Translate this page into:
Novel 3′-diindolylmethane nanoformulation induces apoptosis, and reduces migration and angiogenesis in liver cancer cells
⁎Corresponding authors at: King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia (S. Harakeh). sharakeh@gmail.com (Steve Harakeh), shaker.mousa@acphs.edu (Shaker Mousa)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Liver cancer (LC) ranks as the second most prevalent cause of cancer-related deaths. Herbaceous plants are valuable sources of complementary, adjuvant, or alternative anti-tumor therapy as they contain natural active ingredients with anti-cancer potential. Although the clinical use of 3, 3′-Diindolylmethane (DIM) has been established, its low chemical stability and bioavailability, limits its therapeutic applications. Increasing effort has been undertaken to improve DIM’s biological activity including nanoformulations. Here, we evaluated the efficacy of DIM nanoparticles (DIM-NPs) coated with PEG/chitosan for the treatment of liver cancer and elucidated the underlying molecular mechanisms contributing to its anti-tumor activity. DIM-PLGA-PEG/chitosan NPs were synthesized and characterized using dynamic light scattering (DLS). The effect of newly synthesized DIM-NPs was evaluated in HepG-2 and HUH-7 hepatocarcinoma cells and compared to THLE-2 immortal normal liver cells and WI-38 (normal lung fibroblast cells). These cells were treated with different non-cytotoxic concentrations of DIM-NPs and MTT assay and other functional assays were performed. Compared to normal cells, DIM-NPs induced cytotoxicity in HepG-2 cells at 6.25 µg/mL after 48 h of treatment. Treatment of HepG-2 cells with the 50 % inhibitory concentration (IC50) 12.5 µg/mL of DIM-NPs inhibited cell migration (p < 0.001). Treatment of chicken embryo with 5ug/ml DIM-NPs reduced (p < 0.001) angiogenesis at day 4. Notably, at the molecular level, DIM-NPs upregulated Bax and p53 and downregulated Bcl-2 in a dose-dependent manner. DIM-NPs also induced cell apoptosis in HepG-2 cells. Treatment of hepatic cells with DIM-NPs decreased cell proliferation, migration and angiogenesis, and induced cell death via up-regulation of Bax and p53, and down-regulation of Bcl-2 in HepG-2 cells. Further investigations are necessitated to determine the pharmacokinetics of DIM-NPs using a preclinical cancer model.
Keywords
3, 3′-Diindolylmethane (DIM) nanoparticles
Hepatic cancer cells
Migration
Angiogenesis
Apoptosis
1 Introduction
Cancer has a major impact on the public health worldwide. In 2017, data from the USA alone revealed that around one million and seven hundred new cases of cancer are diagnosed yearly, with projected deaths of about 600,000 (Rebecca et al., 2017). One of these cancers is liver cancer (LC) which has been on the rise since the 1980 s and has been tripled since. Currently, LC is reported to be three-fold more prominent in men compared to women (Alqahtani et al., 2019).LC ranks as the second most prevalent aetiology of cancer-related deaths, more especially hepatocellular carcinoma (HCC), and its prevalence is rising worldwide (Llovet et al., 2022, Rumgay et al., 2022). The Cancer Society in America reported 42,030 new cases of LC in 2019 (29,480 in males and 12,550 in females) resulting in the demise of 31,780 individuals (21,600 men and 10,180 women). Remarkably, this has been predicted to rise higher in the coming years (Alqahtani et al., 2019). In Saudi Arabia, liver cancer, (LC) is ranked as the third most common malignancy in men, and the seventh in women totalling over 800,000 additional cases per annum and resulting in more than 700,000 deaths per year (Alqahtani et al., 2019). HCC is a significant health concern in Saudi Arabia. It is one of the most common types of cancer in the country, with a relatively high incidence rate. Several factors contribute to the prevalence of liver cancer in Saudi Arabia, including viral hepatitis infections, particularly hepatitis B and C, which are known to be major risk factors for the development of HCC. In contrast to primary LC which starts from the liver itself, secondary LC originates from other bodily parts like lungs, ovaries, breasts, colorectal area, pancreas, and reaches the liver through metastases (Shen et al., 2012). In most cases, patients are presented with advanced tumor stages where oncologists have limited treatment options to offer to patients, specifically when the tumor starts to metastasize (Spano et al., 2012, Yamashita and Kaneko 2016, Burton et al., 2021). Although tremendous efforts have been taken toward the successful treatment of primary tumors, this success is challenged by off-site targets, the development of aggressive tumors, drug resistance, and relapse (Anderson, 2019). Consequently, more studies are necessitated to uncover the potency of DIM (3, 3′-Diindolylmethane) anti-cancer agents and/or develop novel targeted therapies against aggressive forms of cancer cells. One of these DIM efforts to treat patients with aggressive tumors is to explore the efficacy of herbal-based nanoformulations.
The use of nanotechnology in medicine to treat cancer is due to the potential that the nanoparticles have. Such features are targeted drug delivery, vaccine development, and imaging and diagnosis (Gowda et al., 2013, Basha et al., 2014, Baetke et al., 2015).
As a targeted drug delivery system, nanoparticles deliver the drug to the specific site where the disease is manifested. As such, smaller amounts of the drugs are needed, and this will result in fewer toxicities and associated side effects. Nanoparticles also have the ability to trigger the therapeutic agent to be released. The ability to carry multiple therapeutic payloads by the same nanoparticle system is one of the unique properties of the nanomedicine approach of delivering drugs which will result in the reduction of resistance (Wang et al., 2011). Nanoparticles can be used as cross-carriers for drugs in order for a drug to cross the blood–brain barrier as an example (Yemisci et al., 2012), and can be used to enhance imaging and for better diagnosis of cancer (Chapman et al., 2013).
Nanoformulations are currently in use to enhance the solubility, stability and increase the shelf life of the therapeutic drugs (Narvekar et al., 2014). Also, they help with the circulations of the drugs (Yoo et al., 2010). One of such novel nanoformulations is DIM. Notably, cruciferous plants like broccoli, cauliflower, and cabbage are rich in DIM. DIM is the main acid product resulting from the condensation of indole-3-carbinol (I3C) (Kristal and Lampe 2002). The acidity of the stomach causes the conversion of the I3C to DIM in the stomach (Brandi et al., 2013). However, adverse effects may occur when DIM is administered at higher concentrations. It was reported that 10 µM concentration induced tumor cell proliferation rather than inhibition through the activation of estrogen receptor α signaling pathway, in the absence of estradiol (Marques et al., 2014). Therefore, there is a risk of using high concentrations of DIM as dietary supplement. As such, further studies are warranted to investigate the inhibition of HCC by DIM encapsulated using nanoparticles.
