Translate this page into:
One-pot, simple and efficient synthesis of novel bioactive 4-aryl-1,2-dihydro-6-(4-hydroxy-2-oxo-2H-chromen-3-yl)-2-oxopyridin-3-carbonitriles via multi-component approach
⁎Corresponding author at: Research Laboratory of Environmental Sciences and Technologies (LR16ES09), Higher Institute of Environmental Sciences and Technology, University of Carthage, Hammam-Lif, Tunisia. naceur.hamdi@isste.rnu.tn (Naceur Hamdi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
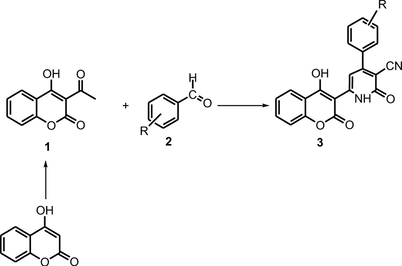
Quantitative multicomponent cyclocondensation of 3-acetyl-4-hydroxycoumarine with ethyl cyanoacetate and aryldehydes has been carried out in ammonium acetate, at reflux.
Abstract
Quantitative multicomponent cyclocondensation of 3-acetyl-4-hydroxycoumarine with ethyl cyanoacetate and aryldehydes has been carried out in ammonium acetate, at reflux. The optimization details of the developed novel protocol are recorded. The structure of newly synthesized compounds have been characterized by spectroscopic data (elemental analysis, IR and 1H NMR spectroscopic studied). Antimicrobial properties of the novel coumarins derivatives 3 are investigated. The acetylcholinesterase inhibition activity (AChEI) was tested for all compounds, and the 3b shows the highest AChEI activity with 48.25% of inhibition.
Keywords
Multicomponent reaction
4-Hydroxycoumarin
Ethyl cyanoacetate
Antibacterial and acetylcholinesterase inhibition activities
- POCl3
-
phosphorus oxychloride
- DMC
-
dimethylcarbonate
- DEC
-
diethylcarbonate
- DCM
-
dichloromethane
- NH4OAc
-
ammonium acetate
Abbreviations
1 Introduction
4H- hydroxycoumarins derivatives have a wide range of biological applications (Doshi et al., 2006; Kemnitzer et al., 2007; Kumar et al., 2007; Gao et al., 2010). In this connexion coumarins derivatives are important in organic synthesis studies (Thompson, 2000; Akbarzadeh et al., 2012; Wang et al., 2000). On the other multicomponent reaction protocol with environmentally solvents and catalytic systems is one of the most suitable strategies, for developing libraries of medicinal scaffolds (Saeedi et al., 2013; Dömling, 2002). One pot, multi-component synthetic protocol is more significant than the step wise approach in terms of efficiency, minimal waste production, energy or cost-effectiveness, and operational simplicity (Li and Chan, 1997; Boubakri et al., 2016; Medyouni et al., 2016). As part of our ongoing studies for the development of an environmentally friendly reaction condition to produce heterocyclic compounds (Hamdi et al., 2017; Al-Ayed and Hamdi, 2014; Hamdi et al., 2011), we now report herein an efficient and clean synthesis method of a novel coumarins derivatives carbonitriles 3. These compounds were subsequently evaluated for their biological activities.
2 Experimental section
2.1 General procedures
1H NMR (300 MHz) and 13C NMR (75 MHz) spectra were recorded on a Varian VXR 300 instrument at 293 K in CDCl3 or DMSO- d6. Spectra were internally referenced to TMS. Peaks are reported in ppm downfield of TMS. The melting points of compounds were determined in open glass capillaries in a paraffin bath and are uncorrected. Elemental microanalysis was obtained by the microanalytical laboratory services, university of Rennes 1 in France.
3-Acetyl-4-hydroxycoumarin (1) was synthesized using our previous work (Al-Ayed and Hamdi, 2014).
