Translate this page into:
Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review
⁎Corresponding authors. gaurav8777@gmail.com (Gaurav Sharma), mnaushad@ksu.edu.sa (Mu. Naushad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Bimetallic nanoparticles (BNPs) are formed by the combination of two different metals. Bimetallic nanoparticles have attracted huge attention as compared to monometallic nanoparticles in both technological and scientific view because BNPs shows better properties. Bimetallic nanoparticles can be synthesized in different shape, size and structure. The nanocomposites of BNPs have been prepared by supporting them on the organic or inorganic counterparts. BNPs nanocomposites have improved properties as compared to those of bimetallic nanoparticles. Due to reduction in size and increase in surface area, these are prominently used as catalyst. Application area of bimetallic nanocomposites includes drug delivery, water purification and catalysis etc.
Keywords
Nanotechnology
Nanoparticles
Monometallic
Bimetallic
Bimetallic nanocomposites
Catalysts
1 Introduction
1.1 Nanotechnology
In the recent years, nanotechnology has emerged as a cutting edge technology interdisciplinary with physics, biology, chemistry, medicine, and material science. The prefix nano is derived from Greek word ‘nanos’ meaning “dwarf” which refers to things of one billionth in size. It was in the mid of 20th century when the basic concept of nanotechnology was put forward. Richard Feynman, an American physicist is known as the “Father of nanotechnology” and he explained in American physical society meeting held in 1959, that how world’s all books can fit in a pamphlet. At that time, his lecture brought a revolution in the field of science. He even explained different methods by which it was possible to transform the individual atoms or molecules from one form to another smaller form by using different sets of tools. Nanotechnology word was coined by Norio Taniguchi of Tokyo University of Science. Much awareness was aroused among the people about nanotechnology when the book written by Eric Drexler named “Engines of creation” was published. He explained nanotechnology in relation to the nanometric scale in this book. So, nanotechnology can be defined as the application of controlling the properties of the matter at the molecular level.
Nanotechnology plays an important role in many technological fields due to the predefined superstructures. This field offers us the power of manipulating the atoms or molecules and transforming them into structures having desired geometry and properties. It has both environmental and health applications which includes effective drug delivery and applications in harvesting solar energyetc. It also helps in reducing the use of industrial chemicals and making the environment healthier, safer and worth living. In addition to this, it can be used for water purification, cancer treatment and food packaging etc.
1.2 Nanoparticles
Nanoparticles (NPs) are the nanometer-sized solid particles engineered at atomic or molecular scale so to form either novel or superior physical properties that are not attainable by conventional bulk solids. These nanosized particles; nanoparticles act like a complete unit in relation to properties. All materials have some critical range or value below which their properties changes drastically. Particles below 100 nm in diameter show properties that are different from those of conventional solids. When all the dimensions of the particle are in nanometer range, it is known as isodimensional nanoparticles, for example spherical nanoparticles of silica. The definition of nanoparticles may differ for different fields and different materials. From theoretical point of view, nanoparticles are frequently called Nano clusters or simply clusters which are defined as the combination of millions of atoms or molecules (Ferrando et al., 2008). These atoms or molecules may be of same or of different kind. Nanoparticles can be of amorphous or crystalline form and their surfaces can act as a carrier for liquid droplets or gases. The drugs can dissolve, entrap, encapsulate or attach to a nanoparticle matrix. NPs have properties in between those of the bulk material and the atomic or molecular structures. Nanoparticles should be considered as a distinct state of matter such as crystalline nanoparticle forms (fullerenes and carbon nanotubes) and traditional crystalline solid forms (graphite and diamond). Many authors limit the size of nano materials between 10 and 100 nm.
Nanoparticles can be synthesized using two techniques: top-down approach and bottom-up approach. In top-down approach, the bulk materials are broken down to the nanoparticles; however, in bottom-up approach, the nanoparticles combine so as to form the bulk material. The bottom-up approach is more convenient than that of the top-down approach because in the later, the chances of contamination are quite high.
Nanoparticles are of great importance in our day to day life. For example, nanoparticles of titanium dioxide are one of the most important components of sunscreen products. It allows only visible light to pass through the skin while scattering the harmful ultraviolet rays. Nanoparticles form the basis of nanotechnology. These are used in different forms depending upon the type of applications.
1.2.1 Monometallic nanoparticles
Monometallic nanoparticles (MNPs), as the name suggests, consist of only single metal. The constituted metal atom determines the properties of these nanoparticles. Monometallic nanoparticles are of different types depending upon the type of metal atom present such as magnetic, metallic and transition metal nanoparticles, etc. They can be prepared by different routes but the most important is the chemical method. Their structurecan be stabilized using various functional groups. Last few decades have marked the greater interest in the field of metallic nanoparticles due to their enhanced physical and chemical properties. For this reason, they are used for a number of applications such as in electronic, optical and catalysis, etc. In addition, they have also been used as antimicrobial agents against a number of microorganisms such as Escherichia coli (Sondi and Salopek-Sondi, 2004), Streptococcus mutans (Espinosa-Cristóbal et al., 2009), Streptococcus pyogens (Nanda and Saravanan, 2009) and Bacillus subtilis (Rai et al., 2009).
1.2.2 Bimetallic nanoparticles
Bimetallic nanoparticles; composed of two different metals have drawn a greater interest than the monometallic nanoparticles from both scientific as well as technological point of view (Sharma et al., 2016b; Toshima and Yonieawa, 1998). Constituting metals and their nanometric size determine the properties of the bimetallic nanoparticles. These are synthesized by the combination of different architectures of metallic nanoparticles. They actually offer us the tendency of optimizing the energy of Plasmon absorption band of metallic mixture which offers us a multipurpose tool for biosensing. These properties may differ from those of pure elemental particles and include unique size dependent optical, electronic, thermal and catalytic effects. Extensive studies in the field of bimetallic nanoparticles started just a decade back. Different methods have been proposed for their preparation and detailed characterization. These days’ researchers are focusing on selectively preparing new bimetallic nanoparticles in different forms, such as alloys, core-shell and contact aggregate. Actually, through bimetallization, the catalytic properties of the resulting nanoparticles can be improved to great extent which may not be achievable by the use of monometallic catalysts. In bimetallic catalysts, the electronic effect plays an important role which describes the charge transfer. Alloying of the constituting elements can result in the structural changes of the bimetallic nanoparticles. From monometallic to bimetallic nanoparticles, an extra degree of freedom is introduced (Sharma et al., 2015). The catalytic activities of different bimetallic nanoparticles have been subsequently compared. Different methods and correlations have been developed with the help of physical and spectroscopic measurements. The preparation conditions determine the structure and miscibility of the two metals in bimetallic nanoparticle. Generally, bimetallic nanoparticles are prepared by simultaneous reduction of two metal ions in the presence of suitable stabilization strategy such as steric hindrance and static-electronic repulsive force. In this method, a particle structure between core shell and homogeneous alloy depending on the reduction condition is obtained. By controlling the size, shape and structure of the nanoparticles, we can have control over the reduction rates of the two components.
2 Methods of synthesis of nanoparticles
Researchers have discovered many new methods to prepare nanoparticles which are of the required size, composition and shape because these factors greatly influence the properties of the material. Some of the methods used for the synthesis of the nanoparticles are:-
2.1 Thermal and photochemical decomposition
It involves the pyrolysis of precursors in boiling solvents at high temperature but the main disadvantage of this process is that under such high temperature, the isolation of unstable nanocrystal phase from the reactive phase becomes very difficult. Usually thermal method is endothermic in nature due to high energy requirement for the bond breakage. Photochemical method facilitates the isolation and study of nanomaterials having unusual size and composition.
2.2 Electrochemical reduction
In this method, electricity is used as the driving or controlling force. The electric current is passed between the two electrodes which are separated by electrolyte (Katwal et al., 2015). Researchers used electrochemical method for the preparation of metallic nanoparticles. They dissolved the metallic anodic sheet and metallic salt formed was reduced by the cathode to metallic particles. These metallic particles formed were stabilized by tetraalkylammonium salts. Merits of electrochemical technique include low cost, high purity of particles, particles size control by optimizing the current density and simple method of operation. This method is mainly used in industrial applications.
2.3 Chemical reduction
This method produces metal nanoparticle in the zero valent state. In this method, two processes namely, reduction and interaction between the metallic and polymeric species operate. Different reducing agents such as sodium borohydride, elemental hydrogen, Tollen’s reagent and ascorbate, etc. are being used. Chemical reduction is a common method used for the synthesis of silver nanoparticles.
Successive reduction is the most promising way or method of synthesizing core-shell structured bimetallic nanoparticles. It involves the deposition of a metal on the synthesized monometallic nanoparticles of other metal. The pre-synthesized monometallic nanoparticles should be chemically surrounded by the deposited metal atom.
2.4 Sputtering
Sputtering is process where nanoparticles are ejected from the surface of target material by using high energy external stimuli. Ejection of nanoparticles takes place only when the amount of energy provided is high as compared to the conventional thermal energies. This method produces nanoparticles of high purity. For example, silicon nanowires are prepared using magnetic sputtering method. This method suffers from certain drawbacks such as less control over the morphology of particle and energy consumption for the ejection of electron is quite high. Since high temperature is required; it can be harmful causing various skin diseases.
2.5 Sol-gel method
The sol-gel method drives from two words; sol and gel. The sol is a stable suspension of colloidal solid particle in liquid. The dispersed phase in sol is so small that there only Vander-Waal forces. In gel, the concentration of solid is more than liquid. It is a semi-rigid mass in which the particles or ions left after the evaporation starts to form a continuous network. In most of the gel systems, there exist the covalent interactions. The combination of these two network functions is called sol-gel method. This method mainly consists of two main reactions, hydrolysis and condensation. Various BNPs are synthesized by sol-gel method, such as Au-Ag, Au-Pd and Au-Pt, etc. This method is quite useful because it is a simple, economic and effective method to produce good quality nanoparticles (Sharma et al., 2016a). It is quite interesting since it is a low temperature technique that offers the ability to control the product’s chemical composition.
2.6 Chemical precipitation method
Chemical precipitation is the process of conversion of a solution into solid by converting the substance into insoluble form or by making the solution a super saturated one. It involves the addition of chemical reagents and then separation of precipitates from the solution (Sharma et al., 2016b). Nanoparticles of ZnO and ZnS can be prepared by this method. Since it is a single step process and helps in large scale production of nanoparticles without any impurities, it is quite a useful technique. It even helps in the purification of water and is long term remedy or produces permanent results.
