Translate this page into:
Neurobehavioral and neurobiochemical changes in arsenic treated male mice
⁎Corresponding author. gmabutaweel@jazanu.edu.sa (Gasem Abu-Taweel) abutaweelbiochem@gmail.com (Gasem Abu-Taweel)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Arsenic is an element that raises much concern from the both environmental and human health. The effects of As on learning behavior, neurotransmitters, and oxidative stress indicators were studied in male Swiss white mice. The purpose of this study was to explore into the preliminary work described concerning memory, biochemical changes, and learning impairment in mice exposed to arsenic. The study results in the Shuttle box, Water-maze, T-Maze tests and body weight measurements, As-treated males demonstrated decreased body weight and learning behavior. Dopamine, DA, serotonin, 5-HA, and cholinesterase, AchE levels were all decreased. When compared to the control, GSH and the enzymes GST, CAT, and SOD activity were decreased, while TBARS was raised. This study concludes as the essential reasons that explain the causes of As influence on learning and other data, based on the results collected and other previous studies in this field. In general, additional research is required to determine As negative effects on various elements of human and animal health.
Keywords
Arsenic (As)
Learning behavior
Shuttle box
Water maze
Mice
Neurotransmitters
Oxidative stress
1 Introduction
Arsenic (As) is the 33rd element in the Periodic Table of Elements, a metalloid found in nature such as water, soil, and air. It can be found in both inorganic and organic forms, and in a variety of oxidation states (3, 0, + 3, + 5) (Singh et al., 2022). As a well-known hazardous metalloid element in the environment and as a human carcinogen (Kaur et al., 2022). As can create both inorganic and organic molecules in the environment and the human body (Sharma et al., 2022). There are numerous anthropogenic uses for as a natural occurring element, such as the production of computer chips, glass, mining waste, agrochemicals (insecticides, rodenticides, herbicides), and wood preservatives, in addition to its widespread occurrence in the Earth crust. Drinking water is the primary method of As exposure, whereas eating is the second (Moreno Ávila et al., 2016). Locomotor activity in rats exposed to As is affected by the dose, route, and time of exposure as well as the strain of rat employed in behavioral experiments. Studies on mice have shown that the dose, duration of exposure, strain, and gender all affect locomotor activity, with both increases and declines. Dopaminergic effects of As exposure have also been studied in these and other studies, and the results show that dopamine (DA) metabolite levels in the striatum are either reduced or increased (DA content in striatal homogenates or decreased) with increasing doses of As exposure. An increase in reactive oxygen species (ROS), nitric oxide, enzyme and mitochondrial dysfunction, as well as a number of stress genes, is a result of exposure. Microorganisms' intracellular survival is increased when As is exposed to in vivo in murine splenic macrophages, according to research. In a previous study, the activity of acetylcholinesterase in the hippocampus was significantly reduced after treatment with As (Aktar et al., 2017). In human, early clinical symptoms at acute arsenic intoxication may be muscular pain, weakness with flusking skin. Severe nausea and vomiting, colicky abdominal pain, and profuse diarrhoea with rice-water stools abruptly ensure (Moreno Ávila et al., 2016). This study examined into memory and learning impairment, as well as biochemical changes in mice exposed to arsenic.
2 Materials and methods
2.1 Animal maintenance
In this study, 12 Swiss white male mice ranging in age from 10 to 12 weeks e (weighing 25–30 g) were recruited. The mice were transported from Central Animal House at King Saud University to Jazan University. The entire animal procedure was carried out in accordance with the National Institutes of Health protocol. This study was also granted ethical approval. Animals are housed in dedicated climatic rooms where the ambient temperature ranges between 18 and 22⁰C and the lighting length is 12 h, from 22.30 to 10.30, followed by darkness for a similar period. The animals were housed in special plastic cages with dimensions of 30 × 12 11 cm, and they were given sawdust to absorb moisture and offer warmth to the mice, as well as to help clean the cages on a regular basis. Food (Pilsburyes Diet, Grain Silos, Riyadh) and water are provided to animals on an ad libitum or as needed basis. Furthermore, all animal care and care, as well as all other behavioral tests, are performed under a dim red light.
