Translate this page into:
Neuropeptide Y may participate in the pathogenesis of cervical vertigo
⁎Corresponding author. zjlqiu@sina.com (Jinliang Zuo)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
The concrete mechanism underlying the pathogenesis of cervical vertigo has not been well illustrated. This study aims to determine the diagnostic and potential therapeutic values of the neurotransmitter neuropeptide Y (NPY) in cervical vertigo.
Methods
Sixty male rabbits were randomly divided into five groups: the blank control, superior cervical sympathetic ganglia (SCSG) stimulation, SCSG sham-operated control (ganglion exposure and electrode placement in SCSG), inferior cervical sympathetic ganglia (ICSG) stimulation, and ICSG sham-operated control (ganglion exposure and electrode placement in ICSG) groups. Electrical stimulation at 30.0 Hz and 10.0 V for 5 min was delivered to expose SCSG or ICSG. Immunochemistry was conducted to determine the expression levels of NPY in all cervical spinal ganglia (C1–C8) dissected immediately after electrical stimulation.
Results
According to the immunohistochemical results, the blank control group only had few NPY-positive cells and was not significantly different with the sham-operated group. However, after SCSG stimulation, more NPY-positive cells in C2, C3, C4, and C5 were observed (C2 and C3 were the most evident). After ICSG stimulation, more NPY-positive cells were found in C6, C7, and C8, particularly in C6 and C7.
Conclusions
The low expression of NPY in all cervical ganglia (C1–C8) can be enhanced by electrical stimulation, whereas the expression of NPY in the cervical sympathetic ganglia was not affected by mechanical stimulation. This study provided new insights into the possible relationship between the neurotransmitter NPY and the pathogenesis of cervical vertigo.
Keywords
Neuropeptide Y
Cervical vertigo
Cervical spinal ganglion
1 Introduction
Cervical vertigo often occurs in adults, but whether it is an independent entity is still controversial due to the lack of specialized diagnostic criteria (Reid and Rivett, 2005). The pathogenesis of cervical vertigo has not been well elucidated. To date, several hypotheses regarding the etiology of cervical vertigo, such as neurovascular, somatosensory input, vascular, and cervical instability, have been proposed (Yacovino and Hain, 2013; Hain, 2015). Studies have suggested that insufficient blood supply to the vertebrobasilar artery may be an important factor in cervical dizziness after whipping injury (Endo et al., 2006). The blood supply in the vertebral artery is primarily dominated by the nerve fibers of the cervical sympathetic ganglia (Yan et al., 2009).
Neuropeptide Y (NPY) is primarily synthesized in the peripheral nervous system by autosympathetic and parasympathetic neurons and widely expressed in the central nervous system (Lundberg et al., 1982). NPY affects many physiological processes, such as cortical excitability, stress response, food intake, circadian rhythm, and cardiovascular function. NPY is considered as a sympathetic transmitter and vasoconstrictor of sympathetic nerves and a common marker of sympathetic neurons (Straub et al., 2009; Morosawa et al., 2017). NPY plays a role in the regulation of canine cerebral circulation (Suzuki et al., 1988). In addition, low-intensity electrical stimulation releases more NPY after nerve injury (Tsai et al., 2009). However, limited information is found regarding the NPY expression in cervical spinal ganglia after cervical vertigo, and the relationship between stress-induced NPY and cervical vertigo is unknown.
This study aims to investigate how electrical stimulation in the sympathetic cervical ganglia affects the expression of NPY in cervical spinal cord ganglia and provide theoretical support for studying the role of NPY in cervical vertigo. The expression of NPY in cervical ganglia is found to be enhanced by electrical stimulation and not by mechanical stimulation. This study suggests that NPY is probably involved in the pathogenesis of cervical vertigo and provides a theoretical basis for the clinical diagnosis of cervical vertigo.