Preparation of coumarins derivatives 3 Fig. 1 (Supplementary material, SS1).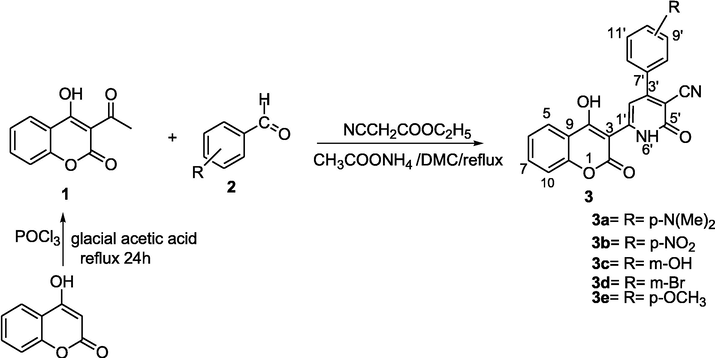
Synthesis of coumarins derivatives carbonitriles 3 catalyzed by NH4OAc.
2.2 Antibacterial activity tests
Antibacterial activities were tested against selected food-borne pathogens and clinical Gram-positive and Gram-negative bacteria strains obtained from local culture collection and International Culture Collections (ATCC). We have selected for this study Staphylococcus aureus (S. aureus) ATCC 6538, Micrococcus luteus (M. luteus) LB14110, Listeria monocytogenes (L. monocytogenes) ATCC 19117 Salmonella typhimurium (S. typhimurium) ATCC 14028 and Agrobacterium tumifaciens (A. tumifaciens).
Analysis of the activities of compounds against these selected bacteria was conducted using the Agar well diffusion method according to Guven et al. (2005) with some modification as reported by Al-Ayed and Hamdi (2014). Minimum inhibitory concentration (MIC) is defined as the lowest concentration of the tested compound for which the bacteria did not growth in the optimal condition of incubation. The procedures for MIC tests of the newly synthesized products were conducted in accordance with NCCLS guideline M7-A6 and M38-P (National Committee for Clinical Laboratory Standard, 2003) and detailed in Al-Ayed and Hamdi (2014).
2.3 Acetylcholinesterase inhibitory potential (AChEI)
AChEI was performed following the spectrophotometric procedures previous reported by Ellman et al. (1961). All obtained compounds were tested at concentration of 100 mg/ml. We have followed the same protocol reported in Al-Ayed and Hamdi (2014).
For all biological teste we have expressed the measurements in average ± standard deviation after triplicate assays.
3 Results and discussions
3.1 Chemistry
Reaction between 4-hydroxycoumarin and phosphorus oxychloride (POCl3) in glacial acetic acid, under reflux for 24 h afforded 3-acetyl-4-hydroxycoumarine 1 in excellent yield (90%) (Hamdi et al., 2011).
The IR spectra of compound 1 revealed a strong band at 3185 cm−1 confirming the presence of OH group and showed band in the region of 1700 cm−1 which is the characteristic for C⚌O of coumarin. The 1H NMR data of compound 1 revealed signal at 2.72 ppm attributed to the methylic protons, the aromatic protons resoned between 7.1 and 7.98 pp. While hydroxylic proton (OH) resonated at δ 17.69 ppm.
The reaction of 3-acetyl-4-hydroxycoumarine 1 with ethyl cyanoacetate and benzaldehyde in was explored using different solvents (DMC, DEC, ethanol, H2O, tetrahydrofuran (THF), dichloromethane and toluene). (Table 1, entries 1–7) in the presence of a catalytic amount of ammonium acetate. The results are summarized in Table 1. aReaction conditions: 3-acetyl-4-hyddroxycoumarine (1 mmol), ethyl cyanoacetate (1 mmol), solvent (5 mL), reflux. bIsolated yield of product.
Entry
Solvent
Temp (°C)
Time (min)
Yield (%)b
1
DMC
90
30
85
2
DEC
128
30
80
3
ethanol
78
120
72
4
THF
66
120
62
5
H2O
100
30
83
6
DCM
40
120
60
7
Toluene
110
120
60
From the obtained results we can say that solvents affected the yields (Table 1, entries 1–7), and the best result was obtained in excellent yield and high purity with DMC.
