Translate this page into:
Native Trichoderma harzianum strains from Argentina produce indole-3 acetic acid and phosphorus solubilization, promote growth and control wilt disease on tomato (Solanum lycopersicum L.)
⁎Corresponding author.
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Trichoderma fungi are considered as suitable biofertilizers, since they could increase the efficiency of the use of nutrients in plants and are used as biocontrol agents against plant pathogen. The aim of this study was to characterize a set of Trichoderma strains isolated from horticultural and near-pristine soils from the Argentine Pampas, and to evaluate their potentiality as a growth promoter and as a biocontroller of Fusarium wilt disease on tomato. Nineteen collected strains, identified as T. brevicompactum, T. gamsii and T. harzianum, were tested for their ability to produce the auxin indole 3-acetic acid (IAA), solubilize phosphate and biocontrol F. oxysporum. Twelve strains reduced growth of pathogenic fungus over 50% and four of them (named FCCT 16, FCCT 58, FCCT 199-2 and FCCT 363-2) exhibited IAA highest production (ranging 13.38–21.14 µg/ml) and were able to solubilize phosphate (ranging 215.80–288.18 µg/ml of calcium phosphate). Tomato plants inoculated with those four strains increased chlorophyll content, shoot length, fresh and dry weight of shoot and roots, and reduced F. oxysporum wilt disease between 10 and 30%. On the basis of our results, we conclude that soils of the Argentine Pampas harbor Trichoderma strains with beneficial dual effects which could be of interest for the development of commercial products.
Keywords
Biological control
Phosphate solubilizing fungi
Phytohormone
Plant growth promotion
Tomato
Trichoderma
1 Introduction
The increase of global demand for agricultural food products over the last 50 years caused the expansion of cultivated areas and resulted in some adverse environmental impacts. At present, crop production involves chemical supplements to increase yield. As an example, fertilizers based on organic nitrate (urea) having a low assimilation, can be dissolved in water or washed from the soil, resulting in excess of nitrogen that ends up in water places causing eutrophication. In general, phosphorus (P) is also supplied to favor plant growth and nutrient uptake; however more than 80% of the P from fertilizers is not available for plant uptake (Kapri and Tewari, 2010). Pesticides are other inputs used in agriculture, resulting in a high cost for producers and causing serious health problems in humans. Moreover, they can produce drastic effects as well as the development of resistant pests (Naseby et al., 2000). At this stage, the reduction or elimination of synthetic products is highly desirable and most promising strategies should be considered. Fortunately, there are some alternatives to increase agricultural production using the beneficial capacities of microorganisms.
Plant growth promoting microbes (PGPM) could contribute increasing the development of plants directly through the improvement of nutrient availability, producing stimulating compounds and releasing phytohormones (Antoun and Prévost, 2005). According to Whipps (2001) diseases caused by pathogens can be indirectly suppressed.
Overall, new tools based on agents of biological control of diseases could be promising strategies to be used alone or in combination with reduced doses of chemicals (Chet and Inbar, 1994; Harman and Kubicek, 1998).
Trichoderma spp. are free living fungi of soil and rhizosphere, plant symbionts and function as parasites of other fungi. They are among the most studied and marketed fungi that are used as biopesticide and biofertilizer soil amendments. Strains with potential for plant growth and health are often isolated from soils (Harman et al., 2004; Lorito et al., 2004).
Many Trichoderma strains are capable to produce compounds as growth regulators that cause sustainable changes in the metabolism of plants. In this sense, Gravel et al. (2007) reported that T. atroviride produced IAA-related indoles under in vitro conditions after the addition of L-tryptophan, suggesting a possible mechanism to increase fresh weight of tomato shoots and roots on tomato. Contreras-Cornejo et al. (2009) suggested that T. virens and T. atroviride synthesize IAA or some derivatives that increase lateral roots in Arabidopsis plants. However, Hoyos-Carvajal et al. (2009) reported that many strains synthesize IAA, but only a few promote plant growth. Thus, the correlation between IAA synthesis and growth promotion in soil-based systems is not clear yet (Nieto-Jacobo et al., 2017).
