Translate this page into:
Natural antioxidant curcumin attenuates NiO nanoparticle-induced cytotoxicity in mouse spermatogonia cells: A mechanistic study
⁎Corresponding author. mahamed@ksu.edu.sa (Maqusood Ahamed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Current research focuses on the effects of nanomaterials on the human reproductive system. Nanostructures can cross the epithelial and blood-testicular barriers and pose risks to the reproductive organs. Oxidative stress has been proposed as a possible mechanism of reproductive toxicity caused by nanomaterials. Dietary curcumin could be a therapeutic drug for nanomaterial-induced reproductive toxicity. Studies on effect of commonly used nickel (II) oxide nanoparticle (NiO NPs) on male reproductive organs and their attenuation by natural antioxidant curcumin is scarce. This work intended to study the attenuating potential of curcumin against NiO NPs-induced toxicity in mouse spermatogonia GC-1 spg cells. Plausible mechanisms of alleviating effect curcumin against NiO induced reproductive toxicity was explored through oxidative stress pathway. NiO NPs was synthesized via chemical co-precipitation route and characterized by SEM, TEM, and XRD. NiO NPs was found to induce dose-dependent cytotoxicity in GC-1 spg cells (10–320 µg/ml for 24 h) whereas curcumin did not exert any effect in concentration range of 1–80 µg/ml. Interestingly, cytotoxic response of NiO NPs in GC-1 spg cells was significantly attenuated by curcumin. The higher expression of caspase-3 gene and loss of mitochondrial membrane potential after treatment with NiO NPs were effectively alleviated by curcumin. The increase in intracellular pro-oxidant levels (hydrogen peroxide, malondialdehyde, and reactive oxygen species) after exposure to NiO NPs was also mitigated by curcumin. Moreover, glutathione depletion and lower activity of several antioxidant enzymes (GPx, SOD, and CAT) after NiO NPs were further almost reverted by curcumin. We believe, this is the first preliminary study showing that NiO NPs induced cytotoxicity in mouse spermatogonia cells was mitigated by curcumin via oxidative stress. The therapeutic effect of dietary antioxidant curcumin against nanomaterial-induced reproductive toxicity is warranted further research.
Keywords
NiO nanoparticles
Reproductive toxicity
Dietary antioxidant
Mitigation
Apoptosis
Oxidative stress
Caspase-3 enzyme
1 Introduction
Nickel oxide nanoparticles (NiO NPs) belong to transition metal oxide group with cubic lattice structure that has exceptional electrical, thermal, and optical properties (Bonomo et al., 2018). Due to these unique properties NiO NPs are being utilized in sensor, battery electrode, fuel cell, ion storage material, electrochromic film, thermoelectric material, and dye-sensitized photocathode (Adinaveen et al., 2019; Diallo et al., 2018). NiO NPs are also applied in medical and household products because of its antimicrobial property (Behera et al., 2019). The high production and diverse application of NiO NPs in industry, biomedical, and personal care products may increase the chance of human exposure and potential health risks. Current research focuses on effect of commonly used NPs on human reproductive organs. Studies have shown that NPs can easily cross the blood-testis barrier (BTB) and affect the spermatogenesis (Lan and Yang, 2012). A recent work showed that TiO2 NPs can expand the BTB gaps and allow the NPs to easily pass BTB and affect spermatogenesis (Ni et al., 2021). Pinho et al. also found that ZnO NPs induce cytotoxicity in mouse spermatogonia cells, which interfere the spermatogenesis process (Pinho et al., 2020). Knowledge on the effect of NiO NPs on male reproductive system and approaches that mitigate the reproductive toxicity of NPs is limited. Development of a novel approach to protect the reproductive organs from NP-induced toxicity is urgently required.
