Translate this page into:
Nepeta paulsenii Briq. inhibits hepatic toxicity in albino rats: Phytochemical analysis and chemical profiling
⁎Corresponding authors. ICIR.2022@uaf.edu.pk (Aqsa Hanif), samina.tanwir@uaf.edu.pk (Samina Tanwir),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Nepeta paulsenii is a less explored plant found in the South-Eastern hilly region of Pakistan. Local people consider it a medicinal plant. The protective effect of methanol extract of N. paulsenii leaves on carbon-tetrachloride (CCl4) tempted liver damage in male rats was assessed. Forty-eight rats were equally divided into 8 groups and various concentrations of extract + CCl4 were induced for 30 days. Crude methanolic extract of N. paulsenii plant was examined through phytochemical analyses and gas chromatograph coupled with mass spectrometre (GC–MS) before biological testing on albino rats. Phytochemical analysis of extract showed coumarins, flavonoids, terpenoids, saponins and betacyanins, while GC–MS results revealed the existence of several compounds belonging to diverse classes, responsible for the hepatoprotective attribute of N.paulsenii. CCl4 exposure substantially decreased the antioxidant enzymes activity while increased the thiobarbituric acid reactive substances and reactive oxygen species levels. A remarkable increase in the aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase levels was observed in CCl4 treated rats. Comet-parameters were observed after CCl4 administration. N. paulsenii extract administration substantially improved the antioxidant enzyme activity, hepatic markers as well as comet parameters and reversed the CCl4 induced histopathological damages. The results revealed that N. paulsenii has therapeutic effects against CCl4-induced liver damages.
Keywords
Carbon tetrachloride
Toxicant
Nepeta paulsenii
Therapeutic
Antioxidant
- OS
-
Oxidative stress
- ROS
-
Reactive oxygen species
- CCl4
-
Carbon tetrachloride
- LP
-
Lipid peroxidation
- PUFA
-
Polyunsaturated fatty acid
- NaOH
-
Sodium Hydroxide
- CHCl3
-
Chloroform or trichloromethane
- H2SO4
-
Sulfuric acid
- HCl
-
Hydrochloric acid
- DNA
-
Deoxyribonucleic acid
- UAF
-
University of Agriculture Faisalabad
- CAT
-
Catalase
- POD
-
Peroxidase
- GSR
-
Glutathione reductase
- TBARS
-
Thiobarbituric acid reactive substance
- NMA
-
Normal melting agarose
- OOCCl3
-
Proxy trichloromethyl
- CCl3
-
Trichloromethyl free radical
- ALP
-
Alanine-transaminase
- ALT
-
Alkaline-phosphatase
- AST
-
Aspartate-transaminase
- GST
-
Glutathione-S-transferase
- GSH
-
Reduced glutathione
- SOD
-
Superoxide dismutase
Abbreviations
1 Introduction
The liver is a vital and second-largest organ in the body, representing 1.4–2.4 % of lean body mass (Dai et al., 2021) that plays an essential role in eradicating toxic materials from the body and aids in the digestion of food stuff (Vancells Lujan et al., 2021). The liver involves excretory, vascular, immunological, secretory and metabolic functions and plays a significant role in fat, carbohydrate and protein metabolism (Panzitt and Wagner 2021). Liver is main organ which is attacked by reactive oxygen species because hepatic parenchymal cells are prone to oxidative stress (Shi et al., 2021). Liver diseases can be caused by drugs, virus infiltration from infection or ingestion of toxic chemicals (Cheemerla and Balakrishnan 2021). Toxins are converted into intermediate free radicals that are very reactive and unstable. These radicals produce hepatotoxic effects (Lu et al., 2021). Presently, drugs that are used against liver diseases are often inadequate and have some severe side effects so there is need to explore the alternative medicines to replace with existing medicines of uncertain efficiency and security (Rubiano et al., 2021).
The models of experiment for hepatotoxicity can be designed by using CCl4, paracetamol or alcohol. CCl4 is a strong hepatotoxic agent, generally used as a standard toxicant to provoke chemical injury (Samad et al., 2020). CCl4 causes accumulation of fat and induces centrilobular necrosis in liver. CCl4 disrupts the triglycerides and total protein levels (Amir et al., 2021). CCl4 induced hepatic injury is because of the free radical production and lipid per-oxidation (Sajid et al., 2016). CCl4 forms CCl3 and OOCCl3 radicals and these radicals bind with PUFA and form peroxy and alkoxy radicals. These radicals induce damages in tissue and alter the activity of different enzymes (Cheng et al., 2022).
Medicinal plants are used to cure many diseases due to their therapeutic potential (Almoshari 2022, Ayidh AlThobaiti 2023). Plants are a source of natural biologically active antioxidant components like alkaloids, flavonoids (15-C compounds), saponins, tannins and these components are present in the different parts of plants which are extracted by proper extraction technique (Pandey et al., 2021). Oxidative stress induces hepatic damage due to many factors such as environmental pollutants, drugs, alcohol, and irradiation which are responsible to cause some liver diseases. Reactive oxygen species (ROS) break the strands of DNA, initiates lipid peroxidation and oxidize all molecules that are present in biological tissues and membranes and cause injury. Due to the high production of ROS and low production of antioxidants, oxidative stress is produced. Antioxidants signify a rational therapeutic strategy to cure and prevent hepatic diseases. A variety of antioxidants present in medicinal and edible plants frequently displays a strong antioxidant abilities (Raeeszadeh et al., 2021).
Genus Nepeta belongs to Lamiaceae family having more than 250 species (Talebi 2021). Due to their anti-asthma, diuretic, anti-spasmodic, diaphoretic, antitussive characteristics various species of Nepeta genus have been used in preparation of traditional medicines (Alkahtani et al., 2022). Plants of Nepeta genus are traditionally used for curative-purposes due to the extensive diversity and high contents of flavonoid, phenolic and terpenoid compounds in Nepeta species. Several species of Nepeta genus contain antioxidant characteristics and also show anti-fungal, anti-viral and anti-bacterial activities (Azizian et al., 2021). Phytochemicals existing in Nepeta plants are beneficial for both animals and humans and most of these phytochemical substances are biologically active compounds which are used for the manufacturing of new drugs (Samad et al., 2020). Many experiments have been carried out on many plants of the Nepeta genus, but the N. paulsenii Briq. plant is hardly ever used in this respect. To explore the phytochemicals and to investigate the hepato-protective and antioxidant functions of N. Paulsenii Briq. Crude methanolic extract against CCl4 intoxicated rats, the present study was planned.