Argentina is mainly an agro-exporting country in which horticultural crop region reaches 409,321 ha with an estimated annual yield of around 10,500,000 tons. The tomato (Solanum lycopersicum L.) is one of the most demanded horticultural crops in our country and its production is mainly done in greenhouses (Argerich, 2011). In this context, the objective is to optimize the yield, the soil resources and minimize diseases. To increase tomato production, continuous nutrient supply and repeated chemical control of diseases are usually applied. In this sense, it was found that the south of the Pampas region had low levels of soil P (<10 ppm P-Bray I), and in most cases, a large amount of P added as fertilizer remains retained in the soil due to its high adsorption capacity and not available for the growth of plants (Silva Rossi et al., 2013). Therefore, strategies for the solubilization of P are sought.
Fungal disease is a crucial problem in intensive cultivation and every year the crop losses are caused by fungal species such as Cladosporium fulvum, Fusarium oxysporum, Alternaria dauci f. sp. solani, Phytophthora infestans and capsici (Adlercreutz et al., 2014). Because laws limit the use of chemicals in agriculture, farmers are looking for other strategies for a sustainable production with the maintenance of productivity, and beneficial microorganisms could represent a promising alternative for the achievement of this goal. The soils of the Argentine Pampas have a high microbial diversity, but little has been explored in their potential as Trichoderma hot spots. In this context, our aim was to isolate, characterize and select strains of Trichoderma spp. native from this region with potential as growth promoters and as biocontrol agents for wilt disease caused by F. oxysporum on tomato.
2 Materials and methods
2.1 Isolation of Trichoderma strains
Trichoderma strains were isolated from horticultural and near-pristine soils from five locations in Buenos Aires Province, in the Pampa region, Argentina (Table 1). Nineteen geo-referenced samples (about 500 g soil, after removing the top 5 cm) were collected on a 50-m grid to a depth of 5–20 cm, and preserved at 4 °C until process.
Strain
Location
Soil source
Closest species match
Accession number
FCCT 16
37° 52′ 40.79″ S
farming
Trichoderma harzianum
KY381958
57° 46′ 50.92″ W
FCCT 34
37° 56′ 40.79″ S
farming
Trichoderma harzianum
KY381962
57° 44′ 50.92″ W
FCCT 37
37° 56′ 41.79″ S
farming
Trichoderma harzianum
KY381966
57° 46′ 51.92″ W
FCCT 42
37° 56′ 42.79″ S
farming
Trichoderma harzianum
KY381967
57° 42′ 50.92″ W
FCCT 45
37° 56′ 39.79″ S
farming
Trichoderma harzianum
KY381968
57° 46′ 50.92″ W
FCCT 58
37° 56′ 40.79″ S
farming
Trichoderma harzianum
KY381969
57° 46′ 50.92″ W
FCCT 63
37° 56′ 40.79″ S
farming
Trichoderma harzianum
KY381970
57° 46′ 50.92″ W
FCCT 187-2
33° 40′ 13.02″ S
farming
Trichoderma gamsii
MH734514
60° 41′ 55.80″ W
FCCT 188-3
33° 54′ 53.15″S
farming
Trichoderma brevicompactum
KY381960
60° 42′ 23.37 W
FCCT 198-1
34° 18′ 6.98″ S
farming
Trichoderma brevicompactum
MH734373
60° 44′ 42.42″ W
FCCT 199-2
34° 0′ 44.18″ S
farming
Trichoderma harzianum
KY381961
60° 48′ 52.82″ W
FCCT 363-1
33° 39′ 40.22″S
pristine
Trichoderma harzianum
KY381963
60° 42′ 23.37″ W
FCCT 363-2
33° 39′ 40.22″S
pristine
Trichoderma harzianum
KF518931
60° 42′ 23.37″ W
FCCT 363-3
33° 39′ 40.22″S
pristine
Trichoderma harzianum
KY381964
60° 42′ 23.37″ W
FCCT 364-2
33° 54′ 50.56″ S
pristine
Trichoderma harzianum
KY381965
60° 38′ 21.28″ W
FCCT 364-4
33°54′50.56″ S
pristine
Trichoderma harzianum
KC403946
60° 38′ 21.28″ W
FCCT 1155-2
34° 38′ 78.17″S
farming
Trichoderma harzianum
KC403945
60° 35′ 31.50″ W
FCCT 1155-4
37° 49′ 00″.00″S
pristine
Trichoderma harzianum
KY381955
58° 15′ 00.00″W
FCCT 1207-2
37° 49′ 00″.00S
farming
Trichoderma brevicompactum
DQ080074
58° 15′ 00.00″W
Fungal strains were isolated by the serial dilution method, using the Trichoderma Selective Medium (TSM) (Elad et al., 1981). Putative colonies were purified by two rounds of subculturing on potato dextrose agar (PDA) and identified according to Samuels et al. (2013).
