Translate this page into:
Nanoencaspsulation of cysteine protease for the management of stored grain pest, Sitotroga cerealella (Olivier)
⁎Corresponding author. qamarsaeed@bzu.edu.pk (Qamar Saeed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Attaining food security is crucial to feed increasing population. Minimizing post harvest losses is a good solution to food scarcity. Hence, cereals are staple food for most of the developing countries, there is a need to develope a biopesticide against stored product pests to avoid post-harvest losses. Current study involves the development of an eco-friendly tool to manage stored product insect pests by nanoencapsulation of 25 kDa cysteine protease isolated from Albizia procera (ApCP). The nanoencapsulation was tested on Sitotroga cerealella. The ApCP was tested as an insecticide (with and without GQDs encapsulation) at three different concentrations (7.0, 3.5 and 1.7 mg of ApCP/g of wheat flour pellets). Sitotroga cerealella was fed to treated diet and tested for life cycle stages. ApCP was 100% more efficient against the test insect after encapsulation with GQDs, at 7.0 and 3.5 mg/g concentrations, resulting in no population build-up, while 1.7 mg/g concentration exhibited fewer population numbers. ApCP exhibited increased insectidial efficacy due to encapsulation with GQDs. The results suggested that ApCP encapsulation with GQDs has the potential to control the insect pest and could be commercially developed as a biopesticide.
Keywords
Cysteine protease
Graphene quantum dots
Nanoencapsulation
Biopesticides
Albizia procera
Sitotroga cerealella
1 Introduction
Although pesticides are responsible for improved agricultural production, but it has been explored that they are global contaminants (Carvalho, 2017). During the application of insecticides, many cases of human intoxication are reported. An unintentional insecticide poisoning kills more than 355,000 people each year globally (Alavanja and Bonner, 2012; Hassaan and El Nemr, 2020). Pesticide remnants in the environment also kill non target organisms like beneficial insects, birds, amphibians, fish along with small mammals, which creates loss in biodiversity and ecosystem (Köhler and Triebskorn, 2013). Besides, pesticides are often volatile compounds and get evaporated soon after application. These chemicals are transported by atmospheric processes, condense in cooler climates and deposit into water catchment areas and lakes (Carvalho, 2017).
Currently, losses caused by stored product pests are kept under threshold by phosphine fumigation. Phosphine is a cost effective and extensively used fumigant which does not leave residues on stored products. Meanwhile, it is highly toxic, inflammable and detoxified within the insect body by enzymes (Schlipalius et al., 2012; El-Shafie, 2019). Grains stay at risk of quantitative or qualitative damages dues to stored products pests reaching up to 88% germination loss and 29% weight loss (Mesterházy et al., 2020; Rajendran, 2020). Angoumois grain moth, Sitotroga cerealella (Olivier) (Lepidoptera: Gelechiidae) is a global pest of stored grains (Ma et al., 2016) whose female can destroy 500 g of hulled cereal if control measures are not implemented (Akinneye and Oyeniyi, 2016). A single larva is accountable for 24% loss in cereal weight and nutrition (Moore et al., 1966). Adults can fly up to 600 m and infest distantly stored grain reserves (Trematerra, 2015).
Use of biopesticides or organic repellents and antifeedants could be a substitute to chemical control in grain reserves (Malaikozhundan and Vinodhini, 2018). Additional to the traditional plant extracts which are used as insecticides, plant proteins of insecticidal nature can be used to manage insect pests (Gressent et al., 2007). The efficiency of these biomolecules can be enhanced by use of nanotechnology (Ditta, 2012; Rai and Ingle, 2012; De bose et al., 2014; Prasad et al., 2014; Ghodake et al., 2018). Nanotechnology can redefine agriculture and crop protection by improved delivery of fertilizers and crop protection chemicals. Nanoencapsulation of pesticides provides increased efficiency and shelf life, improved dispersal, controlled release and protection from harsh environmental conditons (Kumar et al., 2019). Many studies are confirmed for nanoencapsulation of plant originated insecticidal ingredients (azadirachtin, carvacrol, citronella etc.) (Ghodake et al., 2018), but nanoencapsulation of proteins for insecticidal purpose is still at a rudimentary stage. This study involves the nanoencapsulation of 25 kDa cysteine protease extricated by seed coat from Albizia procera (ApCP) against S. cerealella as an ecofriendly insecticide. Cysteine proteases have been reported many times for insecticidal activity due to chitin degradation in insect midgut and skeleton (Harrison and Bonning, 2010; Silva et al., 2016). This study confirmed the targeted delivery of insecticide (ApCP) due to nanoencapsulation with graphene quantum dots (GQDs) and hence improved insecticidal efficacy.