NPs prepared from FDA-approved biodegradable Poly-Lactic-co-Glycolic-Acid (PLGA) and loaded with different therapeutic agents have been extensively evaluated for their anti-tumor activities in a wide variety of malignancies including liver cancer (Verderio et al., 2013, Bian and Guo 2020). The major limitation of using PLGA-formulated NPs is the rapid clearance of NPs-loaded therapy from the blood by the process of phagocytosis. Thus, polymers with more hydrophilic properties acting as surface coating materials such polyethylene glycol (PEG) and chitosan have been used to reduce the phagocytic effect of PLGA (Hu et al., 2008). Towards this goal, NPs coated with PEG and chitosan and loaded with DIM improved the drug release by increasing the retention time of the loaded drug, and hence increasing its therapeutic efficacy (Parveen and Sahoo 2011). Therefore, this study is centred on using a multi-modal approach to evaluate the potential of DIM-NPs coated with PEG/chitosan to elicit an improved anti-tumor effect in liver cancer. We used two cell lines; HepG-2 and immortal normal liver cells HUH-7 to determine both the anti-tumor activity of DIM-NPs against liver cancer cells and the toxicity against normal cells. The result revealed that the use of DIM-NPs to treat cancer cells inhibited cell proliferation, migration, and angiogenesis. At the molecular level, DIM-NPs upregulated Bax and p53 and downregulated Bcl-2 on mRNA level and inhibits ERK1/2 activity on protein level. By utilizing Ehrlich solid carcinoma-induced mouse model, DIM-NPs reduced the tumor effect on hepatic tissues as reflected by improving histological features compared to mice-engrafted Ehrlich solid carcinoma cells.
2 Materials and methods
2.1 Diindolylmethane-nanoparticles-nanoparticles preparations
Diindolylmethane-nanoparticles (DIM-NPs) were synthesized according to the method previously described (Anand et al., 2010). Briefly, PLGA-PEG/DSPE-PEG was used in a 99:1 ratio along with polyvinyl alcohol (PVA) and 0.05 % chitosan was used as the main component of the nanoformulation. The size of NPs was characterized by dynamic light scattering (DLS). The concentration of encapsulated DIM was assayed by UV–Vis spectroscopy at λ440 nm according to a prior report (Anand et al., 2010). Chitosan served as mucoadhesive for long-residence time on the skin layers, vitamin E/Lycopene/Omega 3 fatty acids severs + hyaluronic acid (each at 1–5 mg/ 100–500 mg DIM) as skin protective and skin regeneration bioactive ingredients.
2.2 Cell lines
THLE-2, WI-38, HepG-2, and HUH-7 cell lines were obtained from the American Tissue Culture Collection (ATCC) and maintained under appropriate standard conditions. All cell lines were incubated in RPMI-1640 medium supplemented with 1.0 % penicillin/streptomycin and 10.0 % inactivated Foetal Bovine Serum (FBS) and maintained in a 5% CO2 humidified environment.
2.3 Cell viability
Cell cytotoxicity was evaluated using the MTT assay as previously reported (Kumari, Sharma et al. 2021). Briefly, cells (5x103/well) were seeded into 96-well plates and treated with different concentrations (0––200 µg/ml) of DIM NPs for 24 and 48 h time points. Tetrazolium salt 10-μl (5 mg/ml) were added to each well and incubated at 37 °C for 3–––4 h and the absorbance was read at 570 nM using an ELISA microplate reader (Molecular Devices, Downingtown, PA, USA). For each cell line, the 50% inhibition concentration (IC50) of DIM NPs was calculated according to Reed-Muench method (Saganuwan 2011).
2.4 Cell apoptosis by flowcytometry
Apoptotic and necrotic cell death was confirmed using propidium Iodide and annexin-V method. Briefly, cells were plated in 10 cm3 dishes and treated with effective doses (0, 6.25, 12.5, and 25 mg/mL) of DIM NPs. After 24 h, cells were harvested, and the resulting pellets were fixed in ice-cold 70% ethanol. Those cells were centrifuged, washed, and re-suspended in PBS solution containing RNase A (1 mg/ml), and incubated for 30 min at 37 °C. Annexin-V/propidium iodide (PI) solution at 1.0 mg/mL concentration was added. Analysis of the stained cells was performed using a fluorescence-activated cell sorter (FACS) following the manufacturer’s instructions.
2.5 Gene expression analysis
Cells were trypsinized and collected on ice. Total RNA was extracted using RNeasy kit (Qiagen; Germantown, MD). cDNA was synthesized using M−MuLV RT and random primer mix (New England Biolabs, Ipswich, MA). Quantitative Real-Time PCR (qPCR) was performed using SYBR Green master-mix (Bio-Rad, Hercules, CA). The fold change calculated regarding GAPDH. The sequence of primers were designed using primer-BLAST NCBI tool, for Bax was F: 5′‐CCCGAGAGGGTCTTTTTCCGAG‐3′ and R: 5′‐CCAGCCCATGATGGTTCTGAT‐3′, Bcl-2F: 5′- TCAGAGCTTTGAGCAGGTAG‐3′ and R: 5′‐AAGGGCTCTAGGTCATTC‐3′, Tp53 F: 5′- TGACTGTACCACCATCCACTA‐3′ and R: 5′‐AAACACGCACCTCAAAGC‐3′, and GAPDH F: 5′-TGTCCGTCGTGGATCTGAC‐3′ and R: 5′‐ CCTGCTTCACCACCTTCTTG −3′.
2.6 Wound healing evaluation
The effect of DIM NPs on LC cells migration was evluated by the wound-healing assay as previously described (Gaballa et al., 2020). In brief, wound was created by making a scratch per each well of 6-well plate when cells reached 80–90% confluence using a pipette tip (10 ul). Cells were gently washed with PBS and treated with 0, 6.25, 12.5, and 25 µg/ml of DIM NPs. Images were acquired from at least 5 areas and then analyzed by ImageJ software (NIH, Bethesda, MD, USA).
