Translate this page into:
Nitrogen enriched chemically produced carbon supplementary impacts on maize growth under saline soil conditions
⁎Corresponding author at: Department of Geology and Pedology, Faculty of Forestry and Wood Technology, Mendel University in Brno, Zemedelska1, 61300 Brno, Czech Republic (R. Datta). Department of Botany, The Islamia University of Bahawalpur, Punjab, Pakistan (M. Ramzan). musarrat.ramzan@iub.edu.pk (Musarrat Ramzan), rahulmedcure@gmail.com (Rahul Datta)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Poor organic matter and nitrogen (N) deficiency along with salinity in arid and semiarid region soils are major hurdles to optimization of cereal's yield. In different cereals, maize productivity is significantly decreased due to less availability of N in low organic matter soils. Scientists suggest the incorporation of organic fertilizers to overcome this issue. That’s why the current study was conducted to explore the effectiveness of chemically produced nitrate blended acidified carbon (NBC). There were 3 levels of NBC i.e., 0, 0.50 and 1.00% applied under naturally normal and saline soils having EC 2.75 and 6.19 dS/m respectively. Results showed that the addition of 1.00NBC was significantly better compared to 0NBC for improvement in maize growth attributes i.e., root (32.86 and 77.84%) and shoot (74.17 and 67.57%) length, shoot fresh (53.98 and 97.42%) and dry weight (53.20and 84.20%), root dry (45.97 and 53.66%) and fresh weight (45.62 and 25.14%) in normal and saline conditions respectively. A significant enhancement in chlorophyll a, b, total and carotenoids of maize leaves also validated the imperative role of 1.00NBC than 0NBC in saline and normal soil. Application of 1.00BC also significantly decreases leaves electrolyte leakage and Na concentration in root and leaves over 0NBC in saline soils. In conclusion, 1.00NBC is an effective amendment to improve maize growth in saline soil. More investigations are suggested on different cereal crops under variable agroclimatic zones to declare 1.00NBC as the most effective amendment for alleviation of salinity stress.
Keywords
Biochar
Chlorophyll contents
Electrolyte leakage
Morphological attributes
Carbon amendment
Zea mays
1 Introduction
Soils of arid and semiarid areas of the world have low organic residues, poor fertility status and are salt-affected (Oueriemmi et al., 2021; Qadir et al., 2007). High temperatures with low rainfall are crucial factors in these areas which played a notorious role in the intensive decomposition of organic residues (Xu et al., 2021). Poor organic matter where not only disturb the soil structure but also decreases the bioavailability of essential micronutrients to the plants (Arif et al., 2021; Garcia et al., 2017). It also adversely affects soil health due to the formation of some complexes which decrease the microbial metabolic efficiency under low organic residue (Liu et al., 2021). Furthermore, saline conditions, increase the uptake of Na, Ca, Mg and Cl by decreasing the bioavailability of essential nutrients in plants which causes a significant reduction in growth and yield (Dustgeer et al., 2021). To overcome this problem, the application of organic amendments in the soil is an effective technique that mostly shows positive effects in this regard (Rahi et al., 2021; Sultan et al., 2020; Zafar-ul-Hye et al., 2021).
Many organic compounds are utilized as organic fertilizers in agriculture. These organic amendments include farmyard manure, green manure, humic substances, and compost (De Corato, 2021; Karmegam et al., 2021; Khayat, 2021; Mghaiouini et al., 2021; Radziemska et al., 2021). These organic fertilizers not only improve soil's physical, chemical and biological attributes but also provide essential nutrients to the plant (Lazcano et al., 2021). It also provides sufficient for the microbial population which played a critical role in increasing their positive actions towards improvement in soil ecology and plant productivity (Dahunsi et al., 2021; Viketoft et al., 2021; Zafar-ul-hye et al., 2020). On the other hand, the susceptibility of these organic residues towards fast decomposition is a major concern in arid and semiarid areas of the world. Continuous application of these organic fertilizers in bilk amount not only increase the cost of growers but also is laborious (Ajwa and Tabatabai, 1994). That’s decomposition resistant activated biochar is gaining the attention of scientists (Danish et al., 2020; Danish and Zafar-ul-Hye, 2020; Sultan et al., 2020; Younis et al., 2020).
Activated carbon is an organic amendment that has the potential to improve soil fertility (Danish et al., 2015; Danish and Zafar-ul-Hye, 2019; Hashmi et al., 2019; Yaseen et al., 2021). Its production under high temperatures and limited availability of oxygen by the process of pyrolysis makes it resistant to decomposition (Fang et al., 2021; Feng et al., 2021). It can significantly enhance the nutrient holding capacity of soil due to height and exchange capacity. On the other hand, biochar also provides nutrients to the plants which are an integral part of the structure. These nutrients become part of the soil nutrients labile pool when irrigation is applied (Haider et al., 2022; Joseph et al., 2021). However, the production of biochar under such high temperature and anaerobic conditions is a major problem for industries (Sultan et al., 2020). Scientists are working now on the production of acidified carbon, especially for alkaline nature soils (Sultan et al., 2020). This acidified carbon can significantly decrease soil pH which imperatively regulates the nutrient dynamics in soil (Ahmed et al., 2021). It has also been observed that significant improvement in organic residues of soil also facilitates the bioavailability of the nutrient when acidified carbon is applied to the soil as an amendment (Sultan et al., 2020). Furthermore, the chemical production of this carbon is also cost and time effective that required less precautions compared to thermo pyrolyzed biochar (Sultan et al., 2020).
In addition to the above, to the feeding expanding population of the world, agriculture is also facing intensive crop production issues (AL-Aasmi et al., 2022). Cultivation of high yield cultivars for the achievement of maximum crop yield has become a necessity of time (Madan et al., 2022). These high yielding varieties have also increased the consumption of inorganic fertilizers which are an important part of the green revolution (Madan et al., 2022). Among different inorganic fertilizers nitrogen (N) is the most important one which is usually applied in the form of urea, diammonium phosphate and ammonium nitrate (Bian et al., 2022; Machado et al., 2022; Qi and Pan, 2022; Wakeel et al., 2022). Most farmers like the practice of over nitrogen use for the achievement of maximum yield. However, this increase in the use of N fertilizer where increases the chances of the weeds problem, it also enhances the environmental risks associated with the overuse of N fertilizers (Diacono et al., 2013). It has been observed that more than 33 % of overused N is lost after application as fertilizers either in the form of ammonia or nitrate ions (Diacono et al., 2013; Madan et al., 2022; Yan et al., 2022; Yu et al., 2022).