2 Materials and methods
2.1 Collection of sample and extract-preparation
Nepeta paulsenii Briq, was gathered from Skardu (Gilgit-Baltistan), Pakistan. The plant was identified by Prof. Dr. Mansoor Hameed, and voucher specimen number 1002 was banked at the science herbarium of Quaid-e-Azam University, Department of Botany, Islamabad, Pakistan. The plant sample was collected at mature stage and the identification was carried out on the basis of morphological characteristics of sample. Leaves of N. paulsenii plant were dehydrated under shadow at RT and grinded to a very fine powder. Leaves powder was placed in Soxhlet apparatus for methanolic extraction. Extracted material was dried under vacuum with rotary-evaporator.
2.2 Phytochemical-analysis
Various qualitative trials were performed to recognize the classes of phytochemicals that exists in crude methanolic extract of leaves.
2.2.1 Flavonoids assessment
Concisely, 1 mg of specimen was permitted to react with 2 N NaOH (1 mL). Development of light yellow color was the symbol of existence of flavonoids in sample (Harborne 1998).
2.2.2 Coumarins assessment
Sample (1 mg) was mixed with 1 mL of NaOH (10 %). The confirmation signal of the existence of coumarins in sample was the appearance of yellow color (Harborne 1998).
2.2.3 Saponins assessment
A 2 mg solution was applied to distilled water (2 mL) of and mixed robustly. The development of approximately 1–2 cm soapy layer was a proof of the presence of saponins (Harborne 1998).
2.2.4 Terpenoids assessment
Development of reddish brown layer at the interface of 2 reacting layers verified the presence of terpenoids on mixing ½ mg of sample with 3 mL of CHCl3 and 3 mL of conc. H2SO4 (Harborne 1998).
2.2.5 Assessment of betacyanins and anthocyanins
One mg sample was boiled for 10 min. in 2 mL of 1 N NaOH. A yellow color formation indicate the betacyanin, and formation of bluish green color indicates the anthocyanins (Trease and Evans 1989).
2.2.6 Assessment of anthraquinones
One mg sample was mixed with 2 mL of diluted 2 % HCl and change in color towards red indicated the presence of anthraquinones in the extracted material (Harborne 1998).
2.3 Gas chromatograph coupled with mass spectrometre (GC–MS) analysis
N. paulsenii was scrutinized for the existence of active compounds for mass determination on the gas chromatograph“ Thermo GC-Trace Ultra Ver; 5.0 ” supporting with a“ Thermo MSSDSQ II. The elements were parted on a 60 m long 'ZB 5-MS Capillary Standard Non-polar Pole' with a film thickness of 0.25-μm.-The temp. was increased from 70 − 260 °C at a rate of 6 °C per min. throughout the experiment. The rate of flow of carrier gas was 1 mL per min. of helium, while the sample injection vol. was 1 μL. The-characterization of chemical components was based on analogy with those obtained from genuine sample and Wiley library spectra.
2.4 Experimental design
In the UAF animal house, male albino rats were separated for this trial. Tap water and food chaw ad libitum were given to rats. The rats of different groups were kept in separate cages. Eight-groups (n = 6/ group) of male albino rats were established to scrutinize the hepatoprotective role of N. paulsenii extract. Group-I was referred as control. Group-II was treated with olive oil and dimethyl-sulfoxide (1:1, v/v) at a dose of 1 mL per kg bw orally. Rats in group III were treated with Olive oil + CCl4 (8:2, 1 mL per kg bw). CCl4 + silymarin (50 mg per kg bw) in dimethyl sulfoxide was administered to group IV rats. Doses of 200 and 400 mg/kg bw of N. paulsenii extract were orally administered to group V and VI rats respectively with CCl4. Dose of 200 and 400 mg/kg bw in N. paulsenii extract only were given to VII and VIII group rats respectively.
Trial was carried out for thirty days and after completion; all of the rats were examined then blood sample was stored in sterilized tubes and liver of every single rat was removed out. For removal of plasma, centrifugation of blood carried out for 15 min at 3000 revolution per minutes and kept at −20 °C for further analysis. Liver was washed with ice cold saline. A segment of each liver was packaged in zip lock bag and kept at −20 °C for evaluation of anti-oxidant enzymes and DNA damage studies. And other part of liver was used for histopathological studies. This protocol was certified by the ethical board of Agriculture University, Faisalabad-Pakistan.
2.5 Anti-oxidant enzymes assessment
The assessments of anti-oxidant enzymes were carried out according to the reported procedures (Ijaz et al., 2021, Rehman et al., 2022). Finally, the standard curve was plotted (Hayashi et al., 2007).
2.6 Biochemical studies of serum
Liver marker enzymes, i.e., ALT, ALP and AST were analyzed utilizing relevant commercially available diagnostic kits (Wiesbaden-Germany).
2.7 Comet assay
For the estimation of DNA-injury, process of (Dhawan et al., 2009) was followed. According to this method, a reasonable section of liver tissue was placed and crushed in a number of fragments on autoclaved slides and then immersed in 0.5 % of low melting agarose and 0.6 % of NMA (Normal melting agarose) and let it set at normal room temp. After solidification, these slides were bathed in cold lysis solution for 1 hr. at 4 ℃. For the DNA un-winding, these slides were kept in electrophoretic buffer for 20 min. and then processed for electrophoresis for 30 min. After that, Tris-hydrochloric acid buffer at 25 °C was used to bring pH 7.0 of each slide. Ethidium bromide (1 %) was used for staining of slides before studying under fluorescence microscope. For DNA assessment, injury level image software (TRITEK) was used. Parameters i.e., comet number, olive moment, tail moment, head length, tail length, comet length % DNA contents in head and tail were considered for DNA integrity.
2.8 Histopathological examination
Histopathological analysis was performed to observe hepatic injuries. Samples were fixed in fixative solution (glacial acetic acid10 %, absolute alcohol 70 %, formaldehyde 20 %). For the gradual dehydration process, the tissues were passed through various grades of ethanol (70 %, 90 % and 100 %). For embedding Paraffin wax was used. Thick slices of tissues were cut to the 4–5 μm using spencer rotatory microtome (model 820) was used for this purpose. Lastly stained using the stain (hematoxylin-eosin) which was dissolved in 70 % of alcohol. Light microscope (Nikon model 187–842, Japan) was used to observe these slides at × 40 and microphotography was done using Leica LB microscope was adapted to utilize the photographs.
2.9 Statistical analysis
Results are displayed as Mean ± SEM. The difference between the results of different groups was evaluated as reported earlier (Samad et al., 2020).
3 Results
3.1 Qualitative phytochemical analysis
The phytochemical examination of crude extract is shown in Table 1. The existence of flavonoids, coumarins, saponins, terpenoids and betacyanins in methanol extract of N. paulsenii was confirmed by a qualitative study. (+) present (-) absent.