2.7 Micro-emulsion method
Micro-emulsion is defined as a solution which is composed of at least three components namely, a polar component, a non-polar component and a surfactant. The function of the surfactant is to form a layer between the polar and non-polar component. It is even thermodynamically stable and homogeneous in nature. Micro emulsion can be classified into water-in-oil (w/o) or oil-in-water (o/w), depending upon the type of dispersed and continuous phase. Only a few organic nanoparticles can be prepared using oil in water micro-emulsion. The Pd-Au bimetallic nanoparticles which are supported on nickel foil substrate via in-situ self-assembly of irreversible micro emulsion of water/Triton X-100/n-hexanol/n-hexane. The electrocatalytic performance of these nanoparticles was studied by cyclic voltammetric and chrono amperometric measurements which show that these have good stability for ethanol oxidation in alkaline media. The La/Cd BNPs has been synthesized using micro-emulsion method and used for degradation of organic pollutants (Sharma et al., 2015).
2.8 Hydrothermal method
In this method, nanoparticles are produced under the influence of high temperature, about 470°C and pressure, below 300 MPa. This method allows the dilution of components which are normally non-soluble under normal conditions. The properties of the resulting nanoparticles then depend upon the pH, temperature and pressure of the medium. Further enhancement in this method can be useful because it will help in monitoring the crystal growth. This method is advantageous due to the production of high yield and pure products. In addition to this, it produces crystals of high quality and offers us the ability to control the physical and chemical properties of the resulting nanoparticles. Disadvantages of this method include the high equipment cost and it is not possible to monitor the growth process of crystal. Zeolites and nanoparticles of Lead telluride have been synthesized by this method.
3 Various bimetallic nanoparticles
Researchers are synthesizing novel bimetallic nanoparticles to get desired properties. Various types of bimetallic nanoparticles are:-
3.1 Platinum based bimetallic nanoparticles
Platinum nanoparticles are now being used in the upcoming generation for automotive catalytic converters due to high surface area, thus the amount of platinum required for their fabrication is less. To enhance the effectiveness of Pt-based electrodes, the Pt based bimetallic nanoparticle catalysts have been synthesized (Karthikeyan and Murgavelu, 2012; Patra and Yang, 2009). The Pt-X (X = Cu, Au, or Ag, etc.) alloys are very important due to their high catalytic efficiency. In the recent years, polymer-protected colloidal Pt–Cu particles have been prepared and these can be used for the catalytic hydrogenation of solution, where the bimetallic clusters are used as active and selective agents for the hydration of acrylonitrile to acrylamide as well as for hydrogenation of 1,3-cyclooctadiene to cyclooctene (Toshima and Wang, 1994). It has also been found that Pt-Cu bimetallic catalysts are effective in the reduction of NOx. Studies about Pt-Cu based bimetallic nanoparticles have revealed their heterogeneous catalytic activities for reduction of gas phaseNO with hydrogen as the reducing agent (Obuchi et al., 1993; Odenbrand et al., 1999). In Pt-based catalyst studies, the addition of other metals to Pt catalyst decreases the amount of need of noble metal, Pt. It also helps in improving its catalytic ability (Maiyalagan et al., 2012; Thandavarayan et al., 2012). For example, Hu et al. have concluded that bimetallic Pt–Au nanocatalyst shows better catalytic capability as compared to single metal catalyst of Au or Pt.
3.2 Nickel based bimetallic nanoparticles
Metal nanoparticles such as nickel nanoparticles have been intensively used for its catalytic and the magnetic properties. However, Nickel on combination with other metals shows fascinating properties (Kumar et al., 2014; Shah et al., 2015). Catalysts containing nickel are commonly used due to their low cost, high stability and fast turnover rate. In the past few years, Ni-Sn based bimetallic nanoparticles have been prepared with controlled size and composition. By varying the stoichiometric ratios of Ni and Sn, bimetallic nanoparticles with different composition such as Ni100, Ni74-Sn41, Ni75-Sn25, Ni40-Sn40 and Ni50-Sn50 have been synthesized. The Cu-Ni based bimetallic catalyst has been used as an effective method for improving the efficiency of various reactions. Cu–Ni/Al2O3 catalysts and Ni–Cu/samaria-doped ceria catalysts have been used which are used for the hydrogenation of carbon dioxide and for steam reforming of methane.
3.3 Iron based bimetallic nanoparticles
From the recent studies, it has been found that the Fe-Cu bimetallic catalyst system has attracted tremendous attention (Han et al., 2011; Kumar et al., 2016; Lam and Hu, 2013; Xia et al., 2011). It has been reported that this catalyst when supported on MCM-41 showed higher catalytic activity as compared to Cu or Fe when singly supported on MCM-41 (Lam and Hu, 2013). It shows high catalytic activity even after 10 consecutive runs. Other class of iron based bimetallic nanoparticles includes Pd/Fe nanoparticles which have been prepared by chemical precipitation method in liquid phase. With diameters in the range of 30–50 nm, these nanoparticles exhibit excellent catalytic activity for dechlorination of chlorinated methanes such as dichloromethane (DCM), chloroform (CF) and carbon tetrachloride (CT) (Wang et al., 2009). For the treatment of chlorinated organic pollutants, nanoscale bimetallic particles (Pd/Fe, Pd/Zn, Pt/Fe, Ni/Fe) have been synthesized in the laboratory.
3.4 Palladium based bimetallic nanoparticles
Now-a-days, palladium nanoparticles are being used for various applications due to their low cost and easy availability. The palladium based bimetallic nanoparticles are stable in the acidic medium and are suitable for the oxidation of alcohol in alkaline medium (Seo et al., 2013). Palladium coupled gold nanoparticles has a tendency of acting as a catalyst which is used mainly for the ligand-free Suzuki coupling reactions. These nanoparticles reside their catalytic efficiency even after repeated number of cycles (Nasrollahzadeh et al., 2015). The Pd/Fe bimetallic nanoparticles have great economic importance because they have the tendency of transforming the chlorinated compounds. It has been recently reported that Pd/Ag nanoparticles acts as a sensor which can be used for the electrochemical detection of L-Cysteine (Murugavelu and Karthikeyan, 2014).
3.5 Gold based bimetallic nanoparticles
Gold NPs act as an efficient catalyst and biosensors. It is believed that gold containing nanoparticles can be used for increasing the catalytic activity and selectivity. Au/Pd based bimetallic nanoparticles have been recently prepared and they show interesting catalytic, electrochemical and structural properties (Venkatesan and Santhanalakshmi, 2011). In addition to this, bimetallic nanoparticles of gold/copper have also been prepared. These have many applications but are mainly used in medical sensors and biomedicine. Au/Ni based bimetallic nanoparticles in different shapes and forms are prepared and systematically investigated. Extensive studies have been done for synthesizing Au/Ag BNPs. These bimetallic nanoparticles have extensive applications such that they are used in the detection of glucose and it also exhibits property of chemiluminescence (Yu and He, 2015).
4 Nanocomposites
Nanocomposite is a mixture of two or more materials producing new material with at least one dimension in the nano range. The constituting materials are mixed in such a way that the resulting material exhibits “averaged” properties between the two. The assembly of the two materials is done because they exhibit better properties such as catalytic, thermal stabilityand adsorption properties, etc. than the individual materials (Sharma et al., 2017a; Pathania et al., 2015). Mostly, composite materials are composed of two phases, the discontinuous (reinforcing material) and the continuous (matrix). On the basis of properties, nanocomposites are classified into two broad categories, Functional materials (based on the electrical, magnetic and optical properties) and Structural materials (based on their mechanical properties). The number of applications of nanocomposites is growing at a faster rate. These days nanocomposites are used in medicine, engineering, drug delivery, anti-corrosion barrier protection and UV protection gels, etc (Bozetine et al., 2016; Kumar et al., 2015; Sharma et al., 2017).
5 Bimetallic nanocomposites
Bimetallic nanocomposite materials that comprise inorganic nanoparticles and biopolymers have attracted considerable attention during the last few decades (Sharma et al., 2015). The mechanical, electrical, thermal, optical and catalytic properties of nanocomposites will be different from that of individual components. Size limit of this effect has been proposed to be <5 nm for catalytic activity, <20 nm for making hard magnetic material soft, <50 nm for refractive index and <100 nm for superparamagnetism. These are also found to occur naturally such as in abalone shell. In the framework of this rapid development, the domain of bimetallic nanocomposite materials is attracting researchers (Sharma et al., 2015). The importance of bimetallic nanocomposites lies in their multifunctionality, the possibility of unique combination of properties which are unachievable with traditional materials. Bimetallic nanocomposites are recently gaining momentum due to their large number of applications such as the anti-corrosion barrier coatings; UV Protection gels, lubricants and scratch/abrasion resist materials, etc. It even produces superior strength fibers and films. Bimetallic nanocomposites are becoming very important these days due to their ability to exhibit vast applications. These show better catalytic, thermal, optical, electrical and magnetic properties as compared to those of monometallic as well as bimetallic nanoparticles.
5.1 Carbon supported bimetallic nanocomposites
Bimetallic nanocomposites supported on carbon are of great interest. Carbon supported bimetallic nanoparticles have reduced surface area which enhances their properties to a large extent. Water contamination is one of the major problems faced by human beings now-a-days. The presence of unwanted material, ions, micro-organisms such as fungi, bacteria and virus, etc. contaminates the water. The presence of perchlorate ion makes the water unsafe for drinking. Nanotechnology provides an alternate to the water purification. When ruthenium-palladium bimetallic nanoparticles are supported on the carbon matrix, they efficiently reduce the perchlorate into chloride ions under acidic conditions and thus, helps in treating waste or contaminated water containing perchlorate as the major impurity (Liu et al., 2013a,b). Au/Pt/C nanocomposites have improved catalytic properties. These are formed by the normal synthetic route. The reinforcing material (Pt + Au) is uniformly distributed on the matrix (Carbon). It acts as a catalyst in the electro-oxidation of glucose and helps in increasing the rate of reaction. However, Co/Pt nanoparticles supported on carbon is used for the fuel-cell applications. Ag/Ni-C nanocomposites show higher oxygen reduction reaction capacity as compared to the simple Ag/Ni bimetallic nanoparticles.
Direct methanol fuel cells are in use from many years but it suffers from certain drawbacks such as high level of poisoning due to platinum anode electrocatalyst. In order to overcome it, new nanocomposites Pt/Au supported on platelet carbon nanofibers (pCNF) has been synthesized and these acts as an effective electrodes for DMFCs, thus, reduces its toxic effects and increases the life span of fuel cell (Giorgi et al., 2014). Fig. 1 shows the SEM image of electrodeposition of Pt/Au catalyst on platelet carbon nanofibers (pCNF).