2.7 Blood vessel formation using a chick embryo model
The effects of DIM NPs on blood vessel formation was assessed using the chick embryo model (Lokman et al., 2012). Briefly, Gallus domesticus fertilized eggs were used. All eggs were incubated under appropriate conditions using an electrical thermostatically controlled incubator. Eggs trays and incubator were well cleaned. Fertile eggs were labelled and placed in the trays and placed vertically inside the incubator for 2, 3, and 4 days after treatment with DIM NPs in addition to PBS as a control group. A small window of about 1 cm2 was made in the eggshell. DIM NPs were placed, and the windows were sealed with tape to the end of the experiment (Lokman et al., 2012). By the end of each time point, images were acquired. According to our institutional ethics, no ethical approval was required in the chick embryos up to day 14 of incubation.
2.8 Animals and treatment
Twenty adult male albino mice with ages between six and eight weeks old, and body weights of between 20 and 26 g were reared and habituated for two weeks at the animal facility and provided with ad libitum supply of feed and water. Before initiating preclinical mouse model, the experimental protocol received official approval from the Ethical Committee at KAU University. The induction of Ehrlich solid carcinoma (ESC) was carried out in mice using Ehrlich Ascites Carcinoma (EAC) cells bought from the National Cancer Institute (NCI) from Cairo, Egypt. ESC cells’ viability was evaluated by trypan blue exclusion test. About 2.5 × 106 viable EAC cells were resuspended in 0.2 ml PBS/animal and administered intramuscularly into the thigh of each animal to induce solid tumour formation following the standard protocol (Aldubayan et al., 2019). Tumour growth was reported in 100% of the mice, with a palpable solid tumour mass within 10 days of implantation. The mice bearing tumors were randomly grouped into 3 cohorts; 5 mice each, and in addition, one group of mice with no tumour which served as a controls. The tumour groups were designed as follows; tumour group, tumour + cisplatin (3.5 mg/kg), and tumour + cisplatin + DIM-NPs (3 mg/kg). The selected doses of cisplatin and DIM-NPs were intraperitoneally administered on a daily base for 2 weeks as previously reported (El-Shitany et al., 2019). The tumour group was injected with phosphate buffer saline (PBS) solution and kept as a second control.
2.9 Immunohistochemistry of Cas-3
Immunohistochemistry (IHC) allows us the confirmation of the expression of Cas-3 as apoptotic markers in tumor treated liver. Immunohistochemical staining was performed using the peroxidase-labeled streptavidin–biotin technique (Makhlouf et al., 2014). Anti-caspase-3 antibody (Santa Cruz Biotechnology) was used to investigate apoptosis, and diluted to 1:1,000 using PBS. A light microscope connected to a digital camera (Olympus, BX-61, Los Angeles, CA) was used to examine all the slides.
2.10 Statistical analysis
Data were represented as mean ± standard error of the mean (SEM), and statistically evaluated by two-way ANOVA, followed by Dunnett’s post-hoc multiple comparison test to evaluate differences with control counterparts. Correlations between the protein expression in mice tumor tissues with and without NP treatments was analyzed with a two-tailed Spearman rank correlation (Rho) using GraphPad Prism 6.0 (Intuitive Software for Science, San Diego, CA, USA). The value of p < 0.05 was considered statistically significant.
3 Results
3.1 The effect of different concentrations of DIM-nanoparticles on cell viability
To determine the effect of DIM-NPs on inducing cell toxicity, cell toxicity assay was performed on liver cancer cell lines HepG-2 and HUH-7 compare to normal liver cells. Cells were incubated with different concentrations of DIM-NPs for 24 and 48 hrs. Initially, treatment of cancer cells with DIM-NPs showed an early cytotoxic effect after 24 h and reached the maximum effect after 48 h as shown in Fig. 1. The IC50 of HUH-7 and HepG 2 at 24hr was 51.69 and 17.27 µg/mL, respectively compared to normal HepG 2 (IC50 = 93.74 µg/mL) and WI-38 (IC50 = 335.4 µg/mL) cells.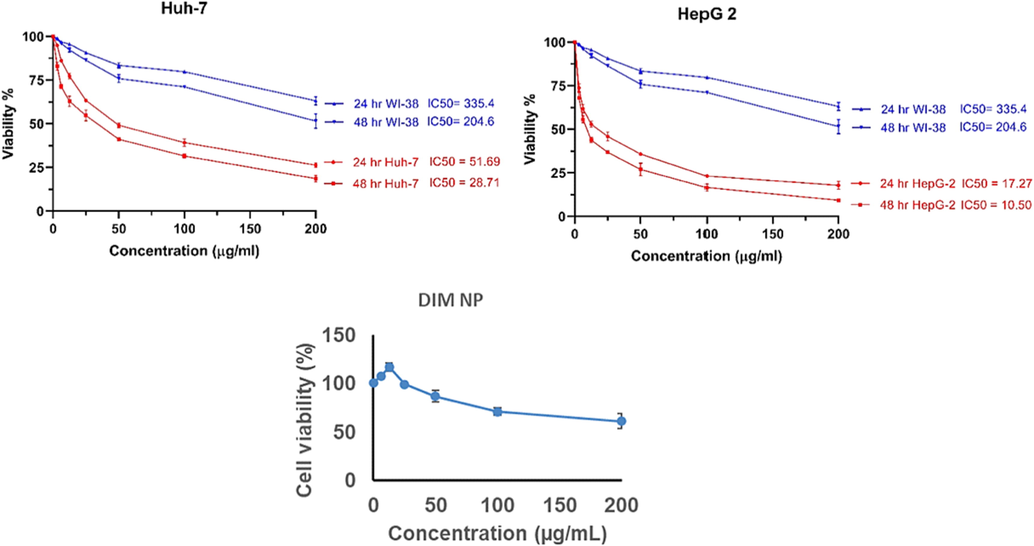
The effects of varied concentrations of DIM nanoparticles on viability of liver cancer HepG-2 cell and immortal normal liver cells HUH-7 cell and were compared to control WI-38 cells. Cells were incubated with the specified concentrations of the DIM nanoparticles for 24 and for 48 h, respectively and the proliferation of cells was evaluated using MTT assay.