Compound class
N. paulsenii extract
Flavonoids
+
Coumarins
+
Saponins
+
Terpenoids
+
Anthraquinones
_
Betacyanins
+
3.2 GC–MS analysis
3.2.1 Identification of components
N. paulsenii crude extract was designated for GC–MS study to reveal the compounds accountable for hepatoprotective activity. GC–MS exploration specified that N. paulsenii contains 10 chemical-components eluted between 2.0 and 10.0 min. (Fig. 1). The documentation of chemical components was based on a comparison of their mass spectra and relative-rt with those attained from reliable samples and Wiley libraries spectra (Table 2). In N. paulsenii, out of these 10 chemical constituents, there was 2-glycerin (4.37 % as per peak area and 2.32 min. R/T), geranic acid (4.87 % and 4.08 min.), 6-octadien-1-ol (2.32 % and 4.22 min.), 2-pentadecanone (3.74 % and 6.53 min.), benzenepropanoic acid (2.20 % and 6.95 min.), ascorbic acid (22.1 % and 7.05 min.), phytol (10.8 % and 7.67 min.), 9, 12, 15-octadecatrienoic acid (36.1 % and 7.80 min.), octadecanoic acid (7.78 % and 7.87 min.) and 1,2-benzenedicarboxylic acid (4.43 % and 9.71 min.) (Fig. 2).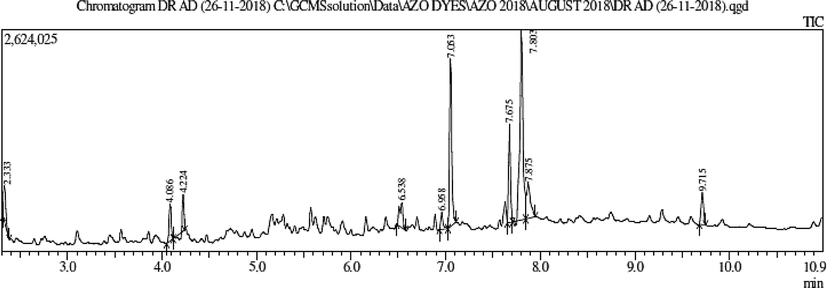
GC–MS chromatogram of leaves methanol extract of N. paulsenii plant.
Sr#
Name of compound
Peak area %
Class
R/T (mins.)
1
glycerin
4.37
Alcohol
2.33
2
geranic acid
4.87
Polyunsaturated fatty acid
4.08
3
2,6-octadien-1-ol
2.34
Alcohol
4.22
4
2-pentadecanone
3.74
Ketone
6.53
5
benzenepropanoic acid
2.20
Carboxylic acid
6.95
6
1-(+)-ascorbic acid
22.1
Vitamin C
7.05
7
phytol
10.8
Diterpene alcohol
7.67
8
9,12,15-octadecatrienoic acid
36.1
Linolenic acid
7.80
9
octadecanoic acid
7.78
Stearic acid ester
7.87
10
1,2-benzenedicarboxylic acid
4.43
Plasticizer
9.71
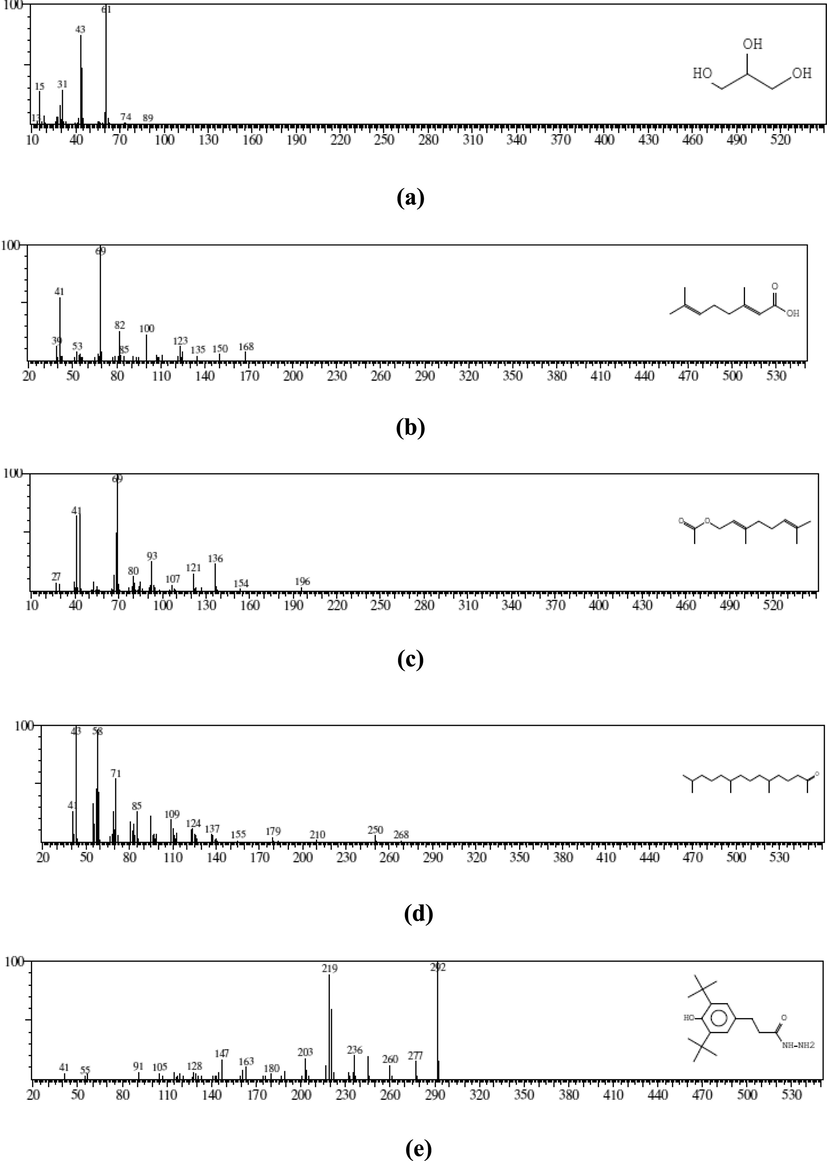
(a) MS of glycerin (RT: 2.33) (b) MS of geranic acid (RT: 4.08) (c) MS of 2, 6-Octadien-1-ol (RT: 4.22) (d) MS of 2-Pentadecanone (RT: 6.53) (e) MS of Benzenepropanoic acid (RT: 6.95) (f) MS of 1-(+)-ascorbic acid (RT: 7.05) (g) MS of phytol (RT: 7.67) (h) MS of 9, 12, 15-octadecatrienoic acid (RT: 7.80) (i) MS of octadecanoic acid (RT: 7.87) (j) MS of 1, 2-benzenedicarboxylic acid (RT: 9.71).