Quantum dots are minute nanocrystals with 2–10 nm diameter. Due to nanometer-size, molecular absorption of nanoencapsulated drugs can be improved. Quantum dots have tendency of fluorescence and can be detected at the target site after delivery of drug and hence, used in bioimaging (Singh and Lillard, 2009; De bose et al., 2014). It is hypothesized that nanoencapsulation of ApCP with GQDs could be proved as a promising biopesticide.
2 Material and methods
2.1 Extraction of ApCP
The study was run parallel to the experimentation of Batool et al., 2020. The ApCP was extracted by separation of seed coat from A. procera, followed by grinding and suspension into Tris buffer (0.1 M, pH 7.0) with 0.15 M NaCl. The seed coat blend was centrifuged at 5300 rpm for 15 min, and the supernatant was obtained. The extract was dialyzed, examined via SDS-PAGE and HPLC and identified by LC-MS/MS. The quantification of ApCP was done through nanodrop (Batool et al., 2020).
2.2 Insect culture
Sitotroga cerealella was cultured till five generations, to get a homogenous population, at sanitized wheat grains by maintaining the temperature at 30 ± 5 °C and relative humidity at 60 ± 5% at the Eco-Toxicology Laboratory in the Department of Entomology, Bahauddin Zakariya University, Multan, Pakistan (30° 11′ 44 N; 71° 28′ 31 E).
2.3 Contact toxicity
Albizia procera seed coat (dialyzed) extract (30 ml) was poured in beakers at three different concentrations of 7.0, 3.5, 1.7 mg/ml of buffer. Filter papers (of 5 cm diameter) were dipped in the extract for 30 s, collected out and air dried. Treated filter papers were placed in pre-sanitized Petri dishes of 5 cm diameter. Ten 3rd instar larvae of S. cerealella were released on treated filter papers. The experiment was replicated five times for each concentration. The mortality data was recorded after 6, 12, 24 and 72 h after treatment and compared with control.
2.4 Feeding toxicity
Pre-sanitized wheat flour (150 g for each concentration) was treated with three concentrations (7.0, 3.5 and 1.7 mg/ml) of ApCP and control (extraction buffer) separately. The treated flour/dough was shaped into pellets. Wheat flour pellets were air dried for four days and divided in to 30 g aliqoutes to make five replications. Each 30 g pellets aliquote was supplied to a plastic jar and fresh adults of S. cerealella (five pairs). Jars were kept in laboratory by maintaining temperature (30 ± 5 °C) and relative humidity (60 ± 5%). Adults were removed from experimental jars after seven days to check the life cycle stages, eggs, larvae, pupae and adults eclosion. Pellets were placed under light microscope to see the galleries made by larvae. Pupae were observed by cutting the pellets. To check the highest activity of ApCP, extraction was done with phosphate buffer solution at pH 7 and distilled water and compared with control (no treatment applied to flour). Best extraction conditions of ApCP were explored by feeding toxicity assays.