3.2 The effect of DIM-nanoparticles on the cell migration using wound healing assay
Treatment of MDA-MB-231 for 48 h with DIM-NPs (2.5, 5 and 10 µg/mL) significantly suppressed cell migration in comparison to the controls (Fig. 2). The suppression of migratory cells was shown at low concentration of DIM-NPs at 2.5 µg/mL and reached the maximum inhibition at 10 µg/mL after treatment for 48hrs (p < 0.0001) for the tested cells.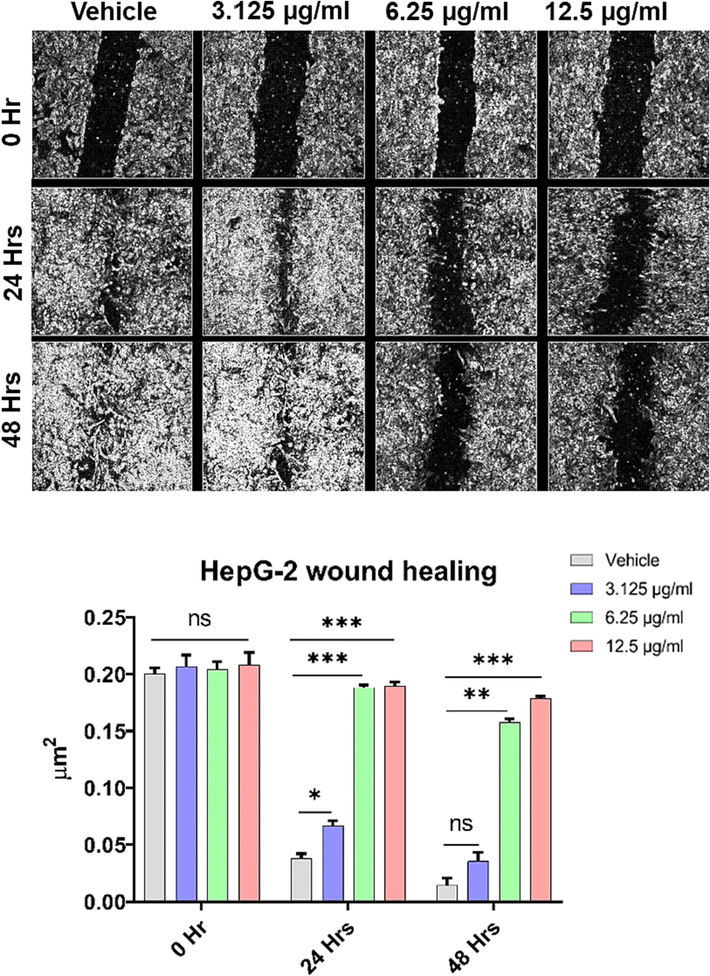
Effects of DIM nanoparticles on cell-migration using wound healing assay. MDA-MB-23 cells were treated with DIM-NPs for 24, and 48 hrs. A converted microscope was used to visualise the Wounds while ImageJ software was used to analyze the images. Data was considered significant at p < 0.05 regarding control non-treated cells and treatment after 24 and 48 hrs. All the data was expressed as mean ± SEM from three separate experiments and analysed with two-way ANOVA, values of ***P ≤ 0.001, and ****P ≤ 0.0001 were considered statistically significant.
3.3 Effects of DIM-nanoparticles on formation of blood vessels in chick embryos
The effects of DIM-NP on the formation of blood vessels using chick embryos was examined over the course of 4 days using one medium concentration of 5 µg/mL. The results indicate that treatment of fertilized eggs with DIM-NPs inhibited the blood vessel formation after 4 days of DIM-NPs application as compared to control embryos (Fig. 3).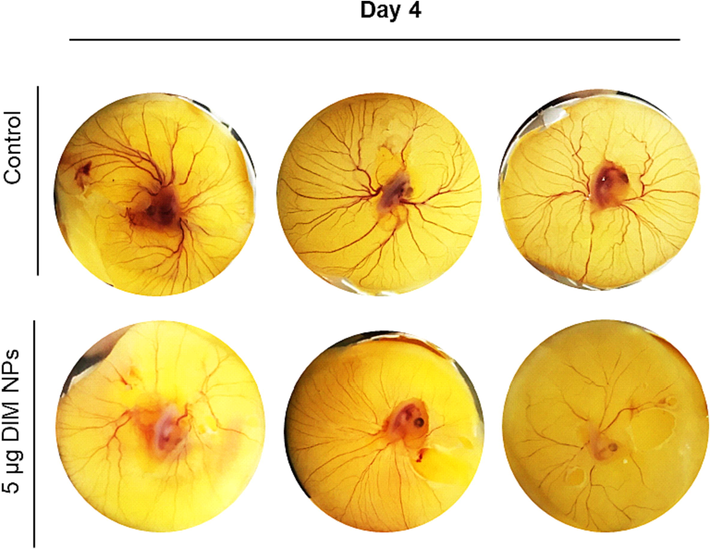
Effects of DIM nanoparticles on the formation of blood vessels using chick embryos. Fertile eggs were treated with and without DIM-NPs and placed vertically in the trays inside the incubator for 4 days.
3.4 Effect of DIM-nanoparticles on inducing cell apoptosis
The effect of treatment of DIM-NPs on apoptosis-related genes expression in liver cancer cell lines; HepG-2 cell, and immortal normal liver cells HUH-7 cell were evaluated by quantitative Real-Time PCR analysis. As revealed in Fig. 4, DIM-NPs induced a significant upregulation of Bax and p53 after 48 h of treatment (P < 0.001). The upregulation of these two genes was concentration dependent. At 25 µg/mL concentration of DIM-NPs, the upregulated genes were increase up to 6-folds in MCF7 and 2.2-folds in MDA-MB-231 for Bax and 8-folds in MCF7 and 2.35-folds in MDA-MB-231 for P53 gene, respectively. Regarding Bcl2, the treatment of MCF7 and MDA-MB-231 cells with DIM-NPs exerted opposite effect by significantly suppressing gene expression at the concentration increased compared to the controls.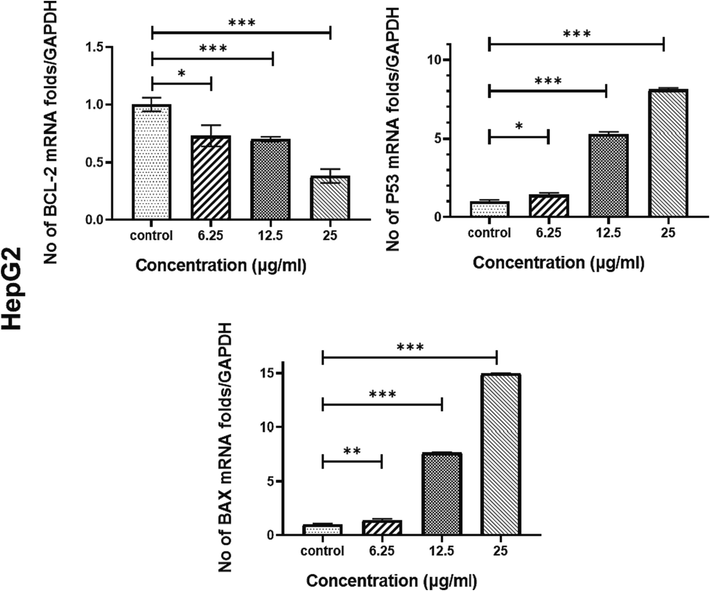
Effects of DIM nanoparticles on gene and protein expressions. HepG 2 cells were treated with DIM-NPs at the indicated concentrations for 48 hrs. After RNA extraction and DNA synthesis, quantitative Real-Time PCR was performed. All data represent the mean ± SD from three independent experiments and analyzed with one-way ANOVA, Data considered statistically significant at (*P ≤ 0.05), (**P ≤ 0.01), (***P ≤ 0.001) and (****P ≤ 0.0001).