Nitrogen deficiency is also a major agriculture issue (Azimi et al., 2021). Limited uptake of nitrogen in plants can cause severe disturbance in root to shoot ratio. It can shorten lateral branches in plants with a decrease in the size of leaves. Under highly N deficient conditions, the disintegration of chloroplast is a common phenomenon that can cause the death of plants (Azimi et al., 2021). As a permanent soil property, it is difficult to modify soil texture. However, the role of texture is also vital in nitrogen management. A sandy texture soil has a low retention potential for nitrogen ions compared to clay. In this soil leaching of nitrate is a dominant mechanism that significantly decreases the bioavailability of N to plants (Kühling et al., 2021).
So far, attempts have been made with sulphuric acid to produce acidified carbon. However, the current is novel where nitric acid is used to produce acidified carbon along with sulphuric acid. The study novelty was to introduce nitrate enrichment in acidified carbon which is an important form of N bioavailable for the plants. The study aimed to explore the effectiveness of acidified chemically produced nitrogen blended carbon against salinity stress. This study will cover the knowledge gap regarding the use of nitrogen blended chemically produced carbon (NBC) as an amendment against salinity stress and improvement in maize growth. It is hypothesized that NBC may be an effective approach to alleviate the salinity stress in maize.
2 Materials and methods
2.1 Experimental site and design
A pot experiment was conducted in the research area (29.3788N and 71.7652E) of the Department of Botany, The Islamia University Bahawalpur. The design of the experiment was a completely randomized design (CRD). Two factorial arrangements of treatment were made i.e., salinity levels and nitrogen blended chemically produced carbon (NBC).
2.2 Production and characterization of NBC
For the production of NBC modification in methods of Sultan et al. (2020) was done. Instead of using H2SO4, 2:1 mixture of HNO3 and H2SO4 was used. When carbon was produced then it was passed through 2 mm sieve. Finally, fine powder of NBC was applied as per the treatment plan in the soil. For pH and EC assessment of NBC, it was mixed in 1:20 w/v ratio in deionized water. Final readings were taken on pre-calibrated pH and EC meter (Shi et al., 2017). Di-acid mixture HNO3:HClO4 in 2:1 ratio was used for the digestion of NBC at 200 °C on a hot plate (Miller, 1998). Yellow color method was used for the assessment of P in NBC on a spectrophotometer (Chapman and Pratt, 1961). Potassium, sodium and calcium were examined in digested material by running it on a flame photometer (Donald and Hanson, 1998). For the determination of N in NBC, digestion was done with H2SO4 at 380 °C. After that Kjeldhal’s distillation apparatus was used for the assessment of total N in NBC (Bremner, 1996). Ash content (AC) and volatile matter (VM) in NBC were examined by heating the sample in a muffle furnace at 550 °C and 450 °C respectively (Danish et al., 2019). The fixed carbon in BC and AAC was calculated using the equation Noor et al. (2012).
The characteristic of NBC is provided in Table 1.
NBC
Soil
Attributes
Units
Values
Attributes
Units
Normal
Saline
pH
–
6.54
Sand
%
30
30
EC
dS/m
3.96
Silt
%
30
30
Volatile matter
%
15.54
Clay
%
40
40
Ash content
%
26.78
Texture
–
Clay Loam
Fixed C
%
57.68
pHs
–
8.02
8.67
Total N
%
3.47
ECe
dS/m
2.75
6.19
Total P
%
0.21
Organic matter
%
0.45
0.30
Total K
%
0.97
Total N
%
0.023
0.015
Total Na
%
0.12
Extractable P
mg kg−1
7.21
3.44
Total Ca
%
0.23
Extractable K
mg kg−1
136
98
2.3 Salinity and soil characterization
Naturally normal and saline soils were collected from nearby research areas. Soil EC was used as the main factor for the assessment of salinity. After analysis, it was noted that normal soil EC was 2.75 dS/m while saline soil EC was 6.19 dS/m. The hydrometer method was used for the assessment of sand, silt and clay. The final soil texture was computed by using USDA textural triangle (Gee and Bauder, 1986). For examination of soil EC and pH, 1:10 and 1:1 w/v ratio of soil and deionized water was mixed. After that pH of the soil paste was analyzed in a pre-calibrated pH meter. However, extraction was done for EC and extracted solution was run on EC meter for final EC determination (Page et al., 1983; Rhoades, 1996). For analysis of total organic matter potassium dichromate and ferrous ammonium sulphate were utilized as per standard protocol (Sparks et al., 1996). Extracting Olsen’s reagent was used for extraction of available P. Final values of P were computed on a spectrophotometer by taking absorbance at 880 nm (Kuo, 1996). Assessment of extractable K was done by using ammonium acetate solution. Final readings were noted by running the extracting solution on a flamephotometer (Donald and Hanson, 1998).
2.4 Treatment plan and NBC application
There were six treatments with 3 replications. The treatments include control (No NBC) + normal soil (2.75 dS/m EC), 2.75 dS/m EC + 0.5 %NBC (0.50NBC), 2.75 dS/m EC + 1.00 %NBC (1.00NBC), saline soil (6.19 dS/m EC), 6.19 dS/m EC + 0.50NBC and 6.19 dS/m EC + 1.00NBC. On w/w basis NBC was applied in soil as per treatment plan manually.
2.5 Irrigation characteristics and application
The moisture in the pots were maintained at 65 % field capacity of soil. For irrigation purpose tap water was used. The characteristics of tap water were pH = 6.89, EC = 0.34 dS/m, carbonates = 0.00 (meq./L), bicarbonates = (3.27 meq./L), chlorides = (0.40 meq./L) and Ca + Mg = (3.21 meq./L) (Estefan et al., 2013).
2.6 Seeds collection and sowing
Seeds of maize YH 1898 variety was collected from a local certified seeds shop. Initially, weak and damaged seeds were screened out manually. After that, 4 seeds were sown in each pot. When seeds get germinated, thinning was done to maintain 2 seedlings per pot for further experiment.
2.7 Fertilizer application
Nitrogen fertilizer was applied at the rate of 227.24 kg ha−1 in three separate aliquots. Phosphorus (143.26 kg ha−1) and K (91.93 kg ha−1) were applied as a basal dose at the time of sowing (Saboor et al., 2021).
2.8 Harvesting and data collection
Plants were harvested at the vegetative phase of maturity (before tillering) (Saboor et al., 2021). Shoot length, root length, shoot fresh and dry weight, root fresh and dry weight were recorded soon after harvesting. For dry weight analysis, samples were oven-dried at 65 °C for 48 h. After the achievement of constant's weight, analytical grade balance was used for measurements.
2.9 Gas exchange attributes
IRGA (infrared gas analyzer) was utilized to the determination of photosynthetic rate, transpiration rate and stomatal conductance on a sunny day (9 and 11 am) (Danish and Zafar-ul-Hye, 2019; Saboor et al., 2021).
