Translate this page into:
Nutritional compositions and antioxidative capacity of the silk obtained from immature and mature corn
∗Corresponding author. Tel.: +60 9 7677779 (Office); fax: +60 9 7677515 (Office) rosliishak@gmail.com (Wan Ishak Wan Rosli) wrosli@kck.usm.my (Wan Ishak Wan Rosli)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Available online 21 November 2013

Abstract
The silks of immature and mature corn were evaluated for their variations in nutritional compositions, mineral content and antioxidant capacity. Both immature and mature silks were good source of nutritional compositions. Immature silks contained significantly higher moisture (89.31%) (fresh basis), lipid (1.27%) and protein (12.96%) content than the mature silk. Mature silks contained higher composition of ash (5.51%), carbohydrate (29.74%) and total dietary fiber (51.25 g/100 g), than the immature silk, but the difference was not significant. In mineral determination, immature silk was rich source of Ca (1087.08 μg/g), Mg (1219.17 μg/g), Cu (5.60 μg/g) and Zn (46.37 μg/g) than the mature silks. In contrast, other minerals such as K (35671.67 μg/g), Na (266.67 μg/g), Fe (4.50 μg/g) and Mn (35.57 μg/g) were found higher in the mature silk. The silks were extracted with ethyl acetate, ethanol and water using the Soxhlet extraction method to determine the polyphenol and ABTS radical scavenging capacity. From this study, the highest content of total polyphenol of immature silks was exhibited by ethanol extract (92.21 mg GAE/g) while water extract (64.22 mg GAE/g) had the highest polyphenol content among mature silk extracts. Total flavonoid content of both immature and mature silks was higher in the water extract at 8.40 mg CAE/g and 2.31 mg CAE/g, respectively. In the ABTS free radical assay method, all immature silk extracts had higher percentage of inhibition compared to the mature silks. Among all three crude extracts, the ethanol extract of immature (EC50 = 0.478 mg/ml) and mature silk (EC50 = 0.799 mg/ml) exhibited the strongest antioxidant capacity followed by the water and ethyl acetate extract.
Keywords
Nutritional compositions
Mineral
Antioxidant capacity
Corn silk
Immature
1 Introduction
Corn is one of the most widely grown cereal crops in the world and has become the third most important cereal crops other than wheat and rice (Ramessar et al., 2008). Production of corn was reported to increase from 713 thousand metric tons in 2006/2007 to nearly 820 thousand metric tons in 2010/2011 (USDA-FAS, 2008). The high production corresponds to increased consumption of corn crop when the world consumption of corn was reported to increase rapidly in 2007/2008 and in the following years (USDA-FAS, 2008).
Corn and its other plant parts are used in various food, agricultural and health applications (Naqvi et al., 2011; Voca et al., 2009). Corn cereal is being a significant staple food for some countries. It contains nutritious components essential for health. Its pericarp contains high crude fiber (about 87%) which constituted with 67% hemicellulose, 23% cellulose and 0.1% of lignin. Its endosperm is rich in starch (87.6%) and protein (8%). The germ however is characterized by high composition of crude fat (33%), protein (18.4%) and minerals (Lunven, 1992). The immature corn (baby corn) contains high protein (25.58%) and dietary fiber (30.4%) content and lower content of crude lipid (3.67%) and 3.74% of ash and appreciable total sugar content (10.07 g/100 g) (Rosli and Anis, 2012). On the other nutrient, immature corn contained 5.43 mg/100 g of ascorbic acid amino acid (0.05 g/g methionine, 2.85 g/g isoleucine and 0.6756 g/g leucine). In addition, some minerals such as Ca, Mg and P were presented at 95, 345 and 898.62 mg/100 g, respectively (Hooda and Kawatra, 2013).
Corn silks are a bundle of silky, long and yellowish strands which could be seen on top of both baby corn and corn fruit. The immature part of a corn plant called baby corn is an unfertilized female flower which is usually harvested after 60 days of planting. Conversely, a corn fruit is developed from a fertilized female flower that is later grown into a corn fruit after 4 months of planting. Corns have been used extensively as foods, feeds and are processed to produce oil, starch and ethanol (Ramessar et al., 2008).
Meanwhile, corn silk is predominantly discarded together with other parts of the plant due to lack of effective utilization. The silks function as a stigma of a female flower and as the fruit develops, the silk elongates beyond the cob covering the edible plant part. Traditionally, the hairs are used as a diuretic agent to ease the passing of stones or gravel in kidneys and urinary bladder (Maksimovic and Kovacevic, 2003). In China, corn silk is well known as an important traditional Chinese medicine in treating several illnesses related to kidney (Zhao et al., 2012). Besides, it also was used to treat edema, cystitis, gout, treat rheumatism and rheumatoid, arthritic and as an antimicrobial agent as well (Maksimovic and Kovacevic, 2003; Velazquez et al., 2005; Fatima et al., 2012). Scientifically, corn silk has been reported to exhibit positive effect on glycemic metabolism by increasing the insulin level whereby the increasing of insulin level and recovery of β-cells were known to be the mechanism involved in the glycemic metabolism (Guo et al., 2009). In other study corn silk polysaccharides were found to exhibit an anti-diabetic effect on streptozotocin (STZ)-induced diabetic rats. The daily treatment of 100–500 mg/kg body weight of the polysaccharide had significantly decreased the blood glucose level and serum lipid. Evidently, the total cholesterol level of the polysaccharide treated group of streptozotocin-induced diabetic rats was found significantly lower compared to the normal and control group (Zhao et al., 2012).