2.2 Administration of arsenic and experimental design
Arsenic was prepared in two concentrations (1.0 and 5.0 mg/L). The experimental animals were separated into three groups, and either distilled water or arsenic dosages were administered orally through a stomach tube for 30 days as follows:
The first group includes: Distilled water was provided as the control.
Arsenic (1.0 mg/L) was given to the second group.
Arsenic (5.0 mg/L) was given to the third group.
2.3 Body mass index of mice
Weight is an indicator of the body's natural growth (Abu-Taweel and Rudayni, 2022). Use an electronic scale (to determine the weights of animals in groups, so that the animals were weighed before exposure, then every five days) in the current study.
2.4 Behavioral assessment
2.4.1 Avoidances of active responses
A “shuttle box” is an automatic reflex conditioner, which was used to measure the animals' active avoidance responses. A stainless-steel wall with a gate allowing entrance to the adjacent compartment divided the rectangular shuttle-box into two equal-sized chambers. Each animal was given two minutes to become acquainted with the shuttle box before the trial sessions began. An LED light (21 W) and a buzzer (670-Hz, 70 dB) were utilized as conditioned stimuli for six seconds each. The conditioned stimulus (CS) began 5 s before the unconditioned stimulus (US). The grid floor of the United States received an electric scrambler shock of 1 mA for four seconds. A conditioned avoidance response was recorded by the shuttle box's microprocessor recorder if the animal ran into another compartment within 5 s of the commencement of the CS and avoided the electric shock. During the experiment, each animal was given a total of 50 trials with a 15-second inter-trial pause. The total number of avoidances during the individual animal's 50-trial session were counted. To avoid shock treatment, each animal's escape latency (also known as latency of avoidance reaction or escape latency) was timed, and the findings were tabulated for each group. The number of crossings between chambers when no shock was present during UCS and CS was also used to assess the mouse's spontaneous migration to the opposite compartment between trials (inter-trial crossing). The automated shuttle box's recorder unit kept track of these variables throughout the duration of each animal's experiment (50 trials). Mice's active avoidance reflexes were previously measured using a “shuttle box,” an automatic reflex conditioner (Abu-Taweel, 2018, Abu-Taweel, 2020, Abu-Taweel et al., 2012, Abu-Taweel et al., 2014, Ahmed et al., 2016).
2.4.2 Water-maze testing by morris
Rat and mouse models have been widely studied using this test (Abu-Taweel, 2018, Abu-Taweel, 2019, Ahmed et al., 2016). A water maze was used to assess the mice visual–spatial memory after the exposure period had ended. The water-maze was a 90-cm-diameter, 50-cm-high galvanized white-water tank filled to a depth of 15 cm with 221 °C clear tap water. A 6x6cm, stainless steel, adjustable, white, escape platform was put 1 cm below the water level and 13 cm from the rim of the tank. The platform couldn't be seen since the water was made opaque by adding 1 L of milk. In order to divide the pool into four halves, four places on the tank's rim were assigned the letters N, S, E, and W. (NW, NE, SE and SW). It was on the first day that each animal was able to swim freely without a platform. The mice were able to acquire accustomed to the training setting since they were able to swim freely. It was on days 24 that animals were trained to identify and escape onto the submerged platform for 24 trials (six trials per day, inter-trial intervals of 30 s). The mouse was thrown into the water tank at the beginning of each experiment to ensure that it was completely submerged. We timed how long it took to get out of the water and onto the hidden platform (with a trial period of a maximum of 120 s), and then analyzed the data. A score of 120 s was recorded for each experiment in which the mouse was terminated by the experimenter because it couldn't identify the platform in 120 s despite the assistance of a glass rod. Each testing day's failure rate was calculated by totaling the number of failed trials. Each mouse was given 30 s of respite after mounting the platform so that it could learn and memorize the spatial clues needed to reach the platform for escape. The animals were permitted to climb onto a wire mesh grid and be transferred to their cage without further handling at the conclusion of the trials in order to reduce handling. The platform was removed from the water for a 120-second probe trial on day five of the experiment. On an electronic time, recorder, time spent in each quadrant (within 120 s of the probe test period) was recorded Probe trials in a water-maze test often show that normal animals spend a lot of time in the quadrant where the platform was previously situated. Tests conducted over a four-day period to discover where the platform was hidden provide an indication of hippocampal-dependent spatial reference memory, while the probe trial measures the strength of spatial learning, the human brain's closest equivalent to episodic memory (Jeltsch et al., 2001, Spiers et al., 2001).