2 Materials and methods
2.1 Animals and experimental design
White rabbits were purchased from Jinan xilingjiao biotechnology co. LTD. Sixty adult healthy male New Zealand White rabbits weighing 2 kg to 2.5 kg were equally and randomly divided into five groups: the blank control (n = 12), superior cervical sympathetic ganglia (SCSG) stimulation (n = 12), SCSG sham-operated control (n = 12), inferior cervical sympathetic ganglia (ICSG) stimulation (n = 12), and ICSG sham-operated control (n = 12) groups. All animal procedures were conducted in accordance with the National Institutes Health guideline and approved by the Animal Experimentation Ethic Committee of Shandong University.
2.2 Electrical stimulation of cervical sympathetic ganglia
Animals were anesthetized with an intraperitoneal injection of 10% chloral hydrate (150 mg/kg body weight) and placed in the supine position on the experimental table. This experiment was conducted under sterile conditions to eliminate disturbance from bacteria or other microorganisms. After the administration of chloral hydrate, the cervical hair was shaved, and the corresponding SCSG or ICSG was exposed unilaterally through a flank incision on the neck. Subsequently, the corresponding ganglion was isolated from the surrounding tissue by using rubber gloves with a stimulating electrode being needled inside (Fig. 1A).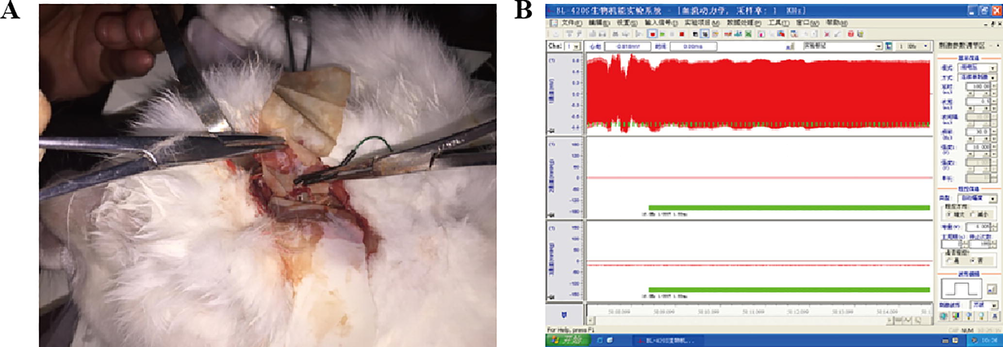
Electrical stimulation of cervical sympathetic ganglion (CSG) in rabbits. A. A stimulating electrode was stabbed into the exposed cervical sympathetic ganglion; B. Parameters regarding the electrical stimulation were presented.
The electrical stimuli (i.e., square-wave pulses of 0.5 ms pulse width at 30.0 Hz and 10.0 V for 5 min) applied in the SCSG and the ICSG groups were the same (Fig. 1B). These stimulus pulses were delivered from a pulse generator (SEN-7103, Nihon Kohden). The rabbits in the corresponding control group experienced the same surgery for cervical sympathetic ganglion exposure and electrode penetration without electrical stimulation.
2.3 Specimen collection and cryosection
Specimens were collected and disposed using the aforementioned method with modifications (Zuo et al., 2014). Immediately after electrical stimulation, the animals were sacrificed using a rapid left ventricular perfusion with physiological saline (37 °C, 300–400 mL) followed by slow perfusion with 4% paraformaldehyde at room temperature. After 1 h perfusion, the ipsilateral cervical spinal ganglia (C1–C8) were dissected and postfixed in 4% paraformaldehyde for 4 h at room temperature. Subsequently, the specimens were successively immersed into 10%, 20%, and 30% sucrose solutions for gradient dehydration until the specimens completely sank. Cryosections at thickness of 8 μm were sliced along the long axis of the ganglia. Three slides of each specimen were chosen from the front, middle, and back of the ganglia for further immunohistochemistry experiments.
