Translate this page into:
Olfactory response of two different Bactrocera fruit flies (Diptera: Tephritidae) on banana, guava, and mango fruits
⁎Corresponding authors at: Department of Entomology, South China Agricultural University, Guangzhou 510642, China. waqar4me@yahoo.com (Waqar Jaleel), yrhe@scau.edu.cn (Yurong He)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Bactrocera dorsalis and B. correcta (Diptera: Tephritidae) are economically important pests of fruits and have caused serious damage to fruits for the last several years worldwide. In China, B. correcta is second economic pest of fruits after B. dorsalis. Considering the importance of Integrated Pest Management (IPM) programs, Information regarding host preference and fitness of both Bactrocera species are necessary for better management strategies. Therefore, the current study explains the response of both Bactrocera species on banana, guava, and mango fruits. The cultivar of banana, guava, and mango fruits used first time in this study. Therefore, the volatile/aromatic components of banana, guava, and mango fruits were determined using porapak Q via gas chromatography-mass spectrometry (GC–MS). Results concluded that the number of male flies of both species on each types of fruits were lower in comparison to female flies. The number of flies and oviposition punctures by female B. dorsalis flies were maximum on mango fruits than those of guava and banana fruits. While in the case of B. correcta, the guava fruits were preferable for visits and oviposition punctures than those of other two fruits. Mango fruits were more favorable for the development and survival of both Bactrocera species than those of other two fruits. The GC/MS results indicated that butanoic acid-3-methylbutyl ester, α-caryophyllene, and 3-carene were the major volatile components of banana, guava, and mango fruits, respectively. Based on the results, mango and guava fruits were more suitable for both Bactrocera species. Future studies are needed to confirm the results of this laboratory study in the fruit orchards.
Keywords
Bactrocera species
Butanoic acid-3-methylbutyl ester
Fruits preference
3-Carene
And α-caryophyllene
1 Introduction
Insect pests, especially Bactrocera species (Diptera; Tephritidae) are important pests and have caused serious damage to fruits for the last several years (Allwood et al., 1999, Jamal et al., 2021). Among Bactrocera species, Bactrocera dorsalis Hendel and Bactrocera correcta Bezzi are economically important pests of fruits in Asia (Jaleel et al., 2019, Jaleel et al., 2018a, Jaleel et al., 2018b, Jaleel et al., 2018c, Jaleel et al., 2020, Jaleel et al., 2021, Allwood et al., 1999). Nowadays, B. correcta is the second to B. dorsalis as serious pest to fruits in China. B dorsalis is one of the most polyphagous pests that can infest more than 250 host plant species, especially fruits (Jaleel et al., 2018b). Mango, guava, papaya, banana, and citrus fruits are the favorite hosts of B. dorsalis (Zhang et al., 2018). Similarly, B. correcta is a serious pest of guava fruits. Other fruits such as mango, cashew nut, orange, banana, cherry, jujube, carambola, and wax apple has also been reported as host (Liu et al., 2013, Jaleel et al., 2020). Both B. dorsalis and B. correcta prefer to oviposit on the most favorable hosts (Cunningham et al., 2016). Female adults of B. dorsalis recognize the suitable host at an optimal distance using visual and olfactory chemical cues (García Gonzalez et al., 2018). However, most of Bactrocera species dislike the unripe fruits, or with a hard skin for oviposition or their immature development (Rattanapun et al., 2009, Jaleel et al., 2018b).
The host preference of female Bactrocera flies usually depends on the host aroma emission rate, softness, (Metcalf et al., 1983, Jamal et al., 2021), and sugar level (Rattanapun et al., 2009, Naeem-Ullah et al., 2020). The physical characteristics of fruits are essential for study the olfactory and ovipositional behavior of Bactrocera species. Because skin toughness and sugar level (Brix) of fruits have a significant impact on the selection behavior of female Bactrocera flies for their immature development (Jaleel et al., 2018b, Rattanapun et al., 2009).
The development and survival rate of Bactrocera species usually varies on different fruits (Rattanapun et al., 2009, García Gonzalez et al., 2018). Soft and juicy skin fruits are more suitable for the survival and development of Bactrocera species (Rattanapun et al., 2009, Jaleel et al., 2018b). The nutrition level of fruits may have a significant effect on the development and survival of the Bactrocera offspring (McGraw et al., 2005, Khan and Ghramh, 2021). However, fruits have a different level of toxins, which may affect the development of the larvae of Bactrocera (Rattanapun et al., 2009). However, to the best of our knowledge, any works has been carried out on the B. correcta preference for mango fruits.