2.10 Chlorophyll contents
For the determination of chlorophyll contents, initially grinding and then extraction was done by using 80 % acetone. After that absorbance was recorded on a spectrophotometer at 645, 663 and 480 nm (Arnon, 1949; Kirk and Allen, 1965; Sims and Gamon, 2002). Where,
OD = Optical density (wavelength).
V = Final volume made.
W = Fresh leaf weight (g).
2.11 Electrolyte leakage
Electrolyte leakage (EL) was measured using the method Lutts et al. (1996). Leaf discs of equal size (1 g) were dipped in 15 ml of deionized water (DI) water and incubated for 2 h at 25 °C in test tubes. Initial EC of the solution (EC1) was taken after incubation. Samples were again autoclaved at 120 °C for 20 min and final EC (EC2) was measured after equilibrium at 25 °C.
2.12 Statistical analysis
All the data were processed by using standard statistical procedure (Steel et al., 1997). Two factorial ANOVA and Tukey’s test were applied for the comparison of treatments. Origin2021Pro software was used for making paired comparisons and Pearson correlation graphs (OriginLab Corporation, 2021).
3 Results
3.1 Shoot and root length
Results showed that treatments effects were significant on shoot and length of maize cultivated under normal (2.75 dS/m EC) and saline (6.19 dS/m EC) soil conditions. Under normal and saline soil conditions, application of 1.00NBC remained significantly best compared to 0.50 and 0NBC for enhancement in the shoot (Fig. 1A) and root length (Fig. 1B) of maize. Treatment 0.50NBC also differed significantly better than 0NBC for improvement in shoot and root length of maize in saline and normal soils. The maximum increase in the root (32.86 and 77.84 %) and shoot (74.17 and 67.57 %) length were observed in normal and saline soil conditions respectively where 1.00NBC was applied compared to 0NBC.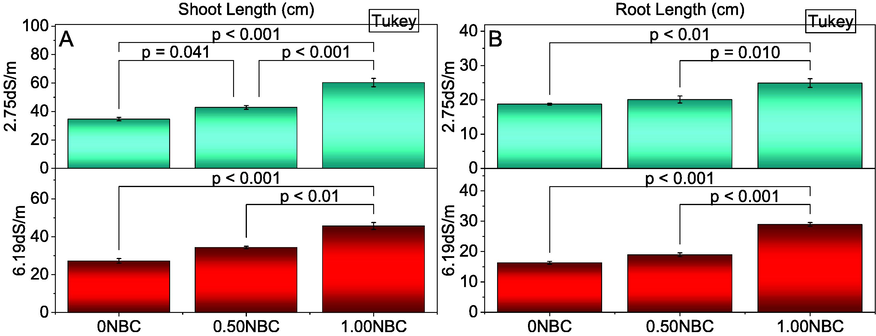
Effect of nitrate blended chemically produced carbon (NBC) different application rates on the shoot (A) and root length (B) of maize under normal (2.75 dS/m EC) and saline (6.19 dS/m EC) soil. Different values on bars are p-values computed by paired comparison Tukey test; p ≤ 0.05. 0NBC (control having no NBC); 0.50NBC (0.50 % w/w NBC applied in soil); 1.00NBC (1.00 % w/w NBC applied in soil). Red bars are indicating salinity stress. Green bars are indicating normal soil conditions.
3.2 Shoot fresh and dry weight
Application of treatments differed significantly for shoot fresh and dry weight of maize grown under normal and saline soil conditions. Treatment 1.00NBC differed significantly best over 0NBC for improvement in shoot fresh (Fig. 2A) and dry weight (Fig. 2B) of maize under normal and saline soil conditions. No significant change was observed between 0.50NBC and 0NBC for shoot fresh weight under normal and saline conditions. It was also observed that 1.00NBC and 0.50NBC remained statistically alike to each other for shoot fresh weight in normal and saline soils. However, 0.50NBC also differed significantly better from 0NBC for enhancement in shoot dry weight of maize in normal and saline soils. Similarly, 1.00NBC performed significantly better than 0NBC for shoot dry weight in normal and saline soil conditions. The maximum increase in shoot fresh (53.98 and 97.42 %) and dry weight (53.20and 84.20 %) were observed 1.00NBC than 0NBC under normal and saline soil conditions respectively.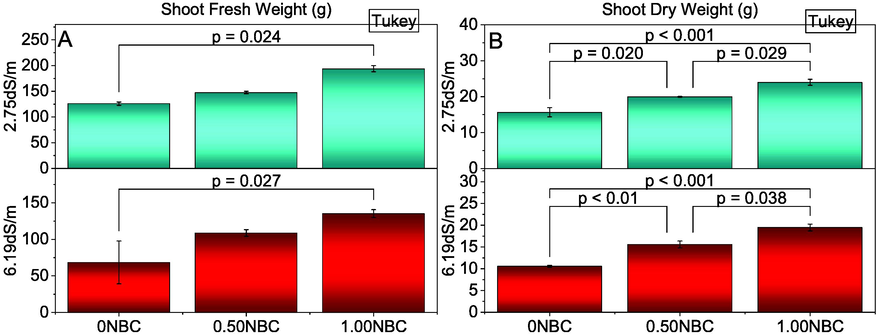
Effect of nitrate blended chemically produced carbon (NBC) different application rates on shoot fresh (A) and dry weight (B) of maize under normal (2.75 dS/m EC) and saline (6.19 dS/m EC) soil. Different values on bars are p-values computed by paired comparison Tukey test; p ≤ 0.05. Bars are means of three replicates. 0NBC (control having no NBC); 0.50NBC (0.50 % w/w NBC applied in soil); 1.00NBC (1.00 % w/w NBC applied in soil). Red bars are indicating salinity stress. Green bars are indicating normal soil conditions.