Several reports suggested that NiO NPs induce toxicity through redox imbalance and oxidative damage of cell macromolecules (Sutunkova et al., 2019; Yu et al., 2018). To certain level, the antioxidant defense system of the cells, eliminate the ROS level and compensate the oxidative stress. However, when the antioxidant defense system of cells is unable to eradicate the higher level intracellular ROS, several health issues may happen (Jadeja et al., 2017). Indeed, oxidative stress has been associated with several reproductive disorders (Sabeti et al., 2016). Studies suggest that oxidative stress mediated injury to spermatozoa is a chief contributor to the pathology of > 50% of male infertility (Bisht et al., 2017). Spermatozoa are most susceptible to the harmful effects of oxidative stress because these cells have limited antioxidant defence systems (Alahmar, 2019). Oxidative stress is an emerging risk factor for male infertility because it can affect the spermatogenesis process by inducing the oxidative damage of DNA protein, and lipid peroxidation (Agarwal et al., 2014). Consequently, natural antioxidants supplementation could be an important treatment for male reproductive toxicity caused by NiO NPs exposure.
Dietary antioxidant curcumin, a polyphenolic compound present in turmeric (Curcuma longa L.) rhizome, has antioxidant and anti-inflammatory properties. Hence, curcumin can be one of the natural antioxidants to treat various health issues including reproductive disorders. Di(2-ethylhexyl) phthalate (DEHP) induced testicular damage in mice was reversed by curcumin treatment (Głombik et al., 2014). Another study reported that turmeric rhizome prevent hypertension mediated male reproductive disorder in rats (Akinyemi et al., 2015). There is limited information of reproductive toxicity of NiO NPs and its attenuation by dietary curcumin. This work intended to study the attenuating effect of curcumin against NiO NPs induced toxicity in mouse spermatogonia GC-1 spg cells. Plausible mechanisms of alleviating effect curcumin against NiO induced reproductive toxicity was also explored through the oxidative stress pathway. This cell line (GC-1 spg) originated from mouse testes and serves as an excellent in vitro model to study the male reproductive toxicity (Ahamed et al., 2021a; Yang et al., 2020).
2 Materials and methods
2.1 Synthesis and characterization of NiO NPs
NiO NPs was synthesized by a chemical co-precipitation method using Ni(NO3)2·6H2O (Sigma-Aldrich) and NaOH as precursors (Atul et al., 2021). X-ray diffraction (XRD), transmission electron microscopy (TEM), and scanning electron microscopy (SEM) were applied to characterize the synthesized sample.
2.2 Cell culture and exposure protocol
Mouse spermatogonia (GC-1 spg) cell line (ATCC, Manassas, VA, USA) was cultured in DMEM (Invitrogen, Carlsbad, CA, USA) with fetal bovine serum (10%), 100 unit/ml penicillin, and 100 µg/ml streptomycin. Cells were grown at 37 °C with 5% CO2 gas. Cells were exposed to different concentrations of NiO NPs (1–320 µg/ml) and curcumin (1–320 µg/ml) for 24 h. In co-exposure study, cells were treated with 10 µg/ml of curcumin before 1 h exposure to a moderate concentration of NiO NPs (40 µg/ml). These concentrations were selected based on the preliminary cytotoxicity results.
2.3 Bioassays
Cytotoxicity was examined by the MTT assay (Mosmann, 1983) with some changes (Ahamed et al., 2022). Caspase-3 gene (mRNA) expression was analysed by a RT-PCR (Applied Biosystems, Waltham, MA, USA) using SYBR green as previously reported (Ahamed et al., 2022). Caspase-3 enzyme assay was done using the BioVision colorimetric kit (Milpitas, CA, USA). Mitochondrial membrane potential (MMP) was estimated by a microplate reader (Synergy-HT, BioTek, Winooski, VT, USA) applying tetramethylrhodamine methyl ester (TMRM) probe as describe before (Ahamed et al., 2021b). ROS generation was assessed with a microplate reader (Synergy-HT, BioTek) utilizing 2 -7 -dichlorodihydrofluorescein diacetate (H2DCFDA) (Ahamed et al., 2021a). Glutathione (GSH) content was assayed through Elman’s protocol (Ellman, 1959). Malonaldehyde (MDA) content was determined as reported by Ohkawa et al. (Ohkawa et al., 1979). Intracellular hydrogen peroxide (H2O2) level was estimated by commercial kit (Sigma-Aldrich). Superoxide dismutase (SOD) activity was examined using a kit from Cayman chemical (Michigan, USA). Glutathione peroxidase (GPx) and catalase (CAT) enzymes activities were tested as previously reported (Rotruck et al., 1973; Sinha, 1972). Protein assay was done by Bradfords method (Bradford, 1976).
