Translate this page into:
Neurotoxicity of aluminium chloride and okadaic acid in zebrafish: Insights into Alzheimer's disease models through anxiety and locomotion testing, and acute toxicity assessment with Litsea garciae bark's methanolic extract
⁎Corresponding author. quahmed@iium.edu.my (Qamar Uddin Ahmed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Alzheimer's disease (AD) is a complicated neurodegenerative disorder that presents significant challenges for the development of effective therapeutic interventions. Understanding disease mechanisms and exploring potential treatments require the use of animal models that accurately replicate the pathology of AD. In this study, we investigated the potential of two neurotoxin inducers, aluminium chloride (AlCl3) and okadaic acid (OKA), to validate the zebrafish as a model organism for AD. AD can impact locomotor activity and induce anxiety-like behaviors. To assess these behaviors, a 6-minute novel tank test was conducted. Zebrafish were administered with low, medium, or high doses of neurotoxic agent (AlCl3 or OKA) intraperitoneally twice weekly for 21 days. Behavioral activities were recorded at three time points: day 7 (short duration), day 14 (moderate duration), and day 21 (extended duration). The behavioral task required the evaluation of four endpoints. Methanolic extract of Litsea garciae bark was selected as a potential plant for the treatment of AD in this study, based on its previously demonstrated antioxidant effect. However, the acute toxicity of this plant has not been previously assessed. Therefore, this research was aimed to investigate the acute toxicity of the L. garciae bark’s methanolic extract in adult zebrafish. The extract was immersed in a static system following OECD Test Guideline No. 203, and the acute toxicity test involved monitoring the adult zebrafish for 96 h for any deaths or apparent abnormalities. Regarding the behavioural task, the groups induced with 100 nM of OKA demonstrated significant differences in all measured parameters compared to the control group at the 21-day time point. In contrast, none of the parameters were significantly different between the AlCl3-induced groups and the control group at any of the three time points (7, 14, or 21 days). Regarding acute toxicity, neither the test group (100 mg/L) nor the control group recorded any deaths or abnormalities. Therefore, no LC50 value could be determined. These findings confirm the acceptance of OKA as an inducer in the zebrafish model of AD and highlight the significance of the safe and non-toxic nature of L. garciae bark's methanolic extract for future ethnopharmacological investigations.
Keywords
Alzheimer's disease
Adult zebrafish
Aluminium chloride
Okadaic acid
Litsea garciae
Acute toxicity
- AD
-
Alzheimer's disease
- AlCl3
-
Aluminium chloride
- OKA
-
okadaic acid
- NTT
-
Novel tank test
- LG
-
Litsea garciae
- CREAM
-
Central Research and Animals Facility
- IACUC-IIUM
-
Institutional Animal Care & Use Committee IIUM
- SBC
-
Sarawak Biodiversity Centre
- OECD
-
Organisation for Economic Cooperation and Development
- LC50
-
lethal concentration
- pH
-
potential of hydrogen
Abbreviations
1 Introduction
Alzheimer's disease (AD) can have secondary effects on anxiety and locomotion in addition to its primary impact on cognitive functions such as memory, thinking, and reasoning. Research has shown that AD is associated with prominent neuropsychiatric symptoms including anxiety (Mendez, 2021). Pottorf et al. (2022) revealed that individuals who are older with AD had lower magnitudes of split-belt locomotor adaptation compared to healthy older adults.
The physiology and immunity of zebrafish (Danio rerio) exhibit specialisations similar to those of mammals, as shown by whole-genome sequencing and new data. As a result, this tiny organism is now a frequently utilised model for research on human diseases (Holtzman et al., 2016). According to Chia et al. (2022), many human neurodegenerative illnesses including AD have been successfully modelled in zebrafish. Based on Tierney (2011), zebrafish are a valuable model for identifying neurological disorders. They offer a practical means of studying neurotoxicity and neurodegeneration. Zebrafish have received significant attention as a good model for studying neurobehavioral studies. Adult zebrafish have been studied for their behaviour in a variety of contexts, including anxiety (Barcellos et al., 2007) and learning and memory (Colwill et al., 2005). Moreover, zebrafish are ideal for drug investigations as medication can be easily added to the water in their tank.
Behaviour is a functional indicator of the net sensory, motor, and integrative processes in the central and peripheral nervous systems, which are thought to be more sensitive in detecting toxicity (Mitchell et al., 1982). So far, scientists have studied around 190 different behaviours in zebrafish using various tests, including the novel tank test and T (Y)-maze test (Petersen et al., 2022). The novel tank test (NTT) is employed as a behavioural assessment to evaluate anxiety-like behaviour and locomotion in zebrafish (Zanandrea et al., 2018). Kysil et al. (2017) opined that a novel tank is a valuable instrument for researching anxiety-like behaviour in zebrafish. During this test, individual fish are placed in a designated aquarium and observed for six minutes. Their position in the tank, specifically their ability to distinguish between the bottom and upper levels, is believed to indicate their level of anxiety. By analysing particular parameters, such as the effects of drugs such as aluminium chloride (AlCl3) and okadaic acid (OKA), it is possible to determine their impact on anxiety-like behaviour in zebrafish.
Aluminium does cause neurotoxicity (Kumar & Gill, 2014). The use of aluminium as a model for neurotoxicity is supported by observable functional impairments resulting from neurodegeneration in the brain of zebrafish. The presence of aluminium interferes with the signalling processes in pathways linked to AD (Sudwarts, 2017). He et al. (2012) found that exposing zebrafish to AlCl3 in acidic conditions affected their memory retention. The study investigated the effects of aluminium exposure on the zebrafish's locomotor activity, learning, and memory abilities. These results indicate a potential link between aluminium exposure, AD, and its impact on memory.