3.3 Root fresh and dry weight
Root fresh and dry weight was significantly affected due to the application of different rates of NBC in normal and saline soils. The addition of 1.00NBC differed significantly best over 0NBC for improvement in root fresh (Fig. 3A) and dry weight (Fig. 3B) of maize in normal and saline soil. Treatments 0.50NBC and 0NBC did not differ significantly for root fresh and dry weight under saline conditions but were significantly different under normal soil. It was also observed that 1.00NBC and 0.50NBC remained statistically similar to each other for root fresh and dry weight in saline soils. However, 1.00NBC remained significantly better than 0.50NBC for shoot fresh and dry weight in normal. The maximum increase in root dry (45.97 and 53.66 %) and fresh weight (45.62 and 25.14 %) were noted in 1.00NBC over 0NBC under normal and saline soil conditions respectively.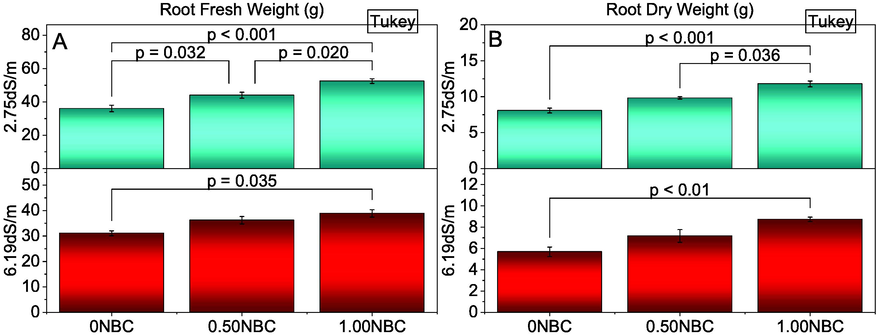
Effect of nitrate blended chemically produced carbon (NBC) different application rates on root fresh (A) and dry weight (B) of maize under normal (2.75 dS/m EC) and saline (6.19 dS/m EC) soil. Different values on bars are p-values computed by paired comparison Tukey test; p ≤ 0.05. Bars are means of three replicates. 0NBC (control having no NBC); 0.50NBC (0.50 % w/w NBC applied in soil); 1.00NBC (1.00 % w/w NBC applied in soil). Red bars are indicating salinity stress. Green bars are indicating normal soil conditions.
3.4 Chlorophyll contents
Chlorophyll contents i.e., chlorophyll a, chlorophyll b and total chlorophyll and carotenoids were differed significantly by different rates of NBC applied in normal and saline soils. Under normal and saline soil conditions, the addition of 1.00NBC remained significantly best over 0.50 and 0NBC for improvement chlorophyll a (Fig. 4A), chlorophyll b (Fig. 4B), total chlorophyll (Fig. 4C) and carotenoids (Fig. 4D) of maize. Application of 0.50NBC also differed significantly over 0NBC for enhancement in chlorophyll b, total chlorophyll and carotenoids of maize in saline and normal soils. However, results showed 0.50NBC did not show any significant change in chlorophyll a under saline conditions. On the other hand, 0.50NBC was significant for improvement in chlorophyll a under normal soil conditions. Maximum enhancement in chlorophyll a (65.97 and 64.95 %), chlorophyll b (72.64 and 76.39 %), total chlorophyll (68.80 and 69.82 %) and carotenoids (43.48 and 44.64 %) were observed in normal and saline soil conditions respectively where 1.00NBC was applied compared to 0NBC.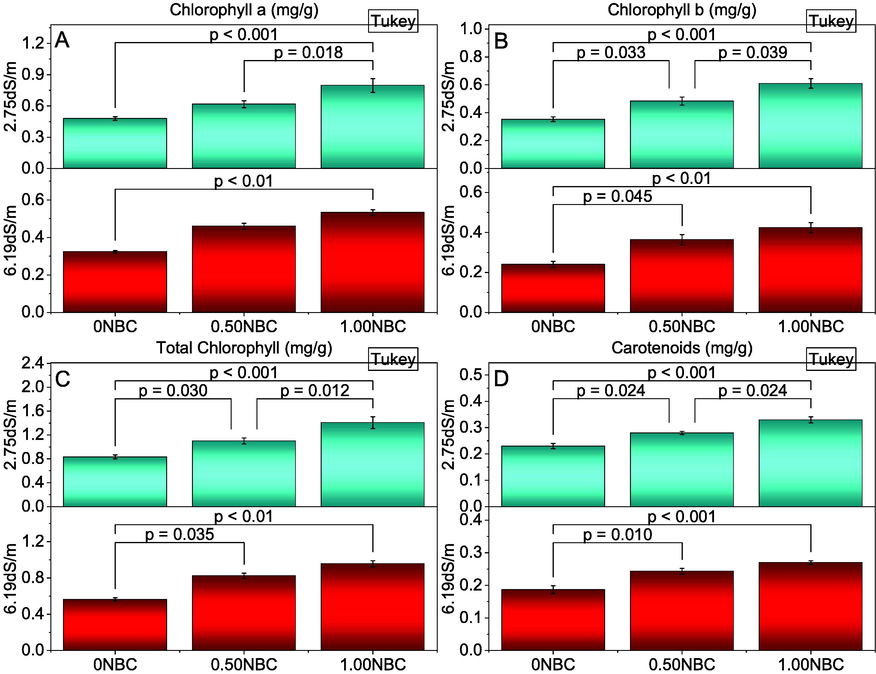
Effect of nitrate blended chemically produced carbon (NBC) different application rates on chlorophyll a (A), chlorophyll b (B), total chlorophyll (C) and carotenoids (D) of maize under normal (2.75 dS/m EC) and saline (6.19 dS/m EC) soil. Different values on bars are p-values computed by paired comparison Tukey test; p ≤ 0.05. Bars are means of three replicates. 0NBC (control having no NBC); 0.50NBC (0.50 % w/w NBC applied in soil); 1.00NBC (1.00 % w/w NBC applied in soil). Red bars are indicating salinity stress. Green bars are indicating normal soil conditions.
3.5 Leaves Na, root Na, electrolyte leakage and leaves N
Results showed that the effect of the treatment was significant for leaves Na, root Na, electrolyte leakage and leaves N of maize grown in saline and normal soils. No significant change in leaves, root Na, electrolyte leakage and leaves N was noted among the treatments in normal soil. Under saline soil conditions, 1.00NBC caused a significant decrease in leave and root Na compared to 0NBC. However, 0.50 differed significantly only for root Na and remained non-significant for leaves Na over 0NBC in saline soil (Table 2). It was observed that both 1.00NBC and 0.50NBC caused a significant decrease in electrolyte leakage and increase in leaves N when applied in saline soil conditions than 0NBC. The maximum decrease in leaves Na (11.26 and 39.72 %), root Na (9.55 and 78.45 %), electrolyte leakage (9.07 and 65.75 %) and increase in leaf N (11.11 and 30.77 %) were observed in normal and saline soil conditions respectively where 1.00NBC was applied over 0NBC. Different letters are showing significant differences computed by paired comparison Tukey test; p ≤ 0.05. Means are an average of three replicates. SE (Means standard error); 0NBC (control having no NBC); 0.50NBC (0.50 % w/w NBC applied in soil); 1.00NBC (1.00 % w/w NBC applied in soil). Red bars are indicating salinity stress. Green bars are indicating normal soil conditions.