2.4.3 The T-maze tests
The three groups of animals were not allowed to eat for the whole night prior to the examination. The elevated T-maze was made up of three closed arms that formed a T shape. The main arm (100 x 10 x 20 cm) and two lateral arms (40 x 10 x 20 cm) at a 20 cm elevation above the floor. The maze's arms form a T-shaped structure. The rodent meal was inserted into the right lateral arm. After each test, the maze was cleaned with 20% ethanol. The hungry animals were put at the far end of the elevated T-main maze's arm, facing the path to the two lateral arms. The mice were allowed to explore the maze for 1 min before being removed and kept in their cage for 2 h. The mice were replaced in the same location in the main arm, and their behavior was observed for 5 min by an experimenter who was not aware of the experimental technique. The frequency and duration of meal and main arm visits were recorded (under red illumination). The time spent exploring the arms to get to the food (seconds) and the time spent in the food arm (seconds) were calculated. According to previous studies, the frequency and timing of accessing the food lateral arm, rather than the other lateral arm, was regarded a memory reflector (Maodaa et al., 2016, Abu-Taweel et al., 2014).
2.5 Biochemical analysis
At the end of experimental, the animals scarified and the forebrain was separated and frozen in liquid nitrogen for biochemical tests to ensure neurotransmitters. Using HPLC, Monoamine neurotransmitters dopamine (DA) and serotonin/5-hydroxytryptamine (5-HT), Acetylcholinesterase (AChE), UV–visible spectrophotometer was used to measure lipid peroxides using thiobarbituric acid-reactive compounds (TBARS), glutathione (GSH), glutathione-S-Transferase (GST), catalase (CAT), and superoxide dismutase (SOD) were measured. Non-enzymatic oxidative stress parameters were also determined (Eghwrudjakpor et al., 1991, Ohkawa et al., 1979, Misra and Fridovich, 1972).
2.6 Statistical analysis
All experimental results were subjected to one-way analysis of variance (ANOVA) between groups (Khan et al., 2019). The student-Newman-Keuls multiple comparison test was then carried out. The significance values were set at P < 0.05, P < 0.01, and P < 0.001 (Abu-Taweel, 2020).
3 Results
3.1 Body mass index
Fig. 1 demonstrated that arsenic exposure resulted in significantly lower body weight of mice in a dose-dependent manner when compared to the control.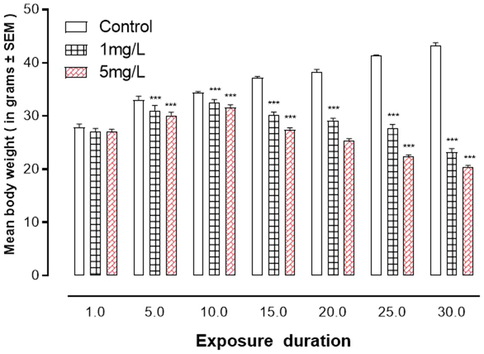
Arsenic exposure of effects art aniinal15. body weight ***represent statistically significant at P < 0.001 from the control group by ANOVA and student's t-test.