2.2 Determination of Trichoderma species
For each isolate, 100 mg of mycelium were harvested from axenic cultures. Genomic DNA was extracted according to Raeder and Broda (1985). PCR amplification of internal transcribed spacers (ITS) of the ribosomal DNA (rDNA) was performed in a BIOER thermocycler (Technology Co.) using the universal primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (White et al., 1990). A total of 25 μL reaction mix was carried out containing 10–20 ng of DNA, buffer 1× (Promega), 1 mM of MgCl2, 0.15 mM of each dNTPs, 5 µM of each primer, and 1 unit of Go Taq polymerase (Promega). PCR conditions involved an initial step at 94 °C (2.5 min), followed by 35 cycles at 94 °C (15 s), 48 °C (1 min), and 72 °C (90 s). A final extension step was included at 72 °C for 10 min. Amplicons were separated by gel electrophoresis in 1.5% w/v agarose supplemented with GelRed™ (Biotium), then purified and sequenced using ITS4 primer (Macrogen Inc.). All sequences were deposited in GenBank under the accession number listed on Table 1.
2.3 In vitro dual confrontation assays
All Trichoderma strains were screened for their ability to inhibit a virulent strain of F. oxysporum (INBIOTEC Culture Collection) by using a dual culture method (Upadhyay and Rai, 1987). Assays were performed on PDA Petri dish by inoculating a block of 5 mm of fresh mycelium of the pathogen; after 48 h, one piece of each Trichoderma isolate was transferred in the same plate separated 3 cm, and observed periodically during 7 days. The percentage of ratio inhibition (RI) was calculated according to Grondona et al. (1997) and the value was the mean of three replicates.
2.4 Chemical quantification of indole 3-acetic acid
In vitro production of IAA for each strain was determined as an indicator of plant growth promotion through Salkowski test (Gordon and Weber, 1951). A standard T. harzianum strain (TH) from our laboratory was used as positive control. Previously, a standard curve was made from 1 to 100 µg/ml of IAA. Measured values for each strain were deduced from Linear Regression (R2 = 0.998, p < 0.0001).
Erlenmeyer flasks containing 50 ml of Tryptic Soy Broth (TSB) were inoculated with 1 × 107 conidia/ml and incubated for a week at room temperature and continue stirring at 100 rpm. Then, cultures were centrifuged at 10,000 rpm for 10 min and the supernatant was collected. One ml of the filtrate was mixed with 2 ml of Salkowski reagent at 28 ± 2 °C for 30 min and the quantification was assessed by absorbance in a spectrophotometer at 530 nm.
2.5 Phosphorus solubilizing screening
The four strains (named FCCT 16, FCCT 58, FCCT 199-2 and FCCT 363-2) that produced the highest IAA, and the TH standard isolate were subjected to an in vitro screening for their capacity to solubilize phosphate. Quantitative estimation of soluble P was carried out by the colorimetric method of blue acetic acid (Bray and Kurtz, 1945). Previously, a calibration curve of KH2PO4 was performed from a stock solution of 20 mg/l. Values for the different strains were deduced from Linear Regression (R2 = 0.988 p < 0.0001).
Erlenmeyer flasks (100 ml) containing 45 ml of National Botanical Research Institute Phosphate (NBRIP) growth medium (Mehta and Nautiyal, 2001) were inoculated with 1 × 107 conidia/ml and incubated at 28 ± 2 °C in an orbital shaker at 120 rpm for 5 d. Then, an aliquot of 2.5 ml from each culture flask was centrifuged (5000 rpm for 10 min). Supernatant was diluted 1:100 and 2.5 ml sample was incubated for 20 min with 0.4 ml of the colorimetric reagent. Absorbance was measured at 880 nm for quantification of orthophosphate ions.
2.6 Growth chamber trials
The four Trichoderma strains selected for the high IAA production and ability to solubilize tri-calcium phosphate were screened for their growth promotion and biocontrol ability. Firstly, tomato seeds (Solanum lycopersicum var. platense), were coated with each Trichoderma strains; secondly, Trichoderma treated seeds were inoculated with F. oxysporum. Appropriate controls and treatment were carried out in triplicate. Plastic pots were filled with 1 kg of substrate, composed by a mixture of an agricultural soil from Balcarce, Buenos Aires Province, Argentina: This soil was composed of organic matter 1% w/v, 6.2 ppm of available P and pH 7.1, and perlite and sand were added in volume proportion 2:1:1. Before inoculation, seeds were surface sterilized with 0.1% sodium hypochlorite and peeled with about 1 × 105 conidia/ml of each selected strain as described by Cordo et al. (2007). Three pre-germinated seeds (48 h on humid Whatman paper) were put in each pot and plants were maintained in a growth chamber (25 ± 2 °C, 16:8 h light/dark, and watered regularly).