2.4 Immunohistochemistry
The immunohistochemistry detection for NPY expression was conducted using the PV-9003 Polink-2 plus® polymer HRP detection system (ZSGB-Bio, Beijing, China) following the manufacturer’s instructions. Cryosections stored at −20 °C were placed aside for 5 min at room temperature, postfixed with 4% paraformaldehyde for 15 min, incubated with 3% H2O2 for 10 min at 37 °C, and washed thrice with PBS buffer. Slides were incubated with goat anti-NPY polyclonal primary antibody (Abcam, USA) at 4 °C overnight. The next day, the section were washed thrice with PBS buffer and successively incubated with polymer adjuvant for 20 min at 37 °C and secondary HRP-conjugated antigoat IgG antibody for 1 h (Sigma, USA). Afterward, the sections were immersed for 3 min in the 3,3ʹ-diaminobenzidine coloring substrate (0.4 mg/mL, DAKO, USA), rinsed with water, counterstained with hematoxylin, dehydrated, and sealed with neutral balsam. All sections were observed under a microscope. Positive areas were stained brown, and the Image-Pro Plus software was used to quantify the average optical density. Ten randomly selected discontinuous fields (200×) per slide were evaluated.
2.5 Statistical analysis
Data were expressed as mean ± SD. Statistical analysis was performed using the SPSS 13.0 (SPSS, Chicago, IL). The values of optical density were compared using the Student’s T-test. P < 0.05 was considered statistically significant.
3 Results
3.1 Low NPY level in normal cervical spinal ganglia
NPY immunohistochemical detection was performed to detect the level of NPY in cervical spinal cord ganglia under physiological conditions. As shown in Figs. 2A and 3A, only a few cells showed positive nucleus staining, indicating that NPY was weakly expressed in all cervical ganglia (C1–C8).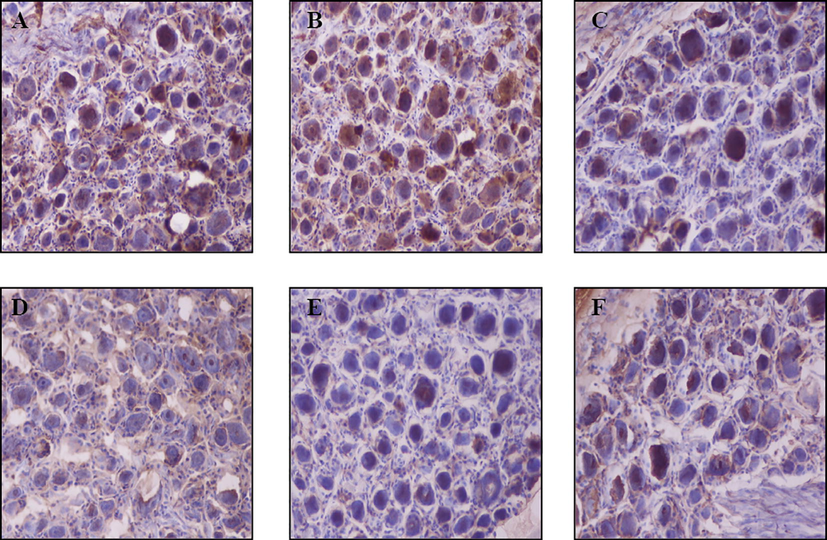
Immunohistochemistry of neuropeptide Y in the C2–C5 cervical spinal ganglia at hours after the electrical stimulation of the superior cervical sympathetic ganglion (SCSG). Representative immunohistochemistry images (200×) of neuropeptide Y in the blank control (A, C2), sham-operated (B, C2), and SCSG-stimulated (C, C2; D, C3; E, C4; F, C5) groups. Brown-colored cells in nuclei are neuropeptide Y-positive cells.
3.2 The mechanical stimuli cannot affect the expression of NPY in cervical spinal ganglia
Ganglion exposure and electrode placement were external mechanical stimuli to the body and may disturb the results in this study. A sham-operated group was established to eliminate the influence of surgery and electrode placement on NPY. Subsequently, the expression levels of NPY in the corresponding ipsilateral cervical spinal ganglia were detected by immunohistochemistry. As shown in Figs. 2B and 3B, few NPY-positive cells were observed in the corresponding sham-operated groups. Quantification analysis revealed that no significant difference was found in the NPY expressions between the sham-operated and blank control groups, indicating that the mechanical stimuli in the cervical sympathetic ganglion did not affect the expression of NPY in the corresponding cervical ganglion.