3.2 Behavioral evaluation
3.2.1 Responses to active avoidance
Male mice exposed to arsenic had a memory and learning problem. Shuttle box tests (Fig. 2A-F) revealed that arsenic exposure significantly decreased learning (P < 0.001) when compared to controls.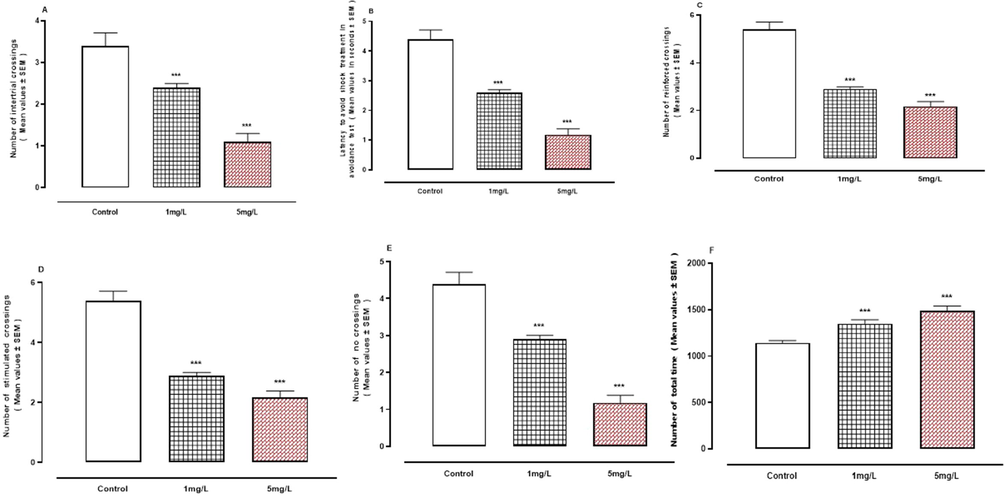
A-F: Exposure effects of arsenic in shuttle box tests. A, Number of intertrial crossings. B, Latency to avoid shock treatment. C, Number of reinforced crossings. D, Number of stimulated crossings. E, Number of no crossings. F, Number of total times. ***represent statistically significant at P < 0.001 from the control group by ANOVA and student's t-test.
3.2.2 Water maze designed by morris
When compared to the control group, Fig. 3A-C reveal that exposure to Ar led in a substantial decrease (P < 0.001) in all elements of learning behavior in the water maze on all test days.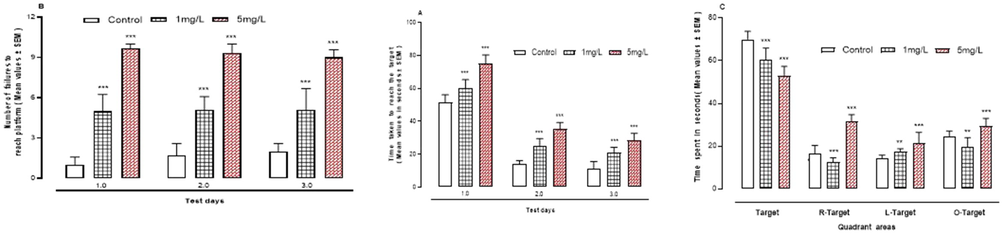
A-C: The effect of arsenic on learning behaviour in the water maze. A, time taken to reach the target. B, number of fallures to reach platform. C, time spent in quadrant areas of apparatus. ***P < 0.001 means compared to control group by ANOVA and student’s t-test.