Typical FEG-SEM micrographs of PtAu catalyst electrodeposited on platelet carbon nanofibers (pCNF) (Giorgi et al., 2014).
5.2 Graphene supported bimetallic nanocomposites
Graphene is the allotropic form of carbon having honeycomb like two dimensional structure. Graphene supported bimetallic nanocomposites exhibit excellent catalytic, thermal and chemical properties. There exists Vander Waals interactions and π-π stacking between them. These two factors lead to irreversible agglomerates and thus, decreasing the surface area and conductivity. This can be prevented by using spacers such as carbon nanotubes (CNTs) (Mittal et al., 2016). These when placed in between the graphene sheets avoids the agglomeration and helps in increasing the surface area, electronic and mechanical stability of graphene. These G-CNTs acts as catalyst support material and increases the catalytic activity of nanoparticles. Pt/Au bimetallic nanocomposites can be successfully synthesized when supported on G-CNTs by simple one step co-reduction method. The resulting nanocomposite, (Pt/Au/G-CNTs) acts as sensor which shows high sensitivity towards the detection of hydrogen peroxide. This sensor has low detection limit (Lu et al., 2013). Fig. 2 shows the schematic representation for the preparation of Pt/Au/G-CNTs nanocomposites.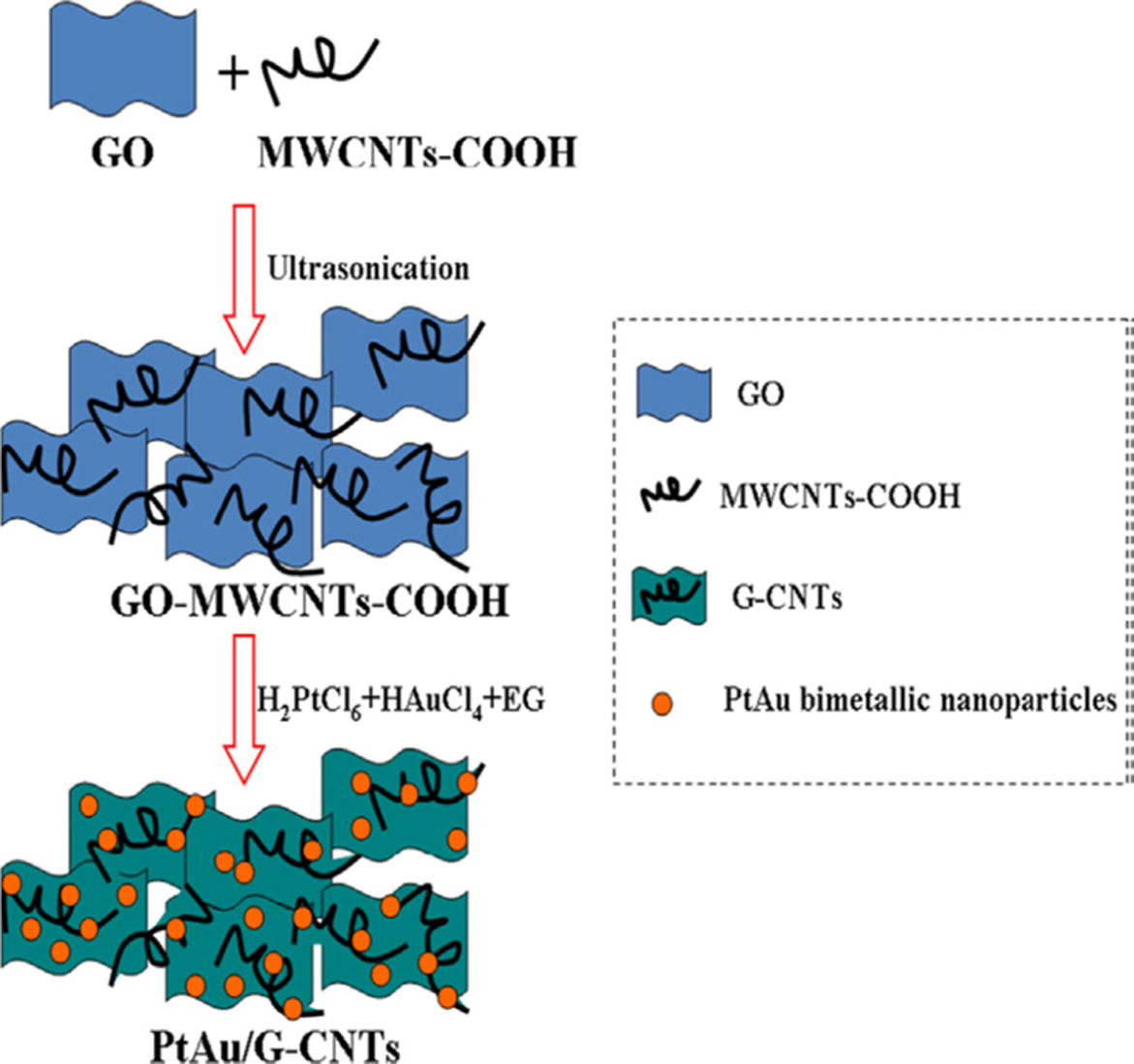
Schematic representation for the synthesis of PtAu/G-CNTs nanocomposites (Lu et al., 2013).
The Pd/Co-G nanocomposites act as a perfect agent for the electrocatalytic oxidation of alcohol and can be prepared by the rapid reducing method. However, the palladium-yttrium supported on graphene is used in the oxygen reduction and ethanol oxidation reactions. Fe/Ag bimetallic nanoparticles supported on graphene shows antibacterial activity and have been the composition of Fe/Ag on graphene sheet synthesized by one step pot method by varying. It has been against number of bacteria such as Bacillus subtilis, E. coli, etc. (Ahmad et al., 2016). The nanocomposites of platinum-ruthenium nanoparticles supported on graphene sheets can be used as electrochemical biosensors for the detection of hydrogen peroxide (Kung et al., 2014).
5.3 Inorganic material supported bimetallic nanocomposites
Under this category, we support the bimetallic nanoparticles on different inorganic materials such as kaolin, zeolites and zirconium, etc. Nanocomposites of Au/Pd nanoparticles supported on titanium oxide have been prepared by micro-emulsion method and being used as an efficient photocatalyst due to their high light absorption capacity. Bimetallic nanocomposites of Fe/Ni-K have the ability of removing Direct Black G (DBG) from the waste water. The tendency of removing DBG in the nanocomposite (Ni/Fe-K) is much higher than that of individual kaolin and the bimetallic nanoparticles (Ni/Fe) (Liu et al., 2013a,b). Fig. 3 is the SEM images that reveal the morphological changes between kaolin, Fe/Ni, K-Fe/Ni, and K-Fe/Ni after their reaction with DBG.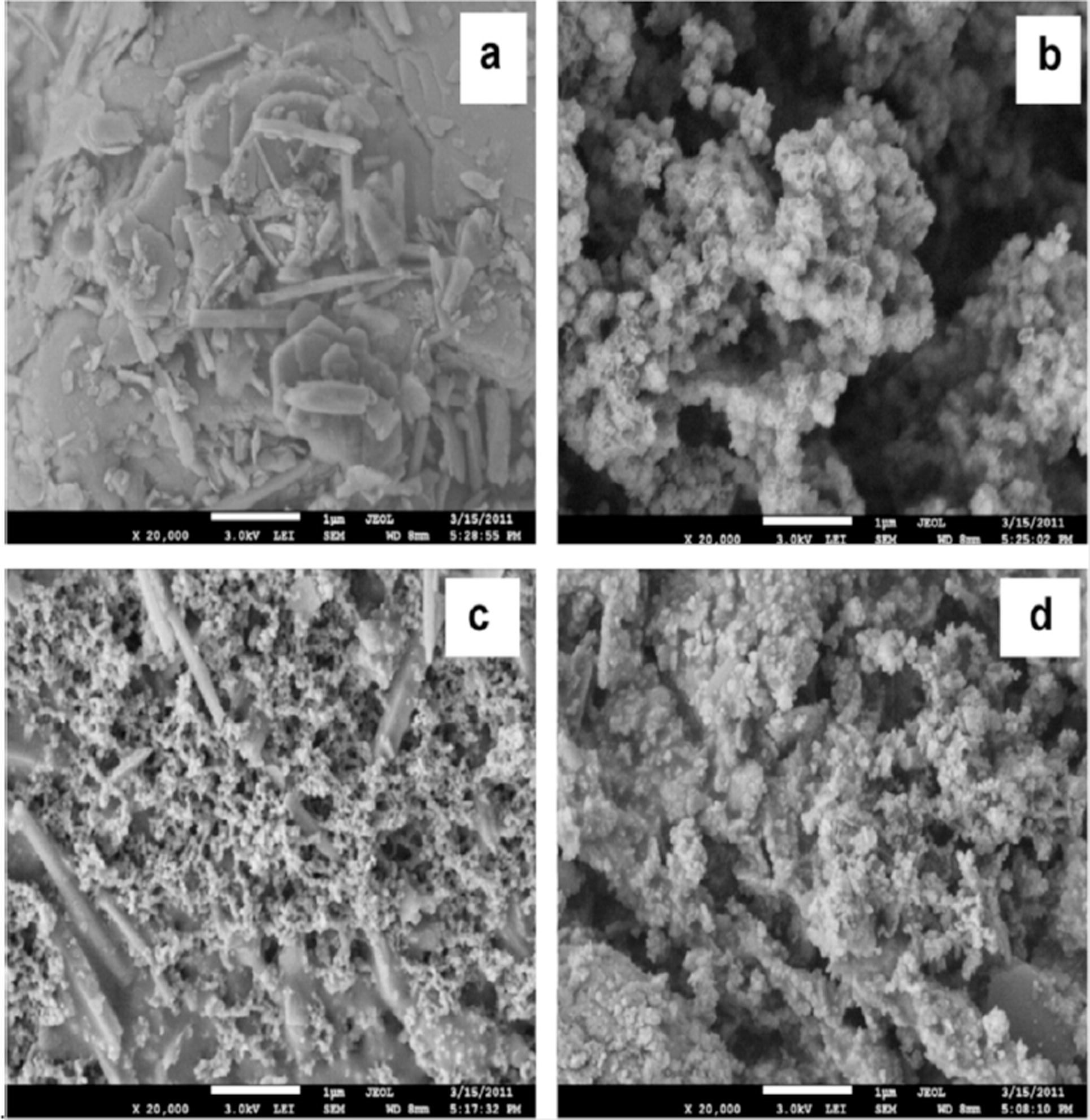
SEM images (a) kaolin, (b) Fe/Ni, (c) K-Fe/Ni, and (d) K-Fe/Ni after reaction with DBG (Liu et al., 2013a,b).