2.4 Statistics
Data were analysed by applying ANOVA followed by Dennett’s multiple tests. The p < 0.05 was assigned as statistically significant. Data were presented as mean ± SD of three individual assays (n = 3).
3 Results and discussion
3.1 Characterization
XRD spectra of NiO NPs showed the sharp diffraction peaks at 2θ values 38.39, 44.37, 63.16, 76.13, and 80.05, indexed as (1 1 1), (2 0 0), (2 2 0), (3 1 1), and (2 2 2) that correspond to face centred cubic NiO, which according to standard data (JCPDS Card No. 65–2901) (Khodair et al., 2022) (Fig. 1A). Crystallite size of NiO NPs was estimated using Scherrer formula (Patterson, 1939). It was found around 53 nm. Low resolution TEM image indicated the almost spherical morphology with average particle size of around 51 nm (Fig. 1B). High resolution TEM micrograph suggests the crystalline nature of prepared sample supporting the XRD data (Fig. 1C). SEM micrograph suggests the smooth morphology of prepared NiO NPs (Fig. 2).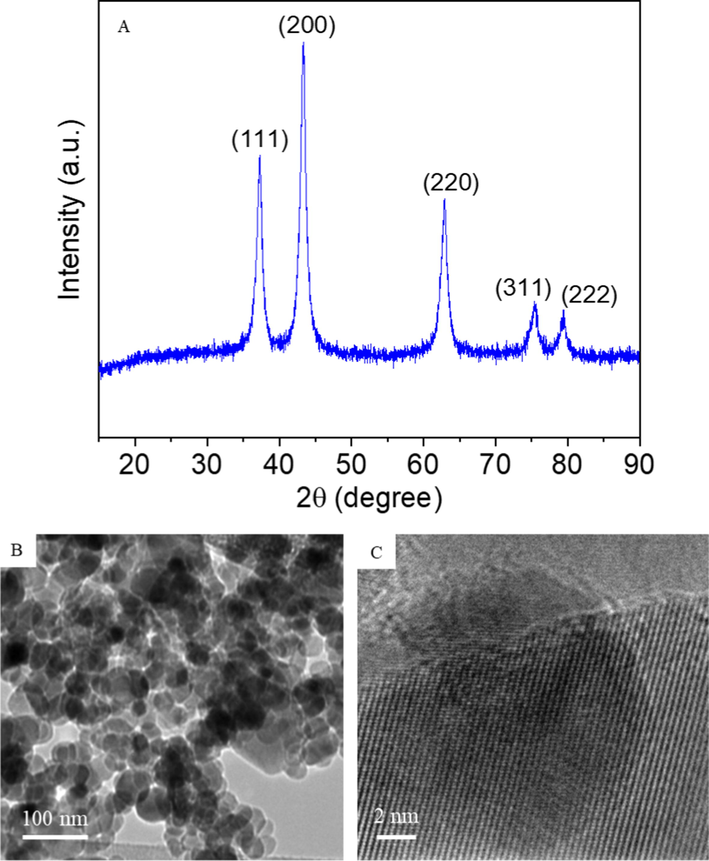
(A) XRD spectra, (B) low resolution TEM image, and (C) high resolution TEM image of NiO NPs.

SEM image of NiO NPs.