It is known that an antioxidant can inhibit or delay oxidation process although at lower concentration than the biomolecule it is protecting (Halliwell, 1995; Gutteridge and Halliwell, 2010). Utilization of synthetic antioxidants as food preservatives has raised consumers’ consciousness toward health due to some adverse effects. For instance, an excessive use of butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) is harmful and accompanied with side effects thus restricted their uses (Ningappa et al., 2008). Natural antioxidants thus are being studied extensively with regards to confer many benefits to health rather than the harmful effects. Other than health implications, natural antioxidants have advantages due to their functionality such as solubility (Moure et al., 2001). The uses of natural antioxidant are varied. Tocopherols have strong capacity to inhibit lipid oxidation and are used in foods while gallic acid derived from Peltiphyllum peltatum has shown nephroprotective effect (Nabavi et al., 2013).
Plants are rich sources of polyphenols and can act as antioxidant. In addition, the harmful effects of free radicals which are known to be associated with several diseases such as cardiovascular disease, diabetes mellitus, cancer, arthrosclerosis, neurodegenerative disease and ageing can be prevented with sufficient intake of polyphenols in the diet (Sugamura and Keaney, 2011; Valko et al., 2007; Williams and Spencer, 2012). These secondary plant metabolites are not only widely distributed in fruits (Zaouay et al., 2012), vegetables (Wootton-Beard and Ryan, 2011), cereals (Qingming et al., 2010), leaves (Costa et al., 2009) and roots (Fu et al., 2012) but the underutilized plant sources like seeds (Tomaino et al., 2013), peels (Wang et al., 2012; Madhava Naidu et al., 2011) and bran (Zhou and Yu, 2004) have been reported to exhibit strong antioxidative effects.
However, the information regarding the nutritional composition such as proximate and mineral composition and antioxidant capacity of the silks from immature and mature corn are still lacking. Therefore, the objectives of this study were to determine moisture, protein, lipid, and total dietary fiber of silks obtained from these two types of silks and to determine the antioxidant capacity of organic and aqueous extracts of these silks as comparison with Trolox standard.
2 Materials and methods
2.1 Sample
Baby corn and sweet corn were purchased from the local wet market in Kota Bharu district, Kelantan state of Peninsular Malaysia. The husks were eliminated and silks were detached from its fruit. Fresh corn silks were chopped and dried in an oven (Memmert, Germany) at 55 °C for 2 days. Dried corn silks were kept in a sealed plastic bag at 4 °C. Prior to analysis, the corn silk was ground into powder form (Waring Commercial, USA).
2.2 Reagents and standards
Folin–Ciocalteu reagent, gallic acid, (+)-catechin, and butylated hydroxytoluene (BHT) were purchased from Sigma–Aldrich (GmbH, Sternheim, Germany). Kalium-hexacyanoferrat (K3Fe(CN)6, 2,2′-azinobis (3-ethylbenzthiazoline)-6-sulfonic acid (ABTS+), Trolox, sulfuric acid, iron (III) chloride anhydrous (FeCl3), nitric acid (65%) and other chemicals were purchased from Merck (Darmstadt, Germany).
2.3 Determination of nutritional composition
Nutritional composition (moisture, protein, lipid and ash) was determined according to (AOAC, 1996). Moisture content of fresh and oven dried silks was determined using an oven-dry technique and expressed as fresh and dry basis, respectively. Protein content was determined by using the Kjeldahl method (Gerhardt, Germany) and nitrogen content of samples was multiplied by a factor of 6.25. Lipid content was determined using a Soxhlet apparatus with petroleum ether as the extracting solvent. Total dietary fiber composition of silk was determined by an enzymatic–gravimetric method, using sequential enzymatic digestions by thermostable α-amylase, protease and amyloglucosidase (Sigma–Aldrich (GmbH, Sternheim, Germany)).
2.4 Mineral composition
Mineral content (Ca, Mg, K, Na, Cu, Fe, Mn and Zn) of corn silk was determined using an atomic absorption spectrophotometer (AAS) (Perkin Elmer Analyst, USA). Samples (0.5 g) were ashed and digested in 2 ml concentrated nitric acid. The acid-digests were filtered through ashless filter paper (Whatman 42) and raised to 10 ml with deionized water.
2.5 Extraction
Plant material (20 g) was extracted with three separate solvents (ethyl acetate, ethanol and water) by the using Soxhlet extraction method. Crude extracts obtained were vacuumed evaporated (Heidolph, Germany) to dryness at 50 °C and kept in a screwed cap bottle at −18 °C. The yield of the extract obtained was reported as percentage and was calculated as follows:
2.6 Determination of total polyphenol content
The total phenolic content of extracts was determined using the Folin–Ciocalteau method (Kaur et al., 2008). One milliliter of crude extract (1 mg/ml) was added with 1 ml of Folin–Ciocalteau reagent (1:1) and 1.5 ml of sodium bicarbonate (20% w/v). The mixture was raised to 10 ml with distilled water. After 2 h of reaction at room ambient the absorbance was recorded at 765 nm and used to calculate the phenolic content by comparing with gallic acid standard. The total phenolic content was then expressed as mg gallic acid equivalent (mg GAE/100 g extract).