2.5 Synthesis of GQDs and encapsulation with ApCP for insecticidal assays
Graphene oxide obtained from graphite powder as previously described by Hummers and Offeman, 1958, was used to synthesize graphene quantum dots. Graphene quantum dots were synthesized by acidic cutting of graphene oxide, characterized by ATR (attenuated total reflectance), AFM (atomic force microscopy) and XRD (X-ray diffraction) and encapsulated with ApCP as described by Batool et al. (2020). Maximum encapsulation of papain was obtained by optimizing parameters such as, weight of GQDs, concentration of serial solutions and temperature. Encapsulation efficiency was calculated by the equation used by Batool et al. (2020). Encapsulation of ApCP was also accomplished by following the same parametrs devised with papain. The stored grain insect pests were exposed to the three concentrations mentioned above, by contact and feeding toxicity assays (following the methods discussed above). Papain (as a reference protein) was also used to compare its insecticidal properties (with ApCP) against the insect pest at a concentration of 3.5 mg/g.
2.6 Data collection
The insect were observed after 6, 12, 24, 48 and 72 h to evaluate the contact toxicity and the data was put to Probit analysis using SPSS software (IBM SPSS Statistics for Windows, Version 23.0, IBM Corp, Armonk, Ny, USA). Insect were checked after seven days interval for the changes in life span stages i.e., eggs, larvae, pupae, and adults eclosion. To record larval and pupal population, flour pellet was kept under microscope and observed for galleries. Data recorded after each life stage of insect pest was analyzed by one way analysis of variance using Statistix 8.1 (Statistix, Tallahassee, FL, USA) for comparison.
3 Results
3.1 Optimizing extraction conditions of ApCP
To discover a good extraction and insecticidal activity of ApCP, extractions in a pH 7 buffer (phosphate) and distilled water were evaluated against S. cerealella life attributes and results are displayed in Fig. 1. Insecticidal activities for both extractions of ApCP were statistically similar in case of S. cerealella eggs (F = 0.04, df = 2, 6, P = 0.9616), larvae (F = 0.08, df = 2, 6, P = 0.923) pupae (F = 0.08, df = 2, 6, P = 0.9246) and adults eclosion (F = 0.14, df = 2, 6, P = 0.8724). The results ensured that ApCP extraction in phosphate and water conditions were non-significant against S. cerealella.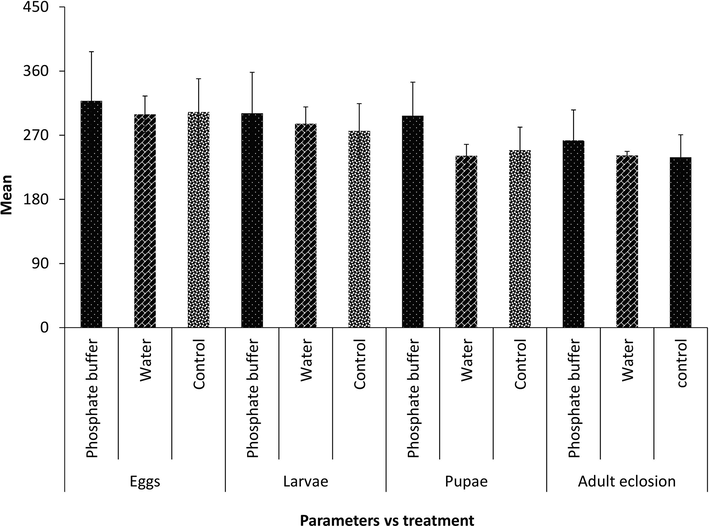
Optimization of insecticidal activity of ApCP against S. cerealella by feeding bioassay. No lettering above bars indicate non-significance of treatments.
3.2 Contact toxicity of ApCP with and without GQDs against S. cerealella
For contact and feeding bioassays, ApCP was encapsulated on GQDs by the same conditions developed by Batool et al., 2020. The mortality data of S. cerealella after 24 h of treatment of ApCP (displayed in Tables 1 and 2) exhibited the the LC50 of 0.744 mg/l with GQDs encapsulation. ApCP caused no mortality of the insect pest without GQDs encapsulation. While, the LC50 was 0.615 mg/l and 1.534 mg/l, after 48 h for the ApCP with and without encapsulation of GQDs, respectively. Parallel toxicity data was recorded at 72 h of treatment, LC50 of ApCP, 0.488 mg/l (with encapsulation) and 0.849 mg/l (without encapsulation). Though all these LC50 values decreased with GQDs encapsulation but stood statistically similar (95% FL overlapped with each other).