2.6.1 Plant growth promotion assays
Plants inoculated with the FCCT 16, FCCT 58, FCCT 199-2 and FCCT 363-2 strains and non-inoculated (controls) were grown during 45 days. At harvest, the relative amount of chlorophyll on leaves was measured by using the SPAD-502PLUS chlorophyll meter (Konica Minolta). Leaf area (Meter Area Elli-3100, LI-COR), stem length, fresh weight and dry matter from shoots and roots were recorded. Dry matter was measured after desiccation in an oven at 65 °C during 72 h.
2.6.2 Pathogenicity assays
A four week plant foliage coming from pelletized seeds with FCCT 16, FCCT 58, FCCT 199-2 and FCCT 363-2 strains, was inoculated with F. oxysporum by spraying the above ground parts with a suspension of 20 ml of 1.14 × 105 macroconidia/ml in water, containing 0.1% Tween 20. Inoculated and control plants (inoculated with the pathogen but not with Trichoderma) were separately covered with polyethylene for 24 h and maintained at 26 ± 2 °C. Disease incidence was recorded 14 days after inoculation and calculated as the proportion of leaves displaying disease symptoms/ total number of leaves per plant.
2.7 Statistical analysis
For dual confrontation assays, tomato plant growth promotion and pathogenicity experiments completely randomized block designs with three replicates were conducted. The experimental data were statistically analyzed by one way analysis of variance (ANOVA). Means and standard deviations were calculated and statistically examined using an analysis of variance and Tukey’s multiple range test at p < 0. For graphics, a GraphPad Prism 5.0 was used.
For IAA analysis and soluble phosphorus quantification a standard Linear Regression Analysis (R Core Team, 2012) was performed for the estimation of values from data set.
3 Results
3.1 Identification of Trichoderma strains from soil samples
Nineteen monoconidial Trichoderma strains were obtained from soils located in the southeast of Buenos Aires Province; thirteen of them were from rhizosphere under agricultural management and six from near pristine soil. According to their morphology and ITS sequence analysis, three strains were confirmed as T. brevicompactum, one as T. gamsii and fifteen as T. harzianum (Table 1).
3.2 Dual confrontation assays between Trichoderma and F. oxysporum
All Trichoderma strains showed in vitro antagonistic effect against F. oxysporum and evidenced inhibition of the pathogeńs growth in the range of 20–100%; in addition, twelve strains inhibited mycelial growth of F. oxysporum by more than 50%. The highest biocontrol effect was obtained for FCCT 42, which showed a 100% of biocontrol efficiency. Strains FCCT 58, FCCT 63, FCCT 187-2 and FCCT363-2 reduced the pathogeńs growth by more than 90% (Fig. 1).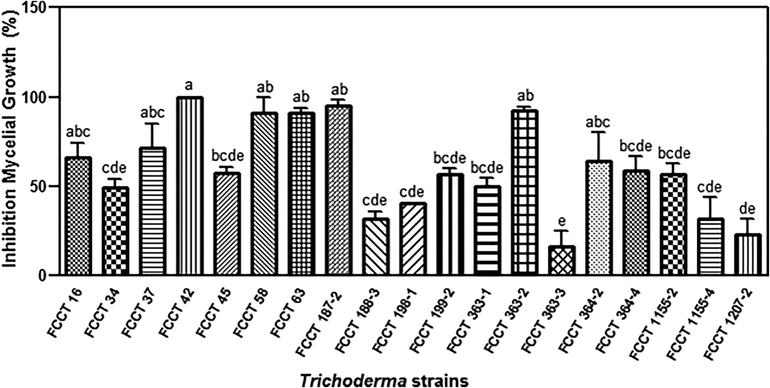
Inhibition effects of Trichoderma strains over Fusarium oxysporum in dual assay after seven days. Mean averages (n = 3). Different letters on bars indicate statistical differences between treatments according to Tukeýs Test (P ≤ 0.05).
In the intermingling zone between the pathogen and each isolate, the plasmolysis of pathogen hyphae or coiling by Trichoderma was evidenced.