2.7 Determination of total flavonoid content
Total flavonoid content was determined by a colorimetric assay described by Ozsoy et al. (2008). An aliquot of 0.25 ml of the extract (1000 μg/ml) was added with 75 μl of NaNO2 (5% w/v) and the mixture was left to stand at room temperature for 6 min. Thereafter, 150 μl of AlCl3 (10% w/v) was added and allowed to react for another 5 min before adding 500 μl of NaOH (1 M). The volume was then adjusted to 2.5 ml with distilled water and mixed thoroughly. The absorbance was measured at 510 nm against blank which contained the same mixture without sample. Total flavonoid content was expressed as mg CAE/g extract, through the (+)-catechin calibration curve.
2.8 ABTS+ free radical scavenging assay
The assay was measured based on the ability of antioxidant from the sample to inhibit the 2,2′-azinobis (3-ethylbenzthiazoline)-6-sulfonic acid or ABTS free radical (ABTS+) by comparing with a reference standard (Trolox) (Re et al., 1999). In this assay, ABTS+ radical was generated by reacting ABTS solution (7 mM) with 2.45 mM potassium persulfate (K2S2O8) which allowed standing for 15 h in the dark at room temperature. The solution was diluted appropriately with ethanol to obtain the absorbance of 0.7 ± 0.2 units at 734 nm. To perform the ABTS+ decolorization assay, 200 μl of plant extract (100 μg/ml) was added with 2000 μl of ABTS free radical solution and reacted for 5 min. After that, the absorbance was measured spectrophotometrically at 734 nm against solvent blanks. The Trolox calibration curve ranging from 0 to 200 μM was prepared according to the sample. The percentage of the ABTS+ inhibition was calculated using formula:
Inhibition (%) = (Ac – As)/Ac × 100 where Ac is the absorbance of control; As is the absorbance of sample or standard. Concentration of the extract that inhibit 50% of free radicals (EC50 values) were also evaluated.
2.9 Statistical
All results were presented as mean (±SD) values of three replicates. Analysis of variance (ANOVA) was performed using SPSS V. 19 (SPSS Inc., Chicago, IL, USA) and mean values were statistically different at P ⩽ 0.05. The significantly different results were further separated using the Tukey multiple range test (SPSS Inc., Chicago, IL, USA).
3 Results
3.1 Nutritional compositions
Nutritional compositions of immature and mature corn silk are shown in Table 1. Overall, the moisture content of immature silks (89.31%) was significantly higher than mature silks (84.42%) (P value <0.05). However, no significant difference of the same component was observed for immature (4.15%) and mature (3.90%) corn of the oven-dried silks. The lipid and protein composition of immature silks were significantly higher than those of mature silks (P value <0.05). Lipid content of immature silks was 1.27% and has dropped to 0.66% in the mature silks. Meanwhile, the protein content of immature silks (12.96%) was also higher than its mature counterpart (8.95%) (P < 0.05). There was no significant difference between the ash and carbohydrate compositions of both immature and mature silks (P > 0.05). The ash (5.28%) and carbohydrate content of immature silks (27.80%) were slightly lower than those of the mature silks. The ash and carbohydrate content of mature silks obtained were 5.51% and 29.74%, respectively. Regarding the TDF composition, both immature and mature silks displayed high content of TDF comprising of 48.50 g/100 g and 51.24 g/100 g, respectively. (a,b)Mean values with different letters in the same row are significantly different (P < 0.05).
Nutritional compound
Corn silks
Immature silks
Mature silks
1Moisture (fresh)
89.31 ± 0.74a
84.42 ± 0.65b
2Moisture (oven-dried)
4.15 ± 0.21b
3.90 ± 0.22b
2Crude lipid
1.27 ± 0.16a
0.66 ± 0.17b
2Crude protein
12.96 ± 0.38a
8.95 ± 0.21b
2Ash (%)
5.28 ± 0.13a
5.51 ± 0.24a
3Carbohydrate
27.80 ± 2.25a
29.74 ± 1.26a
2Total dietary fiber (g/100 g)
48.50 ± 2.88a
51.24 ± 1.50a
3.2 Mineral composition
Mineral contents (Ca, Mg, K, Na, Cu, Fe, Mn and Zn) of the silks from immature and mature corns were shown in Table 2. In general, the content of major elements such as Ca, Mg, K and Na in immature silks was statistically different from the mature silk. Ca and Mg content were much higher in immature silks amounting 1087 and 1219.17 μg/g, respectively compared to the mature silks which contained 707.04 μg/g of Ca and 361.50 μg/g of Mg. However, the content of K and Na was significantly higher in mature silks compared to those of immature silks. K and Na content of mature silks were 35671.67 and 266.67 μg/g, respectively. Immature silks were found to contain lower amount of K (26281.67 μg/g) and Na (190.67 μg/g). Values are mean ± SD of six replicates. (a,b)Mean values with different letters in the same row are significantly different (P < 0.05).