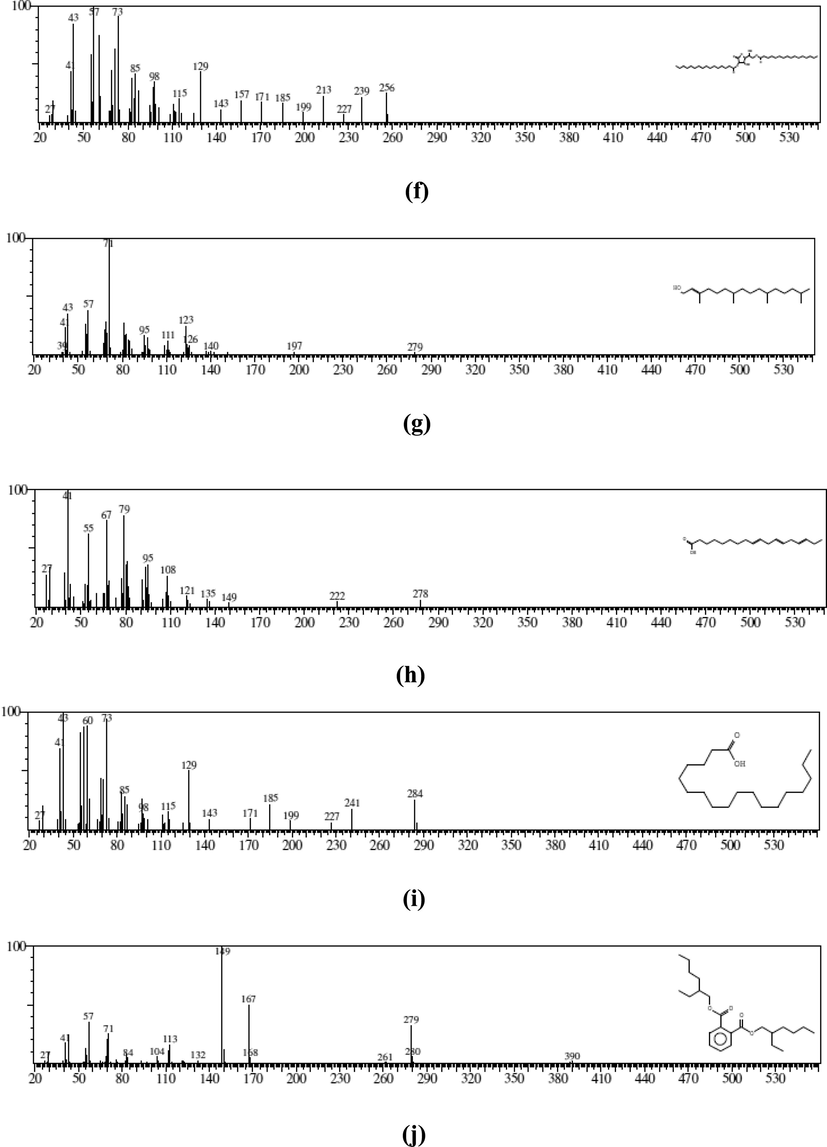
(a) MS of glycerin (RT: 2.33) (b) MS of geranic acid (RT: 4.08) (c) MS of 2, 6-Octadien-1-ol (RT: 4.22) (d) MS of 2-Pentadecanone (RT: 6.53) (e) MS of Benzenepropanoic acid (RT: 6.95) (f) MS of 1-(+)-ascorbic acid (RT: 7.05) (g) MS of phytol (RT: 7.67) (h) MS of 9, 12, 15-octadecatrienoic acid (RT: 7.80) (i) MS of octadecanoic acid (RT: 7.87) (j) MS of 1, 2-benzenedicarboxylic acid (RT: 9.71).
3.3 Effect of crude N. Paulsenii extract on antioxidant enzymes activity
Antioxidant enzymes protect the cell against toxic radical reactions and show defensive effect against cellular damage. Variation in activity of antioxidant enzymes was assessed after CCl4 treatment to illustrate the protecting effect of N. paulsenii extract. Action of CAT, SOD, GST, POD, GSH & GSR were substantially (p < 0.05) reduced in comparison with control after CCl4 administration. N. paulsenii extract reinstated the antioxidant enzymes activities in co-treated groups and it was statistically analogous to the group treated with silymarin at its higher dose. Oral administration of N.paulsenii extract alone demonstrated a marginal difference in the activities compared to control (Table 3). Superscripts with different alphabets in a same column means significantly different.
Group
CAT
(U per min.)
POD
(U per min.)
SOD
(U per mg)
GSH (nMmin./mg/protein)
GSR
(nm NADPH oxidized/min/mg tissue)
GST
(mg per dl)
Control
5.69 ± 0.23a
8.45 ± 0.12a
6.28 ± 0.06a
11.8 ± 0.34a
4.37 ± 0.083a
24.63 ± 0.93a
Vehicle control
5.58 ± 0.14a
8.27 ± 0.07a
6.35 ± 0.04a
11.7 ± 0.43a
4.33 ± 0.06a
24.72 ± 0.77a
CCl4 (1 mL/kg)
2.18 ± 0.10b
3.29 ± 0.06b
2.51 ± 0.06b
5.11 ± 0.13b
0.91 ± 0.04b
10.37 ± 0.37b
Silymarin + CCl4
5.38 ± 0.20a
6.51 ± 1.00c
5.30 ± 0.05c
9.65 ± 0.24c
3.82 ± 0.04c
21.33 ± 0.41c
N.P (200 mg/kg) + CCl4
4.64 ± 0.14c
6.56 ± 0.02c
4.72 ± 0.09d
9.47 ± 0.19c
3.41 ± 0.04c
18.56 ± 0.74ac
N.P (400 mg/kg) + CCl4
4.88 ± 0.04c
6.80 ± 1.70c
5.13 ± 0.03c
9.87 ± 0.13c
3.61 ± 0.07c
21.71 ± 0.65c
N.P (200 mg/kg)
5.68 ± 0.02ac
8.43 ± 0.04a
6.29 ± 0.05ac
11.75 ± 0.22ac
4.35 ± 0.11c
24.67 ± 0.61c
N.P (400 mg/kg)
5.74 ± 0.03a
8.48 ± 0.07a
6.32 ± 0.07a
11.89 ± 0.55a
4.44 ± 0.19a
24.81 ± 1.09a
3.4 Effect of N. Paulsenii extract on ROS and TBARS levels
Compared to control group, the rats treated with CCl4 reported a significant (p < 0.05) eleviation in ROS and TBARS levels. The levels of TBARS and ROS reduced remarkably (p < 0.05) when co-treated with CCl4 + silymarin as opposed to the CCl4 group. As matched with CCl4-intoxicated group, the N. paulsenii + CCl4 groups showed significant decreases in ROS and TBARS levels (dose-dependent). Rats administered with N. paulsenii extract alone showed ROS and TBARS levels within standard range (Table 4). Superscripts with different alphabets in a same column means significantly different.