Researchers have used OKA to study how AD disease works (Malekzadeh et al., 2017). This chemical compound selectively blocks serine/threonine phosphatases 1 and 2 in the rat brain, leading to memory problems and neurotoxicity (Suganuma et al., 1988). In vivo and in vitro investigations have demonstrated that this chemical causes tau hyperphosphorylation (Kamat et al., 2011). Additionally, it raises calcium levels in the brain, which can cause abnormal tau phosphorylation and neuronal death (Kamat et al., 2013). These effects are similar to the symptoms seen in people with AD. According to a study by Nada et al. (2016), the Alzheimer's disease (AD) model induced by OKA in zebrafish can help discover new drugs and conduct preclinical testing for potential AD therapies. Zebrafish is rapidly emerging as an essential model organism for aquatic neuropharmacology and toxicology research (Kalueff et al., 2016). For example, Ni et al. (2019) examined the acute toxicity of maduramicin on zebrafish using a semi-static test for 96 h. The zebrafish model’s adaptability offers the potential for examining the toxicological effects of various chemicals. Zebrafish is an exceptional model organism for toxicological testing because of its distinct features and suitability for various laboratory techniques.
Litsea garciae S. Vidal (locally known in Sarawak as Engkala) belongs to the family Lauraceae. It comes under the endemic category in Indonesia, Taiwan, the Philippines and Malaysia’s southwest Sabah region, Kalimantan. It is a medium-sized tree and its different plant parts (i.e., leaves, bark and wood chips) have been traditionally used in herbal medicine systems in Sarawak, Malaysia to treat various ailments by several tribes in Sarawak (Lim, 2012). Owing to its several traditional medicinal uses and lack of scientific validation for many of its traditional and beneficial uses, this study aimed to investigate the acute toxicity of a methanolic extract of LG bark on adult zebrafish to further confirm and support its safe traditional medicinal uses by different tribes in Sarawak, Malaysia. The harmful substance is primarily emitted through plants, animals, microbes, leaves, fruits, and barks. As a poisonous agent, it will spread the toxic chemical by a variety of mechanisms of transmission, the most common being direct contact. A toxicological test is necessary for allopathic therapy and complementary and alternative medicine. It is designed to discover any adverse effects that do not appear until excessive use causes signs and symptoms (Thiagarajan et al., 2019). A previous study (Raduan et al., 2022) discovered that the methanol extract of LG bark possessed significant antioxidant capabilities compared to the hexane, chloroform, and aqueous extracts. The potential utilization of methanolic bark extract's significant antioxidant properties may be considered in the inhibition of detrimental oxidation during the treatment of neurological disorders, such as AD. As a result, the methanol extract of LG bark was selected in this research study. Before studying its neuroprotective effects in the zebrafish Alzheimer’s model, acute toxicity testing is required to establish a safe dose. The purpose of toxicity testing is to provide dose-dependent responses to the toxicity effect, evaluate the safety of sample components, and verify methods for measuring toxicity (Arome & Chinedu, 2013).
Hence, this study was carried out to validate the experimental design that involved using zebrafish as a model for AD by conducting behavioural assessment involving anxiety and locomotion testing and employing the potent neurotoxin. Considering utilizing LG methanolic crude extract as a potential treatment in the AD-zebrafish model, it is crucial to conduct toxicity assessment to establish its safety and determine the optimal dosage range for treatment.
2 Materials and methods
2.1 Animal care
Adult and healthy wild-type zebrafish of both sexes (Danio rerio; 3–4 months-old; standard short-fin phenotype) were obtained from Central Research and Animals Facility (CREAM), IIUM Kuantan Campus, Pahang, Malaysia. The zebrafish used in this study were housed in the animal facility of the Basic Medical Sciences Department, Kulliyyah of Pharmacy, IIUM Kuantan Campus, Pahang, Malaysia, under standard husbandry conditions. The zebrafish were kept in a controlled environment with a temperature range of 24 °C to 30 °C, a pH range of 6.8 to 8.5, and a light intensity of 250 lx. They were exposed to a 14-hour- and 10-hour dark cycle, with lights turning on at 7 am and off at 9 pm. The zebrafish were given Tetra Bits Complete (Tetra GmbH in Germany) as their daily diet. The fish were fed three times a day to ensure a steady source of nourishment and were allowed to eat freely. The tank used was a standard size and had a circulating water system with constant aeration. It measured 25 × 30 × 30 in. (width × height × length). Male and female fish were mixed-housing in single tank as according to (Norton et al., 2008), no sex differences were made in behaviour in recording setups. All the animal experimentations carried out in this research work were initially approved by The Institutional Animal Care and Use Committee (I-ACUC) IIUM Kuantan Campus, Pahang, Malaysia with approval no of IIUM/IACUC-2019(21).
2.2 Plant material
The bark of Litsea garciae S. Vidal (LG bark) was collected from Kampung Sentosa Salim, Sibu, Sarawak (coordinates: 2.2735800, 111.866599) and authorised by Sarawak Biodiversity Centre (SBC) with voucher specimen number SBC-2018-RDP-15-SZR. The raw plant material was collected, dried, and ground using a commercial blender (Waring Commercial, USA) before extraction.
2.3 Extraction of the plant
The dried powder of LG bark was successively macerated with hexane, chloroform, and methanol in ascending order of polarity. It was initially soaked in hexane (approximately 42 h; 1:10). To extract the bark’s hexane-soluble components; it was soaked three times (until exhausted). Following that, the residue was dried in the fume hood and soaked in chloroform to remove chloroform-soluble compounds. Finally, the dried residue was soaked in methanol after being macerated with chloroform to yield methanolic soluble components. A rotary evaporator was used to dry the filtrate obtained with each solvent (Buchi, Switzerland) (Raduan et al., 2022). The methanolic LG bark crude was then freeze-dried for 72–96 h (Martin Christ, Germany). The residue was thrown away after obtaining all extracts.