Identification of volatile constituents from fruits is necessary because most of the volatile components are good attractants for Bactrocera species (Biasazin et al., 2014, Jaleel et al., 2019). Mango (Mangifera indica L. Anacardiaceae), guava (Psidium guajava Linn. Myrtaceae), and banana (Musa spp. Musaceae) are economically valuable fruits and kept essential vitamins for human nutrition (Paniandy et al., 2000, Maldonado-Celis et al., 2019). Aromatic or volatile compound of fruits are very important to make fruit attractive as a source for pests such as species of Bactrocera. Cyclopentasiloxane and tetradecamethyl- were reported as aromatic compounds of banana fruits (Jaleel et al., 2021). 3-methyl butyl acetate, isoamyl butanoate, and isoamyl isovalerate were the major aromatic components of banana fruits (Schwab et al., 2008). Caryophyllene and humulene were the major volatile components of guava fruits (Jaleel et al., 2021). The 3-carene has been reported one of the aromatic compounds of mango (Tamura et al., 2000, Jaleel et al., 2021). Acetic, butyric, hexanoic acids and ethyl 3-hydroxybutyrate are aromatic components of mango fruits (Sakho et al., 1985). Acetaldehyde, acetone, methanol, ethanol, a-pinene, caryophyllene, 3-carene, b-pinene, myrcene, limonene, terpinolene, a-copaene, and r-cymene were reported from mango fruits (Baldwin et al., 1999, Pino & Mesa, 2006).
In the context of Integrated Pest Management programs, farmers need reliable control methods (Saeed et al., 2019) against both Bactrocera species. Understanding their behavior on fruits is necessary for scheming and applying safe control strategies in the fields. The behavior and fitness of B. dorsalis and B. correcta were yet described on banana, papaya, and guava fruits (Jaleel et al., 2018b). In this study, the mango fruits were selected to study the behavior or host preference of B. correcta in comparison to B. dorsalis on three different fruits 1. banana: Musa acuminata L. var. wn Thang Huanga, 2. guava: Psidium guajava Linn. var. Zhenzhu or Pearl, and 3. mango: M. indica L. Hanana Datai Nong Mang). The cultivar of banana, guava, and mango fruits used first time in this study. The objectives of this work were (1) to find out the aromatic profile and (2) to study the attraction behavior of Bactrocera species.
2 Material and methods
2.1 Organisms of study
Both Bactrocera species (B. dorsalis and B. correcta) were reared according to the methodology described by Jaleel et al. (2018b, c). Colonies of both species were reared up during two generations for acclimatization on each host in the laboratory (26 ± 2 °C, 12:12 h L: D). We used gravid female flies (aged: 15–18 days) in all experiments.
2.2 Characteristics of selected fruits
Banana, guava, and mango fruits were purchased from different orchards located in Guangzhou, Guangdong, China. Based on discussion with farmers, each fruit types were bagged at early ripening stage. A fruit of banana, guava, and mango were kept separately in a plastic jar (23.5 × 15.8 × 10 cm) containing a 3-cm layer of soil in the laboratory. Fifteen replicates were made for each fruit. Each fruit was observed daily for 15 days. There was no infestation by wild insect pests was observed in each type of fruits. Fruit characteristics e.g., Total soluble solids (TSS) of each fruit were measured using a handheld pocket refractometer pal-1 (ATAGO, PR-101a, Brix 0–45%, Tokyo Tech. Japan). The pericarp toughness or firmness of each fruit type was measured using a TMS-Pro texture analyzer (FTC-TV, USA) with probe (1 mm diameter) (Rattanapun et al., 2009, Balagawi et al., 2005, Díaz-Fleischer and Aluja, 2003, Jaleel et al., 2018b).
2.3 Gender
For recognition between male and female flies of B. dorsalis, red permanent marker was used to cover the thorax of male flies. While green color marker was used for female flies. So, ten pairs of B. dorsalis (15–18 days old) were prepared (colored) and released in the cage, and 3 different fruits (one banana, one guava, and one mango) were kept. Observation done for 10 h to record the number of male and female flies present on the fruit surface. Each fruit was observed for 2 min/h. This experiment was replicated six times. Same experiments was done on the B. correcta.
2.4 Time spent
Two types of experiments were conducted to assess the movement behavior B. dorsalis and B. correcta. Firstly, a no-choice test was carried out using a mated female of B. dorslais released into a plastic jar (23.5 × 15.8 × 10 cm) containing one fruit type. Twenty replications were conducted for each fruit. The time spent by female B. dorsalis on each fruit was recorded from 9.00 am to 2.00 pm in a day. Similarly, ths same experiment was done for B. correcta. Second, a multiple-choice test was conducted also using mated females of each species. The B. dorsalis female adult was released into a plastic jar (23.5 × 15.8 × 10 cm) containing banana, guava, and mango fruits. Twenty replications were conducted for this experiment. Similarly, the same experiment was done for B. correcta.
2.5 Number of flies and oviposition punctures
Choice experiments were designed with the following treatments for female B. dorsalis adults, as three different fruits (one banana, one guava, and one mango) were offered in a cage. Twenty gravid female flies of B. dorsalis were released into the cage. The numbers of female flies settling/fruit on each fruit type were recorded, as mentioned above. After 48 h, the number of oviposition punctures/fruit were counted. Each experiment was replicated six times (Jaleel et al., 2018b). Similarly, the same experiment was done for the B. correcta.