Consistent with the gene expression, Flowcytometric analysis showed developed apoptotic and necrotic signals after treatment of HepG 2 cells with DIM-NPs for 48 h in concentration dependent manner as depicted in Fig. 5.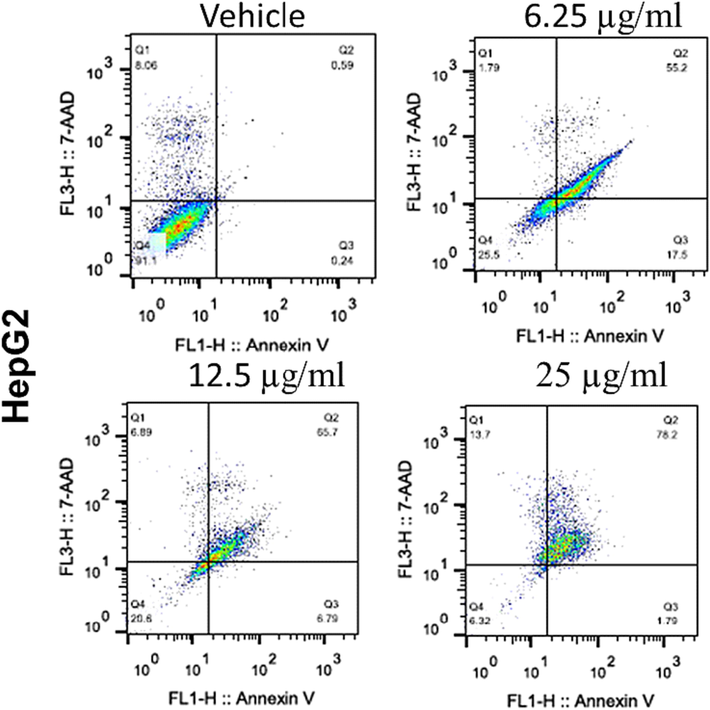
Effects of DIM nanoparticles on cell apoptosis. The effect of different concentrations of DIM-NPs (6.25, 12.5 and 25 µg/mL) on HepG 2 cells. Apoptotic and necrotic cell death of HepG 2 cells were assessed using Annexin V and 7AAD assay after 48 h treatment with DIM NPs.
3.5 Effect of DIM nanoparticles on histopathogy
Consistent with the apoptotic related genes and FACS data, the immune staining of Caspase-3 in liver of Ehrlich carcinoma mice model showed normal negatively stained central (CV) and portal (PV) regions of liver sections in negative control treated with vehicle Fig. 6 (A&E). Tumor mice liver showing metastasizing tumor cells (star) and mild weak Cas-3 immunostained reaction (black arrow) as in Fig. 6 (B&F). Treatment with cisplatin showing less infiltrating neoplastic cells in the portal vein (PV) region (black star) with most hepatocytes showing highly positive caspase-3 expression indicating apoptosis (dotted arrow) as shown in Fig. 6 (C&G). Interestingly, the treatment with cisplatin in combination with DIM-NPs showed few neoplastic infiltrating cells (star) in the normal architecture central vein (CV) and portal vein (PV) areas with more viable active hepatocytes and low cleavage caspase-3 level indicating protection from cis hepatotoxicity as shown in Fig. 6 (D&H).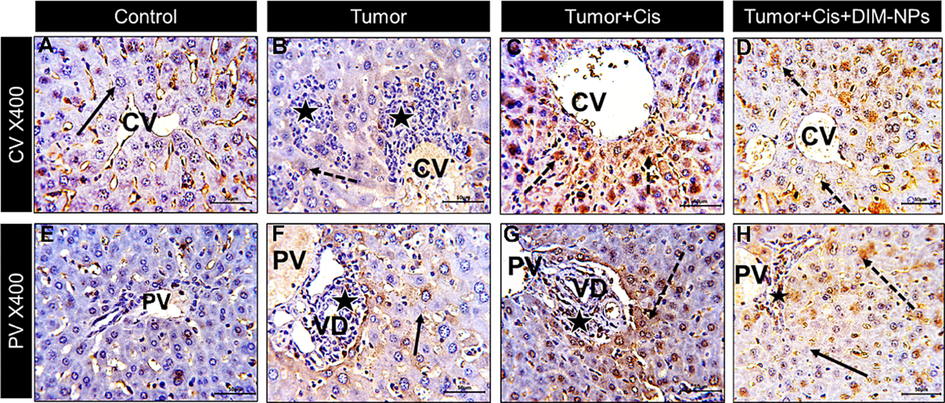
Sections from mice liver at both central (CV) and portal (PV) regions stained for the apoptotic marker caspase-3 and photographed at x400 bar + 50 µm) showing: Control: (A&E) CV and PV regions with negative staining of hepatocyte cytoplasm (black arrow) and non-specific staining of sinusoids, Tumor (Ehrlich model): (B&F) CV and PV regions with metastasizing tumor cells (star) and mild weak immunostained reaction (black arrow), Tumor + Cis: (C&G) CV and PV regions showing less infiltrating neoplastic cells in the portal vein (PV) region (black star) with most hepatocytes showing highly positive caspase-3 expression indicating apoptosis (dotted arrow), Tumor + Cis + DIM-NPs: (D&H) CV and PV regions showing few neoplastic infiltrating cells (star) in the portal vein area (PV) with more viable active hepatocytes with decreased caspase-3 expression indicating protection from cis hepatotoxicity.