3.2 Cytotoxicity
GC-spg cells were treated with different concentrations of NiO NPs (1–320 µg/ml) and curcumin (1–320 µg/ml) for 24 and cytotoxicity was assessed by MTT assay. Fig. 3A demonstrated that NiO NPs induce dose-dependent cytotoxicity in the concentration range of 10–320 µg/ml. NiO NPs decreased the cell viability to 99%, 95%, 92%, 88%, 74%, 53%, 35%, 19%, and 10% for the concentrations of 1, 2, 5, 10, 20, 40, 80, 160, and 320 µg/ml, respectively (p < 0.05). Present data on cytotoxic response of NiO NPs in GC-spg cells was according to other studies where investigators also observed dose-dependent cytotoxicity of NiO NPs in various mammalian cell lines (Ahamed et al., 2013; Ezhilarasi et al., 2016). Fig. 3B demonstrated that curcumin did not reduce the significant viability of GC-1 spg cells up to the concentration of 80 µg/ml. However, at high dosages (160 and 320 µg/ml) curcumin induces moderate toxicity to GC-1 spg cells. Cytocompatibility of curcumin in these concentrations was also observed by other investigators (Ahamed et al., 2022; Ghosh et al., 2020; Siddiqui et al., 2012).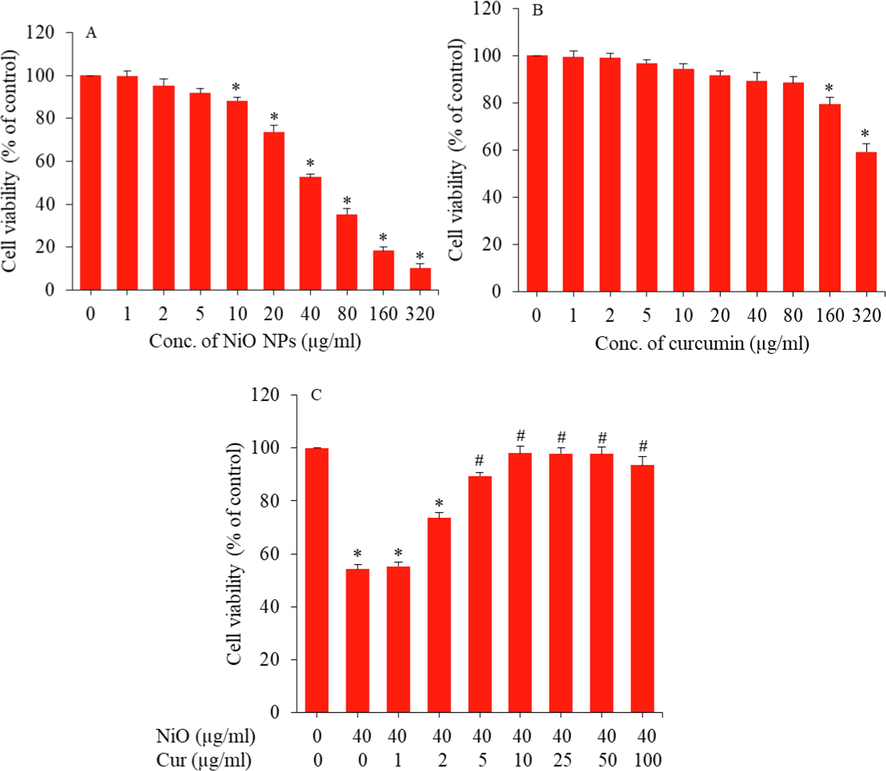
(A) Cytotoxicity of NiO NPs in GC-1 spg cells. (B) Cytocompatibility of curcumin in GC-1 spg cells. (C) Protective effect of curcumin against NiO NPs induced cytotoxicity in GC-1 spg cells. *Significant difference from the control (p < 0.05). #Significant protective effect of curcumin against NiO NPs cytotoxicity (p < 0.05).
For the selection of appropriate concentration of curcumin, which efficiently attenuate the cytotoxicity of NiO NPs, we have taken a moderate cytotoxicity concentration of NiO NPs (40 µg/ml) and co-treated with various cytocompatible concentrations of curcumin (1–80 µg/ml) for 24 h. Fig. 3C exhibited that at a concentration of 10 µg/ml, curcumin received highest attenuating effect on NiO NPs induced toxicity at a concentration of 40 µg/ml. Hence, we have chosen 10 µg/ml concentration of curcumin and 40 µg/ml concentration of NiO NPs to further investigate possible mechanisms of attenuating effect of curcumin against NiO NPs toxicity in mouse spermatogonia cells.