In 2013, Au@Pt-TPAD nanocomposite was synthesized and the preparation was carried out in various steps. Firstly, Au nanoparticles were imparted on the triphenyl amine-based dye (TPAD) by ethanol reduction method and then on the resulting composite, Pt shell nanoparticles were projected by successive two-step reduction method. These nanocomposites were found to be an effective photocatalysts and used as an effective system for the photocatalytic hydrogen evolution (Cheng et al., 2013). Fig. 4shows the photo induced hydrogen evolution under light irradiation by Au core Pt shell nanoparticles. The Cerium dioxide supported Cu/Ni alloy bimetallic nanoparticles were prepared by co-precipitation method and used in the dehydrogenation reactions (Rao et al., 2012).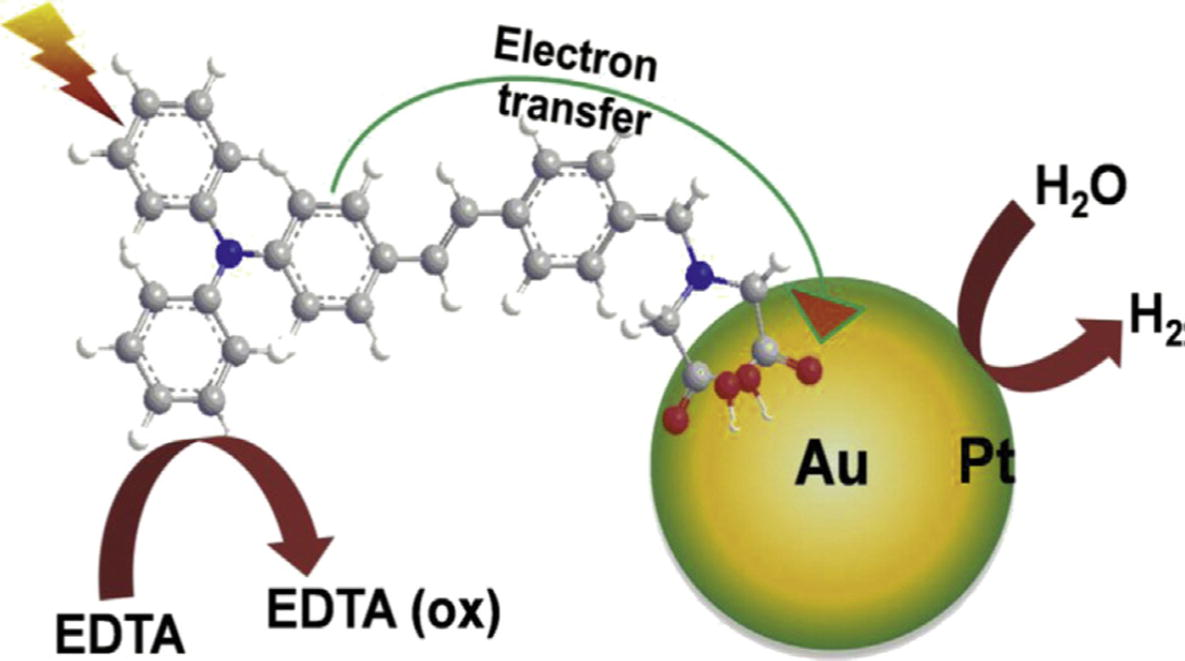
Schematic representation of the electron transfer from a TPAD sensitizer into Au core Pt shell nanoparticle and photoinduced hydrogen evolution under light irradiation (Cheng et al., 2013).
6 Bimetallic nanoparticles over monometallic nanoparticles
In bimetallic nanoparticles, two different metals combine to show novel properties which are the combination of the two metals present in it. This makes them important from industrial as well as from technological point of view. Bimetallic nanoparticles are more important as compared to those of monometallic nanoparticles due to the presence of extra degree of freedom. The catalytic properties of gold can be enhanced to a greater extent by mixing it with some other metals such silver or nickel or cobalt, etc. BNPs have greater surface area which increases their adsorption power and hence acts as efficient catalysts as compared to those of monometallic nanoparticles.
7 Applications of bimetallic nanoparticles and nanocomposites
The use of BNPs makes it possible not only to obtain better catalytic activity but also helps in making new materials having desired properties which cannot be achieved by single metal atom. Bimetallic nanoparticles unveil interesting electronic, chemical, biological, mechanical and thermal properties due to the composition and synergistic effects. The activity as well as the selectivity of catalyst is affected by size of the particle. The use of bimetallic nanoparticles may give rise to synergism, when the particles are used for catalysis. The combination of two different metals enhances their specific properties. These properties may be different to those of pure elemental particles. The elemental arrangement of bimetallic nanoparticles depends stronglyon the method used for their production and the system of two metals is generally not in thermodynamic equilibrium. Core-shell bimetallic particles are among the most studied nano-catalysts. Metallic and bimetallic nanoparticles, especially those containing a few tens or few hundreds of atoms, are excellent catalysts because of their highly active surfaces. These catalytic nanoparticles have improved selectivity, efficiency and recyclability, achieving the modern requirement for green catalysts. In the preparation of bimetallic nanoparticles, the interaction between two metals plays an important role (Sharma et al., 2017b). The bimetallic nanoparticles such as Pt-Ag, Pt-Rh, Ag-Ni, Au-Pt, Pd-Au and Ni-Au, etc. has been already prepared.
Silver and gold have almost identical lattice constant (0.408 for gold and 0.409 for silver) which leads to their strong tendency towards alloy formation. For more than 10 years, nanoparticles and other nanostructures have been used in bio diagnostics (or molecular diagnostics), a field which belongs to biomedicine. Bimetallic nanoparticles are also used in hydrogen storage, environmental catalysts, MRI contrast agents, photo catalytic treatment, antibacterial, and wound dressings, etc. Nanoparticles have been known to be sensitive and selective when used as cellular labels and DNA/protein markers for diagnosing disease. Medical applications of these particles help in the early diagnosis of disease and its treatment. Table 1 gives a short survey of the synthesis and applications of different bimetallic nanoparticles and nanocomposites.
Sr. No.
Bimetallic nanoparticles and their composites
Method of synthesis
Applications
References
1.
Fe-Cu bimetallic nanoparticles embedded within ordered mesoporous carbon
–
Heterogeneous Fenton catalyst for the degradation of organic contaminants
(Wang et al., 2015)
2.
Au/Pd bimetallic nanoparticles
Arc discharge & drop-drying process
Application in the Suzuki coupling reaction
(Nasrollahzadeh et al., 2015)
3.
Fe/Ni nanoparticles
–
Degradation of scarlet 4BS in aqueous solution
(Lin et al., 2012)
4.
Pd/Cu-graphene
Facile synthetic method
Application as novel electrocatalyst for ethanol oxidation in alkaline media
(Dong et al., 2014)
5.
Pd/Co bimetallic nanoparticles supported on graphene
Rapid reducing method
Electrocatalytic alcohol oxidation
(Wang et al., 2014)
6.
Pt/Cu alloy BNPs.
Reverse micelles method
–
(Weihua et al., 2006)
7.
Ni/Fe bimetallic nanoparticles
–
Remediation of polybrominated diphenyl ethers in soil
(Xie et al., 2014)
8.
Bimetallic Pt-Ni and Pt–Co electrodes
Galvanic replacement method
Rotating disc electrode studies of borohydride oxidation
(Tegou et al., 2011)
9
Pd/Fe bimetallic nanoparticles
Chemical precipitation method
Dechlorination of chlorinated methanes
(Wang et al., 2009)
10.
Pd/Fe, Pd/Zn, Pt/Fe, Ni/FeNanoscale bimetallic particles
Reductive deposition
Treatment of chlorinated organic contaminants
(Zhang et al., 1998)
11.
CuAu–ZnO–graphene nanocomposite bimetallic alloy-semiconductor
–
Photocatalytic degradation of synthetic colorants methyl orange (MO), methylene blue (MB), indigotin (IN), sunset yellow (SY), and tartrazine
(Xie et al., 2015)
12.
Ni–Mo and Co–Mo
–
Application for catalytic chemical vapor deposition synthesis of carbon nanotubes
(Lobiak et al., 2015)
13.
Bi–Sn bimetallic nanoclusters
Simultaneous reduction reaction
–
(Frongia et al., 2015)
14.
Ag–Pd bimetallic nanoparticles
Chemical reduction method
Modified glassy carbon electrode for detection of L-cysteine
(Murugavelu and Karthikeyan, 2014)
15.
Fe–Ag nano-particles
Reductive deposition of Ag on ZVI nano-particles
Debromination of decabromodiphenyl ether (BDE-209) and 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47)
(Luo et al., 2012)
16.
Cu/Fe bimetallic nanoparticles
Simultaneous Reduction
Higher alcohol synthesis via syngas
(Xiao et al., 2013)
17.
PVP-protected Au/Ni bimetallic nanoparticles
Chemical reduction
Catalytic activity in the hydrogen generation from hydrolysis of a basic NaBH4 solution
(Wang et al., 2014)
18.
Bi/Fe0 bimetallic nanoparticles
–
Oxidative and reductive degradation of hexahydro-1,3,5-trinitro-1,3,5-triazine
(Gong et al., 2015)
19.
Pt/Ir nanoparticles
–
Electrocatalytic ability for hydrogen peroxide oxidation
(Chang et al., 2014)
20.
Ag/Pd bimetallic nanoparticles
Co-reduction
Electrocatalytic reduction of benzyl chloride.
(An et al., 2011)
21.
Ni/Fe bimetallic nanoparticles
Co-reduction
Used for ground water treatment
(Han and Yan, 2014)
22.
Ni-Pt bimetallic nanoparticles
–
Selective decomposition of hydrous hydrazine to hydrogen at room temperature
(Singh and Xu, 2014)
23.
Ag-Cu bimetallic nanoparticles (NPs)
Synthesized using the fruit latex of Achrassapota Linn
Investigated in vitro toxicity studies
(Thakore et al., 2015)
24.
Au@Pt-TPAD
Successive two-step reduction method
Photocatalytic hydrogen evolution
(Cheng et al., 2013)
25.