2.6 Immure development
To check out the influence of different fruits on the larval performance of both Bactrocera species, eggs of both female flies were collected from banana, guava, and mango by removing the skin with a sharp knife under a stereomicroscope; the soft camelhair brush was used to collect the eggs. Twenty eggs of B. dorsalis were transferred inside of each fruit by the making cut with the sterilized fine sharp scissor on each fruit type (3 × 3 cm). Then each fruit was introduced into a separate plastic jar. Development time (days) from egg to adult emergence and pupal survival were check out for both flies. A similar experiment was done for B. correcta. Each experiment was replicated six times.
2.7 Volatile components
To find out the reasons for the behavior differences conducted by both Bactrocera species on the three kinds of fruits (banana, guava, and mango), as well as the aromatic profile of three different fruits. Ripening influences the softening of the pulp and physiological changes of fruits (Fabi et al., 2019). Each fruit cultivar type was described the first time in this study.
The collection of volatiles from the skin of banana, guava, and mango fruits was done using porapak Q. Before the collection of samples, the porapak Q tube preconditioned at 280 °C for 30 min and washed with dichloromethane, then dried under charcoal purified nitrogen. This apparatus setup was connected with air pump, an airflow meter (AFM) (for controlling the flow of air through the system), water bottle, charcoal, plastic bag (Oven bag, Turkey size, 482 × 596 mm), porapak Q (80–100 mesh; Alltech, Deerfield, IL, USA), and air pump. The air pump connected to AFM then attached to water jar and proceeds to the flask, having activated charcoal (for absorbing any volatile foreign compounds in the air). For activation of charcoal, it preheated at 200 °C for 3 h. The charcoal flask followed by an oven bag containing a specified amount (2 kg) of the sample (fruits). Air after passing through the oven bag then passed through the porapak Q, the absorbent material inside the porapak Q. Volatiles eluted from the adsorbents of porapak Q with the help of 1 ml CH2Cl2 and then stored at − 80 °C. A micro syringe (1000 µl) used to collect the volatile compounds/components from the porapak Q (capacity 2 ml). Experiments were repeated eight times for each fruit type (banana, guava, and mango). The 0.1 μl was taken from a sample of fruits and used for the analysis.
The quantitative and qualitative analysis of GC–MS ran in Agilent GC–MS (7890 N, gas chromatograph, Agilent 5975C, a mass selective detector equipped with an HP-5 MS, capillary column: 30 m × 0.25 mm ID, film thickness: 0.25 μm, and Agilent Technologies, USA). The temperature was programmed from 45 (held for 1 min) to 280 °C at 10 °C / min. The solvent delay kept for 5 min, while the injector temperature was set at 250 °C, and helium gas used as the carrier. Electron ionization mass spectra were recorded from m/z 29 °C to 280 °C at 70 eV with the temperature at 230 °C using an iron source. Quantitative and qualitative constituents' analysis of different fruits (banana, guava, and mango) was done based on their retention times (RT) and mass spectra in the computer library (NIST. 11). The quantity of each fruit component was compared using the area of the peak.
2.8 Statistical analysis
The treatments, including time spent, no. of flies, oviposition punctures, development time from egg to adult, and pupal survival (when having three fruits) were analyzed using one-way ANOVA for each species of Bactrocera. The effect of factors on the explanatory variables was assessed using the Fisher's LSD test (P < 0.05). All analyses were run using SPSS Statistics 15.0 (SPSS Inc., Chicago, IL, USA). Quantitative and qualitative analysis of constituents of fruits was done based on their retention times (Rt) and mass spectra in the computer library (NIST. 11).
3 Results
3.1 Characteristics of selected fruits
Physical parameters, e.g., width (cm), length (cm), thickness (cm), total soluble solids (TSS), and Brix firmness/hardness (N) of banana, guava, and mango fruits have shown in table 1. The pericarp toughness of mango fruits was lower than those of the other two tested fruits (banana and guava). While the Brix level of mango fruits was higher than those of the other two tested fruits (Table 1).