Then, we performed the reaction with different amounts of catalyst at ambient temperature (5 mol%, 10 mol%, 15 mol%, and 20 mol%) in order to optimize the ammonium acetate loading. The results are given in Table 2.
Experience
catalyst
Mol (%)
Time (mn)
Yield (%)b
1
NH4OAc
5
5
60
2
NH4OAc
10
10
85
3
NH4OAc
15
3
82
4
NH4OAc
20
3
95
5
NH4OAc
25
3
92
6
NH4OAc
30
620
88
7
No catalyst
–
620
10
When we increase the percentage of the catalyst the obtained yields were also increased up, but after 20 mol% there is no improvement of the yield of the reaction.
In order to explore more this method, the cyclocondensation reaction with different series of substituted aromatic aldehydes was achieved. The results are given in (Table 3). a3-acetyl-4-hydroxycoumarine (1 mmol), arylaldehydes (1 mmol), ethyl cyanoacetate (1 mmol), DMC (5 mL), NH4OAc (20%), reflux. bIsolated yield of product.
entry
1
2
3
4
5
Product
3a
3b
3c
3d
3e
Time (min)
60
50
45
80
75
Yieldb (%)
95
92
90
–
88
The scope of the method was assayed with a series of substituted aromatic aldehydes after optimization of the reaction conditions. The results are summarized in Table 3. As shown in Table 3, the aromatic aldehydes bearing both the electron withdrawing (inputs 2,3) and electron donor groups (1,5) were subjected to successful condensation with ethyl cyanoacetate and 4-hydroxycoumarin. In the presence of a catalytic amount of ammonium acetate in the refluxing DMC to give the corresponding products in convenient yields. It appears that the electronic effects and the nature of the substituents on the arylaldehyde ring have a small effect on the reaction yield and on the time required to complete the reaction. Electron donor groups somewhat increased the