Salinity
Treatments
Leaves Na (mg/g DW)
Root Na (mg/g DW)
Mean
SE
Labelling
Mean
SE
Labelling
2.75dS/m
0NBC
3.26
0.16
c
2.41
0.07
d
0.50NBC
3.07
0.07
c
2.33
0.13
d
1.00NBC
2.93
0.16
c
2.20
0.11
d
6.19dS/m
0NBC
8.97
0.39
a
13.58
0.49
a
0.50NBC
7.72
0.27
ab
10.36
0.34
b
1.00NBC
6.42
0.55
b
7.61
0.63
c
Salinity
Treatments
Electrolyte Leakage (%)
Leaves N (%)
2.75dS/m
0NBC
16.00
0.58
c
0.18
0.0055
abc
0.50NBC
16.67
0.88
c
0.19
0.0034
ab
1.00NBC
14.67
0.88
c
0.20
0.0071
a
6.19dS/m
0NBC
38.67
2.60
a
0.13
0.0057
d
0.50NBC
26.00
1.00
b
0.16
0.0054
c
1.00NBC
23.33
1.20
b
0.17
0.0052
bc
3.6 Gas exchange attributes
It was noted that the effect of treatments was significant for Pn, E and gs of maize cultivated in saline and normal soils. A significant change in Pn and gs of maize was noted in normal and saline soil where 1.00NBC was applied over 0NBC. Under saline soil conditions, 1.00NBC remained non-significant for E but significant in normal soil compared to 0NBC (Table 3). However, 0.50 did not differ significantly for E over 0NBC in normal and saline soil. It was observed that both 0.50NBC caused a significant increase in gs when applied in saline soil conditions than 0NBC. The maximum increase in Pn (8.36 and 23.66 %), E (25.64 and 14.56 %) and gs (25.00 and 33.33 %) were noted in normal and saline soil conditions respectively where 1.00NBC was applied compared to 0NBC Table 3. Different letters are showing significant differences computed by paired comparison Tukey test; p ≤ 0.05. Means are an average of three replicates. SE (Means standard error); 0NBC (control having no NBC); 0.50NBC (0.50 % w/w NBC applied in soil); 1.00NBC (1.00 % w/w NBC applied in soil). Red bars are indicating salinity stress. Green bars are indicating normal soil conditions.
Salinity
Treatments
Pn (µmol/m2/s1)
E (µmol/m2/s1)
gs (mmol/m2/s1)
Mean
SE
Labelling
Mean
SE
Labelling
Mean
SE
Labelling
2.75dS/m
0NBC
12.56
0.17
ab
1.95
0.05
bc
0.08
0.0015
b
0.50NBC
12.93
0.13
a
2.19
0.06
b
0.09
0.0032
ab
1.00NBC
13.61
0.37
a
2.45
0.07
a
0.10
0.0020
a
6.19dS/m
0NBC
10.61
0.17
c
1.58
0.04
d
0.06
0.0052
c
0.50NBC
11.66
0.20
bc
1.68
0.03
d
0.07
0.0012
b
1.00NBC
13.12
0.23
a
1.81
0.03
cd
0.08
0.0023
b
3.7 Leaves and root K
Leaves and root K were significantly changed when different rates of NBC were applied in normal and saline soils. Treatment 1.00NBC significantly enhanced leaves (Fig. 5 A) and root K (Fig. 5 B) of maize over 0NBC in normal and saline soil. The addition of 0.50NBC and 0NBC did not differ significantly for leaves K and root K under saline conditions and normal soils respectively. However, significantly improved leaves K and root K in normal and saline soils. Pearson correlation showed that electrolyte leakage, root Na and leaves Na were negative in correlation with all other studied attributes of maize in normal and saline soil. Leaves N, leaves K and root K were significantly positive in correlation with chlorophyll contents, morphological growth attributes and gas exchange attributes of maize (Fig. 6).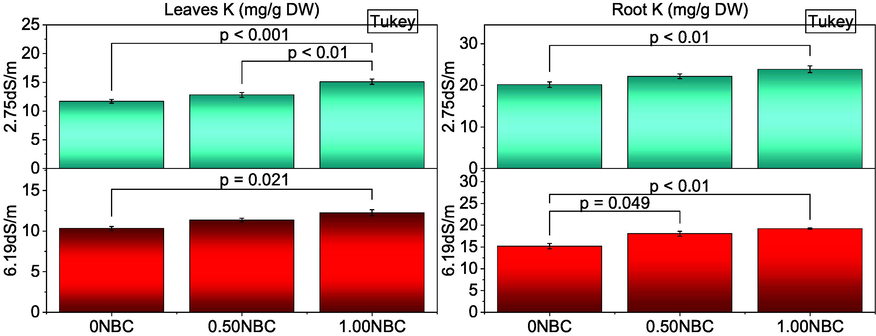
Effect of nitrate blended chemically produced carbon (NBC) different application rates on leaves K (A) and roots K (B) of maize under normal (2.75 dS/m EC) and saline (6.19 dS/m EC) soil. Different values on bars are p-values computed by paired comparison Tukey test; p ≤ 0.05. Bars are means of three replicates. 0NBC (control having no NBC); 0.50NBC (0.50 % w/w NBC applied in soil); 1.00NBC (1.00 % w/w NBC applied in soil). Red bars are indicating salinity stress. Green bars are indicating normal soil conditions.
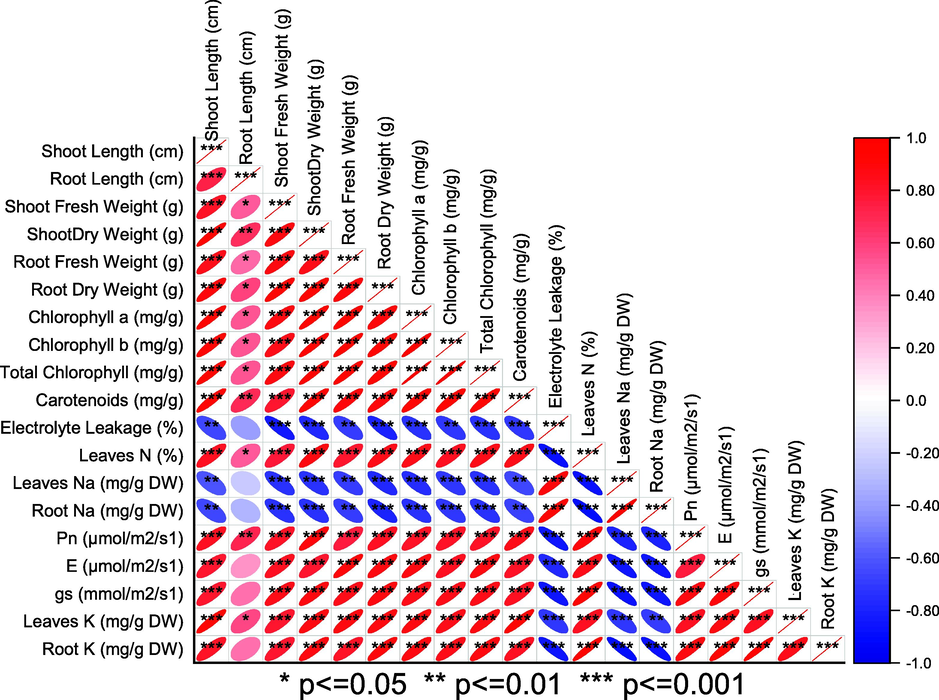
Pearson correlation for different studied attributed amended with different levels of nitrate blended chemically produced carbon (NBC) under normal (2.75 dS/m EC) and saline (6.19 dS/m EC) soil. The blue colour is indicating a negative while the red colour is indicating a positive correlation.