3.3 Apoptosis
Apoptosis (programmed cell death) regulation is a critical factor in determining the germ cells development and function (Aitken and Baker, 2013). A number of factors such as unhealthy lifestyle, radiation, and environmental pollutants might induce apoptosis in germ cells (Bisht et al., 2017). Infertile men have greater level of seminal oxidative stress and increased apoptosis in comparison to fertile men (Manku and Culty, 2015). Earlier, we found that Bi2O3 NPs were able to induce apoptosis in GC-1 spg cells (Ahamed et al., 2021a). Curcumin has also shown potential to overcome the reproductive disorders caused by environmental pollutants (Głombik et al., 2014). In this study, we further assessed apoptotic response of NiO NPs and its mitigation by curcumin in GC-1 spg cells. Fig. 4A showed that NiO NPs-induced up-regulation of mRNA level of caspase-3 gene was significantly alleviated by curcumin. In agreement with RT-PCR mRNA results, NiO NPs induced higher activity of caspase-3 enzyme was successfully reverted by curcumin (Fig. 4B). Male infertility is also associated with mitochondrial membrane potential (MMP) loss in spermatozoa (Agnihotri et al., 2016). Fig. 4C of this study showed that MMP loss in GC-1 spg cells following NiO NPs exposure was significantly abrogated by curcumin.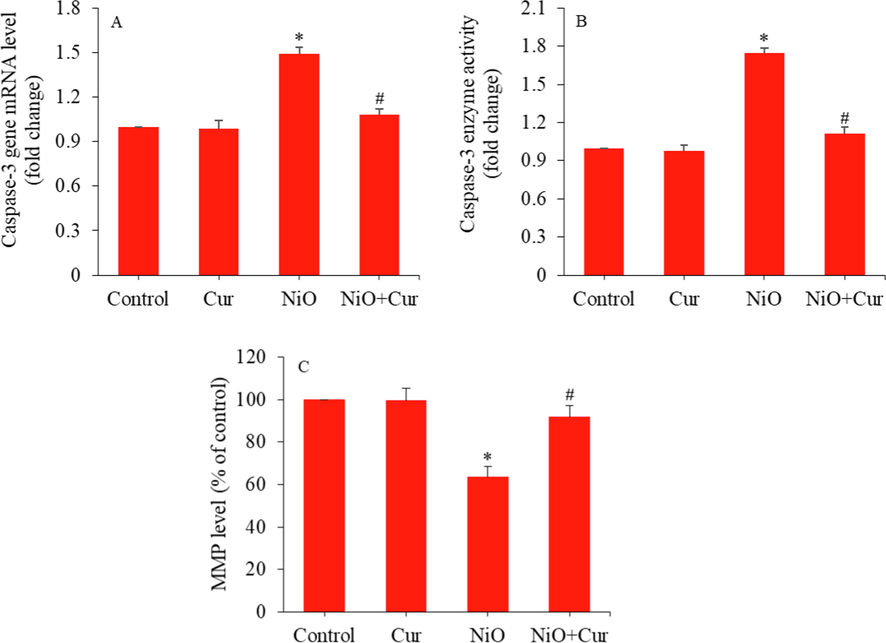
Apoptotic response of GC-1 spg cells treated with NiO NPs (40 µg/ml) and/or curcumin (10 µg/ml) for 24 h. (A) Expression (mRNA) of caspase-3 gene. (B) Caspase-3 enzyme activity. (C) MMP level. *Significant difference from the controls (p < 0.05). #Significant protective effect of curcumin against NiO NPs (p < 0.05).