Hours after treatment
LC50a (95% FLb) (mg/l)
LC90c (95% FL) (mg/l)
Slope (±S.E)
X2 d
Df e
P
24
0.744(0.533–1.038)
1.982(1.420–2.767)
3.095
0.132
2
0.9361309
48
0.615(0.449–0.840)
1.542(1.128–2.109)
3.397
0.041
2
0.9797087
72
0.488(0.361–0.659)
1.169(0.866–1.578)
3.76
0.013
2
0.9935211
Hours after treatment
LC50a (95% FLb) (mg/l)
LC90c (95% FL) (mg/l)
Slope (±S.E)
X2 d
Df e
P
24
–
–
–
–
–
–
48
1.534(0.954–2.466)
5.353(3.329–8.606)
2.365
0.4
1
0.5270893
72
0.849(0.583–1.237)
2.383(1.635–3.469)
2.918
0.395
1
0.5296828
3.3 Feeding toxicity of ApCP with and without GQDs against S. cerealella
When S. cerealella was fed on wheat flour pellet mixed with ApCP (with GQDs encapsulation) for three concentrations 7.0, 3.5 and 1.7 mg/g and control, results were highly significant (F = 161, df = 3, 16, P < 0.0001) as described in Fig. 2. Number of eggs (mean) for three concentrations and control were, 0, 0, 51.2 ± 31.9 and 604.4 ± 33.9 respectively. Feeding bioassay of three concentrations of ApCP (without encapsulation) against S. cerealella showed significantly different (F = 90.7, df = 3, 16, P < 0.0001) responses. Least eggs were recorded at highest concentration (100 ± 1.4). While, 138.6 ± 14.6 eggs were found at 3.5 mg/g concentration. However, the most eggs were recorded in the lowest concentration i.e. 201.2 ± 5.8. All these results were significant with control data (663.2 ± 52.6). Egg laying of S. cerealella was completely inhibited when using 7.5 and 3.5 mg/g of ApCP encapsulated with GQD, while it was delayed by 74.2% when the lowest concentration (1.7 mg/g) was applied.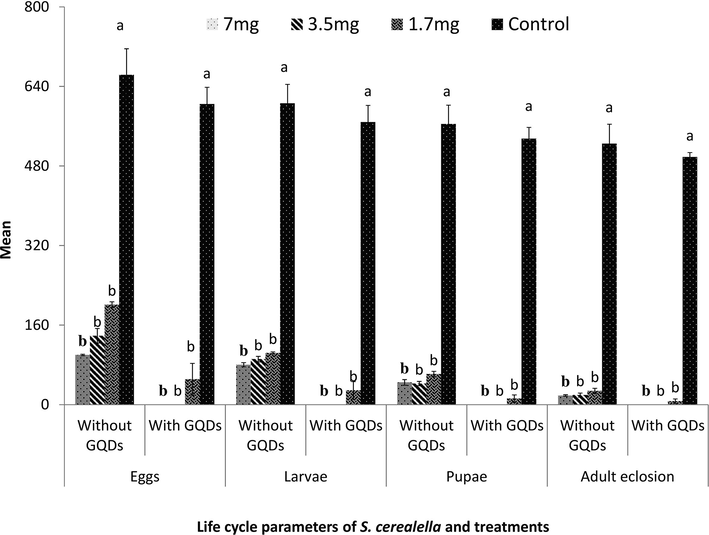
Response of S. cerealella to ApCP with and without Graphene Quantum Dots encapsulation. Different letters above bars describe significance of treatments.