Fruit Properties
Banana fruits
Guava fruits
Mango fruits
Length (cm)
20.29 ± 1.23
8.99 ± 0.36
13.50 ± 1.09
Width (cm)
3.35 ± 0.32
5.71 ± 0.20
10.20 ± 2.05
Thickness (cm)
10.64 ± 0.64
23.78 ± 0.61
22.25 ± 0.05
Pericarp toughness
7.19 ± 0.46
9.25 ± 0.43
3.14 ± 0.09
TSS (°Brix)
8.90 ± 0.31
4.57 ± 0.38
13.2 ± 0.43
3.2 Gender
The number of male B. dorsalis flies was not remarkably different on banana (1.20 ± 0.30 numbers), guava (1.00 ± 0.50 numbers), and mango fruits (1.40 ± 0.30 numbers) (F2,15 = 0.97, P = 0.397). While the number of female B. dorsalis flies was more on mango fruits (4.50 ± 0.50 numbers) than those on other two fruits e.g., guavas (3.50 ± 0.30 numbers) and bananas (1.50 ± 0.40 numbers) (F2,15 = 6.93, P = 0.003; Fig. 1A). While, the female B. correcta flies was more on guava fruits (4.00 ± 0.50 numbers), in comparison to other fruits (F2,15 = 21.40, P < 0.001; Fig. 1B). Female flies of both species were observed more than males on the fruits; based on this result, we used female flies of both species for the next experiments.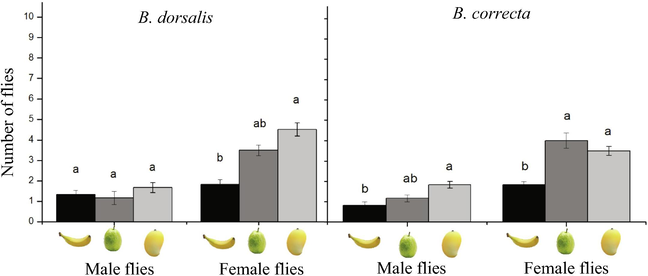
Mean number (±SE) of male and female flies of B. dorsalis and B. correcta in a choice test among three different fruits.
3.3 Time spent
The time spent by the female fly of B. dorsalis was significantly longer on mango fruits (148.33 ± 4.30 min) than those on the other two fruits in a no-choice test (F2,57 = 626, P < 0.001; Fig. 2A). In comparison, the time spent by the female fly of B. correcta was longer on guava fruits (127.16 ± 2.35 min) than those on the other two fruits (F2,57 = 1206, P < 0.001; Fig. 2A).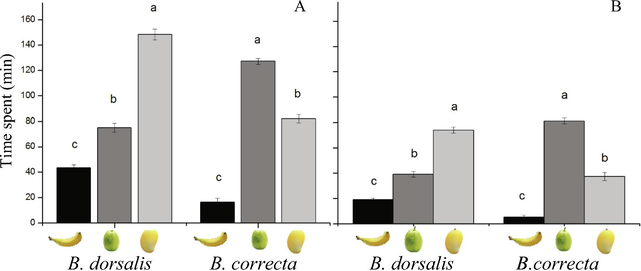
Mean (±SE) time spent by a female of B. dorsalis and B. correcta in the (A) no-choice test and (B) choice test among banana, guava, and mango fruits.
The time spent by the female B. dorsalis fly was significantly longer on mango fruits (74.16 ± 2.45 min) than those on the other two fruits (F2,57 = 790, P < 0.00; Fig. 2B). The time spent by the female fly of B. correcta was significantly longer on guava fruits (81.33 ± 2.40 min) than those on the other two fruits in a choice test (F2,57 = 1021, P < 0.001; Fig. 2B).
3.4 Number of flies and oviposition punctures
In choice test, the no. of female B. dorsalis flies was maximum on mango fruits (7.5 ± 0.51 numbers) than those on other two fruits, e.g., guava (5.5 ± 0.49 numbers) and banana fruits (3.16 ± 0.47 numbers) (F2,15 = 46.4, P < 0.001; Fig. 3A). While, the female B. correcta flies was maximum on guava (4.00 ± 0.25) and mango fruits (3.83 ± 0.30 numbers) than banana fruits (F2,15 = 19.50, P = 0.07; Fig. 3A).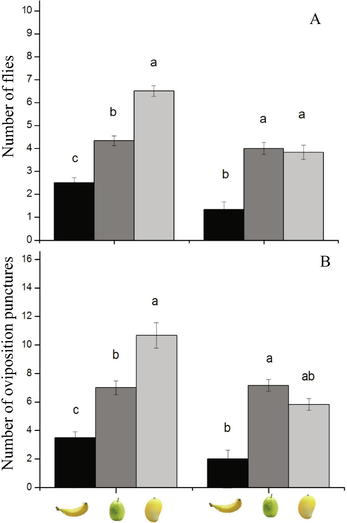
Mean (±SE) (A) no. flies and (B) oviposition punctures done by female B. dorsalis and B. correcta adults in a choice test among banana, guava, and mango fruits.
In choice test, the oviposition punctures by female B. dorsalis flies were more on mango fruits (10.66 ± 0.89 numbers) than guava and banana fruits (F2,15 = 65.50, P < 0.001; Fig. 3B). While, the oviposition punctures by the female B. correcta were more on guava fruits (7.16 ± 0.62 numbers) than mango and guava fruits (F2,15 = 119, P < 0.001; Fig. 3B).