3.2.3 The T-maze test
When compared to the control group, oral As exposure resulted in a substantial decrease (P < 0.001) in all of the elements examined in the T-maze test (Fig. 4A-D).Fig. 5.Fig. 6.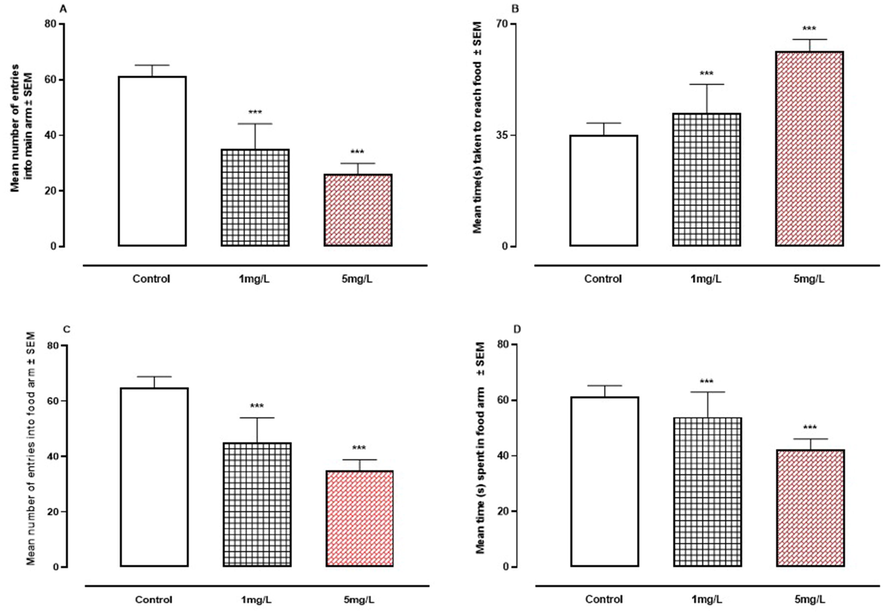
A-D. The effect of arsenic on learning behavior in the T- Maze. A, number of entries into main arm. B, time taken to reach food. C, number of entries into food arm. D, time spent in food arm. *** P < 0.001 means compared to control group by ANOVA and student's t-test.
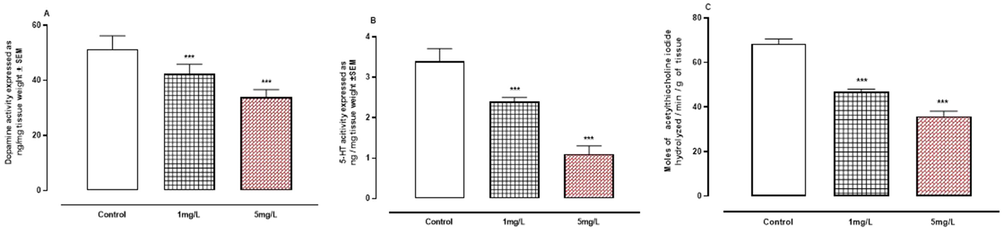
A-C: The effect of arsenic on neurotransmitters. A, dopamine. B, serotonin. C, acetylcholinesterase. *** P < 0.001 means compared to control group by Anova and student’s t-test.
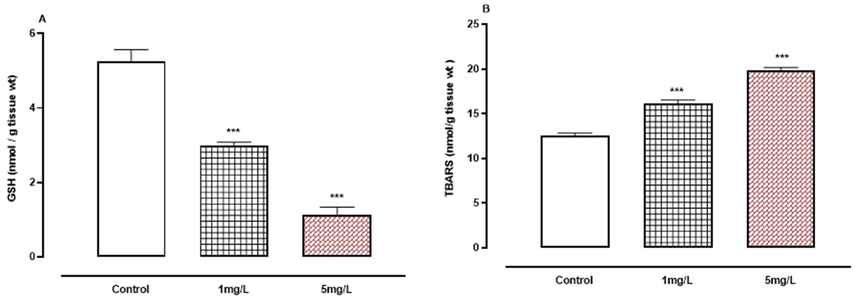
A-B: The effect of arsenic on non-enzymatic oxidative stress indicators. A, GSH.B, TBARS. *** P < 0.001 means compared to control group by ANOVA and student’s t-test.
3.3 Biochemical research
3.3.1 Neurotransmitters
Fig. 5A-C demonstrated that arsenic exposure resulted in a significant (P < 0.001) drop in the levels of neurotransmitters such DA, 5-HA, and AchE enzyme compared to the control group.
3.3.2 Indicators of non-enzymatic oxidative stress
Fig. 6A-B show a drop in GSH levels while TBARS increased significantly (P < 0.001) when compared to the control group.