2.4 AlCl3-induced and OKA-induced Alzheimer model
12 zebrafish were used for both the AlCl3-induced and OKA-induced models. Each group's sample size (n) was calculated based on a power analysis of previous behavioural experiments (Koehler et al., 2019; Kundap et al., 2017).
As for AlCl3-induced Alzheimer’s model, the experimental design consisted of a control group that received normal saline (0 mg/kg AlCl3) and an AlCl3-induced group that received of 75 mg/kg, 150 mg/kg (Maheswari et al., 2014), or 300 mg/kg of AlCl3 twice weekly via intraperitoneal injection for 21 days.
Meanwhile for OKA-induced Alzheimer’s model, the experimental design consisted of a control group that received 90 percent alcohol (0 nM OKA) (Nada et al., 2016; Koehler et al., 2019) and OKA-induced group that received of 25, 50 or 100 nM (Nada et al., 2016; Koehler et al., 2019) of OKA twice weekly via intraperitoneal injection for 21 days. On day 7 (short duration), day 14 (moderate duration), and day 21 (extended duration), a six min-novel tank test (NTT) was carried out.
2.5 Novel tank test (NTT): Anxiety and locomotion test
On days 7, 14 and 21, zebrafish were transferred individually to a novel tank (22 × 26 × 36 cm; width × height × length) [Fig. 2.1(a)] filled with dechlorinated water (up to 20 cm water depth). The novel tank's outer wall had a dividing line that split it into two equal virtual horizontal sections (Egan et al., 2009) and rested on a level, stable surface [Fig. 2.1b]. Once relocated to the novel aquarium, the swimming behaviours of zebrafish were recorded for six minutes. The recording was made using a video recorder with subsequent analysis of traces by Smart V3.0.05 tracking software (Pan Lab, Harvard Apparatus, USA). The four endpoints measured included the latency to reach the upper half of the tank (s), number of transitions (entries) to the upper half, time spent in the upper half (s) and total distance in tank (cm). Locomotor patterns were also documented. These endpoints were selected based on the solid evidence accumulated from past research utilizing the novel tank diving paradigm for zebrafish (Barcellos et al., 2007; Kundap et al., 2017; Levin et al., 2007; Zanandrea et al., 2018).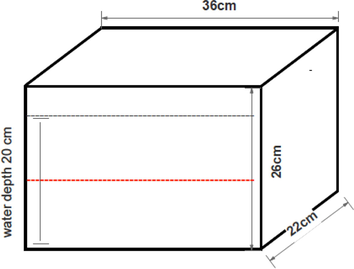
Novel tank.

Novel tank test set up.
2.6 Acute toxicity
Tests on adult zebrafish were performed according to OECD Test Guideline No 203 (OECD, 2019). Conditions of exposure were taken into account during the testing. As for static conditions, a maximum loading of 0.8 g wet weight fish per litre was followed. The photoperiod ranges with a 14 h light: 10 h dark regime (light onset: 7 am; light offset: 9 pm) was adhered. The water temperature was maintained between 21 and 25 °C. The oxygen concentration was maintained at a minimum of 60% of the air saturation level. Throughout the test, aeration was applied. pH was kept between 6.0 and 8.5. Feeding was not allowed. To the maximum extent possible, excessive vibration or noise that may have influenced the fish's behaviour were minimised. Throughout the 96 h testing period, all of the specified conditions were maintained.
The testing groups (7 fish per group) were exposed (by immersion) to the methanolic LG bark crude extract (proposed dose of 6.25, 12.5, 25, 50 and 100 mg/ L) or without methanolic LG bark crude (0 mg/ L) for a period of 96 h under static condition. According to OECD (2019), a limit test may be performed for 96 h at 100 mg/ L in order to demonstrate that the LC50 is greater than this concentration. Thus, the test was started with the 96 h observation on the testing group of 0 mg/L and 100 mg/ L of LG methanolic bark crude extract. Three observations were made within the first 24 h of testing, with a minimum of 3 h between observations. Fish were inspected at 2 ± 0.5 h, 5 ± 1 h and 24 ± 2 h after the start of the exposure (day zero to one). Throughout days two to four of the test, tanks containing live fish were inspected twice daily (early morning and late afternoon). When there was no visible movement (e.g., no opercular motion) and touching the caudal peduncle did not elicit a response, the fish was considered dead. Any deaths and visible abnormalities in equilibrium, swimming behaviour, ventilatory (respiratory) function, skin pigmentation, and other visible characteristics, exophthalmia were observed. The concentrations required to kill 50 percent of the fish were determined wherever possible (LC50).
2.7 Statistical analysis
The statistical analysis used to investigate zebrafish behaviour included employing a two-way analysis of variance (ANOVA) in conjunction with a multiple comparison test. The Turkey post hoc test was specifically employed for additional analysis. In the toxicological study, on the other hand, the weight and pH changes were examined using a one-way ANOVA. All these analyses were conducted using Graph Pad Prism (version 9).
3 Results and discussion
3.1 The latency to reach upper half of the tank (s)
Based on Fig. 3.1(a) , no significant variation was observed in the latency to reach the upper half of the tank between the AlCl3-induced dosage-dependent groups and the control group throughout the 21-day observation period. Conversely, by day 21, the groups induced with 50 nM and 100 nM of OKA exhibited a significant difference compared to the control group [Fig. 3.1(b)].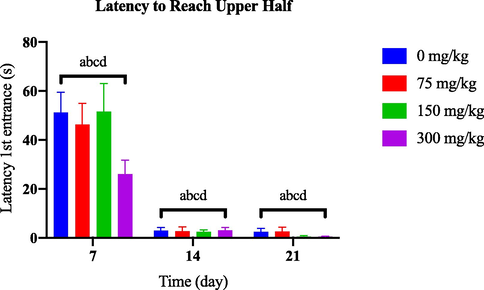
Comparison of latency first entrance to upper half between AlCl3-induced dosage dependent groups within a set of time.