4 Discussion
We used two cell lines; HepG-2 and immortal normal liver cells HUH-7 to determine both the anti-tumor activity of DIM-NPs against liver cancer cells and the toxicity against normal cells. The result revealed that the use of DIM-NPs to treat cancer cells inhibited cell proliferation, migration, and angiogenesis. At the molecular level, DIM-NPs upregulated Bax and p53 and downregulated Bcl-2 on mRNA level and inhibits ERK1/2 activity on protein level. By utilizing Ehrlich solid carcinoma-induced mouse model, DIM-NPs reduced the tumor effect on hepatic tissues as reflected by improving histological features compared to mice-engrafted Ehrlich solid carcinoma cells. Preceding studies have shown that DIM can halt the growth of tumor cells by inhibiting the expression of COX-2 in BC (Degner et al., 2008), increasing the phosphorylation of BRCAl during the oxidative stress (Fan et al., 2009), suppressing angiogenesis-associated genes such as surviving (Rahman et al., 2006) and hypoxia-inducible factor-1 (Riby et al., 2008). Combination of DIM with Herceptin is able to reduce Akt and NF-kB p65 activities, which in turn reduce the expression of FoxM1 in HER-2/Neu among BC cells (Ahmad et al., 2013). Similarly, combining DIM with Taxotere has shown to regulate FoxM1 (Ahmad et al., 2011). Interestingly, DIM has increased the chemosensitivity of tamoxifen through the metabolism of estrogen (Thomson et al., 2017), and induced apoptosis at the G2/M phase. In addition, DIM is capable of sensitizing γ-radiation and increasing the generation of intracellular ROS (Wang et al., 2016). Using a rodent model, DIM significantly reduced the size of tumor and has high efficacy in treating HCC cells (Munakarmi et al., 2020). Although the non-formulated DIM has previously been shown to exhibit anti-tumour effects in different in HCC, its therapeutic efficacy is compromised by its low bioavailability solubility in water, and chemical instability (Munakarmi et al., 2020, Xiang et al., 2021). Hence, nanoparticles (NPs) loaded with DIM have been developed to improve its bioavailability and therapeutic efficacy (Wang et al., 2016, Isabella and Mirunalini 2019, Stainsloss et al., 2021). For example, poly-lactic-co-glycolic-acid (PLGA) NPs loaded with DIM have shown an improved cellular uptake and bioavailability of DIM using tissue culture and animal models (Bhowmik et al., 2017). The present study aimed to investigate the efficacy of DIM nanoparticles coated with PEG/chitosan in improving the anti-neoplastic activity against liver cancer cells using in vitro and in vivo Ehrlich carcinoma mouse model.
Prior studies reported that DIM release from PLGA-formulated nanoparticles was<75% and this decrease may be attributed to high molecular weight of PLGA (Khalil, do Nascimento et al. 2013, Sharma et al., 2021). Thus, surface modification of NPs with various macromolecules, such as chitosan and polyethylene glycol (PEG) exhibited a significant improvement in the bioavailability of DIM (Mattiazzi et al., 2019). Other types of nanoformulations have been developed such as poly-glycerol-malic acid-dodecanedioic acid) nanoparticles (PGMD) which are used for evaluating anti-neoplastic activity against MDA-MB-231 and MCF7 cells (Kumari, Sharma et al. 2021).
The results obtained from this study demonstrated that the average size of the formulated DIM-NPs was 102 nm in diameter which is comparable with other previous studies (Sharma et al., 2021). When the effect of DIM-NPs was assessed on liver cancer cells, DIM-NPs induced significant cytotoxicity in HepG 2 after 48 h of treatment, compared to vehicle controls. Comparable to our results, DIM encapsulated PLGA nanoparticles inhibit growth rate of liver cancer cells (Munakarmi et al., 2020). PEG polylactic acid nanoparticles showed a cytotoxic effect on MDA-MB-231 and HeLa cells (Liang et al., 2017). It has been known that primary liver cancer cells metastasize to distant organs through degradation of extracellular matrix and promoting cell migration, invasion and angiogenesis (Guan 2015). Different approaches, including nanoformulation, have been undertaken to inhibit cancer metastasis and recurrence. Our results showed that treatment of the liver cancer cells with 2.5 to 10 µg/mL of DIM-NPs inhibited cell migration and angiogenesis as evidenced by the results from wound-healing assay and chicken embryo model. Arya et al., reported comparable results of anti-migratory and anti-invasive property when pancreatic cells were treated with PLGA chitosan/PEG curcumin NPs compared to cells treated with native curcumin (Arya et al., 2018). The chick embryo and its chorioallantoic membrane have been used as an accepted model although its reported limitations for evaluating drug delivery loaded in nanoparticles (Vargas et al., 2007, Nowak-Sliwinska et al., 2014). An investigation performed by de Mousa et al., showed that nano-formulations of bioactive compounds obtained from a variety of biological products containing DIM, inhibits pancreatic cancer cell proliferation (Mousa et al., 2020). In addition, a study conducted by Dragostin et al. revealed that treatment of chick embryo with chitosan-sulfadimethoxine (CLC) and chitosan-sulfisoxazole (CLD) NPs exerted an antiangiogenic effect (Dragostin et al., 2020). At the molecular level, our results show that DIM-NPs upregulated Bax and p53 whereas Bcl-2 was down-regulated in a concentration dependent way. Further, we have also shown that DIM-NPs induced cell apoptosis in HepG2 cells. Our findings corroborated preceding experimental studies reporting that DIM-PLGA NPs decreases the activity of multiple drug resistance protein 1 and increases reactive oxygen species (ROS) in cisplatin-resistant oral tumor cells through activation of Caspases 3 and 9 pathways (He et al., 2019, Lee et al., 2019). Chang et al., demonstrate DIM-NPs inhibit cell proliferation, invasion, and angiogenesis by downregulating levels of cyclin-dependent kinases 2 and 6 (CDK2, CDK6) expressions, while upregulating the CDK inhibitor, p27(Kip1) expression (Chang et al., 2005). Another study also verified the crucial role of improved mitochondrial reactive oxygen species release in DIM-induced p21 up-regulation in hominoid LC cells. The potential effect of DIM-NPs on liver tissues is a result of inducing multiple harmonized mechanisms to reduce the deleterious effects of Ehrlich carcinoma and cisplatin on liver tissues in mice models (Rahman et al., 2006, Marques et al., 2014, Verderio et al., 2014, Wang et al., 2016, Lima et al., 2021, Xiang et al., 2021).
5 Conclusions
While native DIM has demonstrated its anticancer potential by inhibiting multiple signaling pathways in liver cancer cells, it does have certain limitations, such as low bioavailability. To address this issue, researchers have utilized PLGA nanoparticles, which have shown promise in improving drug bioavailability and treating various types of cancers. Additionally, coating PLGA with PEG/chitosan has further enhanced the efficacy of the drug and increased its anti-neoplastic effects specifically in liver cancer cells.