4 Discussion
In salt-affected soils, the higher concentration of Na ions competes with K and NO3 ions for their bioavailability to plants. Such conditions also imposed toxic effects on crop growth due to deficiency of essential macronutrients and the development of osmotic stress (Turcios et al., 2021; Wang et al., 2013). In a specific, deficiency of K also facilitates the higher uptake of Na (Botella et al., 1997). According to Amin et al. (2021) sodium chloride is the most important salt which adversely affects the growth of crops under saline conditions. Excessive uptake of Na in plants caused biosynthesis of reactive oxygen species (ROS). These ROS act as a potential toxin at the cellular level, thus inducing oxidative stress (Oi et al., 2020). Disruption of cellular membrane and derailing of homeostasis caused anatomical changes in chloroplast under saline conditions. i.e., oval and expanded mesophyll cells which remained elongated in shape under normal conditions (Oi et al., 2020). Loss in chloroplast structure due to activation of chylase by endogenous stress ethylene is also well documented. Higher salinity stress facilitates the biosynthesis of this ethylene in plants (Matile et al., 1997).
Results of the current study also validated these findings where chloroplast contents were significantly decreased in maize under salinity stress conditions. Application of 0.50 and 1.00NBC caused significant improvement in growth attributes of maize. This enhancement was mainly due to better uptake of N and K. Furthermore, less Na concentration in maize plants root and leaves minimize toxic effects when 1.00NBC was applied as an amendment. The improvement in chloroplast was associated with less uptake of Na and better accumulation of K and N in leaves by the addition of NBC. Furthermore, regulation of gas exchange attributes by NBC also validated the positive effects of K in maize plant leaves.
Better uptake of K caused a reduction in ROS synthesis by minimizing the activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidases and maintenance of photosynthetic electron transport activity (Waraich et al., 2012). Under optimum K uptake, stomatal guard cells become swollen via the absorption of water. Such conditions resulted in stomatal opening and the allowance of gaseous exchange between the environment and plants. Thus, K also controls the evapotranspiration (ET) of water through stomatal pores (Cochrane and Cochrane, 2009). In addition to the above, photosynthesis, carbohydrate translocation and metabolism that eventually increase the crop productivity and improve grain quality are also regulated by the balance uptake of K (Lu et al., 2016; Pettigrew, 2008; Zörb et al., 2014).
Application of 1.00NBC significantly improved N in maize over 0NBC in the current experiment. Better N uptake positively affects the photosynthetic process via facilitating Rubisco synthesis (Heckathorn et al., 1996). The improvement in photosynthetic rate in the current study was also associated with better uptake of N in 1.00NBC and 0.50NBC compared to 0NBC. Improvement in N uptake in plants facilitates mesophyll cell division and epidermal cell elongation. Both of these cells are considered key components of plants which played an imperative role enhancement of morphological growth attributes (MacAdam et al., 1989; Zeiger and Taiz, 2010). It also regulates cell cycle progression, biomass accumulation and growth under kinase activity, thus causing a significant increase in plant growth (Jüppner et al., 2018). The positive correlation of root and shoot fresh and dry weight with N in leaves is in agreement with the above arguments.
5 Conclusion
It is concluded that NBC has the potential to enhance maize growth under salinity stress. Improvement in chlorophyll contents was due to better uptake of N in maize leaves by the application of 1.00NBC than 0NBC. A significant improvement in stomatal conductance and transpiration rates was also associated with better uptake of K in root and shoot by 1.00 NBC under salt-affected soils. The application of 1.00NBC is a more efficacious approach than 0.50 and 0NBC for improvement in maize production in salt-affected soils. Growers are recommended to apply 1.00 % NBC for the achievement of optimum maize productivity in saline conditions. More investigations are suggested in different agroclimatic to declare NBC as the best amendment for alleviation of salinity stress in maize.
Acknowledgement
This project was supported by Researchers Supporting Project number (RSP-2021/230) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Immobilization of Cd in soil by biochar and new emerging chemically produced carbon. J. KSU. - Sci.. 2021;33:101472
- [CrossRef] [Google Scholar]
- Decomposition of different organic materials in soils. Biol. Fertil. Soils. 1994;18:175-182.
- [Google Scholar]
- Impacts of slow-release nitrogen fertilizer rates on the morpho-physiological traits, yield, and nitrogen use efficiency of rice under different water regimes. Agriculture. 2022;12:86.
- [Google Scholar]
- Ion homeostasis for salinity tolerance in plants: a molecular approach. Physiol. Plant.. 2021;171:578-594.
- [Google Scholar]
- Enhancing phosphorus availability, soil organic carbon, maize productivity and farm profitability through biochar and organic–inorganic fertilizers in an irrigated maize agroecosystem under semi-arid climate. Soil Use Manag.. 2021;37:104-119.
- [Google Scholar]
- Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant. Physiol.. 1949;24:1-15.
- [CrossRef] [Google Scholar]
- A deep learning approach to measure stress level in plants due to nitrogen deficiency. Measurement. 2021;173:108650.
- [Google Scholar]
- The long-term effects of microplastics on soil organomineral complexes and bacterial communities from controlled-release fertilizer residual coating. J. Environ. Manage.. 2022;304:114193.
- [Google Scholar]
- Salinity induced potassium deficiency in maize plants. Journal. Plant. Physiol.. 1997;150:200-205.
- [CrossRef] [Google Scholar]
- Bremner, M., 1996. Nitrogen-total. In: Sumner, D.L., A.L., S., P.A., P., R.H., H., N., L.P., A., S.M., T., T.C., E., J.M. (Eds.), Methods of Soil Analysis Part 3. Chemical Methods-SSSA Book Series 5. John Wiley & Sons, Inc., Madison, WI, USA, pp. 1085–1121.
- Methods of Analysis For Soils, Plants and water. Berkeley, CA, USA: University of California, Division of Agricultural Sciences; 1961.