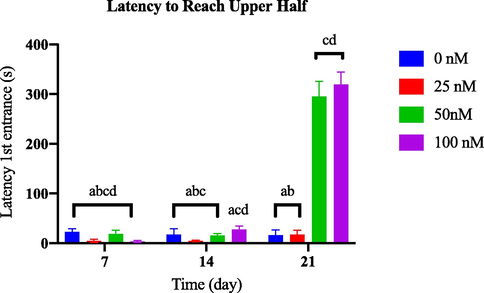
Comparison of latency first entrance to upper half between OKA-induced dosage dependent groups within a set of time.
Time spent at the top and latency to reach the top are zebrafish parameters associated with anxiety-like behaviour (Kysil et al., 2017). Increased latency to enter the upper half of the tank can indicate higher levels of anxiety-like behaviour. Zebrafish that exhibit prolonged latency to explore the upper half may display avoidance or hesitation in response to potential threats or stressful stimuli (Egan et al., 2009). A significant decrease in exploration (longer latency to reach the top, fewer entries to the top) indicates behavioural profiles that reveal elevated anxiety (Barcellos et al., 2007; Levin et al., 2007).
3.2 Number of entries in the upper half
According to Fig. 3.2(a), there was no notable change in the number of entries in the upper half of the tank among the groups induced with different doses of AlCl3 and the control group throughout the 21-day observation period. However, on day 21, the groups induced with varying doses of OKA showed a significant difference compared to the control group. In particular, the group treated with 100 nM OKA demonstrated a significant decrease in entries to the upper half starting from day 7, as shown in Fig. 3.2(b).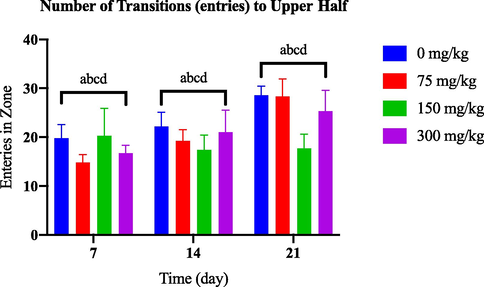
Comparison of entries in upper half between AlCl3-induced dosage dependent groups within a set of time.
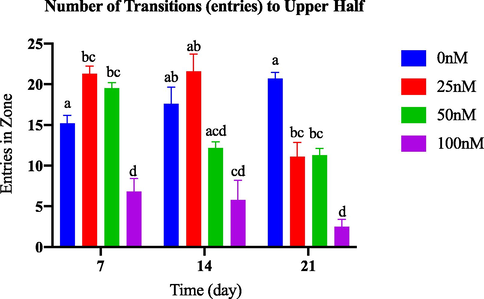
Comparison of entries in upper half between OKA-induced dosage dependent groups within a set of time.
A decrease in the number of entries into the upper half zone of the tank can indicate reduced exploratory behaviour and increased anxiety-like behaviour in zebrafish. Zebrafish that display increased anxiety levels exhibit reduced movement towards the upper area of the tank, as they perceive this region as aversive and strive to avoid it.
When analysing their locomotion and anxiety-like behaviour, it is imperative to consider the effect of mobility changes on zebrafish. Their ability to reach the upper part of the tank is crucial, and impairment caused by neurotoxins can lead to reduced exploration of their surroundings and limit their access to the upper zone (Zanandrea et al., 2018).
3.3 Time spent in the upper half
During the 21-day observation period, no notable changes were observed in the time spent in the upper half among the groups exposed to various concentrations of AlCl3 and the control group, as depicted in Fig. 3.3(a). However, as shown in Fig. 3.3(b), from day 14 onwards, the group treated with 100 nM of OKA spent significantly less time in the tank's upper half than the control group.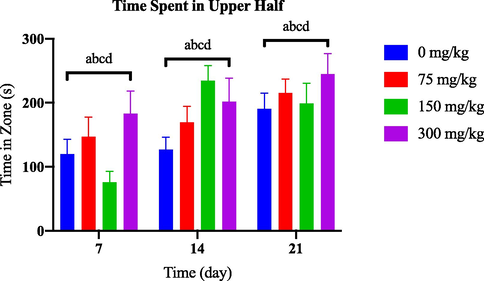
Comparison of time in zone upper half between AlCl3-induced dosage dependent groups within a set of time.
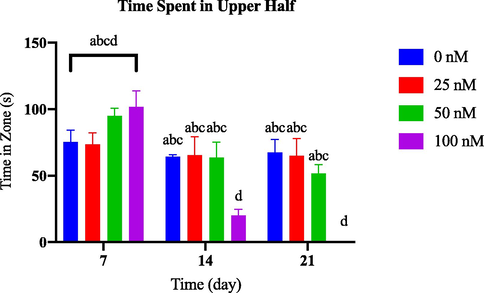
Comparison of time in zone upper half between OKA-induced dosage dependent groups within a set of time.
To assess the impact of neurotoxins on zebrafish behaviour, we can closely monitor the duration of time they spend in the upper portion of their tank. Should their time spent there decrease, it could indicate that they are less inclined to explore, more prone to anxiety, or prefer to remain in the lower half of the tank. It is important to note that if a zebrafish exhibits heightened anxiety levels, it may actively avoid the upper half of the tank, which could potentially put them in danger (Zanandrea et al., 2018).