3.4 Oxidative stress
Germ cells are highly susceptible to oxidative stress owing to limited antioxidant defence capacity and inadequate DNA repair mechanism (Sabeti et al., 2016). Antioxidant supplementation could be a probable therapeutic approach to overcome the oxidative stress-induced male infertility (Gharagozloo et al., 2016). In this work, we further examined the role of oxidative stress in protective effect of curcumin against NiO NPs induced toxicity in GC-1 spg cells by measuring the several parameters of pro-oxidants and antioxidants. Cells were treated for 24 h with NiO NPs (40 µg/ml) and/ or curcumin (10 µg/ml). Fig. 5A and B depicted that NiO NPs induced intracellular ROS and H2O2 levels were effectually reverted by curcumin. Spermatozoa are particularly susceptible to lipid peroxidation they are abundant in polyunsaturated fatty acids that are highly vulnerable to ROS attack (Aitken and Baker, 2013). It has been observed that lipid peroxidation in sperm plasma membrane could be reversed by natural antioxidant (e.g. α-tocopheral), which interrupts the cascade of lipid peroxidation (Gharagozloo et al., 2016). The MDA or 4HNE have been used as biomarkers of lipid peroxidation in germ cells (Aitken et al., 2014). We also found that higher level of MDA in GC-1 spg cells after NiO NPs treatment was reversed by curcumin co-treatment (Fig. 5C).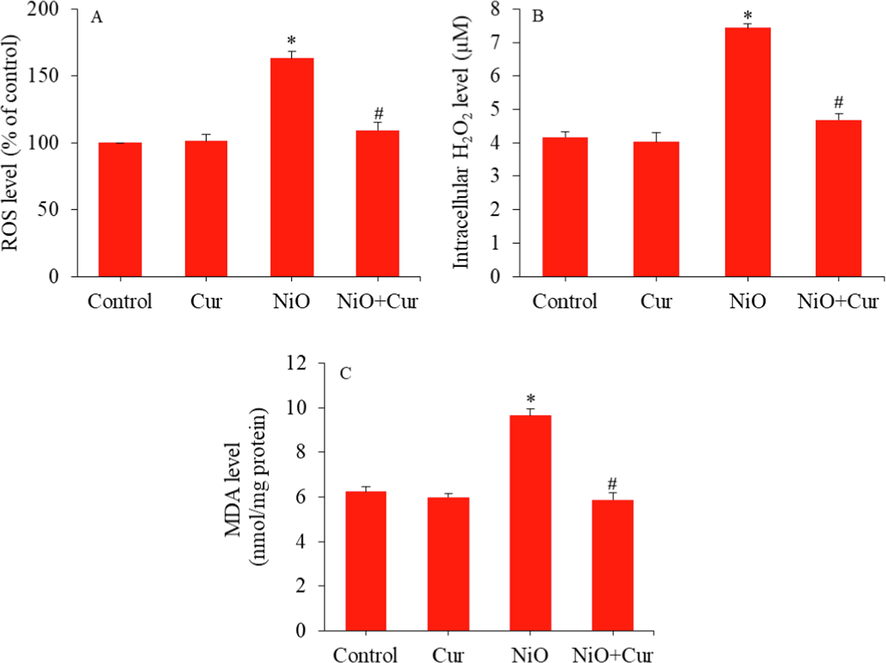
Pro-oxidants generation in GC-1 spg cells treated with NiO NPs (40 µg/ml) and/or curcumin (10 µg/ml) for 24 h. (A) ROS level. (B) H2O2 level. (D) MDA level. *Significant difference from the controls (p < 0.05). # Significant protective effect of curcumin against NiO NPs (p < 0.05).
Curcumin can also protect the spermatozoa from oxidative stress through restoring its antioxidant defense capacity (Kotha and Luthria, 2019). Protective effect of curcumin against NiO NPs induced antioxidants depletion was further assessed in GC-1 spg cells. Results revealed that reduction in antioxidant GSH content and activity of several antioxidant enzymes (e.g. GPx, SOD, and CAT) after NiO NPs treatment was reverted by curcumin co-treatment (Fig. 6A-D).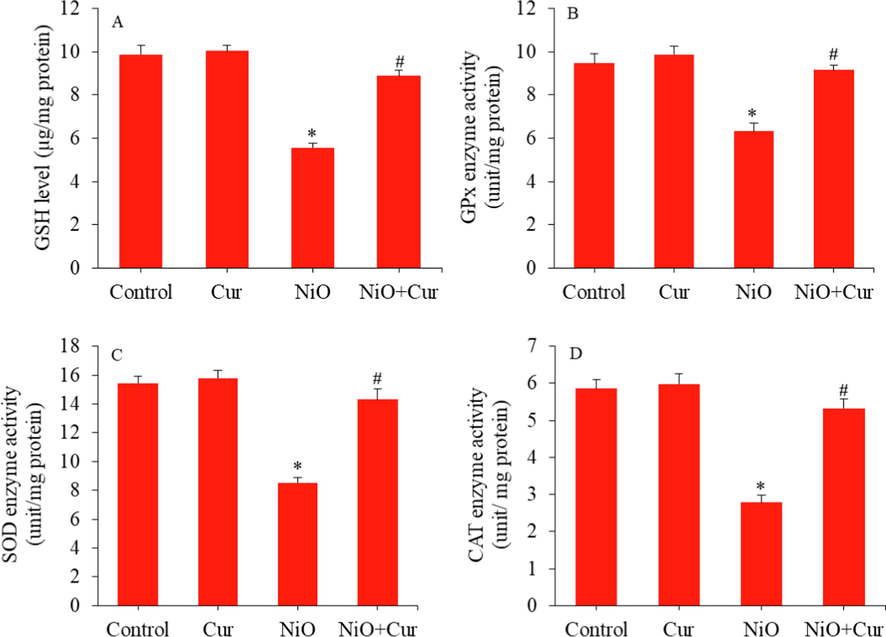
Antioxidants depletion in GC-1 spg cells treated with NiO NPs (40 µg/ml) and/or curcumin (10 µg/ml) for 24 h. (A) GSH level. (B) GPx enzyme activity. (C) SOD enzyme activity. (D) CAT enzyme activity. *Significant difference from the controls (p < 0.05). #Significant protective effect of curcumin against NiO NPs (p < 0.05).