- The vital role of potassium in the osmotic mechanism of stomata aperture modulation and its link with potassium deficiency. Plant. Signal. Behav.. 2009;4:240-243.
- [Google Scholar]
- Crop performance and soil fertility improvement using organic fertilizer produced from valorization of Carica papaya fruit peel. Sci. Rep.. 2021;11:1-16.
- [Google Scholar]
- Effect of foliar application of Fe and banana peel waste biochar on growth, chlorophyll content and accessory pigments synthesis in spinach under chromium (IV) toxicity. Open Agric.. 2019;4:381-390.
- [CrossRef] [Google Scholar]
- Biochar consequences on cations and anions of sandy soil. J. Biodivers. Environ. Sci.. 2015;6:121-131.
- [Google Scholar]
- Co-application of ACC-deaminase producing PGPR and timber-waste biochar improves pigments formation, growth and yield of wheat under drought stress. Sci. Rep.. 2019;9:5999.
- [CrossRef] [Google Scholar]
- ACC-deaminase producing plant growth promoting rhizobacteria and biochar mitigate adverse effects of drought stress on maize growth. PLoS One. 2020;15:e0230615.
- [Google Scholar]
- Combined role of ACC deaminase producing bacteria and biochar on cereals productivity under drought. Phyton (B. Aires). 2020;89:217-227.
- [CrossRef] [Google Scholar]
- Compost and compost tea from on-farm composted agro-wastes improve the sustainability of horticultural organic cropping systems. In: Agri-Based Bioeconomy. CRC Press; 2021. p. :143-162.
- [Google Scholar]
- Precision nitrogen management of wheat. A review. Agron. Sustain. Dev.. 2013;33:219-241.
- [Google Scholar]
- Determination of potassium and sodium by flame emmision spectrophotometery. In: Kalra Y., ed. Handbook of Reference Methods for Plant Analysis. Washington, D.C.: CRC Press; 1998. p. :153-155.
- [Google Scholar]
- Dustgeer, Z., Seleiman, M.F., Imran, K., Chattha, M.U., Alhammad, B.A., Jalal, R.S., Refay, Y., Hassan, M.U., et al., 2021. Glycine-betaine induced salinity tolerance in maize by regulating the physiological attributes, antioxidant defense system and ionic homeostasis. Not. Bot. Horti Agrobot. Cluj-Napoca 49, 12248.
- Methods of Soil, Plant, and Water Analysis : A manual for the West Asia and North Africa region (3rd ed.). Beirut, Lebanon: International Center for Agricultural Research in Dry Areas; 2013.
- Influence of microwave-assisted pyrolysis parameters and additives on phosphorus speciation and transformation in phosphorus-enriched biochar derived from municipal sewage sludge. J. Clean. Prod.. 2021;287:125550.
- [Google Scholar]
- Invasive plants as potential sustainable feedstocks for biochar production and multiple applications: A review. Resour. Conserv. Recycl.. 2021;164:105204.
- [Google Scholar]
- Organic amendments for soil restoration in arid and semiarid areas: a review. AIMS Environ. Sci.. 2017;4:640-676.
- [Google Scholar]
- Gee, G.W., Bauder, J.W., 1986. Particle-size analysis, in: Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods. Madison, pp. 383–411. https://doi.org/10.2136/sssabookser5.1.2ed.c15.
- An overview on biochar production, its implications, and mechanisms of biochar-induced amelioration of soil and plant characteristics. Pedosphere. 2022;32:107-130.
- [Google Scholar]
- Pongamia pinnata L. leaves biochar increased growth and pigments syntheses in Pisum sativum L. exposed to nutritional stress. Agriculture. 2019;9:153.
- [CrossRef] [Google Scholar]
- Nitrogen availability and vegetative development influence the response of ribulose 1,5-bisphosphate carboxylase/oxygenase, phosphoenolpyruvate carboxylase, and heat-shock protein content to heat stress in Zea mays L. Int. J. Plant Sci.. 1996;157:546-553.
- [CrossRef] [Google Scholar]
- How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy. 2021;13:1731-1764.
- [Google Scholar]
- The target of rapamycin kinase affects biomass accumulation and cell cycle progression by altering carbon/nitrogen balance in synchronized Chlamydomonas reinhardtii cells. Plant J.. 2018;93:355-376.
- [CrossRef] [Google Scholar]
- Precomposting and green manure amendment for effective vermitransformation of hazardous coir industrial waste into enriched vermicompost. Bioresour. Technol.. 2021;319:124136.
- [Google Scholar]
- Evaluation Effect of Farmyard Manure (FYM) to improve cereal crop yield. J. Crop Nutr. Sci.. 2021;7:59-67.
- [Google Scholar]
- Dependence of chloroplast pigment synthesis on protein synthesis: effect of actidione. Biochem. Biophys. Res. Commun.. 1965;21:523-530.
- [Google Scholar]
- Effects of adapted N-fertilisation strategies on nitrate leaching and yield performance of arable crops in North-Western Germany. Agronomy. 2021;11:64.
- [Google Scholar]
- Kuo, S., 1996. Phosphorus. In: Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E. (Eds.), Methods of Soil Analysis Part 3: Chemical Methods. John Wiley & Sons, Ltd, SSSA, Madison, Wisconsin, pp. 869–919. https://doi.org/10.2136/sssabookser5.3.c32.
- Effects of organic fertilizers on the soil microorganisms responsible for N2O emissions: A review. Microorganisms. 2021;9:983.
- [Google Scholar]
- Microbial metabolic efficiency and community stability in high and low fertility soils following wheat residue addition. Appl. Soil Ecol.. 2021;159:103848.
- [Google Scholar]
- Anatomical variation of mesophyll conductance under potassium deficiency has a vital role in determining leaf photosynthesis. Plant Cell Environ.. 2016;39:2428-2439.
- [Google Scholar]
- NaCl-induced senescence in leaves of rice (Oryza sativaL.) cultivars differing in salinity resistance. Ann. Bot.. 1996;78:389-398.
- [CrossRef] [Google Scholar]
- Effects of nitrogen on mesophyll cell division and epidermal cell elongation in tall fescue leaf blades. Plant Physiol.. 1989;89:549-556.
- [Google Scholar]
- Influence of nitrogen sources applied by fertigation to an enriched soil with organic compost on growth, mineral nutrition, and phytochemicals content of coriander (Coriandrum sativum L.) in two successive harvests. Plants. 2022;11:22.
- [Google Scholar]
- Crop nitrogen use efficiency for sustainable food security and climate change mitigation. In: Kumar V., Suprasanna A.K., Penna S., eds. Plant Nutrition and Food Security in the Era of Climate Change. Academic Press Inc., Elsevier; 2022. p. :47-72.