Golla et al. (2020) found that stressed fish tend to avoid the upper portion of the tank more than their unstressed counterparts, which may indicate anxious behaviour. This behaviour resembles the one observed in zebrafish exposed to high-tension or anxiety-inducing drugs, as explained by Kalueff et al. (2016), where they exhibit decreased activity at the top and increased activity at the bottom of a novel tank. Furthermore, Collier et al. (2017) also observed that zebrafish in a new tank tend to display anxiety-like behaviour by staying at the bottom and reducing their exploration.
3.4 Locomotor activity
Throughout the 21-day observation period, there was no discernible variation in the total distance travelled by the groups that received varying doses of AlCl3 compared to the control group [as depicted in Fig. 3.4(a)]. However, Fig. 3.4(b) demonstrates a significant decrease in total distance travelled by the 100 nM OKA group from day 14 onwards.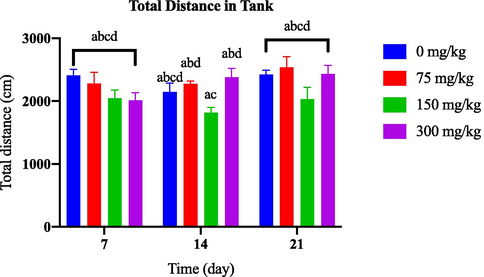
Comparison of total distance in tank between AlCl3-induced dosage dependent groups within a set of time.
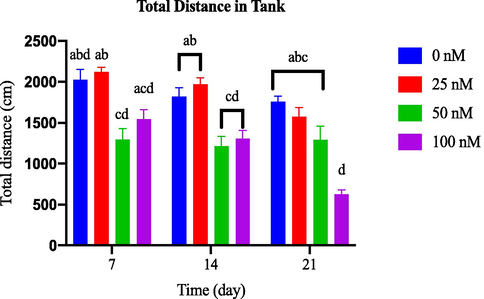
Comparison of total distance in tank between OKA-induced dosage dependent groups within a set of time.
Locomotor activity quantified by total distance travelled (Tran et al., 2016). Increased distance travelled may indicate hyperactivity or heightened exploratory locomotion, while decreased distance travelled may suggest reduced locomotor activity, which can be associated with anxiety-like behaviour in zebrafish (Levin et al., 2007). Zebrafish exhibiting lower anxiety levels tend to swim greater distances compared to those with higher anxiety levels. Consequently, measuring the distance travelled by fish can be a valuable approach for studying the impact of neurotoxins on zebrafish behaviour, particularly in relation to anxiety-like behaviour (Zanandrea et al., 2018).
3.5 Locomotor pattern
The regular movement patterns of the zebrafish in the AlCl3-induced dependent groups were similar to those in the control group [Fig. 3.5(a)]. This similarity was consistent with the insignificant findings in the total distance travelled by the AlCl3-induced dependent groups throughout 21 days of observation.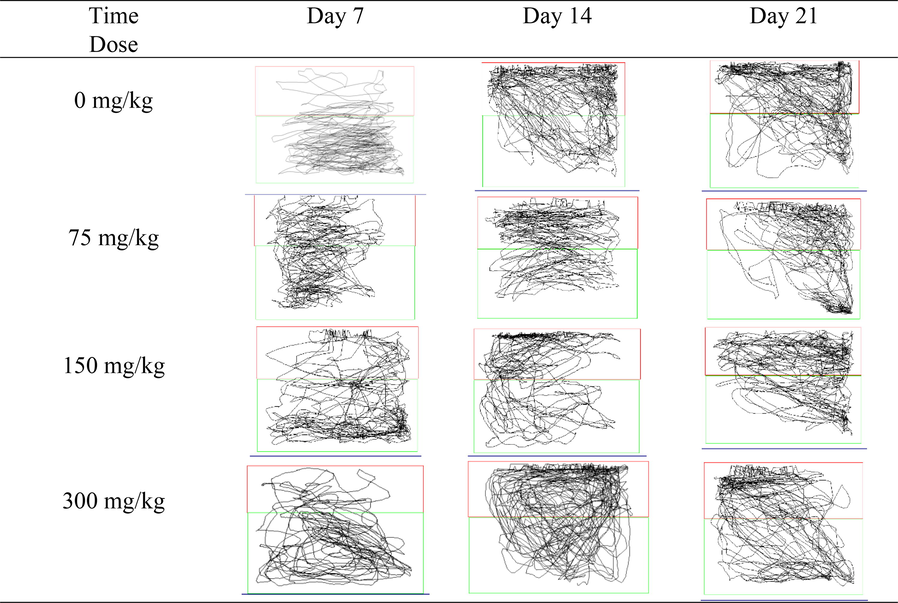
Locomotion tracking patterns in AlCl3-induced dosage dependent groups.