Group
TBARS (nM/min./mg protein)
ROS (U per mg protein)
Control
14.31 ± 0.47 a
0.90 ± 0.10 a
Vehicle control
14.62 ± 1.03 a
0.97 ± 0.92 a
CCl4 (1 mL/kg)
27.59 ± 1.03b
8.41 ± 0.58b
Silymarin + CCl4
17.14 ± 0.39c
1.81 ± 0.08c
N.P (200 mg/kg) + CCl4
19.06 ± 0.16 d
2.09 ± 0.13c
N.P (400 mg/kg) + CCl4
18.28 ± 0.61 cd
1.66 ± 0.11c
N.P (200 mg/kg)
14.37 ± 0.25c
0.92 ± 0.11 a
N.P (400 mg/kg)
14.11 ± 1.46 a
0.85 ± 0.11 a
3.5 Effect of N. Paulsenii extract on serum markers
Levels of ALP, AST and ALT are directly linked to oxidative stress in liver tissues (Table 5). Treatment of CCl4 significantly (p < 0.05) enhanced the level of hepatic serum markers (ALP, AST and ALT) which was diminished significantly (p < 0.05) by co-treatment with N. paulsenii extract. However, in 200 and 400 mg/kg dose of N. paulsenii alone treated groups, the serum markers levels were almost comparable to control group values. Superscripts with different alphabets in a same column means significantly different.
Group
ALT (U per I)
AST(U per I)
ALP(U per I)
Control
41.0 ± 0.40 a
49.5 ± 1.04 a
60.3 ± 1.25 a
Vehicle control
39.5 ± 0.64 a
46.8 ± 2.39 a
58.5 ± 1.19 a
CCl4 (1 mL/kg)
395.8 ± 3.06b
347 ± 4.02b
230.5 ± 4.40b
Silymarin + CCl4
81.75 ± 0.85c
87.8 ± 1.10c
93.0 ± 1.82c
N.P (200 mg/kg) + CCl4
140.3 ± 1.70 d
144 ± 2.67 d
170.5 ± 1.55 bd
N.P (400 mg/kg) + CCl4
113.5 ± 2.53 cd
134.8 ± 2.21 d
141.0 ± 2.48 d
N.P (200 mg/kg)
41.11 ± 1.93c
47.25 ± 1.10 a
60.13 ± 0.91c
N.P (400 mg/kg)
37.33 ± 1.10 a
44.76.0 ± 1.08 a
58.21 ± 1.25 a
3.6 Effect of N. Paulsenii extract on DNA damage
CCl4 intoxication remained significant (p < 0.05) in comet length, comet-numbers, tail length, tail and olive moment, whereas a major decrease was reported in head length and percentage DNA in head as compared with control rats. CCl4 + silymarin treatment exhibited a notable (p < 0.05) reduction in comet number, comet and tail length, % DNA in tail, tail and olive moment and a substantial (p < 0.05) rise in percentage DNA in head and head length was observed as compared to CCl4 intoxicated rats. In identical way, significant reductions in tail length, comet number, comet length, percent DNA in tail, tail moment and olive moment, and a significant increase in percent DNA in head and head length relative to CCl4 administered rats were observed in N. paulsenii + CCl4 administered groups dose dependently. Rats administered with N. paulsenii extract alone showed normal parameters as indicated in the control group (Table 6). Superscripts with different alphabets in a same column means significantly different.
Group
Number of comets
Comet length
Tail length
Head length
% DNA in head
% DNA in tail
Tail Moment
Olive tail moment
Control
22.2 ± 0.51 a
25.61 ± 0.87 a
4.35 ± 0.11 a
40.89 ± 0.86 a
97.17 ± 0.16 a
2.82 ± 0.16 a
1.13 ± 0.13 a
2.41 ± 0.09 a
Vehicle control
23.7 ± 0.71 a
25.78 ± 1.22 a
4.62 ± 0.08 a
40.83 ± 1.02 a
96.74 ± 0.16 a
3.25 ± 0.16 a
1.17 ± 0.08 a
2.43 ± 0.09 a
CCl4 (1 mL/kg)
48.4 ± 1.61b
58.6 ± 3.34b
17.49 ± 0.51b
24.1 ± 0.55b
86.33 ± 0.21b
13.66 ± 0.21b
2.48 ± 0.14b
4.31 ± 0.18b
Silymarin + CCl4
30.9 ± 1.21c
33.94 ± 0.67c
6.61 ± 0.18 ac
35.34 ± 1.54c
95.26 ± 0.14c
4.74 ± 0.14 ac
1.85 ± 0.04 ac
2.91 ± 0.04 ac
N.P (200 mg/kg) + CCl4
32.9 ± 1.14c
36.22 ± 0.59c
7.94 ± 0.19c
33.33 ± 0.73c
94.56 ± 0.23c
5.43 ± 0.23c
1.96 ± 0.03c
3.08 ± 0.07c
N.P (400 mg/kg) + CCl4
32.6 ± 1.06c
35.02 ± 0.22c
6.66 ± 0.11 ac
36.82 ± 0.79c
95.25 ± 0.26c
4.74 ± 0.26 ac
1.90 ± 0.04c
2.94 ± 0.07 ac
N.P (200 mg/kg)
23.4 ± 0.87 ac
25.47 ± 0.51c
4.52 ± 0.12 ac
40.92 ± 1.03 a
96.82 ± 0.08c
2.99 ± 0.08 ac
1.13 ± 0.06 a
2.42 ± 0.04 a
N.P (400 mg/kg)
22.1 ± 1.12 a
24.77 ± 1.46 a
4.12 ± 0.15 a
40.08 ± 0.91 a
95.49 ± 0.27 a
2.56 ± 0.27 a
1.01 ± 0.19 a
2.31 ± 0.09 a
3.7 Effect of N. paulsenii extract on histomorphology of liver