3.3 Auxin production by Trichoderma strains
All tested strains showed in vitro ability to produce IAA that ranged from 7.19 μg/ml to 21.14 μg/ml (within a calibration curve with R2 = 0.9668, data not shown). Strains FCCT 16, FCCT 58, FCCT 199-2 and FCCT 363-2 yielded the highest IAA levels (Table 2) and were selected for the following studies.
Strain
IAA (µg/ml−1)
Soluble P (µg/ml−1)
TH control
11.10 ± 0.030
186.00 ± 0.020
FCCT 16
15.98 ± 0.003
262.46 ± 0.016
FCCT 58
13.38 ± 0.001
250.50 ± 0.001
FCCT 199-2
17.07 ± 0.008
215.80 ± 0.014
FCCT 363-2
21.14 ± 0.003
288.18 ± 0.001
control
7.80 ± 0.006
32.17 ± 0.001
(without inoculum)
3.4 Quantification of soluble phosphorus
The four selected strains previously mentioned showed a very good mycelia growth in NBRIP broth, with simultaneous disappearance of tri-calcium phosphate within 120 h after inoculation. Soluble phosphorus concentration of culture filtrates was between 215.80 and 288.18 µg/ml, which was about six times higher than that determined in the non-inoculated culture and greater than the positive control tested (Table 2).
3.5 Effects of Trichoderma on plant growth and pathogenicity
3.5.1 Influence of Trichoderma on the promotion of tomato plant growth
Tomato plants inoculated with FCCT 16, FCCT 58, FCCT 199-2 and FCCT 363-2 strains increased plant growth (Fig. 2). SPAD units (indirect chlorophyll content indicator) were higher in plants inoculated with FCCT 58 and FCCT 199-2, being the last strain mentioned which showed the highest leaf area. Although the remaining strains also increased the leaf area and the SPAD reading, no significant differences were detected in comparison with the controls (Table 3). *Values are means and standard deviation n = 3 replications and data calculated per plant after 45 days. Different letters in each evaluation indicates difference between treatments after Tukeýs test (p ≤ 0.05).
In vivo plant growth promotion assay of tomato plants (Solanum lycopersicum L. var platense). Shoot and their respective root after 45 days post inoculation of seed with strains FCCT 16 (a) FCCT 58, (b) FCCT 199-2 (c), FCCT 363-2 (d). On left control plants, inoculated on right.
Treatment
Spad units
% over control
Leaf área (cm)
% over control
Stem length (cm)
% over control
Shoot Fresh Weight (g)
% over control
Shoot Dry Weight (g)
% over control
Root Fresh Weight (g)
% over control
Root Dry Weight (g)
% over control
control
19.37 ± 2.41c
73.52 ± 19.15b
22.44 ± 7.37b
1.25 ± 0.41b
0.23 ± 0.05b
0.52 ± 0.14b
0.03 ± 0.01b
FCCT16
21.36 ± 2.51bc
10.2
137.69 ± 17.72ab
87.3
31.9 ± 2.40a
42.1
4.16 ± 0.94a
332
0.55 ± 0.18a
135.6
1.53 ± 0.77a
194.2
0.12 ± 0.06a
400
FCCT58
29.53 ± 3.11a
52.4
140.19 ± 30.86ab
90.7
29.00 ± 4.09a
29.2
3.09 ± 0.83a
247.2
0.48 ± 0.17a
118.1
0.89 ± 0.41ab
71.10
0.07 ± 0.02ab
233
FCCT 199-2
26.70 ± 3.10ab
37.8
176.17 ± 42.08a
139.6
27.5 ± 7.69a
22.7
3.36 ± 1.08a
268.8
0.55 ± 0.07a
135.5
1.54 ± 0.89a
196.1
0.10 ± 0.02ab
333
FCCT 363-2
25.93 ± 4.00abc
33.8
103.66 ± 42.15b
140.9
29.44 ± 1.35a
31.2
2.83 ± 1.06ab
226.4
0.36 ± 0.25b
53.91
0.78 ± 0.45b
50.0
0.05 ± 0.03ab
166
The inoculation of tomato increased shoot length in the range of 22.7–42.1% (for FCCT 199-2 and FCCT 16 treatments, respectively) and no differences among treated plants were detected (Table 3). All inoculated plants showed an increase in the fresh shoot weight (higher than 220% in comparison with the control), and FCCT 16 inoculated plants showed the highest growth. A similar trend was found for the dry weight of the shoot, with a range of 53.91–135.6% (Table 3).