Chitosan stabilized bimetallic Fe/Ni nanoparticles
Liquid-phase reduction method
Used to remove mixed contaminants-amoxicillin and Cd (II) from aqueous solutions
(Weng et al., 2013)
26.
Carbon nanofibers and bimetallic PtAunanocatalysts (PtAu/pCNF electrodes)
–
Methanol electro-oxidation
(Giorgi et al., 2014)
27.
Bimetallic Ni-Pd/graphene composites
Simple chemical method
Hydrogen sensing
(Phan and Chung, 2014)
28.
Graphene foam supported Pt/Ru nanocatalysts
Reduction method
Hydrogen peroxide based electrochemical biosensors.
(Kung et al., 2014)
29.
AuM (M = Pd, Rh, Pt) bimetallic nanocrystals
–
Enhanced electrochemical detection of H2O2
(Han et al., 2015)
30.
Pd–Ag bimetallic colloids in CO2-expanded hexane
–
Application in partial hydrogenation of phenylacetylene
(Wei et al., 2013)
31.
Fe/Ni bimetallic nanoparticles supported on kaolin
Liquid-phase reduction method
Remediation of Direct Black G in wastewater
(Liu et al., 2013a,b)
32.
Ni–Fe bimetallic nanoparticles immobilized on cation exchange resin
–
Pollutants degradation
(Ni and Yang, 2014)
33.
Pt-Ni bimetallic nanoparticles supported on carbon-ceramic
–
Electrocatalyst for electrooxidation of methanol and ethanol
(Habibi and Dadashpour, 2013)
34.
Ni(B)/Fe(B) bimetallic catalytic reductant
Electroless plating method
Catalytic dechlorination of monochlorobenzene
(Han et al., 2008)
35.
Pt/Pd bimetallic on zirconia catalysts
–
Hydro treating purposes
(Devers et al., 2007)
36.
Au/Pt bimetallic nanochains
Mild chemical method
Application in electrochemical immune sensor for the detection of carcino embryonic antigen
(Cao et al., 2013)
37.
Cu/Ni bimetallic particles on CeO2 support
Coprecipitation /Calcination /Hydrogen reduction
Catalytic activity for non-oxidative dehydrogenation of cyclohexanol to cyclohexanone.
(Rao et al., 2012)
38.
Fe/Cu bimetallic films of polymerized porphyrins
–
Study of the electron transfer properties
(Hamer et al., 2012)
39.
Ag-Ni/C bimetallic particles
Simultaneous reduction
Used as cathode catalyst in AFCs (alkaline fuel cells)
(Song and Zhang, 2014)
40.
Pd/Fe bimetallic particles
–
Dechlorination of hexachlorobenzene
(Shih et al., 2009)
41.
Ni/Pt bimetallic polycrystalline surfaces
–
Cyclohexene hydrogenation
(Humbert et al., 2011)
42.
Au/Pd nanoparticles supported on TiO2
Water-in-oil microemulsion
Photocatalytic properties
(Cybula et al., 2014)
43.
Nanocomposite of Ru@Ni-graphene
One-step in situ co-reduction
Hydrolytic dehydrogenation of ammonia borane and methylamine borane
(Cao et al., 2014a)
44.
Pd-Cu alloy nanoparticles
One pot reduction and Co-reduction
Anodic oxidation of ethanol in alkaline media
(Mukherjee et al., 2015)
45.
Co@Pt nanoparticles supported on carbon-ceramic substrate
–
Electrocatalytic activity for fuel cell applications
(Habibi and Ghaderi, 2015)
46.
Ni–Ru supported over hierarchical Beta zeolite
–
Hydro reforming of the oils from LDPE thermal cracking
(Serrano et al., 2015)
47.
Re-Pd bimetallic catalyst
–
Treatment of perchlorate in waste ion-exchange regenerant brine
(Liu et al., 2013a,b)
48.
Cu/Fe clay bimetallic catalyst
–
Photo-assisted degradation of Acid Black 1
(Yip et al., 2007)
49.
Fe–Co/SBA-15 bimetallic catalyst
In situ auto-combustion method
Degradation of Orange II in water
(Cai et al., 2015)
50.
Ag–Au alloy nanoparticles
Reduction method
Antibacterial activity
(Bankura et al., 2014)
51.
Au-Ir catalysts
–
Selective oxidation of ethanol to acetaldehyde
(Guan and Hensen, 2013)
52.
Pt–W bimetallic alloys
–
Electrocatalysts for hydrogen oxidation reaction
(Dai et al., 2013)
53.
Pt-Cu nanoparticles supported on graphene
One-pot synthesis method
Activity for methanol electro-oxidation
(Chen et al., 2015)
54.
Au@Pt/C and AuPt/C alloy-like nanoparticles
Seed growth method
Ethanol electrooxidation.
(Zhou et al., 2014)
55.
Au@Pt core–shell nanoparticles supported on Vulcan XC-72 carbon
Successive reduction
Electrocatalytic activities for methanol oxidation
(Feng et al., 2012)
56.
Palladium-yttrium supported on graphene nanoparticles
–
Oxygen reduction and ethanol oxidation reactions
(Seo et al., 2013)
57.
Ni/Co nanoparticles-doped carbon nanofibers
Electro spinning Method
Electrode for dye-sensitized solar cells
(Motlak et al., 2015)
58.
Cu–Ni/TiO2
Precipitation method
Photodegradation of Orange II under visible light
(Riaz et al., 2012)
59.
Bi-metal Cu–Co from LaCo1 − xCuxO3perovskite supported on zirconia
Citrate complexing method
Synthesis of higher alcohols
(Liu et al., 2014)
60.
Sn–Bi nanostructured alloy
Simultaneous reduction reaction
–
(Frongia et al., 2015)
61.
Cu–Co bimetallic supported on Al2O3
Polyol process
Removal of BTEX and PAH in the incineration flue gas
(Lu et al., 2009)
62.
Pt–Cu bimetallic nanoparticles
Water-in-oil microemulsions method
Application in the reduction of rhodamine B
(Singh et al., 2013)
63.
Au–Ag bimetallic nanoparticles
Green synthesis
Catalysts in the reduction of 2-, 3-, 4-nitrophenols and in the degradation of methyl orange
(Kumari et al., 2015)
64.
Ag-Se bimetallic nanoparticles
Quercetin and gallic acid mediated synthesis
Antitumor and antimicrobial potential
(Kumar et al., 2014)
65.
Ni-Pt nanoparticles supported on MIL-101
Simple liquid impregnation method
Catalysts for hydrogen and chemical hydrogen storage
(Cao et al., 2014b)
66.
Zn/Ag bimetallic doped polyurethane spider net composite nanofibers
Simple electrospinning process
Multipurpose electro spunmat
(Hassan et al., 2013)
67.
Au–Pd bimetallic nanocomposites
–
Catalytic reduction of p-nitrophenol
(Zhao et al., 2009)
68.
Au–Ag reduced graphene oxide based nanocomposites
Sonochemically synthesized
Catalytic reduction of 4-nitorphenol
(Neppolian et al., 2014)
69.
Fe@Aucoreshell nanoparticles anchored graphene oxide
–
Nanocatalyst for the reduction of nitrophenol compounds
(Gupta et al., 2014)
70.
Ag–Ni core–shell nanoparticles
Water-in-oil microemulsions
Reduction of the dye
(Xia et al., 2010)
71.
Dendritic Au–Ag bimetallic nanoparticles
Seed-assisted synthesis
Chemiluminescence activity and their application in glucose detection
(Yu and He, 2015)
72.
Au/Pt bimetallic nanoparticles
–
Counter electrode for quantum-dot-sensitized solar cells
(Dao et al., 2015)
73.
Au/Ag bimetallic alloy nanoparticles
Biogenic synthesis
–
(Mondal et al., 2011)
74.
(Ag/Au) bimetallic nanoparticles
Biogenic synthesis
–
(Jacob et al., 2012)
75.
Au core–Ag shell bimetallic nanoparticles
Biogenic synthesis
–
(Shankar et al., 2004)
76.
Ag–Ni bimetallic nanoparticles
Laser-induced plasma
–
(Xiao et al., 2011)
77.
Pt/Au bimetallic nanoparticles on graphene–carbon nanotube
–
Nonenzymatic hydrogen peroxide sensor
(Lu et al., 2013)
78.
Ni-Sn bimetallic nanoparticles
Colloidal synthesis
Reduction of nitroarenes
(Shah et al., 2015)
79.
Pt/Sn bimetallic nanoparticles
Hydrothermal method
Life time methanol oxidation
(Zheng et al., 2008)
80.
Dendrimer-stabilized bimetallic Pd/Au
Co-precipitation
Application to vinyl acetate synthesis
(Kuhn et al., 2014)
81.
PEG-Pd/Fe, Starch-Pd/Fe, and Guargum-Pd/Fe bimetallic nanoparticles
–
Dechlorination
(Wang et al., 2015)
82.
Pt-Pd bimetallic nanoparticles
–
Direct methanol fuel cells: Enhanced electrocatalytic properties
(Long et al., 2011)
83.
Ag@Au bimetallic nanoparticles
Capacitance coupled RF-PECVD system
Biosensor application
(Ghodselahi et al., 2014)
84.
Au-Ni bimetallic nanoparticles
–
Studied methanol and ethanol oxidation
(Kannan et al., 2015)
85.
Au@Pd core–shell bimetallic nanoparticles
Selective oxidation of benzyl alcohol
(Wang et al., 2015)
86.
Pd–Au bimetallic nanoparticles
Reverse microemulsion method
Ethanol oxidation in alkaline media
(Li et al., 2014)
87.
Ru@Cu core–shell bimetallic nanoparticles
Ionic liquid mediated synthesis
–
(Helgadottir et al., 2013)
88.
Pt/Au-GrCNTs composite
Co-reduction method
Electrochemical sensor for propyl gallate
(Cui et al., 2015)
89.