reactivity and provided higher yields compared to the electron-withdrawing groups. Remarkably, the reactions were clean, and all products were obtained after only filtration and simple washing with water and ethanol. Thus, a simple treatment gives the title products without need of chromatographic purification.
The structure of the new compounds 3 was identified by spectroscopic data and either by their elemental analysis. 13C NMR showed the amide (NH–CO) signal at δ 177.8 ppm, the C1′ signal with a chemical shift of δ = 61.9 ppm, while the C4 signal was assigned further upfield at δ = 96.47 ppm. However, the C2 signal was further upfield, δ = 180.13 ppm, than was the C4′ signal was assigned as δ = 134.48 ppm.
The proposed mechanism of compound 3 is given by the reaction sequence in Fig. 2. First, condensation of ammonium acetate with ethyl cyanoacetate is proposed to give intermediate (i), then 3-acetyl-4-hydroxycoumarine react with arylaldehydes to give intermediate (ii). The Michael addition of intermediate (i) with intermediate (ii) occurs to provide the intermediate (iii) which undergoes aromatization to form the target coumarins carbonitriles 3.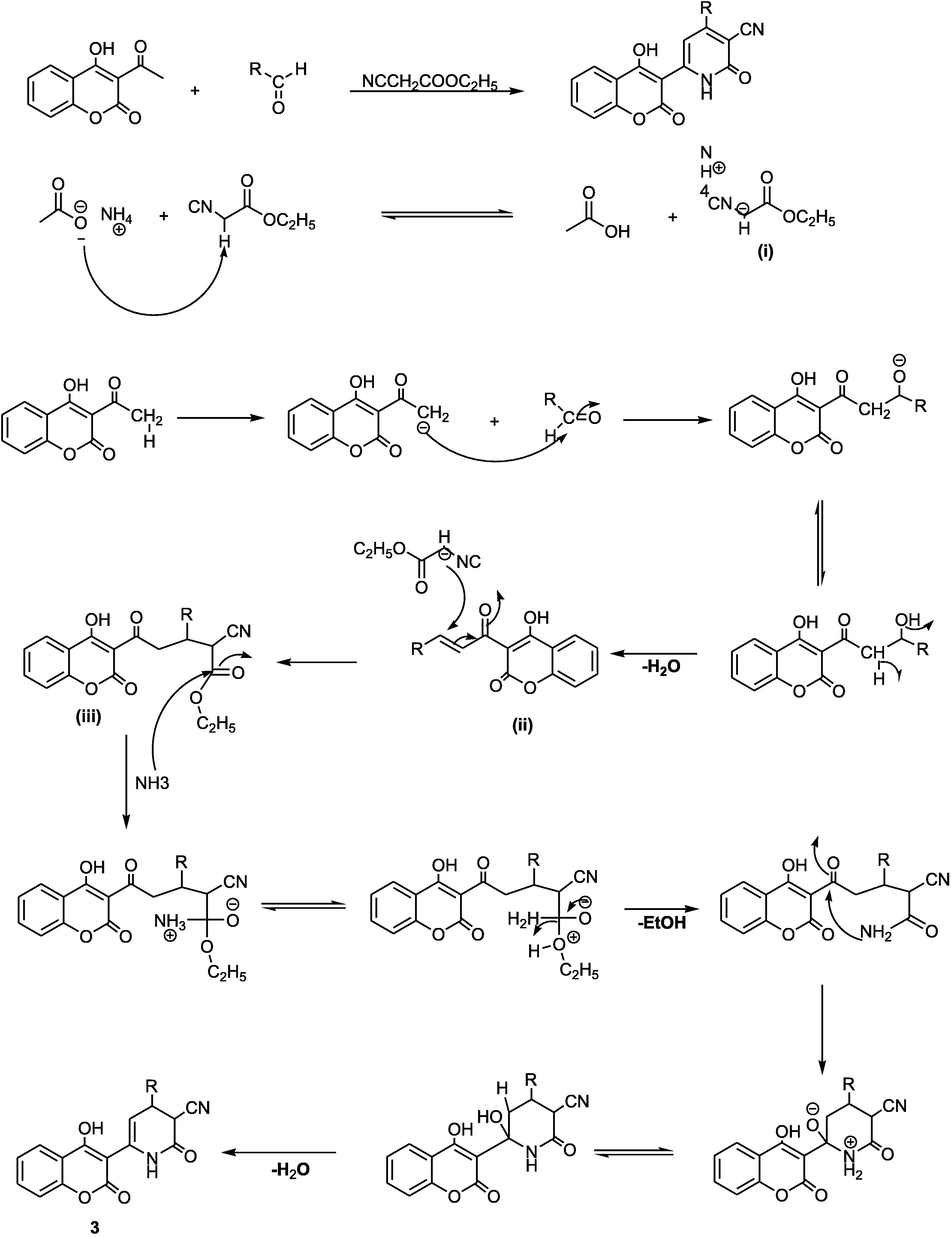
Proposed mechanism for the synthesis of compound 3.
3.2 Biological activities
3.2.1 Antibacterial activity
The coumarins derivatives were screened for their antibacterial as reported by our previous work (Boubakri et al., 2016). The antibacterial activity of the compounds against human pathogenic Gram positive and Gram-negative bacteria of derivatives 3 was estimated by measuring the zone of inhibition in disc diffusion method. The antimicrobial activities of synthesized coumarins are given in Table 4. AMC: Ampicillin.