The inoculation of tomato plants with FCCT 16 and FCCT 199-2 strains increased fresh root weight significantly and FCCT 16 inoculated plants showed the highest percentage in root growth. It is further noted that all the inoculated plants showed greater proliferation of lateral roots in comparison with the control plants (Fig. 2).
3.5.2 Effect of Trichoderma on plants inoculated with F. oxysporum
After 15 days of inoculation with the pathogen, the oldest leaves of the control tomato plants showed the typical symptoms of chlorosis in the main and secondary petioles, as well as the progression of propagation throughout the plant. In contrast, plants obtained from seeds coated with each of the Trichoderma strains, showed a reduction of leaf disease between 10 and 30% (Fig. 3), although tomato plants inoculated with FCCT 363–2 displayed the best performance.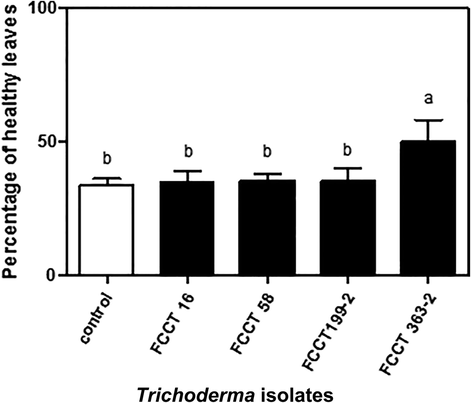
Disease evaluation as percentage of healthy leaves on tomato plants treated with strains FCCT 16, FCCT 58, FCCT 199-2, FCCT 363-2 after 15 days of inoculation with Fusarium oxysporum. Mean averages (n = 3). Different letters on bars indicates statistical differences between treatments after Tuckeýs Test (P ≤ 0.05).
4 Discussion
The Trichoderma genus comprises a large number of soil borne filamentous fungi that are widely used as inoculants for their health benefits to plants, such as conferring better growth, disease resistance or tolerance to abiotic stress for their hosts.
In Argentina, as in the rest of the world, there is a growing interest in developing commercial products based on Trichoderma to be used as amendment and biocontrol agents. However, the study of native strains is still poorly investigated, so the selection of strains could be carefully targeted to improve their potential under field conditions. The beneficial effects of bioinoculants could also depend on the ability of preserving indigenous fungi from invasive microorganisms, in order to keep the native microbial composition finding new strains adapted to local agroecological conditions.
Although the use of chemical pesticides is common in the management of tomato crops and may alter the recovery of fungi from the soil, some Trichoderma species are more tolerant to chemicals than others (Nowara and Radwan, 2017). In this study T. brevicompactum, T. gamsii and T. harzianum were isolated from agricultural soils, usually treated with fungicides, and also from pristine soils. All strains were able to sporulate, and abundant chlamydospores were found which have a potential role as inoculum (Chet, 1987). Nineteen T. harzianum strains were identified and all of them showed inhibition of F. oxysporum growth between 20 and 100%, but four of them FCCT 16, FCCT 42, FCCT 58 and FCCT 363-2, were highlighted because showed values up to 80% (Fig. 1). In agreement with other studies, T. harzianum is reported to be the most aggressive specie against pathogens, suggesting its high potential as an effective agent of biocontrol (Siddiquee et al., 2009).
The production of plant regulators by microorganisms is an important mechanism often associated with growth stimulation, since it is probably a way in which Trichoderma improves the development of plants. The molecular basis that underlies this subject is still unclear. In this sense, our results are being in agreement with studies where Trichoderma species were reported as producers of IAA being part of their metabolism (Contreras-Cornejo et al., 2009, 2011). We determined that all Trichoderma strains produced IAA (data not shown) and interestingly, four of them showed to be the highest IAA producers (between 13.24 μg/ml and 24.3 μg/ml) (Table 2). The biosynthetic pathways of this phytohormone in microbes and plants are highly similar and either tryptophan (Trp)-dependent or Trp-independent pathways were detected. The production of IAA is in general increased several times by the addition of tryptophan or its derivatives in the culture medium. From our results, we highlighted that even without the addition of indole derivative compounds, we measured quantities of IAA as high as reported values, when 200 µg/ml of tryptophan was amended (Gravel et al., 2007). Moreover, it is remarkable that the strains that were higher producers of the phytohormone were also good biocontrollers of F. oxysporum, as confirmed in both in vitro and in vivo assays (Figs. 1 and 3).
