracellular superoxide dismutase attenuates renal oxidative stress through the activation of adenosine monophosphate-activated protein kinase in diabetic nephropathy. Antioxid. Redox Signal. 2017
- [Google Scholar]
- The effective fractionisolated from Radix Astragali alleviates glucose intolerance, insulin resistanceand hypertriglyceridemia in db/db diabetic mice through its anti-inflammatoryactivity. Nutr. Metab.. 2010;7:67.
- [Google Scholar]
- Current challenges and future perspectives of renal tubular dysfunction in diabetic kidney disease. Front. Endocrinol. (Lausanne). 2021;12:661185
- [Google Scholar]
- Myrciaria cauliflora extract improves diabetic nephropathy via suppression of oxidative stress and inflammation in streptozotocinnicotinamide mice. J. Food Drug Anal.. 2016;24:730-737.
- [Google Scholar]
- The hypoglycemic and renal protective effects of Grifola frondosa polysaccharides in early diabetic nephropathy. J. Food Biochem.. 2020;44(12):e13515
- [Google Scholar]
- Protective effects of Croton hookeri on streptozotocin-induced diabetic nephropathy. Food Chem. Toxicol.. 2020;135:110873
- [Google Scholar]
- Diabetic nephropathy – complications and treatment. Int. J. Nephrol. Renov. Dis.. 2014;7:361-381.
- [Google Scholar]
- The anti-inflammatory effects of formononetin and ononin on lipopolysaccharide-induced zebrafish models based on lipidomics and targeted transcriptomics. Metabolomics. 2019;15:153.
- [Google Scholar]
- Effect of arctiin on glomerular filtration barrier damage in STZ-induced diabetic nephropathy rats. Phytother. Res.. 2013;27(10):1474-1480.
- [Google Scholar]
- Klotho ameliorates the onset and progression of cataract via suppressing oxidative stress and inflammation in the lens in streptozotocin-induced diabetic rats. Int. Immunopharmacol.. 2020;85:106582
- [Google Scholar]
- Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem.. 1974;47(3):469-474.
- [Google Scholar]
- Ononin induces cell apoptosis and reduces inflammation in rheumatoid arthritis fibroblast-like synoviocytes by alleviating MAPK and NF-κB signaling pathways. Acta Biochim. Pol.. 2021;68(2):239-245.
- [Google Scholar]
- Oxidative stress in diabetic nephropathy with early chronic kidney disease. J. Diabetes Res.. 2016;2016:1-7.
- [Google Scholar]
- Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat. Rev. Nephrol.. 2011;7:327-340.
- [Google Scholar]
- Nita M, Grzybowski A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell Longev. 2016; Article ID 3164734, 23.
- Lycium chinense leaves extract ameliorates diabetic nephropathy by suppressing hyperglycemia mediated renal oxidative stress and inflammation. Biomed. Pharmacother.. 2018;102:1145-1151.
- [Google Scholar]
- Predictors of chronic kidney disease in type 1 diabetes: a longitudinal study from the AMD Annals initiative. Sci. Res.. 2017;7:3313.
- [Google Scholar]
- Ophiopogonin D of Ophiopogon japonicus ameliorates renal function by suppressing oxidative stress and inflammatory response in streptozotocin-induced diabetic nephropathy rats. Braz. J. Med. Biol. Res.. 2020;53(7):e9628
- [Google Scholar]
- Chrysin suppresses renal carcinogenesis via amelioration of hyperproliferation, oxidative stress and inflammation: plausible role of NF-κB. Toxicol. Lett.. 2013;216(2-3):146-158.
- [Google Scholar]
- Pathogenic perspectives for the role of inflammation in diabetic nephropathy. Clin. Sci.. 2009;116:479-492.
- [Google Scholar]
- Diabetic nephropathy: Is there a role for oxidative stress? Free Radical Biol. Med.. 2018;116:50-63.
- [Google Scholar]
- AGE-RAGE axis blockade in diabetic nephropathy: current status and future directions. Eur. J. Pharmacol.. 2018;833:158-164.
- [Google Scholar]
- Relationship between oxidant/antioxidant markers and severity of microalbuminuria in the early stage of nephropathy in Type 2 diabetic patients. J. Diab. Res.. 2013;2013:1-6.
- [Google Scholar]
- Oxidative stress, apoptosis, and mitochondrial function in diabetic nephropathy. Int. J. Endocrinol.. 2018;2:1-13.
- [Google Scholar]
- Oxidative stress in early diabetic nephropathy: fueling the fire. Nat. Rev. Endocrinol.. 2011;7(3):176-184.
- [Google Scholar]
- Diabetic retinopathy and endothelin system: microangiopathy versus endothelial dysfunction. Eye. 2018;32(7):1157-1163.
- [Google Scholar]
- Association between the interleukin-1beta Gene (IL1B) C-511T polymorphism and the risk of diabetic nephropathy in type 2 diabetes: a candidate gene association study. DNA Cell Biol.. 2014;33:463-468.
- [Google Scholar]
- CXCL6 promotes renal interstitial fibrosis in diabetic nephropathy by activating JAK/STAT3 signaling pathway. Front. Pharmacol.. 2019;10:224.
- [Google Scholar]
- NF-κβ: a potential target in the management of vascular complications of diabetes. Front. Pharmacol.. 2017;8:798.
- [Google Scholar]
- Trace element supplementation in hemodialysis patients: a randomized controlled trial. BMC Nephrol.. 2015;16(52):1-9.
- [Google Scholar]
- Comparison of serum levels of Cystatin-C and traditional renal biomarkers for the early detection of pre-hypertensive nephropathy. J. Pakistan Med. Assoc.. 2019;69:313-319.
- [Google Scholar]
- Influence of different levels of lipoic acid synthase gene expression on diabetic nephropathy. PLoS ONE. 2016;11(10)
- [Google Scholar]
- Ononin, sec-O-b-D-glucosylhamaudol and astragaloside I: antiviral leadcompounds identified via high throughput screening and biological validationfrom traditional Chinese medicine Zhongjing formulary. Pharmacol. Res.. 2019;145:104248
- [Google Scholar]
- Antioxidation, anti-hyperglycaemia and renoprotective effects of extracellular polysaccharides from Pleurotus eryngii SI-04. Int. J. Biol. Macromol.. 2018;111:219-228.
- [Google Scholar]
- Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through down-regulation of PI3Kgamma mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Sci. Rep.. 2018;8:11255.
- [Google Scholar]