Bacteria
Conc. mg/ml
Inhibition zones diamater (mm)
AMC
3a
3b
3c
3d
3e
Micrococcus luteus
LB 14,1100.1
19
0
16
18
19
42
0.3
23
0
16
15
16
0.5
23
24
16
14
15
Staphylococcus aureus
ATCC 65380.1
24
0
40
16
17
0.3
24
0
36
15
16
0.5
33
0
33
15
17
Listeria monocytogenes
ATCC 191170.1
0
0
0
22
23
0.3
0
0
0
23
18
0.5
22
0
0
24
17
Salmonella Typhimurium
ATCC 140280.1
0
0
0
23
16
0.3
0
0
0
24
15
0.5
16
0
22
25
17
Agrobacterium tumifaciens
0.1
21
21
0
15
18
0.3
20
19
16
14
17
0.5
21
18
0
16
16
The synthesized coumarin 3a show activity against LB 1411with three different concentration 0.1, 0.3 and 0.5 mg/ml. Compound 3b show activity against two bacteria Micrococcus luteus LB 14110 and Agrobacterium tumifaciens only at concentration of 0.5 mg/ml. Coumarin derivative 3d show antibacterial activities against all the species of bacteria. The Minimal Inhibitory Concentrations (MICs) values of coumarins derivatives 3 were determined against the same bacteria. The results are given in Table 5.
Microorganism indicator
Compounds
MIC (mg/mL)
Micrococcus luteus LB 1411
Ampicillin
0.0195
3a
0.04
3b
0.03
3c
0.02
3d
0.035
3e
0.042
Listeria monocytogenes ATCC 19117
Ampicillin
0.039
3a
2.5
3b
1.25
3c
2.5
3d
2.4
3e
2.3
Salmonella typhimuriumATCC14028
Ampicillin
0.625
3a
1.25
3b
1.26
3c
0.078
3d
1.5
3e
1.6
The Minimal Inhibitory Concentrations (MICs) values of coumarins derivatives 3 were determined. The most active compound was 3c which presents the highest activity against the Gram-negative bacteria Salmonella typhimurium ATCC14028, often greater than the used standard (ampicillin). Low effect of all substances was observed against the Gram-positive bacteria, Listeria monocytogenes ATCC 19117. All the compounds show efficient effect against the Gram-positive Micrococcus luteus LB 1411
3.2.2 Acetylcholinesterase inhibition
The acetylcholinesterase enzyme (AChE) is hydrolysis of the neurotransmitter acetylcholine allowing the termination of impulse transmission at cholinergic synapses of the central and peripheral nervous systems. Degenerative nervous disease as Alzheimer's disorder has been associated with a deficiency of acetylcholine. Acetylcholinesterase inhibitors (AchEIs) are introduced for symptomatic treatment of Alzheimer disorders (Shah et al., 2008).
The acetylcholinesterase enzyme (AChE) were done as reported work (Kalauni et al., 2002; Atta-ur-Rahman et al., 2004; Ahmad et al., 2003), and they are presented in Table 6.
Compound
(AChEI) (%)
3a
–
3b
48.25
3c
–
3d
37.15
3e
37.80
Three compounds show significant AChEI activity. The compound 3b possesses the most AChEI effect.
4 Conclusion
In conclusion, we have demonstrated a highly efficient method for the synthesis of coumarins carbonitriles 3 via three component reaction of aromatic aldehydes, ethyl cyanoacetoacetate, and 3-acetyl 4-hydroxycumarine using cheap and readily available low toxic organocatalyst ammonium acetate. This proposed method has main advantages to be work-up procedures and simple experimental, small amount of catalyst, solvent-free reaction conditions, high yields, short reaction time, and using reusable and non-expensive catalyst. The obtained compounds show efficient antibacterial activity against pathogenic foodborne bacteria belong either to gram-positive and gram-negative. These notable antibacterial effects confirm the necessity for synthesizing new series derived from these compounds. The availability of these compounds will also facilitate further investigations of their pharmacological properties.
Acknowledgements
This work was funded by the Research Supporting Project (RSP-2019/75), King Saud University, Riyadh Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- In vitro inhibition of acetylcholinesterase, butyrylcholinesterase and lipoxygenase by crude extract of Myricaria elegans Royle. J. Biol. Sci.. 2003;11:1046-1049.
- [Google Scholar]
- A new and efficient method for the synthesis of novel 3-acetyl coumarins oxadiazoles derivatives with expected biological activity. Molecules. 2014;19:911-924.