3.3.3 Indicators of enzymatic oxidative stress
When compared to the control group, oral arsenic exposure resulted in considerably lower activity (P < 0.001) of enzymatic oxidative stress markers such as GST, CAT, and SOD (Fig. 7A-C).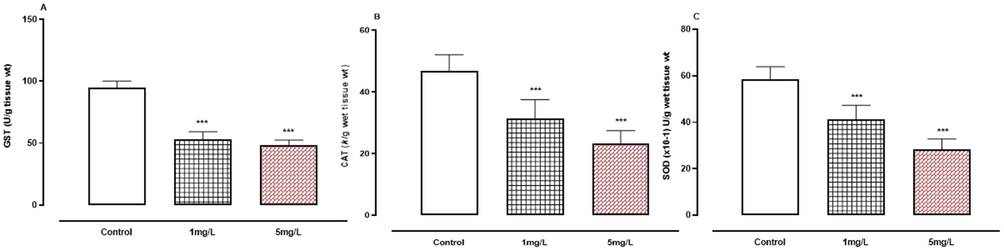
A-C. The effect of arsenic on enzymatic oxidative stress indicators. A, GST.B, CAT.C, SOD. *** P < 0.001 means compared to control group by ANOVA and student’s t-test.
4 Discussion
Arsenic is a well-known harmful metalloid element in the environment, as well as a human carcinogen (Wang et al., 2002). Harmful amounts of As are normally toxic within 30–60 min of ingestion, and severe toxicity has been documented with as low as 1 mg of As2O3 (Cullen et al., 1995). The findings of this study demonstrate that mice exposed to As in DW for one month have lower learning and memory retention, neurotransmitters, while TBARS was increased compared to controls. These results are consistent with earlier research (Martinez-Finley et al., 2009). Body weight was dramatically reduced in treated male mice in a dose-dependent manner when compared to controls. Arsenic's appetite suppressing effects have been documented for millennia (Abebe et al., 2015). Arsenic produces a variety of side effects, including stomach distress, indigestion, dry mouth, nausea (severe or persistent), difficulty swallowing, stomach discomfort after meals, diarrhea, and stomach tenderness. Abdominal or stomach pain, an increase in saliva, sores in the mouth and on the lips, rectal bleeding, a fast, irregular, or pounding heartbeat, and an increase in thirst are all symptoms of a disease that may restrict the appetite and number of foods eaten (Abernathy et al., 2003). The animals' weights dropped after being treated with arsenic, which was most likely due to diabetes. A dose–response association between cumulative As exposure and diabetes (Lai et al., 1994). A similar link was discovered in a study conducted in Bangladesh (Rahman et al., 1998), which employed the presence of keratosis as an indicator of As exposure and found that people who were exposed to As in their drinking water had an increased chance of developing diabetes.
In the Shuttle box, Water-maze, and T-Maze tests, the As-treated males demonstrated lower learning behavior. According to Abu-Taweel studies, the brain and other factors influence learning and other behaviors. Pyramidal cell neurons are few in number, and some of them are atrophic or degenerative (Abu-Taweel, 2020). With vacuolation of their myelinated axons, their cytoplasm displayed varying degrees of cellular degeneration. Pyramidal neurons in recovery animals recovered nearly completely (Abdelhady, 2020, Abernathy et al., 2003). The author came to the conclusion that chronic arsenic exposure caused reversible morphological abnormalities in pyramidal neurons in the frontal brain of adult male rats. The hippocampus may have a role in these disruptions, as most scientific sources state that this region is vital in the process of learning and remembering, and that damage to this part results in memory loss (Nayak, 2002). Memory impairments have been connected by a group of scientists to a flaw in the form of neurons caused by the buildup of aluminum and other oxidants such as arsenic and metabolic products in the cell body or its branches, or when these branches are diminished. The axons of neurons are converted into a spherical or cordlike shape, which impacts the critical synapse in the memory and learning processes (Gonçalves and Silva, 2007). It was discovered that exposure to aluminum and other oxidants, such as As, reduces the size of the synapse and the thickness and density of the pre-synapse, resulting in the expansion of the synaptic gap and the reduction of curvature, which affects the process of nervous conduction and the mechanism of the work of neurotransmitters in the brain, such as serotonin, dopamine, and AchE, Amino acids and adrenaline (Jia et al., 2001, Tsunoda and Sharma, 1999). Arsenic has a profound influence on