Larvae of S. cerealella in ApCP (at 1.7 mg/g concentration) encapsulated with GQDs treatments were found statistically low in number (28.8 ± 17.7) (F = 219, df = 3, 16, P < 0.0001), while maximum number was observed in control (568.4 ± 33.4). On the other hand, 7.0 and 3.5 mg/g of ApCP exhibited no larvae in the experimental units. Conversely, in ApCP treatments without encapsulation of GQDs, larvae were observed unlike encapsulated treatments units where larvae were observed in zero. Larval population was dependant on ApCP concentrations as higher concentrations tend to zero or a few larvae. Larvae with ApCP treatments without GQD encapsulation were found concentration dependant (significant decrease in number of larvae was observed in all evaluated concentrations, with increase in concentration) (F = 175, df = 3, 16, P < 0.0001). Insecticidal efficiency was improved by 100%, 100% and 71.3%, respectively, after ApCP encapsulation with GQDs at the three concentrations.
Significantly lower pupae (12.2 ± 7.5) (F = 219, df = 3, 16, P < 0.0001) were recorded in lowest concentration (1.7 mg/g) of ApCP encapsulated with GQDs related to without encapsulation treatments (61.6 ± 5.4) (F = 175, df = 3, 16, P < 0.0001). No pupa was observed in 7.0 and 3.5 mg/g (concentrations of ApCP encapsulated with GQDs). Pupal population decreased by 100%, 100% and 76.9% at the three tested concentrations, respectively after GQDs encapsulation.
Adults eclosion at all three tested concentrations of ApCP with encapsulation were minimal (F = 2388, df = 3, 16, P < 0.0001). No adult insects were observed when using 7.0 and 3.5 mg/g concentrations of ApCP encapsulated with GQDs. While only 7 ± 4.6 adult were found at 1.7 mg/g concentration. Insecticidal assay of tested three concentrations of ApCP (without encapsulation) against S. cerealella showed statistically significant results (F = 160, df = 3, 16, P < 0.0001). Adult count was 18.4 ± 1.6 at the highest concentration. Adult number was recorded as 19.3 ± 4 and 27.6 ± 5.3 at 3.5 and 1.7 mg/g concentrations respectively. All these counts were statistically similar with each other but different from control data. Adult population was decreased by 100%, 100% and 87% for the tested ApCP encapsulated with GQDs concentrations respectively.
3.4 Feeding toxicity of papain against S. cerealella
Papain was found significant against S. cerealella eggs (F = 136, df = 1, 8, P < 0.0001) larvae (F = 182, df = 1, 8, P < 0.0001) pupae (F = 269, df = 1, 8, P < 0.0001) and adults (F = 2664, df = 1, 8, P < 0.0001). Papain was found more insecticidal when it was encapsulated with GQDs by 22.1% (eggs), 19.7% (larvae), 49.8% (pupae) and 66.79% (adults eclosion) related to without GQDs encapsulation (Fig. 3).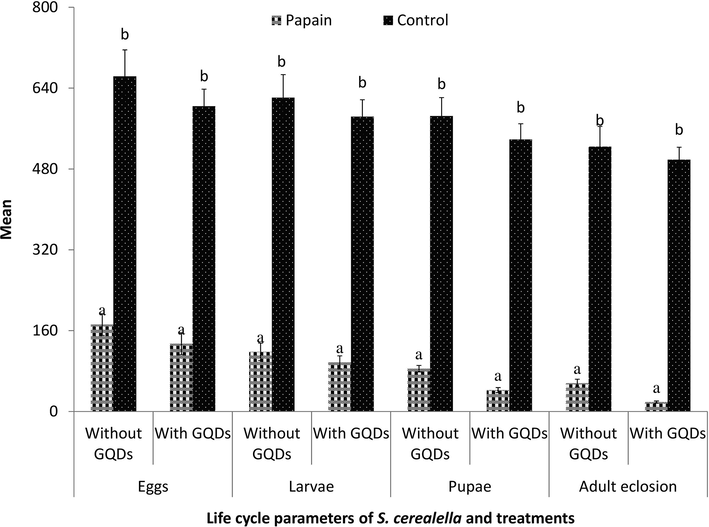
Response of S. cerealella to Papain with and without Graphene Quantum Dots encapsulation. Different letters above bars describe significane of treatments.