Lanthanum/Cadmium/Polyaniline bimetallic nanocomposite
Reverse microemulsion method
Photodegradation of organic pollutant
(Sharma et al., 2015)
In pharmaceutical sciences, these are used to reduce toxicity and side effects of the drugs. Nanoparticles can also be used in our everyday life. For instance, BNPs are used in many popular sunscreens. Nanotechnology is a refractive index for various surfaces and also provides light based sensors for use in diagnosing cancer. Underlying are some major application fields of bimetallic nanoparticles and nanocomposites:
7.1 Electrochemical sensing
The (Pt/Ir-C) nanocomposites are used for the electrochemical sensing of the hydrogen peroxide (H2O2). When Pt and Ir are supported on the carbon, they exhibit greater sensing capability. The function of iridium is to facilitate the oxidation kinetics. It even decreases the adsorption of oxygenated intermediates. There are many other nanocomposites which have been prepared for this but it is the most commonly used nanocomposite (Chang et al., 2014). Pt/Au-GrCNTs based bimetallic nanocomposite has been successfully synthesized by co-reduction method. Prepared nanocomposite was used for the electrochemical sensing of propyl gallate (Cui et al., 2015).
7.2 Biomedical
Special nanoparticles have been prepared that helps in early diagnosis of disease in the human body. These can also be used for the determination of nutrient level in the body. Magnetic bimetallic nanoparticles or nanocomposites have been used for the controlled drug delivery. Controlled drug delivery systems have some specific goals such as maintaining biocompatibility during action, therapeutic processes and faster production. The release rate of these drugs depends upon the diffusion coefficient and biodegradation rate. Bimetallic nanocomposites offer an alternative to the traditionally used drug delivery system due to their high specificity. Magnetic bimetallic nanocomposites when injected inside the body reach their target site under the influence of external magnetic field and helps in eliminating infected cells (Pankhurst et al., 2003). Conjugation of specific proteins such that antibodies to the bimetallic nanoparticle surface have abundant potential for greater specificity and immunologically directed targeting.
7.3 Mechanical
These days’ bimetallic nanocomposites have been prepared which change their properties under the influence of external force. Such type of nanocomposites has anti-corrosion abilities and induces new properties. The mechanical properties of nanocomposites or nanoparticles depend upon the hardness, chemical composition, arrangement of atoms, size of particles, elastic modulus, friction and interfacial adhesion, etc. Bimetallic nanocomposites show different mechanical properties relative to the monometallic nanoparticles, providing an efficient method for surface modification for improving the quality of nanomanufacturing and nanofabrication, etc. Mechanical effects of bimetallic nanoparticles reinforce the composite coatings.
7.4 Electronic
The tiny size of BNPs offers them greater surface area which in turn provides them high conductivity. As a result, these are used in the production of electrical devices. These particles are easily available and are of the low cost, hence, are accessible to all. Due to high surface area, it helps in the production of super-capacitors. Some major advantages of bimetallic nanoparticles in electronic includes increase in density of memory chips, reduced weight, reduced thickness, improving display screens, minimizing the size of transistors used in integrated circuits and reduced power consumption, etc.
7.5 Magnetic
Nanoparticles have the potential of increasing the density of various storage media devices and when magnetized they can improve the detail and contrast of magnetic resonance imaging (MRI) images as previously alluded to. Iron based bimetallic nanoparticles can be used for improving MRI of cancerous cells. These nanoparticles are coated with peptide that specifically binds to the tumor cells. After their attachment, the iron based bimetallic nanoparticles helps in improving the MRI imaging. These are also used in capacitors for storing energy. Magnetic bimetallic nanocomposites containing iron oxide allows the nanoparticles to be directed towards the stents by the magnetic field. This allows the specific delivery of these drugs to the stents placed in arteries.
7.6 Dechlorination
BNPs of nickel and iron have been prepared which helps in the dechlorination of monochlorobenzene. These have been prepared by electroless plating method. In this method, the nanoparticles of nickel are deposited on the surface of iron by chemical reduction. The dechlorination capability of BNPs is high as compared to the individual nickel and iron NPs. Dechlorination by Ni(B)/Fe(B) takes place via pseudo-first order. Complete mechanism followed the dechlorination of MCB using Ni(B)/Fe(B) bimetallic nanoparticles have been presented in Fig. 5.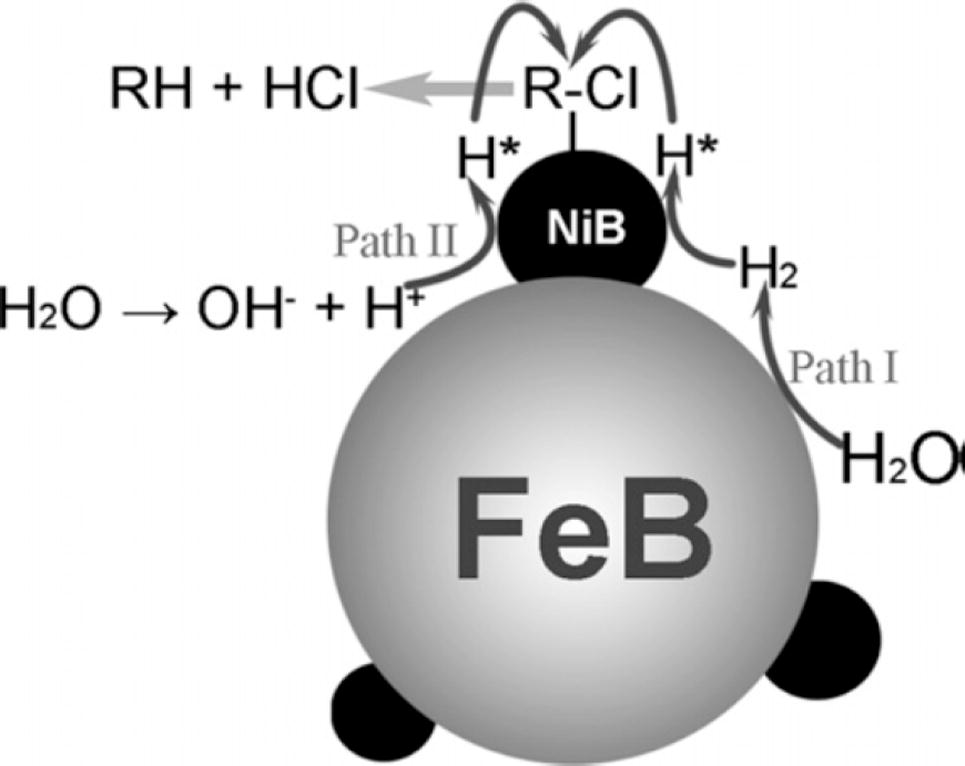
Proposed mechanism for the dechlorination of MCB using Ni(B)/Fe(B) nanoparticles (Han et al., 2008).
8 Conclusion
It is evident from the above discussion that bimetallic nanoparticles are the multifunctional nanomaterials with applications in different fields. The basic reason behind multifunctional behavior is the “synergistic effect” that exists between the two metals. Researchers are trying to synthesize more and more new BNPs with desired and controlled geometrical as well as magnetic properties. It can also be concluded that bimetallic nanoparticles are of greater importance as compared to monometallic nanoparticles because of enhanced properties. These particles have greater surface area, as a result of which they act as catalyst and helps in effectively catalyzing various reactions.
In order to increase the effectiveness and properties of BNPs, these are converted into nanocomposites which are formed by supporting them on the organic (carbon or graphene) or inorganic (kaolin or zeolites) counterparts. Brief discussion about bimetallic nanocomposites exhibits that these are more important than bimetallic and monometallic nanoparticles. Bimetallic nanocomposites acts as sensors and helps in the early diagnosis of the disease. It can even detect only 2–3 cancerous cells present in the body and is thus emerging as the most promising field in which more and more researchers are working.
Acknowledgements
The authors are thankful to the Shoolni University, Solan. This work was supported by the Deanship of Scientific Research, King Saud University for funding through Vice Deanship of Scientific Research Chairs.
References
- Antibacterial activity of graphene supported FeAg bimetallic nanocomposites. Colloids Surf. B Biointerfaces. 2016;143:490-498.
- [Google Scholar]
- Study on Ag–Pd bimetallic nanoparticles for electrocatalytic reduction of benzyl chloride. Electrochem. Commun.. 2011;13:1413-1416.
- [CrossRef] [Google Scholar]
- Antibacterial activity of Ag–Au alloy NPs and chemical sensor property of Au NPs synthesized by dextran. Carbohydr. Polym.. 2014;107:151-157.
- [CrossRef] [Google Scholar]
- Green chemistry approach for the synthesis of ZnO–carbon dots nanocomposites with good photocatalytic properties under visible light. J. Colloid. Interface Sci.. 2016;465:286-294.
- [Google Scholar]
- Ultrasound enhanced heterogeneous activation of peroxymonosulfate by a bimetallic Fe–Co/SBA-15 catalyst for the degradation of Orange II in water. J. Hazard. Mater.. 2015;283:70-79.
- [CrossRef] [Google Scholar]
- Bimetallic AuPt nanochains: synthesis and their application in electrochemical immunosensor for the detection of carcinoembryonic antigen. Biosens. Bioelectron.. 2013;39:226-230.
- [CrossRef] [Google Scholar]
- Hydrolytic dehydrogenation of ammonia borane and methylamine borane catalyzed by graphene supported Ru@Ni core–shell nanoparticles. Int. J. Hydrogen Energy. 2014;39:426-435.
- [CrossRef] [Google Scholar]
- Ni–Pt nanoparticles supported on MIL-101 as highly efficient catalysts for hydrogen generation from aqueous alkaline solution of hydrazine for chemical hydrogen storage. Int. J. Hydrogen Energy. 2014;39:9726-9734.
- [CrossRef] [Google Scholar]
- Bimetallic catalyst of PtIr nanoparticles with high electrocatalytic ability for hydrogen peroxide oxidation. Sens. Actuators B, Chem.. 2014;190:55-60.
- [CrossRef] [Google Scholar]
- Star-like PtCu nanoparticles supported on graphene with superior activity for methanol electro-oxidation. Electrochim. Acta 2015
- [CrossRef] [Google Scholar]
- Enhanced photocatalytic hydrogen evolution based on efficient electron transfer in triphenylamine- based dye functionalized Au@Pt bimetallic core/shell nanocomposite. Int. J. Hydrogen Energy. 2013;38:8631-8638.
- [CrossRef] [Google Scholar]
- Molecularly imprinted electrochemical sensor for propyl gallate based on PtAu bimetallic nanoparticles modified graphene – carbon nanotube composites. Biosens. Bioelectron.. 2015;68:563-569.
- [CrossRef] [Google Scholar]
- The effect of calcination temperature on structure and photocatalytic properties of Au/Pd nanoparticles supported on TiO2. Appl. Catal. B. 2014;153:202-211.
- [CrossRef] [Google Scholar]
- Pt–W bimetallic alloys as CO-tolerant PEMFC anode catalysts. Electrochim. Acta. 2013;89:744-748.