3.5 Immature development
The developmental time (egg to adult) of B. dorsalis was longer on banana fruits (14.16 ± 0.75 d) than those of other two fruits (F2,15 = 77.1, P < 0.001; Fig. 4). While in a case of B. correcta, there were no statistical difference in developmental time between guava (11.00 ± 0.63 d) and mango fruits (10.50 ± 0.51 d) but was significantly longer on a banana fruits (15.00 ± 0.63 d) (F2,15 = 24.80, P < 0.001; Fig. 4).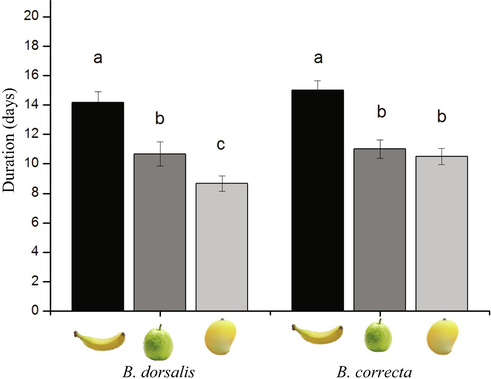
Mean (±SE) developmental time (d) from egg to adult of B. dorsalis and B. correcta reared on banana, guava, and mango fruits.
Pupae (%) of B. dorsalis (F2,15 = 51.67, P < 0.001) and B. correcta (F2,15 = 24.60, P < 0.001) were higher on mango fruits e.g., 92.00 ± 2.44% and 89.00 ± 1.51% respectively than other two fruits, e.g., banana and guava fruits (Fig. 5).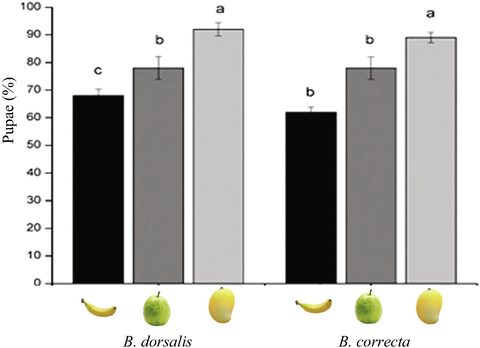
Mean % (±SE) pupae of B. dorsalis and B. correcta when reared on banana, guava, and mango fruits.
3.6 Volatile components
The volatile components of bananas have presented in table 2. Overall, 99.99% of constitutes identified through the retention index and NIST 11. The major dominating constitutes are butanoic acid, 3-methyl butyl ester (21.80%), benzaldehyde, 4-ethyl- (7.89%), 2-pentanol, acetate (7.79%), acetic acid, pentyl ester (5.53%) and 1-butanamine, 3-methyl- (5.49%), which accounts (48.50%) of total constitutes. While other minor constitutes, which make up the balance have given in the table 2. The GCMS analysis of the volatile components of guava fruits has shown in table 3. Overall, 99.99% of constitutes identified through the retention index and NIST 11. The major dominating constitutes were α-caryophyllene (39.88%), 9-octadecenamide, (Z)-(16.86%), α-copaene (10.71%), which overall accounts (67.50%) of total constitute identified. While other minor constitutes which makeup, the balance has presented in table 3. The GCMS analysis of the volatile composition of mangoes has shown in table 4. Overall, 99.99% of constitutes identified through the retention index and NIST 11. The major dominating constitutes were 3-carene (24.98%), hexanoic acid, ethyl ester (20.35%), butanoic acid, ethyl ester (10.47%), which overall accounts (55.95%) of total constitute identified. While other minor constitutes which makeup, the balance has presented in the table 4.
Peak #
RTa
Components nameb
Relative %
KI(Exp)c
AI(Exp)d
1
4.955
1-Butanamine, 3-methyl-
5.439
898
898
2
5.003
2-Pentanol, acetate
7.700
902
902
3
5.351
1-Butanol, 3-methyl-, acetate
3.793
924
927
4
5.392
Acetic acid, pentyl ester
5.523
927
930
5
6.562
1,3-Butadiyne
3.277
1003
1004
6
6.585
Butanoic acid, 2-methylpropyl ester
3.850
1005
1006
7
7.201
Butanoic acid, butyl ester
1.709
1045
1047
8
7.658
Butanoic acid, 1-methylbutyl ester
2.594
1074
1076
9
7.923
2-Heptanol, acetate
0.975
1091
1092
10
8.174
Butanoic acid, 3-methylbutyl ester
21.804
1108
1108
11
8.666
Benzene, 1-ethenyl-4-ethyl-
1.223
1141
1143
12
8.909
Butanoic acid, 3-methyl-, 3-methylbutyl ester
1.667
1157
1159
13
9.882
Benzaldehyde, 4-ethyl-
7.897
1223
1224
14
10.105
Isophthalaldehyde
2.616
1239
1240
15
10.911
1,4-Benzenedicarboxaldehyde
0.781
1295
1296
16
11.320
m-Ethylacetophenone
1.712
1326
1327
17
11.593
Ethanone, 1-(4-ethylphenyl)-
0.828
1346
1347
18
12.319
1H-Indol-4-ol
1.700
1400
1400
19
12.632
1-Propanone
0.750
1425
1426
20
13.609
Ethanone
0.535
1502
1502
Peak #
RTa
Components nameb
Relative %
KI(Exp)c
AI(Exp)d
1
12.663
α-Copaene
10.715
1427
1428
2
13.275
α-Caryophyllene
39.877
1475
1476
3
13.526
Aromandendrene
6.762
1495
1495
4
13.649
cis-Muurola-3,5-diene
3.515
1505
1505
5
13.711
α-Humulene
3.785
1510
1511