4 Conclusion
NiO NPs induced cytotoxicity, caspase-3 activation, and mitochondrial membrane potential loss in mouse spermatogonia (GC-1 spg) cells were effectively abrogated by dietary curcumin. The elevation of intracellular pro-oxidants (e.g·H2O2, MDA, and ROS) and depletion of antioxidants (e.g. GSH, GPx, SOD, and CAT) was efficiently attenuated by curcumin. Overall, the protective effect of curcumin against NiO NPs induced toxicity in mouse spermatogonia cells was mediated via oxidative stress. This study warranted future investigations on mitigating potential natural antioxidants such as curcumin against nanomaterial-induced reproductive toxicity in appropriate in vivo models.
Acknowledgment
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project no. (IFKSURG-2-589).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Photocatalytic and optical properties of NiO added Nephelium lappaceum L. peel extract: an attempt to convert waste to a valuable product. Heliyon. 2019;5
- [CrossRef] [Google Scholar]
- Effect of oxidative stress on male reproduction. World J. Men’s Health. 2014;32:1.
- [CrossRef] [Google Scholar]
- Mitochondrial membrane potential (MMP) regulates sperm motility. In Vitro Cell. Dev. Biol. Anim.. 2016;52:953-960.
- [CrossRef] [Google Scholar]
- Nickel oxide nanoparticles exert cytotoxicity via oxidative stress and induce apoptotic response in human liver cells (HepG2) Chemosphere. 2013;93
- [CrossRef] [Google Scholar]
- Co-exposure of Bi2O3 nanoparticles and bezo[a]pyrene-enhanced in vitro cytotoxicity of mouse spermatogonia cells. Environ. Sci. Pollut. Res. 2021
- [CrossRef] [Google Scholar]
- SnO2-Doped ZnO/Reduced graphene oxide nanocomposites: synthesis, characterization, and improved anticancer activity via oxidative stress pathway. Int. J. Nanomed.. 2021;16:89-104.
- [CrossRef] [Google Scholar]
- Dietary antioxidant curcumin mitigates CuO nanoparticle-induced cytotoxicity through the oxidative stress pathway in human placental cells. Molecules (Basel, Switzerland). 2022;27:7378.
- [CrossRef] [Google Scholar]
- Causes and consequences of apoptosis in spermatozoa; contributions to infertility and impacts on development. Int. J. Dev. Biol.. 2013;57:265-272.
- [CrossRef] [Google Scholar]
- Oxidative stress and male reproductive health. Asian J. Androl.. 2014;16:31.
- [CrossRef] [Google Scholar]
- Dietary supplementation of ginger and turmeric improves reproductive function in hypertensive male rats. Toxicol. Rep.. 2015;2:1357-1366.
- [CrossRef] [Google Scholar]
- Role of oxidative stress in male infertility: an updated review. J. Hum. Reproductive Sci.. 2019;12:4.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of NiO nanoparticles by chemical co-precipitation method: an easy and cost-effective approach. Brazilian. J. Phys.. 2021;52(1):1-6.
- [CrossRef] [Google Scholar]
- Oxidative stress generated at nickel oxide nanoparticle interface results in bacterial membrane damage leading to cell death. RSC Adv.. 2019;9:24888-24894.