- [CrossRef] [Google Scholar]
- A facile synthesis of bimetallic AuPt nanoparticles as a new transparent counter electrode for quantum-dot-sensitized solar cells. J. Power Sources. 2015;274:831-838.
- [CrossRef] [Google Scholar]
- Bimetallic PtPd on zirconia catalysts for hydrotreating purposes. Appl. Catal. A Gen.. 2007;322:172-177.
- [CrossRef] [Google Scholar]
- Pd/Cu bimetallic nanoparticles supported on graphene nanosheets: facile synthesis and application as novel electrocatalyst for ethanol oxidation in alkaline media. Int. J. Hydrogen Energy. 2014;39:14669-14679.
- [CrossRef] [Google Scholar]
- Antibacterial effect of silver nanoparticles against Streptococcus mutans. Mater. Lett.. 2009;63:2603-2606.
- [CrossRef] [Google Scholar]
- Synthesis of core–shell Au@Pt nanoparticles supported on Vulcan XC-72 carbon and their electrocatalytic activities for methanol oxidation. Colloids Surf., A. 2012;406:6-12.
- [CrossRef] [Google Scholar]
- Ferrando, R., Jellinek, H., Johnston, R.L., 2008. Nanoalloys: from theory to applications of alloy clusters and nanoparticles.
- Synthesis and melting behaviour of Bi, Sn and Sn–Bi nanostructured alloy. J. Alloys Compd.. 2015;623:7-14.
- [CrossRef] [Google Scholar]
- Synthesis and biosensor application of Ag@Au bimetallic nanoparticles based on localized surface plasmon resonance. Appl. Surf. Sci.. 2014;301:230-234.
- [CrossRef] [Google Scholar]
- Innovative electrodes for direct methanol fuel cells based on carbon nanofibers and bimetallic PtAu nanocatalysts. Int. J. Hydrogen Energy. 2014;39:21601-21612.
- [Google Scholar]
- Novel self-assembled bimetallic structure of Bi/Fe0: the oxidative and reductive degradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) J. Hazard. Mater.. 2015;286:107-117.
- [CrossRef] [Google Scholar]
- Selective oxidation of ethanol to acetaldehyde by Au/Ir catalysts. J. Catal.. 2013;305:135-145.
- [CrossRef] [Google Scholar]
- A novel magnetic Fe@Au core–shell nanoparticles anchored graphene oxide recyclable nanocatalyst for the reduction of nitrophenol compounds. Water Res.. 2014;48:210-217.
- [CrossRef] [Google Scholar]
- Carbon-ceramic supported bimetallic Pt–Ni nanoparticles as an electrocatalyst for electrooxidation of methanol and ethanol in acidic media. Int. J. Hydrogen Energy. 2013;38:5425-5434.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and electrocatalytic activity of Co@Pt nanoparticles supported on carbon-ceramic substrate for fuel cell applications. Int. J. Hydrogen Energy. 2015;40:5115-5125.
- [CrossRef] [Google Scholar]
- Study of the electron transfer properties of nanostructured bimetallic films of polymerized porphyrins. Electrochim. Acta. 2012;78:302-307.
- [CrossRef] [Google Scholar]
- Bimetallic nickel–iron nanoparticles for groundwater decontamination: effect of groundwater constituents on surface deactivation. Water Res.. 2014;66:149-159.
- [CrossRef] [Google Scholar]
- Catalytic dechlorination of monochlorobenzene with a new type of nanoscale Ni (B)/Fe (B) bimetallic catalytic reductant. Chemosphere. 2008;72:53-58.
- [CrossRef] [Google Scholar]
- Copper- iron bimetal modifies PAN fiber complexes as novel heterogeneous Fenton catalyst for the degradation of organic dye under visible light irradiation. J. Hazard. Mater.. 2011;189:241-248.
- [Google Scholar]
- Monodisperse AuM (M = Pd, Rh, Pt) bimetallic nanocrystals for enhanced electrochemical detection of H2O2. Sens. Actuators B, Chem.. 2015;207:404-412.
- [CrossRef] [Google Scholar]
- Bimetallic Zn/Ag doped polyurethane spider net composite nanofibers: a novel multipurpose electrospun mat. Ceram. Int.. 2013;39:2503-2510.
- [CrossRef] [Google Scholar]
- Synthesis of bimetallic nanoparticles in ionic liquids: chemical routes vs physical vapor deposition. Microelectron. Eng.. 2013;107:229-232.
- [CrossRef] [Google Scholar]
- Bridging the materials gap between single crystal and supported catalysts using polycrystalline Ni/Pt bimetallic surfaces for cyclohexene hydrogenation. J. Catal.. 2011;280:96-103.
- [CrossRef] [Google Scholar]
- A simple approach for facile synthesis of Ag, anisotropic Au and bimetallic (Ag/Au) nanoparticles using cruciferous vegetable extracts. Mater. Sci. Eng. C. 2012;32:1827-1834.
- [CrossRef] [Google Scholar]
- Shape-controlled synthesis of gold–nickel bimetallic nanoparticles and their electrocatalytic properties. Mater. Chem. Phys.. 2015;156:1-8.
- [CrossRef] [Google Scholar]
- Nano bimetallic Ag-Pt system as efficient opto and electrochemical sensing platform towards adenine. Sens. Actuators B, Chem.. 2012;163:216-223.
- [Google Scholar]
- Electrochemical synthesized copper oxide nanoparticles for enhanced photocatalytic and antimicrobial activity. J. Ind. Eng. Chem.. 2015;31:173-184.
- [CrossRef] [Google Scholar]
- Dendrimer-stabilized bimetallic Pd/Au nanoparticles: preparation, characterization and application to vinyl acetate synthesis. Catal. Commun.. 2014;57:78-82.
- [CrossRef] [Google Scholar]
- Polyacrylamide/Ni 0. 02 Zn 0. 98 O Nanocomposite with High Solar Light Photocatalytic Activity and Efficient Adsorption Capacity for Toxic Dye Removal. Ind. Eng. Chem. Res.. 2014;53:15549-15560.
- [Google Scholar]
- SPION/β-cyclodextrin core-shell nanostructures for oil spill remediation and organic pollutant removal from waste water. Chem. Eng. J.. 2015;280:175-187.
- [CrossRef] [Google Scholar]
- Magnetically recoverable ZrO2/Fe3O4/chitosan nanomaterials for enhanced sunlight driven photoreduction of carcinogenic Cr(VI) and dechlorination & mineralization of 4-chlorophenol from simulated waste water. RSC Adv.. 2016;6:13251-13263.
- [CrossRef] [Google Scholar]
- Green synthesis and applications of Au–Ag bimetallic nanoparticles. Spectrochim. Acta, Part A. 2015;137:185-192.
- [CrossRef] [Google Scholar]
- Biosensors and Bioelectronics Preparation and characterization of three dimensional graphene foam supported platinum – ruthenium bimetallic nanocatalysts for hydrogen peroxide based electrochemical biosensors. Biosens. Bioelectron.. 2014;52:1-7.
- [CrossRef] [Google Scholar]
- Lam, F.L.Y., Hu, X., 2013. pH-Insensitive Bimetallic Catalyst for the Abatement of Dye Pollutants by Photo-Fenton Oxidation.
- Facile preparation of Pd–Au bimetallic nanoparticles via in-situ self-assembly in reverse microemulsion and their electrocatalytic properties. Colloids Surf., A. 2014;463:55-62.
- [CrossRef] [Google Scholar]
- Degradation of scarlet 4BS in aqueous solution using bimetallic Fe/Ni nanoparticles. J. Colloid Interface Sci.. 2012;381:30-35.
- [CrossRef] [Google Scholar]
- Application of a Re–Pd bimetallic catalyst for treatment of perchlorate in waste ion-exchange regenerant brine. Water Res.. 2013;47:91-101.
- [CrossRef] [Google Scholar]
- Remediation of Direct Black G in wastewater using kaolin-supported bimetallic Fe/Ni nanoparticles. Chem. Eng. J.. 2013;223:764-771.
- [CrossRef] [Google Scholar]
- Bi-metal Cu–Co from LaCo1−xCuxO3 perovskite supported on zirconia for the synthesis of higher alcohols. Fuel Process. Technol.. 2014;128:289-296.
- [CrossRef] [Google Scholar]
- Ni–Mo and Co–Mo alloy nanoparticles for catalytic chemical vapor deposition synthesis of carbon nanotubes. J. Alloys Compd.. 2015;621:351-356.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of Pt–Pd alloy and core-shell bimetallic nanoparticles for direct methanol fuel cells (DMFCs): enhanced electrocatalytic properties of well-shaped core-shell morphologies and nanostructures. Int. J. Hydrogen Energy. 2011;36:8478-8491.
- [CrossRef] [Google Scholar]
- Al2O3-supported Cu–Co bimetallic catalysts prepared with polyol process for removal of BTEX and PAH in the incineration flue gas. Fuel. 2009;88:340-347.
- [CrossRef] [Google Scholar]
- Synthesis of PtAu bimetallic nanoparticles on graphene – carbon nanotube hybrid nanomaterials for nonenzymatic hydrogen peroxide sensor. Talanta. 2013;112:111-116.
- [CrossRef] [Google Scholar]
- Improved debromination of polybrominated diphenyl ethers by bimetallic iron–silver nanoparticles coupled with microwave energy. Sci. Total Environ.. 2012;429:300-308.
- [CrossRef] [Google Scholar]
- Fabrication of MWCNTs/ThO2 nanocomposite and its adsorption behavior for the removal of Pb(II) metal from aqueous medium. Desalin. Water Treat.. 2016;57
- [CrossRef] [Google Scholar]
- Biogenic synthesis of Ag, Au and bimetallic Au/Ag alloy nanoparticles using aqueous extract of mahogany (Swietenia mahogani JACQ) leaves. Colloids Surf., B. 2011;82:497-504.
- [CrossRef] [Google Scholar]
- High performance of NiCo nanoparticles-doped carbon nano fi bers as counter electrode for dye-sensitized solar cells. Electrochim. Acta. 2015;160:1-6.
- [CrossRef] [Google Scholar]
- Improved catalysis of room temperature synthesized Pd-Cu alloy nanoparticles for anodic oxidation of ethanol in alkaline media. Electrochim. Acta. 2015;154:447-455.
- [CrossRef] [Google Scholar]
- Study of Ag-Pd bimetallic nanoparticles modified glassy carbon electrode for detection of L-cysteine. Superlattices Microstruct.. 2014;75:916-926.