3.3 The electrical stimuli increases the expression of NPY in some cervical spinal ganglia
The SSCG or ISCG suffered from electrical stimulation because the mechanical stimuli did not affect the NPY expression level, and the expression of NPY in cervical spinal ganglion was detected using immunohistochemistry. As shown in Figs. 2C–E and 4A, the SCSG stimulation group had more NPY-positive cells in C2, C3, C4, and C5 compared with the sham-operated group, and the increases in C2 and C3 were the most evident. As shown in Figs. 3C–D and 4B, the ICSG stimulation group had more NPY-positive cells in C6, C7, and C8 compared with the sham-operated group, and those in C6 and C7 presented significant increases. These results suggested that electrical stimulation can increase the expression levels of NPY in certain cervical spinal ganglia, which were primarily controlled by the corresponding cervical sympathetic ganglion.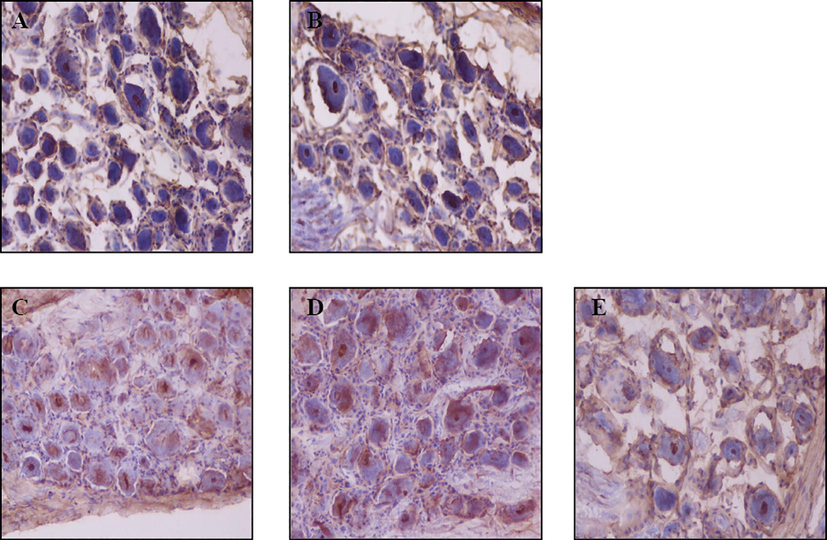
Immunohistochemistry of neuropeptide Y in C6–C8 cervical spinal ganglia after the electrical stimulation of the inferior cervical sympathetic ganglion (ICSG). Representative immunohistochemistry images (200×) of neuropeptide Y in the blank control (A, C6), sham-operated (B, C6), and ICSG-stimulated (C, C6; D, C7; E, C8) groups. Brown-colored cells in nuclei are neuropeptide Y-positive cells.
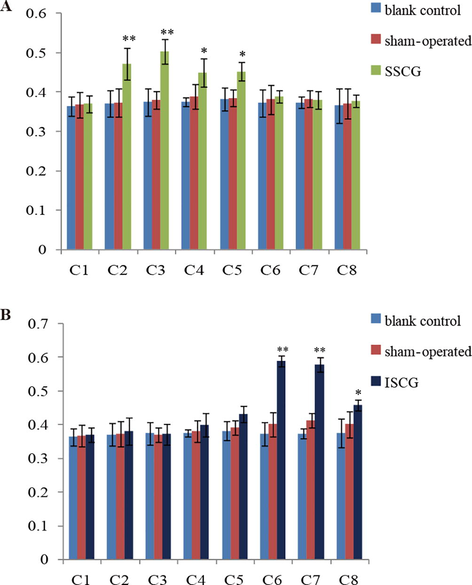
Quantification analysis of neuropeptide Y in the ipsilateral cervical spinal ganglia (C1–C8) after electrical stimulation of unilateral superior (A) and inferior (B) cervical sympathetic ganglion.