Phosphorus is an essential nutrient required for plants, and its bioavailability is associated with increases in plant growth (Richardson, 2001). In our study, the in vitro disappearance of tri-calcium phosphate could indicate a good potential of the strains to solubilize the inorganic bound phosphate. The four selected strains as higher IAA producers (FCCT 16, FCCT 58, FCCT 199-2 and FCCT 363-2), showed this ability in liquid culture reaching 215.8–288 µg/µl of soluble phosphorus (Table 2); comparable to that of other studies carried out by Kapri and Tewari (2010). We demonstrate the sequestration of tri-calcium phosphate by Trichoderma and hypothesize that an available form of the nutrient could be present in close proximity to the roots. Probably, this ability to make available insoluble nutrients could have been supported by the production of different organic acids and phosphatase enzymes secreted by the fungus (Gravel et al., 2007). This indicates an additional key property to select more efficient strains. We found that the four Trichoderma strains that effectively produced IAA and were able to solubilize inorganic phosphorus also contributed to improve tomato plant growth after inoculation (Table 3). In plant assays, each strain increased the potential capacity for photosynthesis, since the inoculated plants showed SPAD readings of approximately 10.2–52.4% and the leaf area between 1.4 and 2.3 times over the control. These data could indicate a more efficient photosynthesis. All the inoculated plants showed growth promotion on shoots and roots and, surprisingly, the most outstanding effect was found in the dry weight of the roots (between 133 and 400%). We found changes in root development as a modification of root architecture, probably increasing the area suitable for the microbial colonization that extends the root system for nutrient uptake (Berg, 2009; Contreras-Cornejo et al., 2009). As previously reported, Trichoderma interferes with plant development, disturbing their auxin balance. So, based on our results, we could suggest that there is a link between IAA secreted by the fungus and growth of tomato plants.
Trichoderma interacts with other rhizosphere microbes influencing the protection against diseases, plant growth and yield. It is reported that some species are able to produce several plant defenses eliciting microbe associated molecular patterns (MAMPs) such as xylanases, swollenins, peptaibols and low molecular weight compounds (Druzinhina et al., 2011; Zeilinger et al., 2016). There is a special interest in those compounds with antibiotic activity, since they are more possibly effective in biological control. We found that the Trichoderma selected strains were able to reduce the F. oxysporum disease symptoms on tomato plants between 10 and 30% (Fig. 3). Probably, a priming effect could be exerted by the fungus associated with the root system. It is reported that in primed plants, defense responses are not directly activated, but are enhanced upon pathogens or insects, resulting in faster and stronger resistance reaction (Van Wees et al., 2008). Successfully, the benefits of priming have emerged as a promising strategy in disease management (Beckers and Conrath, 2007).
In summary, our results suggest that the Trichoderma strains from the Argentine Pampas stimulate the growth of tomato plants through the production of phytohormones, increasing the leaf area and probably photosynthesis and the uptake of phosphorus. As a result, a more vigorous plant could show a priming effect capable to favor the biocontrol against F. oxysporum. Although we consider that further experiments should be carried out to evaluate the effects of Trichoderma on adult plants and fruit yield, both the FCCT 16 strain and the FCCT 363-2 strain can be highly recommended for their properties. These can be properly formulated and applied in a sustainable tomato production.
Acknowledgments
Special thanks to Prof. Ana M. Tassi (former Professor of National University of Mar del Plata) for critical revision of the manuscript.
Funding
This research was supported by National Agency for Scientific and Technological (ANPCyT), PICT 2011-1413, PICT 2015-0392, PIP 2015-0424 (CONICET) and National University of Mar del Plata 15/E692 – EXA742/15.
References
- Szczesny A., ed. Producción Hortícola bajo Cubierta. Instituto Nacional de Tecnología Agropecuaria; 2014. p. :149.
- Ecology of plant growth promoting rhizobacteria. In: Siddiqui Z.A., ed. PGPR: Biocontrol and Biofertilization. Dordrecht: Springer; 2005. p. :1-38.
- [Google Scholar]
- Manual de Buenas Prácticas Agrícolas en la cadena Tomate. ED.FAO Argentina; 2011. p. :11-28. Cap 1
- Priming for stress resistance: from the lab to the field. Curr. Opin. Plant Biol.. 2007;10:425-431.
- [Google Scholar]
- Plant–microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl. Microb. Biotechnol.. 2009;84:11-18.