Minerals level (μg/g)
Minerals
Immature silk
Mature silk
Macro elements
Calcium (Ca)
1087.08 ± 105.51a
707.04 ± 94.41b
Magnesium (Mg)
1219.17 ± 143.07a
361.50 ± 20.53b
Potassium (K)
26281.67 ± 1379.7a
35671.67 ± 2466b
Sodium (Na)
190.67 ± 22.61a
266.67 ± 15.65b
Minor elements
Copper (Cu)
5.60 ± 0.4a
4.12 ± 0.38b
Iron (Fe)
2.17 ± 0.15a
4.50 ± 0.49b
Manganese (Mn)
32.17 ± 3.14a
35.57 ± 2.26a
Zinc (Zn)
46.37 ± 4.21a
35.92 ± 4.24b
Both immature and mature silks contained appreciable amounts of trace elements (Table 2). The content of Cu, Fe, Mn and Zn varied from 4.12 to 5.60, 2.17 to 4.50, 32.17 to 35.57 and 35.92 to 46.37 μg/g, respectively. Cu and Zn were found significantly higher in immature silks which were 5.60 and 46.37 μg/g, respectively. For mature silks, the content of these minerals was 4.12 and 35.92 μg/g, respectively. In contrast, Fe content was found to be significantly higher in mature silks (4.50 μg/g) compared to the immature silks (2.17 μg/g). Mature silks also had a slightly higher content of Mn (35.57 μg/g) than the immature ones (32.17 μg/g), but the contents were not statistically different (P > 0.05).
3.3 Extract recovery, total polyphenol and flavonoid content
The percentage of recovery, total phenolic and flavonoid contents of immature and mature silks using water, ethanol and ethyl acetate solvent are shown in Table 3. Apparently, the water, ethanol and ethyl acetate extracts of immature silks exhibited significantly higher percentage of recovery (P < 0.05) compared to the mature silks. The highest recovery of immature silks was recorded in ethanol (27.73%) extract followed by water extract (14.63%). Conversely, mature silk displayed the highest percentage of recovery in water extract (13.69%) compared to ethanol extract (3.29%). Both immature and mature silks extracted with ethyl acetate recorded the lowest percentage of recovery that was 3.27% and 1.25%, respectively. (a–b)The different letters in the same row are significantly different (P < 0.05).
Recovery of extract (%)
Extract
Immature silks (mean + SD)
Mature silks (mean + SD)
Water
14.63 ± 0.36a
13.69 ± 0.55b
Ethanol
27.73 ± 0.40a
3.29 ± 0.27b
Ethyl acetate
3.27 ± 0.08a
1.25 ± 0.07b
The immature and mature silks had showed significant differences in total polyphenol and flavonoid content (P < 0.05). Total polyphenol content of crude extracts of immature and mature silks varied from 6.70–92.21 mg GAE/g extract to 4.96–64.22 mg GAE/g extract, respectively (Table 4). As for immature silks, the ethanol extract displayed the highest polyphenol (92.21 mg GAE/g extract) content followed by the water (35.35 mg GAE/g extract) and ethyl acetate (6.70 mg GAE/g extract) extract. In contrast, the highest total polyphenol content in mature silk was displayed by water extract (64.22 mg GAE/g extract) followed by the ethanol (49.88 mg GAE/g extract) and ethyl acetate (4.96 mg GAE/g extract) extract. (a–c)The different letters in the same row are significantly different (P < 0.05).
Total polyphenol (mg GAE/g extract)
Extract
Immature silks (mean + SD)
Mature silks (mean + SD)
Water
35.35 ± 2.17b
64.22 ± 2.55a
Ethanol
92.21 ± 3.59a
49.88 ± 2.87b
Ethyl acetate
6.70 ± 0.51c
4.96 ± 0.53c
As for total flavonoid content, the immature silks exhibited higher content compared to the mature silks (Table 5). In addition, the water extract of immature and mature silks gave the highest flavonoid content that was 8.40 mg CAE/g extract and 2.31 mg CAE/g extract, respectively compared to other extracting solvents employed. Meanwhile, the ethanol and ethyl acetate extract of immature and mature silks showed lower content of flavonoid. The flavonoid content of ethanol extract of the immature silk was 7.55 mg CAE/g extract and significantly higher than that of the mature silk (1.96 mg CAE/g extract). For ethyl acetate extract, the flavonoids obtained from mature silks (2.10 mg CAE/g) were higher compared to the immature silk (0.66 mg CAE/g extract) (see Table 6). (a–b)The different letters in the same row are significantly different (P < 0.05). (a–c)Different letters in the same row are significantly different (P < 0.05).