From the 14th day onward, it was observed that the zebrafish generally preferred to remain near the bottom of the tank while in motion [Fig. 3.5(b)]. This behaviour aligns with the findings of the 100 nM OKA group, which exhibited a significant reduction in total distance.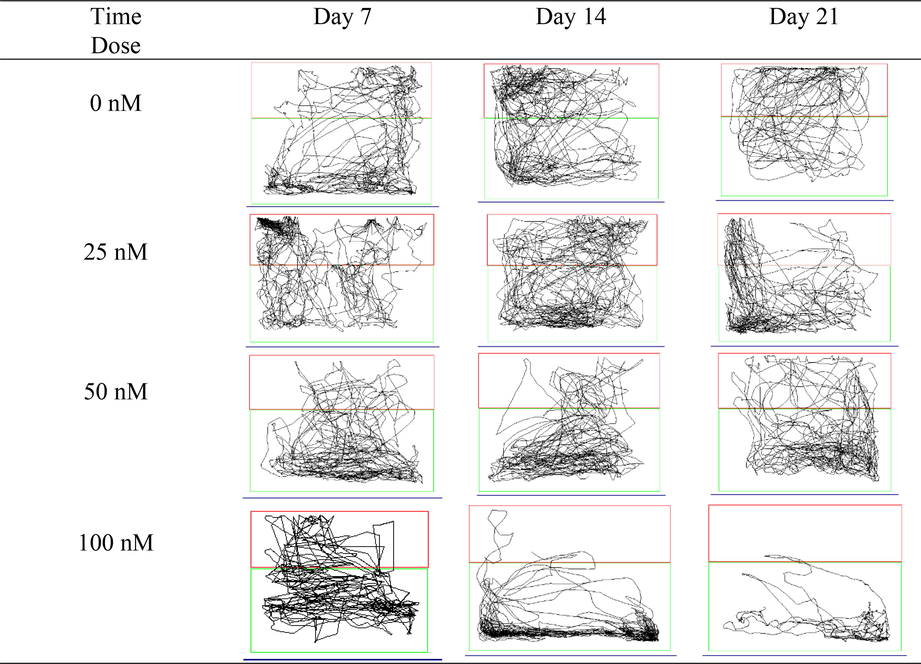
Locomotion tracking patterns in OKA-induced dosage dependent groups.
The complexity of locomotor activity can be measured in an animal model. It includes where, how, and at what speed zebrafish begin to swim when exposed to a new environment and when the fish has become acclimated to this location (Vásquez et al., 2012). Changes in locomotion, as reflected by alterations in total distance travelled (Anichtchik et al., 2004), can provide insights into behavioural modifications. By closely examining the movement patterns of zebrafish, we can understand a typical behaviour triggered by OKA. By analysing their swimming trajectories, we can accurately pinpoint the reasons behind the unusual swimming behaviour of OKA-induced zebrafish in the tank.
OECD (2019) advised utilising the juvenile stage when testing for acute toxicity. Nonetheless, adult zebrafish were used in this investigation because of the current research design's requirements, which will depend on the respective acute toxicity result. The use of adult zebrafish in acute toxicity testing has become a standard method to evaluate the toxicity levels of various chemicals. In the research conducted by Mak et al. (2019), adult zebrafish were exposed to individual polyethylene microplastics at a concentration of 2 mg/L for 96 h. It was to determine the upper and lower limits of microplastic ingestion and its effects. Hill et al. (2005) exposed adult zebrafish to various toxicants, including lead, uranium, malathion, metronidazole, and anilines, to determine acute toxicity and adverse effects.
The concentration limit of fish acute toxicity is 100 mg/L (OECD, 2019). Hence, no mortalities were reported at the 100 mg/L concentration. According to the up-and-down test (Sunderam, 2004), if the fish survives after 24 h, they will be exposed to a concentration that is 1.3 times higher and vice versa. Survival of the test group at the concentration limit of 100 mg/L demonstrated that higher concentrations should not be used. The observed effects are utilised to establish the chemical's lethal concentration (LC50), defined as the concentration at which fifty per cent of the exposed fish die (Xiong et al., 2022). The LC50 was not established since the concentration limit had reached during testing. In addition, no noticeable abnormalities were observed in fish exposed to the 100 mg/L concentration limit. All fish found to have typical orbital sockets and no abnormal skin pigmentation (darker, lighter, or mottled). The findings are illustrated in Fig. 3.6 and presented in Table 3.1. Specific clinical symptoms of toxicity may vary depending on the chemical being studied. However, some common signs of acute toxicity in zebrafish include lethargy, loss of equilibrium, alterations in swimming behaviour, and respiratory distress (Ni et al., 2019).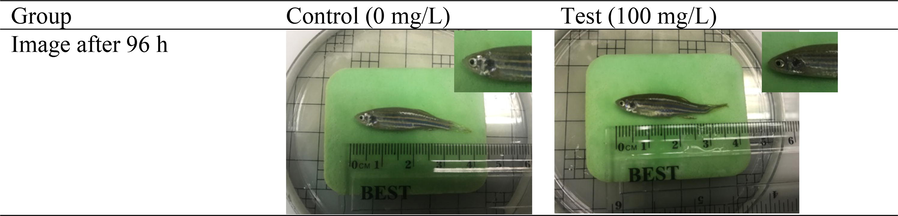
Image of adult zebrafish treated with Litsea garciae methanolic bark crude extract after 96 h of clinical signs observation.
Group
Control (0 mg/ L)
Test (100 mg/L)
Clinical sign
Visible movement
+
+
Touching caudal peduncle
nil
nil
Loss of equilibrium
–
–
Abnormal swimming behaviour
–
–
Abnormal respiratory/ ventilatory function
–
–
Abnormal skin pigmentation
–
–
Exophthalmia
–
–
Respective findings manifested that the LG methanolic bark crude extract is safe to use (with the appropriate extract preparation). Furthermore, 100 mg/L of LG methanolic bark crude extract is the maximum concentration for immersion in an upcoming experiment using adult zebrafish as an animal model.
In addition, zebrafish wet body weight and pH level were recorded before and after 96 h LG methanolic bark crude extract exposure. Based on Fig. 3.7(a), no significant weight changes were detected after 96 h exposure with LG methanolic bark crude extract. However, it showed a small decreasing pattern on the testing group (exposure to 100 mg/L of LG methanolic bark crude extract) compared to the control group. While weight loss is not typically quantified as a defined endpoint in zebrafish toxicity studies, alterations in growth, development, and behaviour can serve as markers of toxicity (Zhang et al., 2003). Consequently, weight loss or other changes in body composition may occur in zebrafish exposed to toxicants. These changes may be utilised as a part of a suite of endpoints to determine toxicity. Additional research may be required to completely comprehend the association between toxicant exposure and zebrafish weight reduction.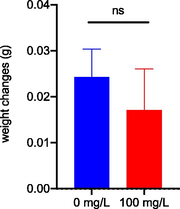
Weight changes on zebrafish after 96 h exposure to Litsea garciae methanolic bark crude extract.