neurotransmitters such as dopamine, serotonin, and AchE, all of which have been found to be drastically reduced in the current study. Both enzymatic and non-enzymatic oxidative stress indicators were altered. In the structure and shape of neurons producing these neurotransmitters in most areas of the brain, the amount of glutathione decreased and the activity of indicators of enzymatic oxidative stress (GST, CAT, SOD) decreased, while the amount of TBARS increased in arsenic-treated males compared to healthy animals (Jia et al., 2001). According to Tusonda studies, oxidative alterations in the blood–brain barrier might cause changes in the levels of neurotransmitters such as dopamine, serotonin, and adrenaline (Tsunoda and Sharma, 1999). Previous studies attribute the lack of neurotransmitters to changing the shape of nerve cells when exposed to aluminum and other oxidative states due to the accumulation of substances in the body of the nerve cell or its branches, or the decrease in the number of branches and the shifting of the axes to a spherical or similar cords (Kawahara, 2005, Polizzi et al., 2002). Because oxidants work to reduce the thickness or density of the pre-synapse region, this leads to the expansion of the intertwining gap and the mitigation of curvature, which negatively affects the neurotransmitters and leads to a decrease in the quantity of the conductor or a decrease in its activity (Schousboe, 2003, Sharma et al., 2007).
5 Conclusion
The new findings have established beyond a shadow of a doubt the dangers of arsenic exposure on learning behavior and neurotransmitter levels in the body. Arsenic and its powerful compounds necessitate additional prospective studies to clarify and investigate their harmful impacts on behavior and biological features.
Acknowledgement
The author is thankful to the Deanship of Scientific Research at Jazan University for supporting this research.
Declaration of Competing Interest
The author declare that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chronic Administration of Sildenafil Citrate (Viagra) on the Frontal Cortex of Adult Male Rats: An Ultrastructural Study. Forensic Medicine and Anatomy Research. 2020;8:38-44.
- [Google Scholar]
- Health effects and risk assessment of arsenic. The Journal of nutrition. 2003;133:1536S-1538S.
- [Google Scholar]
- ABU-TAWEEL, G. M. 2018. Cardamom (Elettaria cardamomum) perinatal exposure effects on the development, behavior and biochemical parameters in mice offspring. Saudi journal of biological sciences, 25, 186-193.
- Neurobehavioral protective properties of curcumin against the mercury chloride treated mice offspring. Saudi journal of biological sciences. 2019;26:736-743.
- [Google Scholar]
- ABU-TAWEEL, G. M. 2020. Celery ameliorating against neurobehavioral and neurochemical disorders of perinatal lipopolysaccharides exposure in mice offspring. Journal of King Saud University-Science, 32, 1764-1771.
- Neurobehavioral toxic effects of perinatal oral exposure to aluminum on the developmental motor reflexes, learning, memory and brain neurotransmitters of mice offspring. Pharmacology Biochemistry and Behavior. 2012;101:49-56.
- [Google Scholar]
- Curcumin ameliorated the mercuric chloride induced depression and anxiety in female mice offspring. Environmental Research. 2022;204:112031
- [Google Scholar]
- Cognitive and biochemical effects of monosodium glutamate and aspartame, administered individually and in combination in male albino mice. Neurotoxicology and Teratology. 2014;42:60-67.
- [Google Scholar]
- The risk of aluminium neurooxicity for young animals and humans due to multiple exposure opportunities, particularly perinatal. Aluminium neurotoxicity. Nova Science Publishers. Inc.; 2016.
- Individual and combined effects of arsenic and lead on behavioral and biochemical changes in mice. Biological Trace Element Research. 2017;177:288-296.
- [Google Scholar]
- CULLEN, N. M., WOLF, L. R. & ST CLAIR, D. 1995. Pediatric arsenic ingestion. The American journal of emergency medicine, 13, 432-435.