6
13.809
Caryophillene
1.785
1518
1519
7
14.204
(+)-epi-Bicyclosesquiphellandrene
2.366
1551
1552
8
14.234
Naphthalene
1.732
1554
1555
9
14.517
Isoledene
7.695
1577
1578
10
14.649
γ-Langene
2.778
1588
1589
11
15.331
Globulol
2.127
1648
1649
12
22.793
9-Octadecenamide, (Z)-
16.864
2432
2433
Peak #
RTa
Components nameb
Relative %
KI(Exp)c
AI(Exp)d
1
3.564
Butanoic acid, butyl ester
1.660
798
799
2
3.644
Butanoic acid, ethyl ester
10.479
800
800
3
3.804
Propanoic acid, 2-methyl-, ethyl ester
3.741
812
814
4
4.134
2-Butenoic acid, ethyl ester, (E)-
0.422
837
841
5
4.194
Oxazole
3.843
841
845
6
6.505
Hexanoic acid, ethyl ester
20.353
1000
1000
7
6.738
Octanoic acid, ethyl ester
10.240
1015
1016
8
7.012
Limonene
0.888
1033
1035
9
7.169
2-Hexenoic acid, ethyl ester
0.570
1043
1045
10
7.942
(+)-4-Carene
11.739
1093
1093
11
8.414
Octanoic acid, methyl ester
0.630
1124
1125
12
9.381
4-Octenoic acid, ethyl ester, (Z)-
1.203
1188
1189
13
9.544
3- Carene
24.985
1199
1199
14
10.207
Ethyl (E)-2-octenoate
1.470
1246
1248
15
12.034
Ethyl trans-4-decenoate
4.328
1379
1380
16
12.736
β-Ylangene
1.007
1433
1434
17
12.872
β-Copaene
0.603
1444
1445
18
13.066
Cedrene
0.212
1459
1460
19
13.189
γ-Muurolene
0.271
1469
1470
20
13.536
Isoledene
0.786
1496
1496
21
13.617
α-Guaiene
0.334
1502
1503
22
15.067
Cedrol
0.235
1625
1625
4 Discussion
The olfactory and ovipositional response of both flies (B. dorsalis and B. correcta) is very important for the bait development study. In China, no detailed studies have been carried out on the susceptibility of banana, guava and mango fruits to B. dorsalis in comparison to B. correcta, information which is required for both production and export systems. The preference of Bactrocera species for fruits may be affected due to the differences in pericarp toughness and TSS ratio. Most of Bactrocera species prefer to lay eggs into soft skin fruits (Jaleel et al., 2018b, Rattanapun et al., 2009). However, it is not right for all Bactrocera species and other insects (Verghese et al., 2011, Ghramh et al., 2019).
Biasazin et al. (2014) reported the behavior of Bactrocera invadens on mango and guava fruits. They found female flies were more attracted than male flies on both fruits. Similarly, in this study, female flies of both Bactrocera species were more attracted in comparison to male flies on all types of fruits. The host preference depends on volatiles emission, texture, and skin toughness of fruits (Rattanapun et al., 2009, Jaleel et al., 2018b). Rattanapun et al. (2009) have reported that B. dorsalis preferred soft skin mango. Jaleel et al. (2018b) have reported that B. dorsalis attracted to soft skin fruits. Rattanapun et al. (2009) explained that when female Bactrocera flies try to inject their eggs into hard skin fruits, the resin comes out immediately and pushes the eggs outside the fruit. The resin inside the mango has a high level of phenol (Keil et al., 1946); this may cause the mortality of immatures of Bactrocera species. While Seo et al. (1982) have been observed that female B. papaya flies were more attracted to papaya fruits having hard skin (Jang & Light, 1991). Oviposition may depend on the pericarp toughness and availability of fruits. In the current study, mango and guava fruits were more suitable for oviposition by B. dorsalis and B. correcta, respectively. Fitness of B. dorsalis was less than 20% in hard skin fruits that indicating the poor host (Rattanapun et al., 2009). Larval diets have a significant impact on adult fitness (Jaleel et al., 2018b). In our study, pupal survival (%) of both species was lower in banana than those of the other two fruits, e.g., guava and mango fruits.