Meanwhile, Fig. 3.7(b) showed no significant changes on pH level after 96 h immersion of LG methanolic bark crude extract compared to the control group. Changes in pH can have toxicological consequences for zebrafish. The lower and upper lethal limits of pH for zebrafish were determined to be 3.0 and 12.0, respectively, and two hours of exposure to these pH levels induced fish death (Zahangir et al., 2015). In addition, exposure to low pH levels can have physiological effects on freshwater fish, including zebrafish, such as inhibition of active sodium ion uptake and increased rates of passive sodium ion losses, resulting in a drop in plasma sodium ion concentration. Most examined fish species exhibit these effects at pH levels between 4.0 and 4.5 (Kwong et al., 2014). In conclusion, exposure to changed pH levels can have toxicological and physiological impacts on zebrafish, emphasising the significance of monitoring and maintaining appropriate pH values in aquatic habitats to preserve the health of aquatic species.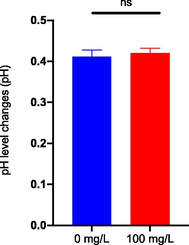
Changes in pH level on treatment water after 96 h of Litsea garciae methanolic bark crude extract immersion.
In a case study on the effects of changing the pH of numerous drugs of abuse, it was discovered that even a single pH unit change could result in significant locomotor alterations in zebrafish at a constant drug concentration (Cleal et al., 2020). These results demonstrate the necessity of managing environmental parameters in zebrafish husbandry to reduce experimental variability.
4 Conclusion
Administering at least 100 nM of OKA appears to be a neurotoxic agent. This dose resulted in significant differences across all measured endpoints and could induce anxiety when administered for at least 21 days. The crude methanolic extract of LG bark was safe and non-toxic based on OECD 203 guideline for acute toxicity test on adult zebrafish. Investigations of acute toxicity typically determine the selection of doses. In most instances, at least three doses of a test substance are administered or exposed to demonstrate dose dependence. The concentration limit of 100 mg/L of LG methanolic bark crude extract should be used as a guideline to set the dose-dependent experimental design. Moreover, it is recommended to do further observations on the effects of LG methanolic bark crude extract by histology, including crude accumulation in fish tissues. Further toxicity studies can be carried out with a sub-acute toxicity test (14 days) in zebrafish. To summarize, a 100 nM of OKA is recommended as the standard amount to induce AD in a zebrafish AD model. This dosage should be administered alongside the methanolic extract of LG bark for treatment purposes.
Acknowledgment
This work was funded by Researchers Supporting Project number (RSPD2023R728), King Saud University, Riyadh, Saudi Arabia and Joint Research Project (UMP-IIUM-UiTM Sustainable Research Collaboration Grant 2020, Malaysia; ID: SRCG20-002-0002). The authors are also grateful to Sarawak Biodiversity Centre (SBC), Sarawak, Malaysia for the approval of research permit (SBC-2018-RDP-15-SZR), Faculty of Medicine and Health Sciences (FMHS), UNIMAS, Sarawak, Malaysia, Kulliyyah of Pharmacy, IIUM Kuantan Campus, Pahang, Malaysia and Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia, Selangor, Malaysia for the administrative and technical support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Neurochemical and behavioural changes in zebrafish Danio rerio after systemic administration of 6-hydroxydopamine and 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine. J. Neurochem.. 2004;88(2):443-453.
- [Google Scholar]
- Whole-body cortisol increases after direct and visual contact with a predator in zebrafish, Danio rerio. Aquaculture. 2007;272(1):774-778.
- [CrossRef] [Google Scholar]
- Zebrafish as a model organism for neurodegenerative disease. Front. Mol. Neurosci.. 2022;15:940484
- [CrossRef] [Google Scholar]
- The importance of pH: How aquarium water is affecting behavioural responses to drug exposure in larval zebrafish. Pharmacol. Biochem. Behav.. 2020;199:173066
- [CrossRef] [Google Scholar]
- Zebrafish Models of Anxiety-Like Behaviors. In: Kalueff A.V., ed. The Rights and Wrongs of Zebrafish: Behavioral Phenotyping of Zebrafish. Springer International Publishing; 2017. p. :45-72.
- [CrossRef] [Google Scholar]
- Visual discrimination learning in zebrafish (Danio rerio) Behav. Process.. 2005;70(1):19-31.
- [CrossRef] [Google Scholar]
- Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res.. 2009;205(1):38-44.
- [CrossRef] [Google Scholar]
- Chronic unpredictable stress induces anxiety-like behaviors in young zebrafish. Sci. Rep.. 2020;10(1):10339.
- [CrossRef] [Google Scholar]
- He, X., Zhong, Z.-M., Che, Y., 2012. Locomotor activity and learning and memory abilities in Alzheimer’s disease induced by aluminum in an acid environment in zebrafish.
- Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci.. 2005;86(1):6-19.
- [CrossRef] [Google Scholar]
- Learning to fish with genetics: a primer on the vertebrate model Danio rerio. Genetics. 2016;203(3):1069-1089.
- [CrossRef] [Google Scholar]
- 2016/01/01/). Zebrafish neurobehavioral phenomics for aquatic neuropharmacology and toxicology research. Aquat. Toxicol.. 2016;170:297-309.
- [CrossRef] [Google Scholar]
- Mitochondrial dysfunction: a crucial event in okadaic acid (ICV) induced memory impairment and apoptotic cell death in rat brain. Pharmacol. Biochem. Behav. 2011;100(2):311-319.