Total flavonoid (mg CAE/g extract)
Extract
Immature silks (mean + SD)
Mature silks (mean + SD)
Water
8.40 ± 0.48a
2.31 ± 0.12b
Ethanol
7.55 ± 0.37a
1.96 ± 0.20b
Ethyl acetate
0.66 ± 0.02b
2.10 ± 0.19a
EC50 value (mg/ml)
Ethanol extract
Water extract
Ethyl acetate extract
Immature silk
0.478 ± 0.030c
0.751 ± 0.240b
2.870 ± 0.110a
Mature silk
0.799 ± 0.100c
1.489 ± 0.166b
6.290 ± 0.830a
Trolox
0.038 ± 0.005
3.4 ABTS free radical scavenging capacity
The antioxidant capacity of immature and mature silk extracts as measured by using the ABTS+ free radical scavenging method is shown in Fig. 1(a–c). Overall, all extracts had showed a dose-dependent response when exposed to different concentration of extracts (0.1–1.6 mg/ml). Higher percentage of inhibition was observed at higher concentration. Apparently, the water, ethanol and ethyl acetate extract of immature silk demonstrated a higher percentage of inhibition compared to the same extracts of mature silk. Among all three extracts, the ethanol extract of both immature and mature silks exhibited the highest scavenging capacity followed by the water and ethyl acetate extract. These results indicated that antioxidant compounds present in immature silk extracts are prone to donate hydrogen atom to ABTS free radical giving rise to its scavenging capacity. The percentage of inhibition of ethanol extract was ranged from 23.50% to 78.40% in immature silk and 17.40% to 67.40% in mature silk (EC50 value of 0.478 mg/ml and 0.799 mg/ml, respectively) (Table 4). A lower scavenging capacity was observed in water and ethyl acetate extract of both immature and mature silks. The percentage of inhibition of immature silk water extract (19–57%) was however slightly higher than that of mature silk (15.44–53.5%) recording the EC50 value of 0.751 mg/ml and 1.489 mg/ml, respectively. The lowest antioxidant capacity of both silks was exhibited by ethyl acetate extract. The ethyl acetate extract of immature silk however showed lower EC50 value (2.87 mg/ml) compared to the mature silk (6.29 mg/ml). The inhibition of immature and mature silks was ranged from 13.4% to 35% and 15.56% to 44.36%, respectively.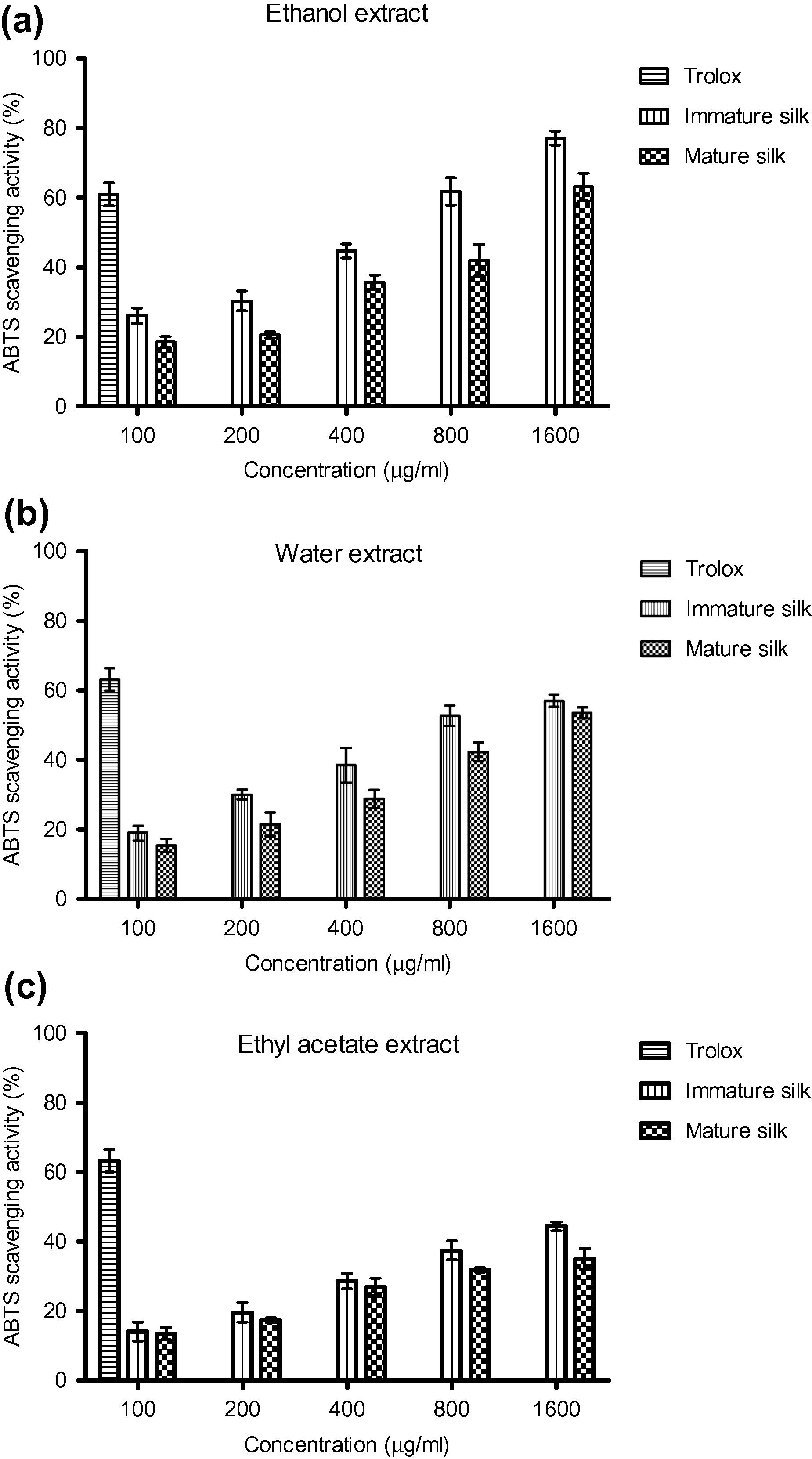
(a–c): ABTS scavenging capacity of ethanol, water and ethyl acetate extract of immature and mature silks.