Effect of N. paulsenii extract in various groups with different doses and CCl4 treatment on histology of liver is presented in Fig. 3 (40X). Vehicle control and Control group indicated normal histomorphology and did not show any disruption in central venule and hepatocytes. CCl4 administration resulted in severe variations that occur in histomorphology of liver tissues. The substantial variations noticed in CCl4 intoxicated group were deterioration of lobule, fatty change, congested blood vessels and severe steatosis. Co-treatment of N. paulsenii extract with CCl4 exhibited tissue recovery and abolition of chronic damages at different doses. N. paulsenii extract (200 mg/kg b.w.) treatment showed a visible level of tissue recovery.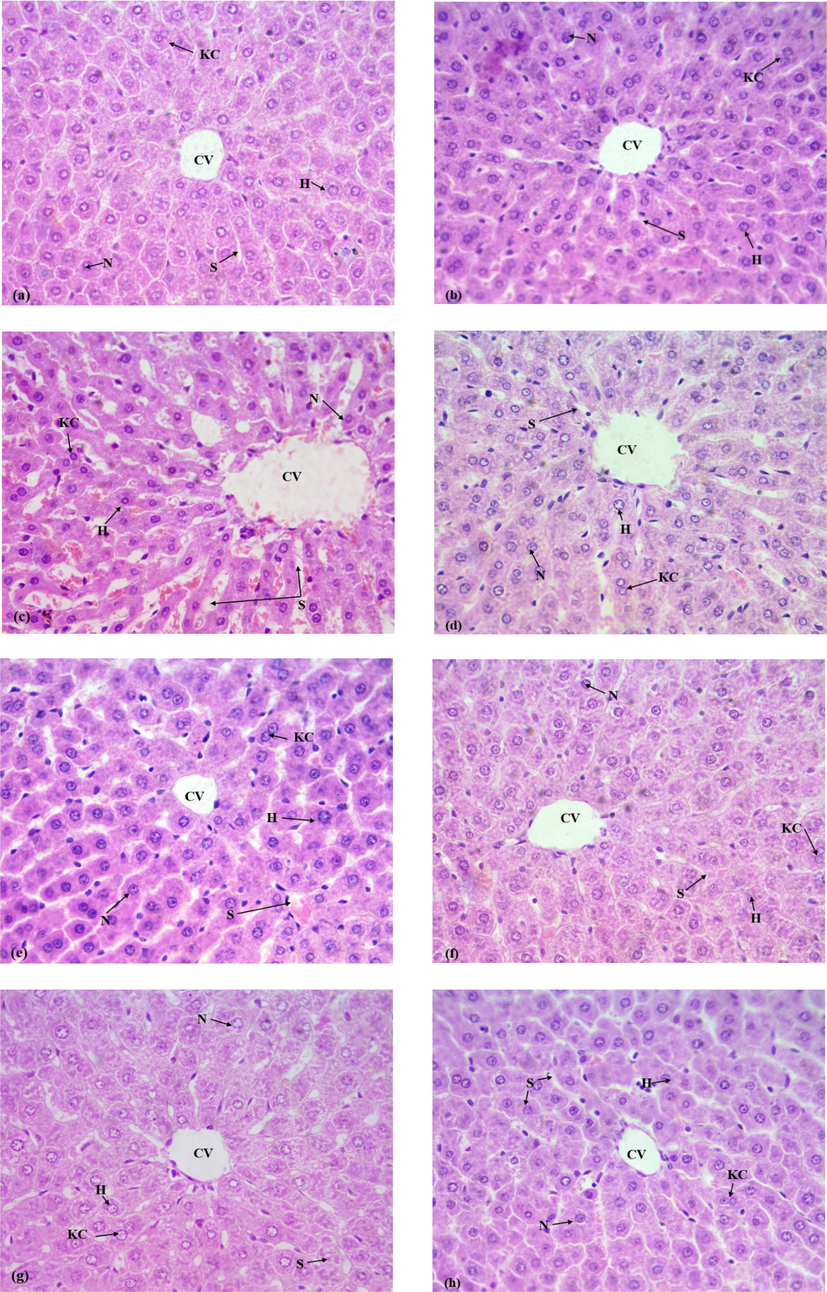
Microscopic images of liver tissues were obtained from different groups (H&E 40X). (a) Normal histoarchitecture (control) (b) Vehicle control (normal histoarchitecture) (c) CCl4 (1 mL/kg) Tissues show extensive and marked necrosis (d) CCl4 + Silymarin (decrease in necrosis throughout the liver tissues and recovery of damaged tissues (e) N.P (200 mg/kg) + CCl4 (decreased necrosis) (f) N.P (400 mg/kg) + CCl4 (shows recovery in damaged tissues) (g) N.P (200 mg/kg) shows normal histoarchitecture (h) N.P (400 mg/kg)shows normal histomorphology: H; Hepatocytes, CV; Central venule, S; Sinusoids, KC; Kupffer cells, N; Nucleus.
3.8 Discussion
Liver performs metabolic functions in the body. Hepatic diseases are a major problem even in advanced countries (Adewusi and Afolayan 2010). Hepatic injury is related with distortion of these metabolic functions. There is a lack of effective medicines which stimulate the functions of liver and protect the liver from damage and regenerate the hepatic cells so there is need to replace the current medicines with new effective medicines (Adewusi and Afolayan 2010). Mostly new drugs are being synthesized from natural products (secondary metabolites) and from those compounds that are obtained from natural products Genus Nepeta have medicinal properties because many species of genus Nepeta are used in folk medicines and components found in species of Nepeta genus have biological activities (Karaman 2007). Herbal medicines that are synthesized from plants have been used against hepatic diseases for a long time and some preparations are accessible in market. Many active components are present in plants from where herbal medicines are synthesized. Therefore, the present investigation emphasizes the hepatoprotective potential of N. paulsenii and its active ingredients, which can be further studied as a pharmacore for reducing the toxic effects of carbon tetrachloride.
Many phytocomponents identified in preliminary phytochemical analysis of extract like coumarins, terpenoids, flavonoids, saponins and betacyanins. Among these phytochemicals, flavonoids, coumarins, saponins and betacyanins have increased a certain importance because of their wide range antioxidant-activities (Sajid et al., 2016). Ten components identified in further analysis (GC–MS) which have medicinal properties these components are glycerin, geranic acid, phytol, octadecanoic acid, 2,6-octadien-1-ol, 2-pentadecanone, benzene propanoic acid, 1-(+)-pscorbic acid, 1,2-benzene-dicarboxylic acid and 9,12,15-octa-decatrienoic acid. benzenepropanoic and 1-(+)-ascorbic acids have anti-oxidant activity (Akpuaka et al., 2013). So benzenepropanoic acid and 1-(+)-ascorbic acid are foremost components providing to the antioxidant activity of the plant. Benzenepropanoic acid is used to extend the shelf life of foods as an antioxidant, and it is also used to protect the shelved foods from deterioration by microorganisms. Moreover, 9,12,15-octadecatrienoic acid has hepatoprotective effect and it is the one of major constituents of the plant (Akpuaka et al., 2013).