The use of DIM-loaded PLGA nanoparticles has resulted in the inhibition of cell proliferation and demonstrated anti-migratory and anti-angiogenic properties, surpassing the effects of native DIM alone. Moreover, these nanoparticles have been found to induce cell apoptosis by up-regulating Bax and p53, while down-regulating Bcl-2, further highlighting their potential as an effective therapeutic strategy.
Institutional review board statement
The animal study protocol was approved by the Animal Care and Use Committee at King Fahd Medical Research Center, KAU (protocol code 02-CEGMR-Bioeth-2022 on 24.8.2021.
Funding
This research work was funded by Institutional Fund Projects under grant no (IFPRC-052-248-2020). Therefore, authors gratefully acknowledge technical and financial support from The Ministry of Education and King Abdulaziz University, Jeddah, Saudi Arabia.
Data availability statement
Not applicable.
CRediT authorship contribution statement
Steve Harakeh: Conceptualization, Resources, Supervision. Saber H. Saber: Methodology, Validation. Turki alamri: Software, Writing – review & editing. Rajaa Al-Raddadi: Software, Writing – review & editing. Soad Al-Jaouni: Formal analysis, Investigation. Hanaa Tashkandi: Validation, Investigation. Mohammed Qari: Formal analysis, Project administration, Funding acquisition. Yousef Qari: Data curation, Project administration. Isaac O. Akefe: Methodology, Data curation. Zakariya Y. Abd Elmageed: Validation, Investigation. Shafiul Haque: Writing – original draft. Anwar M Hashem: . Eram Albajri: . Shaker Mousa: Conceptualization, Resources, Visualization, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 3, 3′-diindolylmethane enhances taxotere-induced growth inhibition of breast cancer cells through downregulation of FoxM1. Int. J. Cancer. 2011;129(7):1781-1791.
- [Google Scholar]
- 3, 3′-Diindolylmethane enhances the effectiveness of herceptin against HER-2/neu-expressing breast cancer cells. PLoS One. 2013;8(1):e54657.
- [Google Scholar]
- Antineoplastic activity and curative role of avenanthramides against the growth of ehrlich solid tumors in mice. Oxid. Med. Cell. Longev.. 2019;2019:5162687.
- [Google Scholar]
- Hepatocellular carcinoma: molecular mechanisms and targeted therapies. Medicina. 2019;55(9):526.
- [Google Scholar]
- Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem. Pharmacol.. 2010;79(3):330-338.
- [Google Scholar]
- A framework for the development of effective anti-metastatic agents. Nat. Rev. Clin. Oncol.. 2019;16(3):185-204.
- [Google Scholar]
- Evaluation of curcumin loaded chitosan/PEG blended PLGA nanoparticles for effective treatment of pancreatic cancer. Biomed. Pharmacother.. 2018;102:555-566.
- [Google Scholar]
- Applications of nanoparticles for diagnosis and therapy of cancer. Br. J. Radiol.. 2015;88(1054):20150207
- [Google Scholar]
- Anti-SSTR2 peptide based targeted delivery of potent PLGA encapsulated 3,3'-diindolylmethane nanoparticles through blood brain barrier prevents glioma progression. Oncotarget. 2017;8(39):65339-65358.
- [Google Scholar]
- Targeted therapy for hepatocellular carcinoma: Co-delivery of sorafenib and curcumin using lactosylated pH-responsive nanoparticles. Drug Des. Devel. Ther.. 2020;14:647-659.
- [Google Scholar]
- Antitumoral activity of indole-3-carbinol cyclic tri-and tetrameric derivatives mixture in human breast cancer cells: in vitro and in vivo studies. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents). 2013;13(4):654-662.
- [Google Scholar]
- Primary liver cancer in the UK: Incidence, incidence-based mortality, and survival by subtype, sex, and nation. JHEP Rep. 2021;3(2):100232
- [Google Scholar]
- 3,3'-Diindolylmethane inhibits angiogenesis and the growth of transplantable human breast carcinoma in athymic mice. Carcinogenesis. 2005;26(4):771-778.
- [Google Scholar]
- Nanoparticles for cancer imaging: The good, the bad, and the promise. Nano Today. 2013;8(5):454-460.
- [Google Scholar]
- Targeting of aryl hydrocarbon receptor-mediated activation of cyclooxygenase-2 expression by the indole-3-carbinol metabolite 3, 3′-diindolylmethane in breast cancer cells. J. Nutr.. 2008;139(1):26-32.
- [Google Scholar]
- Designing of chitosan derivatives nanoparticles with antiangiogenic effect for cancer therapy. Nanomaterials (Basel). 2020;10(4)
- [Google Scholar]
- Nanoparticles ellagic acid protects against cisplatin-induced hepatotoxicity in rats without inhibiting its cytotoxic activity. Int. J. Pharmacol.. 2019;5(4):465-477.
- [Google Scholar]
- Low concentrations of diindolylmethane, a metabolite of indole-3-carbinol, protect against oxidative stress in a BRCA1-dependent manner. Cancer Res.. 2009;69(15):6083-6091.
- [Google Scholar]
- Exosomes-mediated transfer of Itga2 promotes migration and invasion of prostate cancer cells by inducing epithelial-mesenchymal transition. Cancers (Basel). 2020;12(8)
- [Google Scholar]
- Use of nanotechnology to develop multi-drug inhibitors for cancer therapy. Journal of Nanomedicine & Nanotechnology. 2013;4(6)
- [Google Scholar]
- Cancer metastases: challenges and opportunities. Acta Pharm. Sin. B. 2015;5(5):402-418.
- [Google Scholar]
- 3,3'-Diindolylmethane mitigates lipopolysaccharide-induced acute kidney injury in mice by inhibiting NOX-mediated oxidative stress and the apoptosis of renal tubular epithelial cells. Mol. Med. Rep.. 2019;19(6):5115-5122.
- [Google Scholar]
- PEGylated chitosan-based polymer micelle as an intracellular delivery carrier for anti-tumor targeting therapy. Eur. J. Pharm. Biopharm.. 2008;70(3):749-757.
- [Google Scholar]
- 3, 3'-Diindolylmethane-encapsulated chitosan nanoparticles accelerate molecular events during chemical carcinogen-induced mammary cancer in sprague dawley rats. Breast Cancer. 2019;26(4):499-509.
- [Google Scholar]
- Pharmacokinetics of curcumin-loaded PLGA and PLGA-PEG blend nanoparticles after oral administration in rats. Colloids Surf. B Biointerfaces. 2013;101:353-360.