4 Discussion
In this study, the expression of NPY in cervical spinal ganglia from C1 − C8 was first explored, and a low level of NPY-positive cells in all cervical ganglia cryosections was found. Moreover, this study was a useful supplement to the previous study, which stated that NPY is expressed in superior cervical ganglion (Tajti et al., 1999). The NPY expression in normal cervical ganglia provided a feasible premise for the following verification of the speculation that NPY may be involved in cervical vertigo in this study.
Subsequently, the NPY expressions in the sham-operated groups were detected to observe whether the mechanical stimuli, ganglion exposure, and electrode placement can influence the NPY expression in cervical spinal ganglia. Results showed that the mechanical stimuli in the cervical sympathetic ganglion did not affect NPY expression in all the cervical spinal ganglia immediately after surgery. Zhang et al. have shown that the expression of NPY is increased slightly in murine dorsal root ganglia three days after tibial fracture (Zhang et al., 2016). Suggesting that the mechanical stimulation of ganglia cannot increase the NPY expression in a short time, which was consistent with our results.
Cold stress (Han et al., 2005), long-term hypoxia (Poncet et al., 1998; Tilan and Kitlinska, 2016), and chronic electrical stimulation (Yan et al., 2016; Chen et al., 2012), modulate NPY expression in different cells or tissues. Here, the NPY expression in response to the continuous electrical stimulation of sympathetic ganglia was evaluated. Immunohistochemistry and quantification analysis showed that more NPY-positive cells were observed in C2, C3, C4, and C5 (C2 and C3 were the most evident) after SCSG stimulation, and more NPY-positive cells were observed in C6, C7, and C8 (C6 and C7 presented the remarkable increases) after ICSG stimulation, suggesting that electrical stimulation in the sympathetic ganglion can enhance NPY expression in the cervical spinal neurons segmentally. NPY is a potent vasoconstrictive substance. On the one hand, NPY can directly act on vascular smooth muscle cells, leading to vasoconstriction (Ahmed et al., 1993; Hammond et al., 2011). On the other hand, NPY can potentiate the effect of other vasoconstrictive agents (noradrenaline) on the blood vessels (Wahlestedt et al., 1985). An increase in the NPY expression level in response to chronic stimuli may induce continuous constriction, thickening, and stenosis of the vertebrobasilar artery, which may be one of the pathogenetic factors of cervical vertigo. Studies have suggested that NPY can act as a therapeutic target in many diseases, such as stress-related psychiatric diseases (Enman et al., 2015).
This study indicated that NPY expression was increased in specific cervical spinal ganglia in response to corresponding cervical sympathetic ganglion stimulation. However, the blood flow dynamics of the vertebral artery in response to electrical stimulation must be conducted in the future to complete the verification of a cervical vertigo animal model by different electrical stimulations of SCSG or ICSG. In addition, the NPY inhibitor is needed to be applied to elucidate whether NPY can influence the pathogenesis of cervical vertigo.
In summary, this study indicated that NPY was physically expressed in all cervical spinal ganglia (C1–C8) at a low level. The mechanical stimuli in the cervical sympathetic ganglion did not affect the expression of NPY in the cervical spinal ganglia. Electrical stimulation can increase the expression levels of NPY in some cervical spinal ganglia, which were primarily controlled by corresponding cervical sympathetic ganglion. Furthermore, this study provided a new insight into the possible relationship between neurotransmitter NPY and the pathogenesis of cervical vertigo.
Acknowledgements
We thank the teammates involved in the study. This study was supported by National Natural Science Foundation of China (Study on the structure and physiological function of external reflex arc between cervical sympathetic ganglion and spinal nerve internode.) (No. 81472138).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Neuropeptide Y, tyrosine hydroxylase and vasoactive intestinal polypeptide-immunoreactive nerve fibers in the vertebral bodies, discs, dura mater, and spinal ligaments of the rat lumbar spine. Spine. 1993;18:268-273.