Antioxidant enzymes are considered to be the foremost species to defend the biological molecules. The metabolic function of the liver is to detoxify the effect of xenobiotics on ROS development, where CAT, POD, SOD, GSH, GSR and GST play a crucial role in oxidative stress deterrence in liver (Khan et al., 2013). CAT reacts with hydrogen peroxide to form molecular oxygen and water. Administration of CCl4 influenced the activity of POD, CAT, SOD, GSH, GST and GSR while the levels of ROS and TBARS were elevated in CCl4 administered group. Lipid peroxidation of hepatocytes caused when CCl4 is metabolized into highly reactive metabolites by liver. The observed decline in the level of CAT, SOD, POD due to CCl4 exposure is in consensus with former observation of (Sajid et al., 2016), who reported that treatment with CCl4 reduced the level of these antioxidant enzymes. Reduction in the level of these antioxidant enzymes indicated the oxidative damage in liver (Cheng et al., 2013). Administration of plant extract instigated a noteworthy recovery in activities of enzymatic antioxidants CAT, SOD, POD, GSH, GST and GSR, however, ROS and TBARS levels were significantly reduced. This demonstrates that plant extract can scavenge free radicals that might cause reduction in oxidative damage to liver tissue and restore the antioxidant enzymes activities. The existence of bioactive phytochemicals in the methanolic extract of the N. paulsenii may be a major cause of its curative potential.
CCl4-administration significantly increased the AST, ALP and ALT levels in treated rats. The biochemical damage and cellular function defects can be detected by the levels of these enzymes (Khan et al., 2011). Previous studies have shown that high levels of serum ALT, AST, and ALP have a strong linkage with oxidative liver damage (Sharifi-Rigi et al., 2019). Aminotransferases including ALT, ALP and AST are enzymes ked with liver parenchyma cells. These enzymes are normally present in the cytosol, but due to the damage in plasma membrane of hepatic cells, these markers are released into the bloodstream, which indicates liver damage. So, the level of these enzymes is considered as an important marker that is used in the diagnosis of liver diseases (Carobene et al., 2013). Deleterious effects of CCl4 may be linked with its reactive metabolites which are formed intermediately during its metabolism in the body. Previous investigations showed that over production of ROS impairs the structural integrity of hepatic cells, as indicated by the unusual increase in hepatic serum markers. CCl4 treatment caused noteworthy hepatic injury as evident by increased levels of ALP, ALT and AST that are indicator of disturbance in functional-integrity of liver. Treatment with N. paulsenii extract reduced the hepatic markers level which is the sign of stabilization in hepatic functions. It has been reported earlier, phytochemicals (flavonoids, coumarins, saponins and betacyanins) act as an antioxidant which acts as both free radical scavengers and antioxidant (Engwa 2018). This reduction in the level of hepatic serum markers can be accredited to antioxidant potential of N. paulsenii extract.
DNA can be damaged by several exogenous and endogenous factors. Common factors include ROS produced during the normal metabolic functions, spontaneous or enzymatic conversions and errors during DNA replication (Tiwari and Mishra 2017). It was previously reported that several cytotoxic agents pose genotoxic impacts through the generation of ROS, which results in single/double-stranded breakdowns and disrupt the DNA integrity (Srinivas et al., 2019). In the current investigation, the outcomes of the comet parameters show significant variations in comet parameters. CCl4 administration induced a remarkable reduction % DNA in the head and in head length compared to the standards, on the other hand, increased the comet length, comet numbers, tail length, olive-tail moment, and % DNA in tail. It has been reported that CCl4 potentially generates ROS, which leads toward DNA fragmentation (Alkreathy et al., 2014). However, N. paulsenii plant extract recovered the DNA injury provoked by CCl4. Our study evaluated that N. paulsenii plant extract is a potent bioactive agent to avert CCl4-induced DNA damage by decreasing OS in the liver tissues which is further supported by (Samad et al., 2020), who observed that N. paulsenii plant extract decreased oxidative stress produced due to CCl4 administration in the testicular tissues of rats.
Histopathological study of liver showed the severe damages like hepatic damages, inflammatory cell infiltration, fatty changes, degeneration of lobular structure and degeneration of nuclei in CCl4 treated group. Level of fatty degeneration and necrosis were obvious after treatment of CCl4. CCl4 induced hepatic tissue injury is due to the production of free radicals and lipid peroxidation that induces cellular damage. CCl4 forms a CCl3 and OOCCl3 radicals then these radicals bind with polyunsaturated fatty acids (PUFA) and form peroxy and alkoxy radicals. These radicals produce injury in cell membrane, hepatic damage and altered the activity of different enzymes. These radicals bind with macromolecules and become source of oxidative degradation of cellular membrane leading to necrosis of hepatocytes (Ranawat et al., 2010). Administration of high and medium dose of N. paulsenii plant extract reduced the injury and fatty degeneration. This may be due to the presence of phytochemical components like coumarins, terpenoids, flavonoids, saponins and betacyanins. These components exhibited hepatoprotective effect and work individually or combined action against hepatotoxicity. Histological study exhibited a defensive effect of N. paulsenii plant extract on CCl4 induced hepatoxicity which may be attributed to the active components of the plant extract.
3.9 Conclusion
The current study revealed that the hepatoprotective nature of N. paulsenii leaves extract is possibly due to the endogenous antioxidant-molecules and scavenging of free radicals. Natural antioxidants tend to promote this effect in N. paulsenii plant, which greatly decreased oxidative stress and contributed to normal liver functions. Further research is needed to expose the pathways concerning the hepatoprotective function of N. paulsenii at molecular level. Moreover, ethanolic extract of N. paulsenii needs to be studied in future as for the intake of oral drugs ethanolic extracts may be relatively safer compared to methanolic extracts. Also, LC-MS based studies needs to be carried out for N. paulsenii.
Acknowledgement
Dr. Samina is thankful to the HEC - Pakistan for awarding research grant NRPU-6379.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A review of natural products with hepatoprotective activity. J. Med. Plant Res.. 2010;4(13):1318-1334.
- [Google Scholar]
- Biological activities of characterized isolates of n-hexane extract of Azadirachta indica A. Juss (Neem) leaves. Nature and science.. 2013;11(5):141-147.
- [Google Scholar]
- Alkahtani, J., A. Asma, M. Adil, et al., 2022. Phytochemical Investigation and Antimicrobial Potential of Medicinal Plant Nepeta distans Royle ex Benth. Journal of Food Quality. 2022.