4 Discussion
4.1 Nutritional compositions
In moisture content, the oven-dried silks which were dried at 55 °C for 48 h recorded the moisture content of 3.90–4.15%. The differences in moisture content of oven-dried samples in our study with other samples may be due to the differences in the temperature used and duration of drying. The moisture content obtained here was lower than the dried pericarp and seeds of bitter melon (5.0–13.4%) of different maturity stages which had been dried at 40 °C for only 24 h (Horax et al., 2010). The moisture content of both immature and mature silks however was found higher than some dried apple and orange residues (2–4%) (Figuerola et al., 2005), but lower than mango peel powder (10.5%) (Ajila et al., 2008) and its seed (8.5%) (Abdalla et al., 2007).
Among other plant byproducts, the lipid content of maize silks was lower than tomato peel (Elbadrawy and Sello, in press) but comparable with some plant pods (Mateos-Aparicio et al., 2010). Maize silk cuticle was composed of hydrocarbons of silk waxes that was involved in certain hydrocarbon biosynthesis (Perera et al., 2010). Therefore, the accumulation of hydrocarbons in different maturity stage may influence lipid composition.
Immature silk was obtained from unpollinated cob while the mature silk was taken from fully ripen and developed corn fruit. The protein content of immature and mature silks may be influenced by the functions and biosynthesis of amino acids taking place during developmental processes of pollinated and unpollinated silk tissue. It has been reported that amino acids were actively metabolized in immature cob at early stage of silk emergence in order to regulate the seed growth (Seebauer et al., 2004). The protein content of both immature and mature maize silks in the present study was slightly higher than some vegetables (2–4%) (Odhav et al., 2007) and underutilized plant parts such as mango peels (3.6%) and pods (Mateos-Aparicio et al., 2010).
The TDF content of maize silks was higher than some other underutilized plant parts such as seed (Hainida et al., 2008) peel (Elbadrawy and Sello, 2011), and brans (Abdul-Hamid and Luan, 2000). Therefore, maize silks could be considered as a rich source of dietary fiber thus may confer positive health benefits.
4.2 Mineral content
Mineral composition of the silks immature and mature parts was varied. The variations could be attributable to the difference cultivar, plant nutrition, climate and soil conditions (Hamurcu et al., 2010). The distribution of mineral composition is related to certain functions of the plant part during development. Ca is one of essential mineral which is required for structuring of cell wall and membranes (Evans et al., 2001). K ion is very important in biophysical and biochemical functions as it is highly mobile in plants (Szczerba et al., 2009). In stigma, K ion involves in regulation growth of a pollen tube (Holdaway-Clarke and Hepler, 2003; Zienkiewicz et al., 2011). Zn is required in protein synthesis, genetic entities stability and metabolism of carbohydrate and lipid. Mn plays the role as enzymes activator in tricarboxylic acid cycle (Xue et al., 2004), photosynthetic function (Lidon et al., 2004) photosynthesis, fatty acid and carotenoid synthesis (Santandrea et al., 2000). Mg is abundant in plant tissue and is associated with a wide range of cellular functions including photosynthesis (Gardner, 2003), protein synthesis which involves in ribosomal structures and functions (Maathuis, 2009).
4.3 Extract recovery, total polyphenol and flavonoid content
These results demonstrated clearly that the choice of varying polarities solvent had influence on the extractability of bioactive compounds (Trabelsi et al., 2010). The nature of solvent may explain on its capacity to recover as many antioxidants as possible and solvent having higher polarity exerted higher percentage of recovery. In addition, the different affinities of the extraction solvent such as extraction conditions, solvent polarity and temperature toward chemical constituents in plant materials could attribute to the extract recoveries (Moure et al., 2001). In other study, some exotic fruits showed higher yield of polyphenol when combination of ethanol with other solvent was used (Martinez et al., 2012). Successive isolation of flavonoid compounds from tea leaves (Camellia sinensis) was reported by using ethanol and water solvents (Yang et al., 2009). Maize silks extracted with ethyl acetate had little recoveries and found to be lower than the same solvent used to extract tea leaves (Farhoosh et al., 2007).
Maturity stage may influence accumulation of bioactive compounds. In such case, older plant having lower extract recovery than the immature ones had has also been observed in tea leaves extracts (Farhoosh et al., 2007). In apple for instance, the accumulation of total flavonoid and chlorogenic acid was higher in immature fruit but the level had decreased as the fruit reaches the mature and ripening stage (Awad et al., 2001). Some other reasons are due to the physiological and structural changes taking place during the growth period and fertilization requirements (Salvador et al., 2007).
4.4 ABTS free radical scavenging capacity
In this assay, the antioxidant from extract acts as hydrogen donors to ABTS+ free radical thus inhibits oxidation process. Polyphenols include many classes of compounds (phenolic acids, flavanols, xanthones, kaempferol etc.) and act as free radical scavengers. Flavonoid which is the major class of polyphenols exhibits strong antioxidant capacities (Fraga and Oteiza, 2011). However, the antioxidant capacity has decreased during maturity stage and this may be associated with apparent decrease in the total polyhenols and flavonoid contents as compared to the immature part. As the plant grew older, new biosynthesis of polyphenols may interrupt or end thus decrease the content of polyphenols. The reduction of polyphenol content has also been attributed by oxidation of polyphenols during the maturity stage (Shwartz et al., 2009).