- [Google Scholar]
- Activation of NPY receptors suppresses excitatory synaptic transmission in a taste-feeding network in the lower brain stem. Am. J. Physiol. Regul. Integr. Comp. Physiol.. 2012;302:R1401-R1410.
- [Google Scholar]
- Cervical vertigo and dizziness after whiplash injury. Eur. Spine J.. 2006;15:886-890.
- [Google Scholar]
- Targeting the neuropeptide Y system in stress-related psychiatric disorders. Neurobiol. Stress. 2015;1:33-43.
- [Google Scholar]
- Influence of cold stress on neuropeptide Y and sympathetic neurotransmission. Peptides. 2005;26:2603-2609.
- [Google Scholar]
- Scaffolds containing growth factors and extracellular matrix induce hepatocyte proliferation and cell migration in normal and regenerating rat liver. J. Hepatol.. 2011;54:279-287.
- [Google Scholar]
- Neuropeptide Y (NPY)-like immunoreactivity in peripheral noradrenergic neurons and effects of NPY on sympathetic function. Acta physiologica Scandinavica. 1982;116:477-480.
- [Google Scholar]
- Neuropeptide Y neuronal network dysfunction in the frontal lobe of a genetic mouse model of schizophrenia. Neuropeptides. 2017;62:27-35.
- [Google Scholar]
- Effects of carotid sinus nerve transection on changes in neuropeptide Y and indolamines induced by long-term hypoxia in rats. Pflugers Archiv: Eur. J. Physiol.. 1998;437:130-138.
- [Google Scholar]
- Manual therapy treatment of cervicogenic dizziness: a systematic review. Manual Ttherapy. 2005;10:4-13.
- [Google Scholar]
- Effects of neuropeptide Y on canine cerebral circulation. Eur. J. Pharmacol.. 1988;146:271-277.
- [Google Scholar]
- Acute cold stress in rheumatoid arthritis inadequately activates stress responses and induces an increase of interleukin 6. Ann. Rheum. Dis.. 2009;68:572-578.
- [Google Scholar]
- Neuropeptide Y modulates c-Fos protein expression in the cuneate nucleus and contributes to mechanical hypersensitivity following rat median nerve injury. J. Neurotrauma. 2009;26:1609-1621.
- [Google Scholar]
- The human superior cervical ganglion: neuropeptides and peptide receptors. Neurosci. Lett.. 1999;263:121-124.
- [Google Scholar]
- Neuropeptide Y (NPY) in tumor growth and progression: lessons learned from pediatric oncology. Neuropeptides. 2016;55:55-66.
- [Google Scholar]
- Neuropeptide Y potentiates noradrenaline-evoked vasoconstriction: mode of action. J. Pharmacol. Exp. Therapeutics. 1985;234:735-741.
- [Google Scholar]
- Clinical characteristics of cervicogenic-related dizziness and vertigo. Semin. Neurol.. 2013;33:244-255.
- [Google Scholar]
- The terminal insertional segments and communications of the vertebral nerve in the human cervical region. Surg. Radiol. Anatomy: SRA. 2009;31:165-171.
- [Google Scholar]
- Chronic gastric electrical stimulation leads to weight loss via modulating multiple tissue neuropeptide Y, orexin, alpha-melanocyte-stimulating hormone and oxytocin in obese rats. Scand. J. Gastroenterol.. 2016;51:157-167.
- [Google Scholar]
- Neural reflex pathway between cervical spinal and sympathetic ganglia in rabbits: implication for pathogenesis of cervical vertigo. Spine J.. 2014;14:1005-1009.
- [Google Scholar]
- Orthopedic surgery modulates neuropeptides and BDNF expression at the spinal and hippocampal levels. Proc. Natl. Acad. Sci. U. S. A.. 2016;113:E6686-E6695.
- [Google Scholar]