- CCl4 induced genotoxicity and DNA oxidative damages in rats: hepatoprotective effect of Sonchus arvensis. BMC Complement Altern. Med.. 2014;14(1):1-7.
- [Google Scholar]
- Medicinal plants used for dermatological disorders among the people of the kingdom of Saudi Arabia: A narrative review. Saudi Journal of Biological Sciences.. 2022;29(6):103303
- [CrossRef] [Google Scholar]
- Hepatoprotective effect of a polyherbal formulation (Aab-e-Murawaqain) against CCl4 induced liver toxicity in Wistar albino rat model by suppressing proinflammatory cytokines. South African Journal of Botany 2021
- [Google Scholar]
- Protective effect Spirulina against Monosodium glutamate-induced hepatic dysfunction: A biochemical, molecular, and histopathological study. Journal of King Saud University - Science.. 2023;35(2):102464
- [CrossRef] [Google Scholar]
- Phytochemical analysis of selected Nepeta species by HPLC-ESI-MS/MS and GC–MS methods and exploring their antioxidant and antifungal potentials. Journal of Food Measurement and Characterization.. 2021;15(3):2417-2429.
- [Google Scholar]
- A systematic review of data on biological variation for alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transferase. Clin. Chem. Lab. Med.. 2013;51(10):1997-2007.
- [Google Scholar]
- Global epidemiology of chronic liver disease. Clinical Liver Disease.. 2021;17(5):365.
- [Google Scholar]
- Heparanase Expression Propagates Liver Damage in CCL4-Induced Mouse Model. Cells.. 2022;11(13):2035.
- [Google Scholar]
- Antioxidant and hepatoprotective effects of Schisandra chinensis pollen extract on CCl4-induced acute liver damage in mice. Food Chem. Toxicol.. 2013;55:234-240.
- [Google Scholar]
- Fat mass to fat-free mass ratio and the risk of non-alcoholic fatty liver disease and fibrosis in non-obese and obese individuals. Nutrition & metabolism.. 2021;18(1):1-12.
- [Google Scholar]
- Comet assay: a reliable tool for the assessment of DNA damage in different models. Cell Biol. Toxicol.. 2009;25(1):5-32.
- [Google Scholar]
- Engwa, G. A., 2018. Free radicals and the role of plant phytochemicals as antioxidants against oxidative stress-related diseases. Phytochemicals: Source of Antioxidants and Role in Disease Prevention. BoD–Books on Demand. 7 49-74.
- Phytochemical methods a guide to modern techniques of plant analysis. springer science & business media; 1998.
- High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat. Res. Genet. Toxicol. Environ. Mutagen.. 2007;631(1):55-61.
- [Google Scholar]
- Vitexin attenuates cisplatin-induced renal toxicity by reducing oxidative stress and inflammation. Journal of King Saud University - Science.. 2021;33(8):101657
- [CrossRef] [Google Scholar]
- Essential Oil Composition of Nepeta cilicia Boiss. Botany: Apud Bentham and Phlomis viscosa Poiret from Turke. Int. J; 2007.
- Evaluation of antioxidant and fertility effects of Digera muricata in male rats. Afr. J. Pharmacy Pharmacol.. 2011;5(6):688-699.
- [Google Scholar]
- Attenuation of CCl4-induced hepatic oxidative stress in rat by Launaea procumbens. Exp. Toxicol. Pathol.. 2013;65(3):319-326.
- [Google Scholar]
- Recent advances in the development of in vitro liver models for hepatotoxicity testing. Bio-Design and Manufacturing.. 2021;4(4):717-734.
- [Google Scholar]
- Essential oil compositions, pharmacological importance and agro technological practices of Patchouli (Pogostemon cablin Benth.): A review. Journal of Essential Oil Bearing Plants.. 2021;24(6):1212-1226.
- [CrossRef] [Google Scholar]
- FXR in liver physiology: Multiple faces to regulate liver metabolism. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease.. 2021;1867(7):166133
- [Google Scholar]
- Raeeszadeh, M., M. Moradi, P. Ayar, et al., 2021. The antioxidant effect of Medicago sativa L.(alfalfa) ethanolic extract against mercury chloride (HgCl2) toxicity in rat liver and kidney: an in vitro and in vivo study. Evidence-Based Complementary and Alternative Medicine. 2021.
- Hepatoprotective activity of ethanolic extracts of bark of Zanthoxylum armatum DC in CCl4 induced hepatic damage in rats. J. Ethnopharmacol.. 2010;127(3):777-780.
- [Google Scholar]
- Protective effects of aucubin against nonylphenol-induced liver toxicity by improving biochemical, inflammatory and histopathological indices. Journal of King Saud University - Science.. 2022;34(4):102033
- [CrossRef] [Google Scholar]
- Characterizing the reproducibility in using a liver microphysiological system for assaying drug toxicity, metabolism, and accumulation. Clinical and translational science.. 2021;14(3):1049-1061.
- [Google Scholar]
- Phytochemical, antioxidant and hepatoprotective effects of Alnus nitida bark in carbon tetrachloride challenged Sprague Dawley rats. BMC Complement Altern. Med.. 2016;16(1):1-17.
- [Google Scholar]
- Methanolic extract of Nepeta paulsenii as an ameliorative agent against CCl4 induced testicular damage in male albino rats. Journal of King Saud University-Science.. 2020;32(1):1168-1174.
- [Google Scholar]
- Protective and anti-inflammatory effects of hydroalcoholic leaf extract of Origanum vulgare on oxidative stress, TNF-α gene expression and liver histological changes in paraquat-induced hepatotoxicity in rats. Arch. Physiol. Biochem.. 2019;125(1):56-63.
- [CrossRef] [Google Scholar]
- The protective effect of taurine on oxidized fish-oil-induced liver oxidative stress and intestinal barrier-function impairment in juvenile Ictalurus punctatus. Antioxidants.. 2021;10(11):1690.
- [Google Scholar]
- Leaf Anatomical Evaluation of Some Nepeta L., Taxa in Iran. Iranian Journal of Science and Technology, Transactions A. Science.. 2021;45(4):1211-1222.
- [Google Scholar]
- Tiwari, P. and Mishra, 2017. Role of Flavonoids in DNA Damage and Carcinogenesis Prevention’. J. Carcinog. Mutagen. 8 (4) 1000297.
- Pharmacognsy (11th edn.). Brailliar Tiridel Can: Macmillian publishers; 1989.
- Overview of non-alcoholic fatty liver disease (NAFLD) and the role of sugary food consumption and other dietary components in its development. Nutrients.. 2021;13(5):1442.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102542.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