- [CrossRef] [Google Scholar]
- Localization of chlorophyllase in the chloroplast envelope. Planta. 1997;201:96-99.
- [Google Scholar]
- Formulation of new biostimulant of plant and soil correction based on humic acids extracted by magnetized water from compost from the waste of coffee marc and cattle manure. Waste Biomass Valor. 2021:1-13.
- [Google Scholar]
- Nitric-perchloric acid wet digestion in an open vessel. In: Kalra Y., ed. Reference Methods for Plant Analysis. Washington, D.C.: CRC Press; 1998. p. :57-62.
- [Google Scholar]
- Slow pyrolysis of cassava wastes for biochar production and characterization. Iran. J. Energy Environ.. 2012;3:60-65.
- [Google Scholar]
- Three-dimensional ultrastructural change of chloroplasts in rice mesophyll cells responding to salt stress. Ann. Bot.. 2020;125:833-840.
- [Google Scholar]
- OriginLab Corporation, 2021. OriginPro. OriginLab, Northampton, MA, USA.
- Oueriemmi, H., Kidd, P.S., Trasar-Cepeda, C., Rodr\’\iguez-Garrido, B., Zoghlami, R.I., Ardhaoui, K., Prieto-Fernández, Á., Moussa, M., 2021. Evaluation of composted organic wastes and farmyard manure for improving fertility of poor sandy soils in arid regions. Agriculture 11, 415.
- Page, A.L., Miller, R.H., Keeny, D.R., 1983. Soil pH and lime requirement. In: Page, A.L. (Ed.), Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 9.2.2/Agronomy Monographs. American Society of Agronomy, Inc. and Soil Science Society of America, Inc., Madison, pp. 199–208. https://doi.org/10.2134/agronmonogr9.2.2ed.
- Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physio. Plantar. 2008:670-681.
- [CrossRef] [Google Scholar]
- Responses of shoot biomass accumulation, distribution, and nitrogen use efficiency of maize to nitrogen application rates under waterlogging. Agric. Water. Manag.. 2022;261:107352.
- [Google Scholar]
- Insight into metal immobilization and microbial community structure in soil from a steel disposal dump that was phytostabilized with composted, pyrolyzed or gasified wastes. Chemosphere. 2021;272:129576.
- [CrossRef] [Google Scholar]
- Yield enhancement and better micronutrients uptake in tomato fruit through potassium humate combined with micronutrients mixture. Agriculture. 2021;11:357.
- [CrossRef] [Google Scholar]
- Salinity: electrical conductivity and total dissolved solids. In: Sparks D.L., Page A.L., Helmke P.A., Loeppert R.H., Soltanpour P.N., Tabatabai M.A., Johnston C.T., Sumner M.E., eds. Methods of Soil Analysis, Part 3, Chemical Methods. Madison, WI, USA: Soil Science Society of America; 1996. p. :417-435.
- [CrossRef] [Google Scholar]
- Effect of arbuscular mycorrhizal fungi on the physiological functioning of maize under zinc-deficient soils. Sci. Rep.. 2021;11:18468.
- [CrossRef] [Google Scholar]
- Mechanisms for increasing the pH buffering capacity of an acidic ultisol by crop residue-derived biochars. J. Agric. Food. Chem.. 2017;65:8111-8119.
- [CrossRef] [Google Scholar]
- Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote. Sens. Environ.. 2002;81:337-354.
- [CrossRef] [Google Scholar]
- Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Nelson, D.W., Sommers, L.E., 1996. Total carbon, organic carbon, and organic matter. In: Methods of Soil Analysis Part 3—Chemical Methods. Soil Science Society of America, American Society of Agronomy, pp. 961–1010. https://doi.org/10.2136/sssabookser5.3.c34.
- Principles and Procedures of Statistics: A Biometrical Approach (3rd ed.). Singapore: McGraw Hill Book International Co.; 1997.
- Chemical production of acidified activated carbon and its influences on soil fertility comparative to thermo-pyrolyzed biochar. Sci. Rep.. 2020;10:595.
- [CrossRef] [Google Scholar]
- Potassium, an important element to improve water use efficiency and growth parameters in quinoa (Chenopodium quinoa) under saline conditions. J. Agron. Crop Sci. 2021
- [Google Scholar]
- Type of organic fertilizer rather than organic amendment per se increases abundance of soil biota. Peer. J.. 2021;9:e11204.
- [Google Scholar]
- Wakeel, A., Kiran, A., Shahid, M.R., Bano, Z., Zia, M.H., 2022. Trends in nitrogen use and development in Pakistan. In: Aziz, T., Wakeel, A., Watto, M.A., Sanaullah, M., Maqsood, M.A., Kiran, A. (Eds.), Nitrogen Assessment: Pakistan as a Case-Study. Academic Press Inc. Elsevier, pp. 73–97. https://doi.org/10.1016/B978-0-12-824417-3.00002-2.
- The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013
- [CrossRef] [Google Scholar]
- Alleviation of temperature stress by nutrient management in crop plants: A review. J. Soil Sci. Plant Nutr.. 2012;12:221-244.
- [CrossRef] [Google Scholar]
- Variations in precipitation extremes in the arid and semi-arid regions of China. Int. J. Climatol.. 2021;41:1542-1554.
- [Google Scholar]
- Nitrate removal by alkali-resistant Pseudomonas sp. XS-18 under aerobic conditions: Performance and mechanism. Bioresour. Technol.. 2022;344:126175.
- [Google Scholar]
- Supplemental effects of biochar and foliar application of ascorbic acid on physio-biochemical attributes of barley (Hordeum vulgare L.) under cadmium-contaminated soil. Sustainability. 2021;13:9128.
- [Google Scholar]
- Role of cotton sticks biochar in immobilization of nickel under induced toxicity condition and growth indices of Trigonella corniculata L. Environ. Sci. Pollut. Res.. 2020;27
- [CrossRef] [Google Scholar]
- Mechanisms underlying nitrous oxide emissions and nitrogen leaching from potato fields under drip irrigation and furrow irrigation. Agric. Water. Manag.. 2022;260:107270.
- [Google Scholar]
- Effect of cadmium-tolerant rhizobacteria on growth attributes and chlorophyll contents of bitter gourd under cadmium toxicity. Plants. 2020;9:1386.
- [CrossRef] [Google Scholar]
- Compost mixed fruits and vegetable waste biochar with ACC deaminase rhizobacteria can minimize lead stress in mint plants. Sci. Rep.. 2021;11:6606.
- [Google Scholar]
- Plant Physiology. Sunderland, MA, USA: Sinauer Associates Inc.; 2010.
- Potassium in agriculture–status and perspectives. J. Plant. Physiol.. 2014;171:656-669.
- [Google Scholar]