5 Conclusions
In conclusion, both immature and mature silks could be considered as good source of nutritional compositions and antioxidant capacity. The immature silks contained significantly higher content of moisture (P < 0.05) than the mature silks. Lipid and protein content decreased nearly 50% and 31%, respectively in mature silks compared to those in immature silks. The composition of ash, carbohydrate and total dietary fiber of both silks was not significantly different (P > 0.05). Immature silks contained significantly higher level of Ca, Mg, Cu and Zn while other minerals such as K, Na and Fe were significantly higher in the mature silks (P < 0.05). Immature silks had significantly higher content of polyphenol and flavonoid content (P < 0.05) than the mature silk which was shown by the ethanol and water extract, respectively. Both ethanol extracts of immature and mature silks possessed strong free radical scavenging capacity compared to the water and ethyl acetate extract. The ethanol extract of immature silk however had the highest antioxidant capacity compared to same extracts of mature silks.
Acknowledgement
The authors acknowledge Universiti Sains Malaysia research fund (1001/PPSK/813057).
References
- Egyptian mango by-product 1. Compositional quality of mango seed kernel. Food Chem.. 2007;103:1134-1140.
- [Google Scholar]
- Functional properties of dietary fibre prepared from defatted rice bran. Food Chem.. 2000;68:15-19.
- [Google Scholar]
- Improvement of dietary fiber content and antioxidant properties in soft dough biscuits with the incorporation of mango peel powder. J. Cereal Sci.. 2008;48:319-326.
- [Google Scholar]
- Official Methods of Analysis of AOAC International (16th ed). Maryland, USA: AOAC International; 1996.
- Flavonoid and chlorogenic acid changes in skin of ‘Elstar’ and ‘Jonagold’ apples during development and ripening. Sci. Hortic.. 2001;90:69-83.
- [Google Scholar]
- Evaluation of free radical-scavenging and antihemolytic activities of quince (Cydonia oblonga) leaf: a comparative study with green tea (Camellia sinensis) Food Chem. Toxicol.. 2009;47:860-865.
- [Google Scholar]
- Elbadrawy, E., Sello, A., 2011. Evaluation of nutritional value and antioxidant activity of tomato peel extracts. Arab. J. Chem., (in press).
- Antioxidant activity of various extracts of old tea leaves and black tea wastes (Camellia sinensis L.) Food Chem.. 2007;100:231-236.
- [Google Scholar]
- Herbal option for diabetes: an overview. Asian Pac. J. Trop. Dis.. 2012;2:S536-S544.
- [Google Scholar]
- Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem.. 2005;91:395-401.
- [Google Scholar]
- Dietary flavonoids: role of (−)-epicatechin and related procyanidins in cell signaling. Free Radic. Biol. Med.. 2011;51:813-823.
- [Google Scholar]
- Antioxidant activity of Garcinia xanthochymus leaf, root and fruit extracts in vitro. Chin. J. Nat. Med.. 2012;10:129-134.
- [Google Scholar]
- Antioxidants: molecules, medicines, and myths. Biochem. Biophys. Res. Commun.. 2010;393:561-564.
- [Google Scholar]
- Nutritional and amino acid contents of differently treated Roselle (Hibiscus sabdariffa L.) seeds. Food Chem.. 2008;111:906-911.
- [Google Scholar]
- Antioxidant characterization: methodology and mechanism. Biochem. Pharmacol.. 1995;49:1341-1348.
- [Google Scholar]
- Mineral and heavy metal levels of some fruits grown at the roadsides. Food Chem. Toxicol.. 2010;48:1767-1770.
- [Google Scholar]
- Control of pollen tube growth: role of ion gradients and fluxes. New Phytol.. 2003;159:539-563.
- [Google Scholar]
- Proximate composition and amino acid and mineral contents of Mormordica charantia L. pericarp and seeds at different maturity stages. Food Chem.. 2010;122:1111-1115.
- [Google Scholar]
- Antioxidant activity of the phenol rich fractions of leaves of Chukrasia tabularis A. Juss. Bioresour. Technol.. 2008;99:7692-7698.
- [Google Scholar]
- Manganese accumulation in rice: implications for photosynthetic functioning. J. Plant Physiol.. 2004;161:1235-1244.
- [Google Scholar]
- Lunven, P., 1992. Maize in Human Nutrition. FAO Food and Nutrition Series 25, Rome.
- Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol.. 2009;12:250-258.
- [Google Scholar]
- Chemical composition and antioxidant activity of the husk and endosperm of fenugreek seeds. Food Sci. Technol.-LEB. 2011;44:451-456.
- [Google Scholar]
- Preliminary assay on the antioxidative activity of Maydis stigma extracts. Fitoterapia. 2003;74:144-147.
- [Google Scholar]
- Chemical, technological and in vitro antioxidant properties of mango, guava, pineapple and passion fruit dietary fibre concentrate. Food Chem.. 2012;135:1520-1526.