Mixtures of volatile components have a significant role in calling or attracting Bactrocera adults (Jaleel et al., 2019). Cyclopentasiloxane and tetradecamethyl- were reported as the major aromatic compound of banana fruits (Jaleel et al., 2021). 3-methyl butyl acetate, isoamyl butanoate, and isoamyl isovalerate considered major volatile components of banana fruits (Schwab et al., 2008). Butyl acetate, isoamyl acetate, ethyl acetate, butyl butanoate, and isoamyl isobutanoate called major aromatic components of banana fruits (Cano et al., 1997, de Vasconcelos Facundo et al., 2012, Bugaud et al., 2009). In our study, butanoic acid was the major aromatic components of banana fruits. Caryophyllene was reported as the major aromatic components of guava fruits (Jaleel et al., 2021). Caryophyllene and humulene were the major volatile components of guava fruits. Both were found best attractant of Bactrocera species (Nishimura et al., 1989, Tamura et al., 2000, Jaleel et al., 2019). In our study, the α-caryophyllene, α-copaene, and aromadendrene were the main volatile components of guava fruits. In the Coche mango, the predominant components were 3-carene, b-selinene, terpinolene, and limonene (Malo et al., 2012). The 3-carene considered a major fruity order of mango fruits (Tamura et al., 2000, Jaleel et al., 2021). Acetic, butyric, hexanoic acids and ethyl 3-hydroxybutyrate considered main aromatic components in mango fruits (Sakho et al., 1985). Acetaldehyde, acetone, methanol, ethanol, a-pinene, caryophyllene, 3-carene, b-pinene, myrcene, limonene, terpinolene, a-copaene, and r-cymene were reported in the aromatic profile of mango fruits (Baldwin et al., 1999, Pino & Mesa, 2006). In our study, the octanoic acid, ethyl ester, (+)-4-carene, and 3-carene were the main volatile components of mango fruits. Jaleel et al. (2021) reported that 3-carene and the mixture of β-caryophyllene and α-humulene were good attractants for female B. dorsalis and B. correcta flies, respectively in laboratory tests. Based on study results, we recommend that mango and guava fruits are favorable and containing most important volatile attractants for Both flies. This study will be more useful for field study to confirm the efficacy of attractant against both flies.
5 Conclusion
In current study, we concluded that mango and guava fruits were favorite hosts of B. dorsalis and B. correcta respectively in the laboratory. It might be that both fruits (mango and guava) have soft skin as compared to banana fruits. Both fruits have important volatile components that are good attractant for Bactrocera fruit flies. Butanoic acid-3-methylbutyl ester, α-caryophyllene, and 3-carene were the major volatile components of banana, guava, and mango fruits, respectively, and can be used for future studies at field level.
Acknowledgments
This research work was supported by the Guangdong Provincial Special Fund for Modern Agriculture Industry Technology Innovation Teams (2019KJ125), Innovation Team of Modern Agricultural Industry Generic Key Technology R & D of Guangdong (2019KJ134). KAK and HAG appreciate the support of the Research Center for Advanced Materials Science at King Khalid University Abha, Saudi Arabia through a grant RCAMS/ KKU/002-21.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Host plant records for fruit flies (Diptera: Tephritidae) in Southeast Asia. Raffles Bull. Zool.. 1999;47:1-92.
- [Google Scholar]
- Influence of fruit traits on oviposition preference and offspring performance of Bactrocera tryoni (Froggatt)(Diptera: Tephritidae) on three tomato (Lycopersicon lycopersicum) cultivars. Austral Entomol.. 2005;44:97-103.
- [Google Scholar]
- Effect of two edible coatings with different permeability characteristics on mango (Mangifera indica L.) ripening during storage. Postharvest Biol. Technol. 1999;17:215-226.
- [Google Scholar]
- Identification of host blends that attract the African invasive fruit fly, Bactrocera invadens. J. Chem. Ecol.. 2014;40:966-976.
- [Google Scholar]
- Comparison of the physico-chemical characteristics of a new triploid banana hybrid, FLHORBAN 920, and the Cavendish variety. J. Sci. Food Agri.. 2009;89:407-413.
- [Google Scholar]
- Differences among Spanish and Latin-American banana cultivars: morphological, chemical and sensory characteristics. Food Chem.. 1997;59:411-419.
- [Google Scholar]
- Do fruit ripening volatiles enable resource specialism in polyphagous fruit flies? J. Chem. Ecol.. 2016;42:931-940.
- [Google Scholar]
- Influence of different banana cultivars on volatile compounds during ripening in cold storage. Food Res. Int.. 2012;49:626-633.
- [Google Scholar]
- Clutch size in frugivorous insects as a function of host firmness: the case of the tephritid fly Anastrepha ludens. Ecol. Entomol.. 2003;28:268-277.
- [Google Scholar]
- Fast and furious: ethylene-triggered changes in the metabolism of papaya fruit during ripening. Frontiers Plant Sci.. 2019;10:535-539.
- [Google Scholar]
- Undetected infection by maize bushy stunt phytoplasma enhances host-plant preference to Dalbulus maidis (Hemiptera: Cicadellidae) Environ. Entomol.. 2018;47:396-402.