- [CrossRef] [Google Scholar]
- 2013/05/15/). Okadaic acid-induced Tau phosphorylation in rat brain: role of NMDA receptor. Neuroscience. 2013;238:97-113.
- [CrossRef] [Google Scholar]
- 2019/01/01/). The GSK3β inhibitor, TDZD-8, rescues cognition in a zebrafish model of okadaic acid-induced Alzheimer's disease. Neurochem. Int.. 2019;122:31-37.
- [CrossRef] [Google Scholar]
- 2014/03/01/). Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: a review. Neurotoxicology. 2014;41:154-166.
- [CrossRef] [Google Scholar]
- Zebrafish as a model for epilepsy-induced cognitive dysfunction: a pharmacological, biochemical and behavioral approach. Front. Pharmacol.. 2017;8
- [CrossRef] [Google Scholar]
- The physiology of fish at low pH: the zebrafish as a model system. J. Exp. Biol.. 2014;217(Pt 5):651-662.
- [CrossRef] [Google Scholar]
- Comparative analyses of zebrafish anxiety-like behavior using conflict-based novelty tests. Zebrafish. 2017;14(3):197-208.
- [Google Scholar]
- Anxiolytic effects of nicotine in zebrafish. Physiol. Behav.. 2007;90(1):54-58.
- [CrossRef] [Google Scholar]
- Aluminium induced cholinotoxicity in zebra fish brain-A sequel of oxidative stress. Int. J. Adv. Res.. 2014;2:322-335.
- [Google Scholar]
- Acute toxic effects of polyethylene microplastic on adult zebrafish. Ecotoxicol. Environ. Saf.. 2019;182:109442
- [CrossRef] [Google Scholar]
- Drugs induced Alzheimer’s disease in animal model. Galen Med. J.. 2017;6(3):185-196.
- [CrossRef] [Google Scholar]
- The relationship between anxiety and Alzheimer's disease. J. Alzheimers Dis. Rep.. 2021;5(1):171-177.
- [CrossRef] [Google Scholar]
- Behavioral toxicology in risk assessment: problems and research needs. CRC Crit. Rev. Toxicol.. 1982;10(4):265-274.
- [CrossRef] [Google Scholar]
- E Nada, S., E Williams, F., A Shah, Z., 2016. Development of a novel and robust pharmacological model of okadaic acid-induced Alzheimer’s disease in zebrafish. In: CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders), 15(1), pp. 86-94.
- 2019/01/30/). Effects of maduramicin on adult zebrafish (Danio rerio): Acute toxicity, tissue damage and oxidative stress. Ecotoxicol. Environ. Saf.. 2019;168:249-259.
- [CrossRef] [Google Scholar]
- Comparative analysis of serotonin receptor (HTR1A/HTR1B families) and transporter (slc6a4a/b) gene expression in the zebrafish brain. J. Comp Neurol.. 2008;511(4):521-542.
- [Google Scholar]
- OECD, 2019. Test No. 203: Fish, Acute Toxicity Test. Doi: doi:Doi: 10.1787/9789264069961-en.
- Standardizing zebrafish behavioral paradigms across life stages: an effort towards translational pharmacology. Front. Pharmacol.. 2022;13
- [CrossRef] [Google Scholar]
- Locomotor adaptation deficits in older individuals with cognitive impairments: a pilot study. Front. Neurol.. 2022;13
- [CrossRef] [Google Scholar]
- Antioxidant capabilities of Litsea garciae bark extracts and their relation to the phytochemical compositions. Malays. Appl. Biol.. 2022;51(1):99-118.
- [CrossRef] [Google Scholar]
- Zebra fish as a model for translational neurobiology: Implications for drug discovery and development. Queen Mary University of London]; 2017.
- Okadaic acid: an additional non-phorbol-12-tetradecanoate-13-acetate-type tumor promoter. Proc. Natl. Acad. Sci.. 1988;85(6):1768-1771.
- [CrossRef] [Google Scholar]
- Use of the up-and-down acute toxicity test procedure to generate LC50 data for fish. Bull. Environ. Contam. Toxicol.. 2004;72(5)
- [CrossRef] [Google Scholar]
- Evaluation of the Effect of Aqueous <i>Momordica charantia</i> Linn. Extract on zebrafish embryo model through acute toxicity assay assessment. Evidence-Based Complementary Alternative Med.. 2019;9152757
- [CrossRef] [Google Scholar]
- Behavioural assessments of neurotoxic effects and neurodegeneration in zebrafish. Biochimica et Biophysica Acta (BBA) – Mol. Basis Dis.. 2011;1812(3):381-389.
- [CrossRef] [Google Scholar]
- Neurochemical factors underlying individual differences in locomotor activity and anxiety-like behavioral responses in zebrafish. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;65:25-33.
- [CrossRef] [Google Scholar]
- Zebrafish: a model for behavioural pharmacology. Rev. Farmacol. Chile. 2012;5:27-32.
- [Google Scholar]
- Zebrafish larvae acute toxicity test: a promising alternative to the fish acute toxicity test. Aquat Toxicol.. 2022;246:106143
- [CrossRef] [Google Scholar]
- Secondary stress responses of zebrafish to different pH: evaluation in a seasonal manner. Aquacult. Rep.. 2015;2:91-96.
- [CrossRef] [Google Scholar]
- Lithium prevents scopolamine-induced memory impairment in zebrafish. Neurosci. Lett.. 2018;664:34-37.
- [CrossRef] [Google Scholar]
- Zebrafish: an animal model for toxicological studies. Curr. Protoc. Toxicol.. 2003;7
- [CrossRef] [Google Scholar]