- [Google Scholar]
- Determination of total, organic, and available forms of phosphorus in soils. Soil Sci.. 1945;59:39-46.
- [Google Scholar]
- Trichoderma-application, mode of action and potential as biocontrol agent of soilborne plant pathogenic fungi. In: Chet I., ed. Innovative Approaches to Plant Disease Control. New York: Wiley; 1987. p. :137-160.
- [Google Scholar]
- Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Phys.. 2009;149:1579-1592.
- [Google Scholar]
- Trichoderma-induced plant immunity likely involves both hormonal and camalexin-dependent mechanisms in Arabidopsis thaliana and confers resistance against necrotrophic fungi Botrytis cinerea. Plant Signal. Behav.. 2011;6:1554-1563.
- [Google Scholar]
- Trichoderma spp. as elicitors of wheat plant defense responses against Septoria tritici. Biocontrol Sci. Technol.. 2007;17:687-698.
- [Google Scholar]
- Trichoderma: the genomics of opportunistic success. Nat. Rev. Microbiol.. 2011;9:749-759.
- [Google Scholar]
- A selective medium for improving quantitative isolation of Trichoderma spp. from soil. Phytoparasitica. 1981;71:59-67.
- [Google Scholar]
- Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: possible role of indole acetic acid (IAA) Soil Biol. Biochem.. 2007;39:1968-1977.
- [Google Scholar]
- Physiological and biochemical characterization of Trichoderma harzianum, a biological control agent against soil borne fungal plant pathogens. Appl. Environ. Microbiol.. 1997;63:3189-3198.
- [Google Scholar]
- Trichoderma species-opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol.. 2004;2:43-56.
- [Google Scholar]
- Trichoderma and Gliocladium. London: Taylor & Francis; 1998. p. :278.
- Growth stimulation in bean (Phaseolus vulgaris L.) by Trichoderma. Biol. Control. 2009;51:409-416.
- [Google Scholar]
- Phosphate solubilization potential and phosphatase activity of rhizospheric Trichoderma spp. Braz. J. Microbiol.. 2010;41:787-795.
- [Google Scholar]
- Le biotecnologie utili alla difesa sostenibile delle piante: i funghi. Agroindustria. 2004;3:181-195.
- [Google Scholar]
- An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr. Microbiol.. 2001;43:51-56.
- [Google Scholar]
- Effect of biocontrol strains of Trichoderma on plant growth, Pythium ultimum population, soil microbial communities and soil enzyme activities. J. Appl. Microb.. 2000;88:161-169.
- [Google Scholar]
- Environmental growth conditions of trichoderma spp. affects indole acetic acid derivatives, volatile organic compounds, and plant growth promotion. Front. Plant Sci. 2017:8,102.
- [Google Scholar]
- Impact of pesticides on Trichoderma harzianum and on its possible antagonistic activity against Fusarium oxysporum under in vitro conditions. Asian J. Agric. Biol.. 2017;5:291-302.
- [Google Scholar]
- R: A Language and Environment for Statistical Computing. Austria: R Foundation for Statistical Computing. Vienna; 2012. (accessed Feb 2017)
- Rapid preparation of DNA from filamentous fungi. Lett. Appl. Microbiol.. 1985;1:17-20.
- [Google Scholar]
- Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Funct. Plant Biol.. 2001;28:897-906.
- [Google Scholar]
- Trichoderma online, systematic mycology and microbiology laboratory. ARS, USDA; 2013. Verified Oct 2017
- In vitro studies on the potential Trichoderma harzianum for antagonistic properties against Ganoderma boninense. J. Food Agric. Environ.. 2009;7:970-976.
- [Google Scholar]
- Phosphorus availability in the central area of the Argentine Pampean region. 1: Relationship between soil parameters, adsorption processes and wheat, soybean and corn yields in different soil and management environments. Spanish J. Soil Sci.. 2013;3:45-55.
- [Google Scholar]
- Studies on antagonism between Fusarium cudum Butler and root region microflora of pigeon-pea. Plant Soil. 1987;101:79-93.
- [Google Scholar]
- Plant immune responses triggered by beneficial microbes. Curr. Opin. Plant Biol.. 2008;11:443-448.
- [Google Scholar]
- Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot.. 2001;52:487-511.
- [Google Scholar]
- Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols. 1990;18:315-322.
- [Google Scholar]
- Secondary metabolism in Trichoderma-Chemistry meets genomics. Fungal Biol. Rev.. 2016;30:74-90.
- [Google Scholar]