- Central nervous system bioaminergic responses to mechanical trauma: an experimental study. Surgical neurology. 1991;35:273-279.
- [Google Scholar]
- Does neurotransmission impairment accompany aluminium neurotoxicity? Journal of Inorganic Biochemistry. 2007;101:1291-1338.
- [Google Scholar]
- Cognitive performances and locomotor activity following dentate granule cell damage in rats: role of lesion extent and type of memory tested. Neurobiology of learning and memory. 2001;76:81-105.
- [Google Scholar]
- Effects of aluminum on amino acid neurotransmitters in hippocampus of rats. Zhonghua yu Fang yi xue za zhi [Chinese Journal of Preventive Medicine]. 2001;35:397-400.
- [Google Scholar]
- A Clinical Perspective on Arsenic Exposure and Development of Atherosclerotic Cardiovascular Disease. Cardiovascular Drugs and Therapy 2022:1-8.
- [Google Scholar]
- KAWAHARA, M. 2005. Effects of aluminum on the nervous system and its possible link with neurodegenerative diseases. Journal of Alzheimer's disease, 8, 171-182.
- Genetic confirmation of T2DM meta-analysis variants studied in gestational diabetes mellitus in an Indian population. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2019;13:688-694.
- [Google Scholar]
- Ingested inorganic arsenic and prevalence of diabetes mellitus. American Journal of Epidemiology. 1994;139:484-492.
- [Google Scholar]
- Effect of parsley (Petroselinum crispum, Apiaceae) juice against cadmium neurotoxicity in albino mice (Mus musculus) Behavioral and Brain Functions. 2016;12:1-16.
- [Google Scholar]
- Learning deficits in C57BL/6J mice following perinatal arsenic exposure: consequence of lower corticosterone receptor levels? Pharmacology Biochemistry and Behavior. 2009;94:271-277.
- [Google Scholar]
- The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. Journal of Biological chemistry. 1972;247:3170-3175.
- [Google Scholar]
- MORENO ÁVILA, C. L., LIMÓN-PACHECO, J. H., GIORDANO, M. & RODRÍGUEZ, V. M. 2016. Chronic exposure to arsenic in drinking water causes alterations in locomotor activity and decreases striatal mRNA for the D2 dopamine receptor in CD1 male mice. Journal of Toxicology, 2016.
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical biochemistry. 1979;95:351-358.
- [Google Scholar]
- Neurotoxic effects of aluminium among foundry workers and Alzheimer’s disease. Neurotoxicology. 2002;23:761-774.
- [Google Scholar]
- Diabetes mellitus associated with arsenic exposure in Bangladesh. American Journal of epidemiology. 1998;148:198-203.
- [Google Scholar]
- SCHOUSBOE, A. 2003. Role of astrocytes in the maintenance and modulation of glutamatergic and GABAergic neurotransmission. Neurochemical research, 28, 347-352.
- Role of combined administration of Tiron and glutathione against aluminum-induced oxidative stress in rat brain. Journal of Trace Elements in Medicine and Biology. 2007;21:63-70.
- [Google Scholar]
- Removal of volatile organic compounds and heavy metals through the biological-based process. In: An Innovative Role of Biofiltration in Wastewater Treatment Plants (WWTPs). Elsevier; 2022. p. :45-64.
- [Google Scholar]
- Arsenic: a Culpable Element and a Possible Menace for HIV/AIDS Patients. Biological Trace Element Research 2022:1-12.
- [Google Scholar]
- Bilateral hippocampal pathology impairs topographical and episodic memory but not visual pattern matching. Hippocampus. 2001;11:715-725.
- [Google Scholar]
- Altered dopamine turnover in murine hypothalamus after low-dose continuous oral administration of aluminum. Journal of trace elements in medicine and biology. 1999;13:224-231.
- [Google Scholar]
- A review of animal models for the study of arsenic carcinogenesis. Toxicology letters. 2002;133:17-31.
- [Google Scholar]