- [Google Scholar]
- Synthesis of gold nanoparticles (AuNPs) using Ricinus communis leaf ethanol extract, their characterization, and biological applications. Nanomaterials. 2019;9(5):765.
- [Google Scholar]
- The response of two Bactrocera species (Diptera: Tephritidae) to fruit volatiles. J. Asia-Pacific Entomol.. 2019;22:758-765.
- [Google Scholar]
- Jaleel, W., Lu, L.He, Y. 2018a Biology, taxonomy, and IPM strategies of Bactrocera tau Walker and complex species (Diptera; Tephritidae) in Asia: a comprehensive review. Environ. Sci. Pollut. Res. 25, 9346–1936.
- Using GCMS to find out the volatile components in the aroma of three different commercial fruits in China. J. Anim. Plant Sci.. 2021;31:166-174.
- [Google Scholar]
- Using two-sex life table traits to assess the fruit preference and fitness of Bactrocera dorsalis (Diptera: Tephritidae) J. Eco. Entomol.. 2018;111:2936-2945.
- [Google Scholar]
- Evaluating the repellent effect of four botanicals against two Bactrocera species on mangoes. PeerJ. 2020;8:e8537
- [Google Scholar]
- Using two-sex life tables to determine fitness parameters of four Bactrocera species (Diptera: Tephritidae) reared on a semi-artificial diet. Bull. Entomol. Res.. 2018;108:707-714.
- [Google Scholar]
- Fitness parameters of Plutella xylostella (L.)(Lepidoptera; Plutellidae) at four constant temperatures by using age-stage, two-sex life tables. Saudi. J. Biol. Sci.. 2019;26(7):1661-1667.
- [Google Scholar]
- Future expansion of small hive beetles, Aethina tumida, towards North Africa and South Europe based on temperature factors using maximum entropy algorithm. J. King Saud Univ. Sci.. 2021;33(1):101242
- [Google Scholar]
- Behavioral responses of female oriental fruit flies to the odor of papayas at three ripeness stages in a laboratory flight tunnel (Diptera: Tephritidae) J. Insect Beh.. 1991;4:751-762.
- [Google Scholar]
- An investigation of the efficacy of hygienic behavior of various honey bee (Apis mellifera) races toward Varroa destructor (Acari: Varroidae) mite infestation. J. King Saud Univ. Sci. 2021101393
- [Google Scholar]
- Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann. Allergy. 1946;4:268-281.
- [Google Scholar]
- Recent spread and climatic ecological niche of the invasive guava fruit fly, Bactrocera correcta, in mainland China. J. Pest Sci.. 2013;86:449-458.
- [Google Scholar]
- Chemical composition of mango (Mangifera indica L.) fruit: nutritional and phytochemical compounds. Front. Plant Sci.. 2019;10:1073.
- [Google Scholar]
- Attraction of the West Indian fruit fly to mango fruit volatiles. Entomol. Exp. Appl.. 2012;142:45-52.
- [Google Scholar]
- Maternally derived carotenoid pigments affect offspring survival, sex ratio, and sexual attractiveness in a colorful songbird. Naturwissenschaften. 2005;92:375-380.
- [Google Scholar]
- Olfactory receptors in the melon fly Dacus cucurbitae and the oriental fruit fly Dacus dorsalis. Proceed. Nat. Acad. Sci.. 1983;80:3143-3147.
- [Google Scholar]
- Toxicity of four different insecticides against Trilocha varians (Bombycidae: Lepidoptera) J. King Saud Univ. Sci.. 2020;32(3):1853-1855.
- [Google Scholar]
- Volatile constituents of guava fruits (Psidium guajava L.) and canned puree. J. Agri. and Food Chem.. 1989;37:139-142.
- [Google Scholar]
- Chemical composition of the essential oil and headspace solid-phase microextraction of the guava fruit (Psidium guajava L.) J. Ess. Oil Res.. 2000;12:153-158.
- [Google Scholar]
- Contribution of volatile compounds to mango (Mangifera indica L.) aroma. Flavour Fragr J. 2006;21:207-213.
- [Google Scholar]
- Bactrocera dorsalis preference for and performance on two mango varieties at three stages of ripeness. Entomol. Exp. Appl.. 2009;131:243-253.
- [Google Scholar]
- Volatile components of the essential oils in the pulp of four yellow mangoes (Mangifera indica L.) in Thailand. Food Sci. Technol.. 2000;6:68-73.
- [Google Scholar]
- Evidence of a random ovipositional strategy by female fruit fly Bactrocera dorsalis (Tephritidae: Diptera) with reference to host quantum. Curr. Sci.. 2011;100:246-249.
- [Google Scholar]
- Identification and expression profiles of novel odorant binding proteins and functional analysis of OBP99a in Bactrocera dorsalis. Arch. Insect Biochem. Physiol.. 2018;98:e21452
- [Google Scholar]