- [CrossRef] [Google Scholar]
- Oxidative stress and male infertility. Nat. Rev. Urol.. 2017;8(14):470-485.
- [CrossRef] [Google Scholar]
- Electrochemical and photoelectrochemical properties of nickel oxide (NiO) with nanostructured morphology for photoconversion applications. Front. Chem. 2018
- [CrossRef] [Google Scholar]
- A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72:248-254.
- [CrossRef] [Google Scholar]
- Structural, optical and photocatalytic applications of biosynthesized NiO nanocrystals. Green Chem. Lett. Rev.. 2018;11:166-175.
- [CrossRef] [Google Scholar]
- Green synthesis of NiO nanoparticles using Moringa oleifera extract and their biomedical applications: Cytotoxicity effect of nanoparticles against HT-29 cancer cells. J. Photochem. Photobiol. B Biol.. 2016;164:352-360.
- [CrossRef] [Google Scholar]
- A novel antioxidant formulation designed to treat male infertility associated with oxidative stress: promising preclinical evidence from animal models. Hum. Reprod.. 2016;31:252-262.
- [CrossRef] [Google Scholar]
- Unveiling the Behavior of Curcumin in Biocompatible Microemulsion and Its Differential Interaction with Gold and Silver Nanoparticles. J. Phys. Chem. C. 2020;124:3905-3914.
- [CrossRef] [Google Scholar]
- Curcumin influences semen quality parameters and reverses the di(2-ethylhexyl)phthalate (DEHP)-induced testicular damage in mice. Pharmacol. Rep. : PR. 2014;66:782-787.
- [CrossRef] [Google Scholar]
- Oxidative stress in liver diseases: pathogenesis, prevention, and therapeutics. Oxid. Med. Cell. Longev. 2017
- [CrossRef] [Google Scholar]
- Synthesis and characterization of nickel oxide (NiO) nanoparticles using an environmentally friendly method, and their biomedical applications. Chem. Phys. Lett.. 2022;797:139564
- [CrossRef] [Google Scholar]
- Curcumin: biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules. 2019;24
- [CrossRef] [Google Scholar]
- Lan, Z., Yang, W.X., 2012. Nanoparticles and spermatogenesis: how do nanoparticles affect spermatogenesis and penetrate the blood–testis barrier. 12.20 7, 579–596. https://doi.org/10.2217/NNM.12.20.
- Dynamic changes in the expression of apoptosis-related genes in differentiating gonocytes and in seminomas. Asian J. Androl.. 2015;17:403.
- [CrossRef] [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55-63.
- [CrossRef] [Google Scholar]
- Titanium dioxide nanoparticles perturb the blood-testis barrier via disruption of actin-based cell adhesive function. Aging (Albany NY). 2021;13:25440.
- [CrossRef] [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [CrossRef] [Google Scholar]
- The Scherrer formula for X-Ray particle size determination. Phys. Rev.. 1939;56(10):978-982.
- [CrossRef] [Google Scholar]
- In vitro cytotoxicity effects of zinc oxide nanoparticles on spermatogonia cells. Cells. 2020;9
- [CrossRef] [Google Scholar]
- Selenium: biochemical role as a component of glatathione peroxidase. Science. 1973;179:588-590.
- [CrossRef] [Google Scholar]
- Etiologies of sperm oxidative stress. Int. J. Reproductive Biomed.. 2016;14:231.
- [CrossRef] [Google Scholar]
- Nickel oxide nanoparticles induce cytotoxicity, oxidative stress and apoptosis in cultured human cells that is abrogated by the dietary antioxidant curcumin. Food Chem. Toxicol.. 2012;50
- [CrossRef] [Google Scholar]
- Toxic effects of low-level long-term inhalation exposures of rats to nickel oxide nanoparticles. Int. J. Mol. Sci.. 2019;20
- [CrossRef] [Google Scholar]
- Involvement of oxidative stress in ZnO NPs-induced apoptosis and autophagy of mouse GC-1 spg cells. Ecotoxicol. Environ. Saf.. 2020;202:110960
- [CrossRef] [Google Scholar]
- Role of oxidative stress in liver toxicity induced by nickel oxide nanoparticles in rats. Mol. Med. Rep.. 2018;17:3133-3139.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102624.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