- [CrossRef] [Google Scholar]
- Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomedicine Nanotechnology. Biol. Med.. 2009;5:452-456.
- [CrossRef] [Google Scholar]
- Journal of Industrial and Engineering Chemistry Synthesis of Au/Pd bimetallic nanoparticles and their application in the Suzuki coupling reaction. J. Ind. Eng. Chem.. 2015;21:746-748.
- [CrossRef] [Google Scholar]
- Sonochemically synthesized mono and bimetallic Au–Ag reduced graphene oxide based nanocomposites with enhanced catalytic activity. Ultrason. Sonochem.. 2014;21:1948-1953.
- [CrossRef] [Google Scholar]
- Cation exchange resin immobilized bimetallic nickel–iron nanoparticles to facilitate their application in pollutants degradation. J. Colloid Interface Sci.. 2014;420:158-165.
- [CrossRef] [Google Scholar]
- Properties of Pd/Au and Pt/Cu alloy surface for the adsorption and catalytic reduction of O2 and NO by H-2. Colloids Surf., A. 1993;80:121-126.
- [Google Scholar]
- Learn NOx reduction in real diesel exhaust with copper and titaniabased monolithic catalys. Appl. Catal. A Gen.. 1999;23:37-44.
- [Google Scholar]
- Applications of magnetic nanoparticles in biomedicine. J. Phys. D. Appl. Phys.. 2003;36:R167.
- [Google Scholar]
- Pectin@zirconium (IV) silicophosphate nanocomposite ion exchanger: photo catalysis, heavy metal separation and antibacterial activity. Chem. Eng. J.. 2015;267:235-244.
- [CrossRef] [Google Scholar]
- Synthesis of trimetallic Au@Pb@Pt coreshell nanoparticles and their electrocatalytic activity toward formic acid and methanol. Bull. Korean Chem. Soc.. 2009;30:1485-1488.
- [Google Scholar]
- Reliability of hydrogen sensing based on bimetallic Ni–Pd/graphene composites. Int. J. Hydrogen Energy. 2014;39:20294-20304.
- [CrossRef] [Google Scholar]
- Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv.. 2009;27:76-83.
- [CrossRef] [Google Scholar]
- Nature and catalytic activity of bimetallic CuNi particles on CeO 2 support. Catal. Today. 2012;198:140-147.
- [CrossRef] [Google Scholar]
- Photodegradation of Orange II under visible light using Cu–Ni/TiO2: effect of calcination temperature. Chem. Eng. J.. 2012;185–186:108-119.
- [CrossRef] [Google Scholar]
- The graphene-supported palladium and palladium – yttrium nanoparticles for the oxygen reduction and ethanol oxidation reactions: experimental measurement and computational validation. Appl. Catal. B. 2013;129:163-171.
- [CrossRef] [Google Scholar]
- Hydroreforming of the oils from LDPE thermal cracking over Ni–Ru and Ru supported over hierarchical Beta zeolite. Fuel. 2015;144:287-294.
- [CrossRef] [Google Scholar]
- The colloidal synthesis of unsupported nickel - tin bimetallic nanoparticles with tunable composition that have high activity for the reduction of nitroarenes. Catal. Commun.. 2015;65:85-90.
- [CrossRef] [Google Scholar]
- Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci.. 2004;275:496-502.
- [CrossRef] [Google Scholar]
- Lanthanum/Cadmium/Polyaniline bimetallic nanocomposite for the photodegradation of organic pollutant. Iran. Polym. J. (English Ed.). 2015;24:1003-1013.
- [CrossRef] [Google Scholar]
- Polyacrylamide@Zr (IV) vanadophosphate nanocomposite: ion exchange properties, antibacterial activity, and photocatalytic behavior. J. Ind. Eng. Chem.. 2016;33:201-208.
- [Google Scholar]
- Fabrication and characterization of Fe@MoPO nanoparticles: ion exchange behavior and photocatalytic activity against malachite green. J. Mol. Liq.. 2016;219:1137-1143.
- [CrossRef] [Google Scholar]
- Fabrication and characterization of chitosan-crosslinked-poly(alginic acid) nanohydrogel for adsorptive removal of Cr(VI) metal ion from aqueous medium. Int. J. Biol. Macromol.. 2017;95:484-493. doi.org/10.1016/j.ijbiomac.2016.11.072
- [Google Scholar]
- Fabrication and characterization of a nanocomposite hydrogel for combined photocatalytic degradation of a mixture of malachite green and fast green dye. Nanotechnol. Environ. Eng.. 2017;2:4.
- [CrossRef] [Google Scholar]
- Revolution from monometallic to trimetallic nanoparticle composites, various synthesis methods and their applications: a review. Mater. Sci. Eng. C. 2017;71:1216-1230.
- [Google Scholar]
- Dechlorination of hexachlorobenzene by using nanoscale Fe and nanoscale Pd/Fe bimetallic particles. Colloids Surf., A. 2009;332:84-89.
- [CrossRef] [Google Scholar]
- Highly-dispersed surfactant-free bimetallic Ni–Pt nanoparticles as high-performance catalyst for hydrogen generation from hydrous hydrazine. Int. J. Hydrogen Energy. 2014;39:9128-9134.
- [CrossRef] [Google Scholar]
- Synthesis of bimetallic Pt–Cu nanoparticles and their application in the reduction of rhodamine B. Colloids Surf., A. 2013;416:43-50.
- [CrossRef] [Google Scholar]
- Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci.. 2004;275:177-182.
- [CrossRef] [Google Scholar]
- Bimetallic Ag–Ni/C particles as cathode catalyst in AFCs (alkaline fuel cells) Energy. 2014;70:223-230.
- [CrossRef] [Google Scholar]
- Rotating disc electrode studies of borohydride oxidation at Pt and bimetallic Pt–Ni and Pt–Co electrodes. Catal. Today. 2011;170:126-133.
- [CrossRef] [Google Scholar]
- Sapota fruit latex mediated synthesis of Ag, Cu mono and bimetallic nanoparticles and their in vitro toxicity studies. Arab. J. Chem. 2015
- [CrossRef] [Google Scholar]
- Electrodeposited Pt on three-dimensional interconnected graphene as a free-standing electrode for fuel cell application. J. Mater. Chem.. 2012;1–6
- [CrossRef] [Google Scholar]
- Preparation and catalysis of novel colloidal dispersions of copper/noble metal bimetallic clusters. Langmuir. 1994;10:4574-4580.
- [Google Scholar]
- Bimetallic nanoparticles:- noval materials for physical and chemical applications. New J. Chem.. 1998;11:1179-1201.
- [Google Scholar]
- Core-shell bimetallic Au-Pd nanoparticles: synthesis, structure, optical and catalytic properties. Nanosci. Nanotechnol.. 2011;1:43-47.
- [Google Scholar]
- Dechlorination of chlorinated methanes by Pd/Fe bimetallic nanoparticles. J. Hazard. Mater.. 2009;161:815-823.
- [CrossRef] [Google Scholar]
- Synthesis and electrocatalytic alcohol oxidation performance of Pd–Co bimetallic nanoparticles supported on graphene. Int. J. Hydrogen Energy. 2014;39:1325-1335.
- [CrossRef] [Google Scholar]
- Iron-copper bimetallic nanoparticles embedded within ordered mesoporous carbon as effective and stable heterogeneous Fenton catalyst for the degradation of organic contaminants. Appl. Catal. B. 2015;164:396-406.
- [CrossRef] [Google Scholar]
- Synthesis of bimetallic Pd–Ag colloids in CO2-expanded hexane and their application in partial hydrogenation of phenylacetylene. J. Supercrit. Fluids. 2013;81:1-6.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of Pt–Cu bimetallic alloy nanoparticles by reverse micelles method. Colloids Surf., A. 2006;273:35-42.
- [CrossRef] [Google Scholar]
- Chitosan stabilized bimetallic Fe/Ni nanoparticles used to remove mixed contaminants-amoxicillin and Cd(II) from aqueous solutions. Chem. Eng. J.. 2013;229:27-34.
- [CrossRef] [Google Scholar]
- A one-step facile synthesis of Ag–Ni core–shell nanoparticles in water-in-oil microemulsions. Colloids Surf., A. 2010;367:96-101.
- [CrossRef] [Google Scholar]
- A highly active bimetallic oxides catalyst supported on Al- containing MCM-41 for Fenton oxidation of phenol solution. Appl. Catal. B. 2011;110:118-125.
- [Google Scholar]
- Synthesis and characterization of Ag–Ni bimetallic nanoparticles by laser-induced plasma. Thin Solid Films. 2011;519:7116-7119.
- [CrossRef] [Google Scholar]
- Unsupported CuFe bimetallic nanoparticles for higher alcohol synthesis via syngas. Catal. Commun.. 2013;40:154-157.
- [CrossRef] [Google Scholar]
- Remediation of polybrominated diphenyl ethers in soil using Ni/Fe bimetallic nanoparticles: in fl uencing factors, kinetics and mechanism. Sci. Total Environ.. 2014;485–486:363-370.
- [CrossRef] [Google Scholar]
- CuAu–ZnO – graphene nanocomposite: a novel graphene-based bimetallic alloy-semiconductor catalyst with its enhanced photocatalytic degradation performance. J. Alloys Compd.. 2015;636:40-47.
- [CrossRef] [Google Scholar]
- Novel bimetallic catalyst for the photo-assisted degradation of Acid Black 1 over a broad range of pH. Chem. Eng. Sci.. 2007;62:5150-5153.
- [CrossRef] [Google Scholar]
- Seed-assisted synthesis of dendritic Au – Ag bimetallic nanoparticles with chemiluminescence activity and their application in glucose detection. Sens. Actuators B, Chem.. 2015;209:877-882.
- [CrossRef] [Google Scholar]
- Treatment of chlorinated organic contaminants with nanoscale bimetallic particles. Catal. Today. 1998;40:387-395.
- [Google Scholar]
- Thermosensitive and pH-sensitive Au–Pd bimetallic nanocomposites. J. Colloid Interface Sci.. 2009;331:104-112.
- [CrossRef] [Google Scholar]
- Capping agent free synthesis of PtSn bimetallic nanoparticles with enhanced electrocatalytic activity and lifetime over methanol oxidation. Catal. Commun.. 2008;9:624-629.
- [CrossRef] [Google Scholar]
- Supported PtAu catalysts with different nano-structures for ethanol electrooxidation. Electrochim. Acta. 2014;123:233-239.
- [CrossRef] [Google Scholar]