- [Google Scholar]
- 2-Amino-3-cyano-4-(5-arylisoxazol-3-yl)-4H-chromenes: synthesis and in vitro cytotoxic activity. Arch. Pharm.. 2012;345:386-392.
- [Google Scholar]
- New cholinesterase inhibiting bisbenzylisoquinoline alkaloids from Cocculuspendulus. Chem. Pharm. Bull. 2004;52:802-806.
- [Google Scholar]
- Novel pyrano[3,2-c]chromene derivatives via a green one-pot three component: synthesis, characterization, antioxidant, antibacterial and anti-inflammatory activities. Med. Chem.. 2016;5:387-394.
- [Google Scholar]
- Recent advances in isocyanide-based ulticomponent. Chem. Curr. Opin. Chem. Biol.. 2002;6:306-313.
- [Google Scholar]
- Structure-activity relationship studies of ethyl2-amino-6-bromo-4-(1-cyano-2-ethoxy-2-oxoethyl)-4h-chromene-3-carboxylate (HA 14–1), an antagonist for antiapoptotic Bcl-2 proteins to overcome drug resistance in cancer. Chem. Abstr.. 2006;49:7731-7739.
- [Google Scholar]
- Biochemical Pharmacology. 1961;7:88-95.
- Synthesis of carbon-11-labeled 4-aryl-4H-chromens as new PET agents for imaging of apoptosis in cancer. Appl. Radiat. Isot.. 2010;68:110-116.
- [Google Scholar]
- A rapid access to new coumarinyl chalcone and substituted chromen[4,3—c] pyrazol-4-ones and their antibacterial and DPPH radical scavenging activities. Med. Chem. Res.. 2011;20:522-530.
- [Google Scholar]
- Synthesis, characterization, aggregation properties, antioxidant and antimicrobial activity of novel Unmetalled and metallophthalocyanines bearing coumarin derivatives. J. Heterocyclic Chem.. 2017;54:2342-2351.
- [Google Scholar]
- New cholinesterase inhibiting steroidal alkaloids from the leaves of Sarcococcacoriacea of Nepalese origin. Chem. Pharm. Bull. 2002;50:1423-1426.
- [Google Scholar]
- Discovery of 4-Aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. 3. Structure−activity relationships of fused rings at the 7,8-position. J. Med. Chem.. 2007;50:2858-2864.
- [Google Scholar]
- An atom efficient, solvent-free, green synthesis and antimycobacterial evaluation of 2-amino-6-methyl-4-aryl-8-[(E)-arylmethylidene]- 5,6,7,8-tetrahydro-4H-pyrano[3,2-c]pyridine-3-carbonitriles. BioorgMem. Chem. Lett.. 2007;17:6459-6462.
- [Google Scholar]
- Organic Reactions in Aqueous Media. New York NY USA: Wiley; 1997.
- One-pot three-component Biginelli-type reaction to synthesize 3,4-dihydropyrimidine-2-(1H)-ones catalyzed by Co phthalocyanines: Synthesis, characterization, aggregation behavior and antibacterial activity. Spectrochim. Acta A. 2016;167:165-174.
- [Google Scholar]
- National Committee for Clinical Laboratory Standard, 2003. Method for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically Approved Standard, M7-A6. Wayne, Pa, USA.
- Synthesis of novel fused 4,5-dihydro-1,2,3-triazolo[1,5-a][1,4]benzodiazepine derivatives via four-component Ugi–Smiles-type reaction. Tetrahedron. 2013;69:3506-3510.
- [Google Scholar]
- Current approaches in the treatment of Alzheimer's disease. Biomed. Pharmacother.. 2008;62:199-207.
- [Google Scholar]
- Recent applications of polymer-supported reagents and scavengers in combinatorial, parallel, or multistep synthesis. Curr. Opin. Chem. Biol.. 2000;4:324-337.
- [Google Scholar]
- Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc. Natl. Acad. Sci. U.S.A.. 2000;97:7124-7129.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2019.11.023.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