- [Google Scholar]
- Brassica vegetables and prostate cancer risk: a review of the epidemiological evidence. Nutr. Cancer. 2002;42(1):1-9.
- [Google Scholar]
- PGMD/curcumin nanoparticles for the treatment of breast cancer. Sci. Rep.. 2021;11(1):3824.
- [Google Scholar]
- 3,3'-Diindolylmethane promotes BDNF and antioxidant enzyme formation via TrkB/Akt pathway activation for neuroprotection against oxidative stress-induced apoptosis in hippocampal neuronal cells. Antioxidants (Basel). 2019;9(1)
- [Google Scholar]
- Fabrication of biodegradable PEG-PLA nanospheres for solubility, stabilization, and delivery of curcumin. Artif. Cells Nanomed. Biotechnol.. 2017;45(2):297-304.
- [Google Scholar]
- Global breast cancer incidence and mortality trends by region, age-groups, and fertility patterns. EClinicalMedicine. 2021;38:100985
- [Google Scholar]
- Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol.. 2022;19(3):151-172.
- [Google Scholar]
- Chick chorioallantoic membrane (CAM) assay as an in vivo model to study the effect of newly identified molecules on ovarian cancer invasion and metastasis. Int. J. Mol. Sci.. 2012;13(8):9959-9970.
- [Google Scholar]
- Ginkgo modulates noise-induced hippocampal damage in male albino rats: a light and electron microscopic study. Egyptian Journal of Histology. 2014;37(1):159-174.
- [Google Scholar]
- Low levels of 3, 3′-diindolylmethane activate estrogen receptor α and induce proliferation of breast cancer cells in the absence of estradiol. BMC Cancer. 2014;14(1):524.
- [Google Scholar]
- Incorporation of 3,3'-Diindolylmethane into Nanocapsules Improves Its Photostability, Radical Scavenging Capacity, and Cytotoxicity Against Glioma Cells. AAPS PharmSciTech. 2019;20(2):49.
- [Google Scholar]
- Nanoformulated Bioactive Compounds Derived from Different Natural Products Combat Pancreatic Cancer Cell Proliferation. Int. J. Nanomed.. 2020;15:2259-2268.
- [Google Scholar]
- Indole-3-Carbinol Derivative DIM Mitigates Carbon Tetrachloride-Induced Acute Liver Injury in Mice by Inhibiting Inflammatory Response, Apoptosis and Regulating Oxidative Stress. Int. J. Mol. Sci.. 2020;21(6)
- [Google Scholar]
- Nanocarrier for poorly water-soluble anticancer drugs—barriers of translation and solutions. AAPS PharmSciTech. 2014;15(4):822-833.
- [Google Scholar]
- The chicken chorioallantoic membrane model in biology, medicine and bioengineering. Angiogenesis. 2014;17(4):779-804.
- [Google Scholar]
- Long circulating chitosan/PEG blended PLGA nanoparticle for tumor drug delivery. Eur. J. Pharmacol.. 2011;670(2–3):372-383.
- [Google Scholar]
- Gene expression profiling revealed survivin as a target of 3, 3′-diindolylmethane-induced cell growth inhibition and apoptosis in breast cancer cells. Cancer Res.. 2006;66(9):4952-4960.
- [Google Scholar]
- Rebecca L. Siegel, M.P.H., Kimberly D. Miller, M.P.H. and P. Ahmedin Jemal DVM, 2017. “Cancer statistics.” CA: a cancer journal for clinicians.
- 3, 3′-Diindolylmethane reduces levels of HIF-1α and HIF-1 activity in hypoxic cultured human cancer cells. Biochem. Pharmacol.. 2008;75(9):1858-1867.
- [Google Scholar]
- Global, regional and national burden of primary liver cancer by subtype. Eur. J. Cancer. 2022;161:108-118.
- [Google Scholar]
- A modified arithmetical method of Reed and Muench for determination of a relatively ideal median lethal dose. Afr. J. Pharm. Pharmacol. 2011;51(12):1544-1546.
- [Google Scholar]
- Effects of curcumin-loaded poly(lactic-co-glycolic acid) nanoparticles in MDA-MB231 human breast cancer cells. Nanomedicine (Lond.). 2021;16(20):1763-1773.
- [Google Scholar]
- Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol.. 2012;13(8):817-826.
- [Google Scholar]
- Molecular networks that regulate cancer metastasis. Semin. Cancer Biol.. 2012;22(3):234-249.
- [Google Scholar]
- Correction to: 3,3'-diindolylmethane encapsulated chitosan nanoparticles accelerates inflammatory markers, ER/PR, glycoprotein and mast cells population during chemical carcinogen induced mammary cancer in rats. Indian J. Clin. Biochem.. 2021;36(3):382-383.
- [Google Scholar]
- A randomized, placebo-controlled trial of diindolylmethane for breast cancer biomarker modulation in patients taking tamoxifen. Breast Cancer Res. Treat.. 2017;165(1):97-107.
- [Google Scholar]
- The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv. Drug Deliv. Rev.. 2007;59(11):1162-1176.
- [Google Scholar]
- Intracellular drug release from curcumin-loaded PLGA nanoparticles induces G2/M block in breast cancer cells. Biomacromolecules. 2013;14(3):672-682.
- [Google Scholar]
- Antiproliferative effect of ASC-J9 delivered by PLGA nanoparticles against estrogen-dependent breast cancer cells. Mol. Pharm.. 2014;11(8):2864-2875.
- [Google Scholar]
- Development of novel application of 3, 3′-diindolylmethane: sensitizing multidrug resistance human breast cancer cells to γ-irradiation. Pharm. Biol.. 2016;54(12):3164-3168.
- [Google Scholar]
- Enhanced anti-tumor efficacy by co-delivery of doxorubicin and paclitaxel with amphiphilic methoxy PEG-PLGA copolymer nanoparticles. Biomaterials. 2011;32(32):8281-8290.
- [Google Scholar]
- 3,3'-Diindolylmethane enhances paclitaxel sensitivity by suppressing DNMT1-mediated KLF4 methylation in breast cancer. Front. Oncol.. 2021;11:627856
- [Google Scholar]
- Transport of a caspase inhibitor across the blood-brain barrier by chitosan nanoparticles. Methods Enzymol.. 2012;508:253-269.
- [Google Scholar]
- Factors that control the circulation time of nanoparticles in blood: challenges, solutions and future prospects. Curr. Pharm. Des.. 2010;16(21):2298-2307.
- [Google Scholar]