- [Google Scholar]
- High hydrostatic pressure improves the functionality of dietary fibre in okara by-product from soybean. Innov. Food Sci. Emerg. Technol.. 2010;11:445-450.
- [Google Scholar]
- Protective effect of gallic acid isolated from Peltiphyllum peltatum against sodium fluoride-induced oxidative stress in rat’s kidney. Mol. Cell. Biochem.. 2013;372:233-239.
- [Google Scholar]
- Antioxidant and free radical scavenging activities of polyphenol-enriched curry leaf (Murraya koenigii L.) extracts. Food Chem.. 2008;106:720-728.
- [Google Scholar]
- Preliminary assessment of nutritional value of traditional leafy vegetables in Kwa Zulu-Natal, South Africa. J. Food Compos. Anal.. 2007;20:430-435.
- [Google Scholar]
- Antioxidant activity of Smilax excelsa L. leaf extracts. Food Chem.. 2008;110:571-583.
- [Google Scholar]
- Biological origins of normal-chain hydrocarbons: a pathway model based on cuticular wax analyses of maize silks. Plant J.. 2010;64:618-632.
- [Google Scholar]
- Antioxidant activities of malt extract from barley (Hordeum vulgare L.) toward various oxidative stress in vitro and in vivo. Food Chem.. 2010;118:84-89.
- [Google Scholar]
- Maize plants: an ideal production platform for effective and safe molecular pharming. Plant Sci.. 2008;174:409-419.
- [Google Scholar]
- Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med.. 1999;26:1231-1237.
- [Google Scholar]
- The potential of Zea mays ears and it extracts as an alternative food nutritive ingredients. APCBEE Procedia. 2012;2:141-147.
- [Google Scholar]
- Physiological and structural changes during ripening and deastringency treatment of persimmon fruit cv. ‘Rojo Brillant’. Postharvest Biol. Technol.. 2007;46:181-188.
- [Google Scholar]
- A physiological characterization of Mn-tolerant tobacco plants selected by in vitro culture. Plant Sci.. 2000;150:163-170.
- [Google Scholar]
- Amino acid metabolism in maize earshoots. Implications for assimilate preconditioning and nitrogen signaling. Plant Physiol.. 2004;136:4326-4334.
- [Google Scholar]
- Changes in chemical constituents during the maturation and ripening of two commercially important pomegranate accessions. Food Chem.. 2009;115:965-973.
- [Google Scholar]
- Reactive oxygen species in cardiovascular disease. Free Radic. Biol. Med.. 2011;51:978-992.
- [Google Scholar]
- K+ transport in plants: physiology and molecular biology. J. Plant Physiol.. 2009;166:447-466.
- [Google Scholar]
- Antioxidant activity and phenolic profile of pistachio (Pistacia vera L., variety Bronte) seeds and skins. Biochimie. 2013;92:1115-1122.
- [Google Scholar]
- Solvent effects on phenolic contents and biological activities of the halophyte Limoniastrum monopetalum leaves. Food Sci. Technol.-LEB. 2010;43:632-639.
- [Google Scholar]
- World Corn Production, Consumption and Stocks Report. Washington DC: USDA; 2008.
- Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol.. 2007;39:44-84.
- [Google Scholar]
- Zea mays L. extracts modify glomerular function and potassium urinary excretion in conscious rats. Phytomedicine. 2005;12:363-369.
- [Google Scholar]
- Progress in ethanol production from corn kernel by applying cooking pre-treatment. Bioresour. Technol.. 2009;100:2712-2718.
- [Google Scholar]
- Studies on bioactivities of tea (Camellia sinensis L.) fruit peel extracts: antioxidant activity and inhibitory potential against α-glucosidase and α-amylase in vitro. Ind. Crops Prod.. 2012;37:520-526.
- [Google Scholar]
- Flavonoids, cognition, and dementia: actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic. Biol. Med.. 2012;52:35-45.
- [Google Scholar]
- Improving public health? The role of antioxidant-rich fruit and vegetable beverages. Food Res. Int.. 2011;44:3135-3148.
- [Google Scholar]
- Manganese uptake and accumulation by the hyperaccumulator plant Phytolacca acinosa Roxb. (Phytolaccaceae) Environ. Pollut.. 2004;131:393-399.
- [Google Scholar]
- Isolation and identification of compounds from the ethanolic extract of flowers of the tea (Camellia sinensis) plant and their contribution to the antioxidant capacity. Food. Sci. Technol.-LEB. 2009;42:1439-1443.
- [Google Scholar]
- Antioxidant activity and physico-chemical properties of Tunisian grown pomegranate (Punica granatum L.) cultivars. Ind. Crops Prod.. 2012;40:81-89.
- [Google Scholar]
- Comparison of anti-diabetic effects of polysaccharides from corn silk on normal and hyperglycemia rats. Int. J. Biol. Macromol.. 2012;50:1133-1137.
- [Google Scholar]
- Effects of extraction solvent on wheat bran antioxidant activity estimation. Food Sci. Technol.-LEB. 2004;37:717-721.
- [Google Scholar]
- Characterization of a caleosin expressed during olive (Olea europaea L.) pollen ontogeny. BMC Plant Biol.. 2011;11:122.
- [Google Scholar]