4 Discussion
This study explored the potent insecticidal activity of A. procera seed coat cysteine protease of 25 kDa. ApCP was found effective against S. cerealella, which is one of the harmfull pests of warehouse commodities worldwide. The protein treated experimental units of insect pest presented a clear decrease at life cycle attributes (egg, larvae, pupae and adults eclosion) as compared to control. Insecticidal efficacy of ApCP was improved with GQDs encapsulation to 100% at highest concentration for tested life attributes of S. ceraelella. Insecticidal results of GQDs encapsulated cysteine protease have been reported against Tribolium castaneum and Rhyzopertha dominica at same concentrations (Batool et al., 2020). The ApCP was found more effective against S. cerealella as compared to T. castaneum and R. dominica because of 100% population loss of S. cerealella. Moreover, Papain was used as a reference protein to confirm insecticidal efficiency of ApCP. Papain insecticidal effectiveness was also enhanced after encapsulation with GQDs. Population of S. cerealella after papain encapsulation with GQDs, was reduced by 22.1%, 19.7%, 49.8% and 66.7% in terms of eggs, larvae, pupae and adults as compared to papain without encapsulation. Several studies have shown the insecticidal efficacy of cysteine proteases (Konno et al., 2004; Macalood et al., 2013; Silva et al., 2016). Callosobruchus maculatus could not bore into soybean seeds due to seed coat contents (Oliveira et al., 2009). Albizia lebbeck seed coat protein was mixed with feed of C. maculatus and found defensive against egg laying and larval feeding (Silva et al., 2016). ApCP is found promising against the insect pest at less concentrations as compared to likewise reports, by minimizing the number at different life stages. Nanoencapsulation of ApCP with GQDs resulted in no population. The LC50 of protein with GQDs and without GQDs assured the promising control of insect pest of the study as treatments showed valuable decreases in LC50 when treated to ApCP encapsulated with GQDs. Different studies witness for nanoformulations as emerging trend for pest management strategies which support the results of increased insecticidal efficacy of ApCP after nanoencapsulation. Nanoencapsulation of a biopesticide could make it more potent by providing protection through environmental aggressions and improved/controlled release to the target site (Campolo et al., 2017; Kamaraj et al., 2018; Kumar et al., 2019).
Insecticidal activity of cysteine proteases has been well reported from papain and others (Macalood et al., 2013; George et al., 2014). Meanwhile cysteine proteases are also produced in plants in response of herbivory and confer death of caterpillars (Pechan et al., 2000). Active site of proteolysis by cysteine proteases in the insect body might be basement membrane, peritrophic matrix of insect midgut and cuticular proteins (Harrison and Bonning, 2010). There are many reports of insect death from Lepidoptera and Coleoptera due to peritrophic matrix disruption after subjected to cysteine protease (Pechan et al., 2000; Mohan et al., 2006; Silva et al., 2016). Peritrophic matrix is a semipermeable non-cellular membrane of insect midgut. It plays important role in insect digestion and defence from absorption of viruses and other pathogens (Mohan et al., 2006). Peritrophic membrane is either produced by epithelial cells of midgut or cardia and extended throughout the length of midgut forming 0.5–1.0 µm thick layer with delicate differences in some insect orders (Hegedus et al., 2009).
ApCP is similar to other cysteine proteases extracted from Cajanus cajan, Hordeum vulgare and Carica papaya. Hydrolytic activity of cysteine proteases is due to three amino acids (Cys 25, His 159, and Asn 175) which are arranged in a “v” shape cleft forming the two sphere shape of protein. The active site is evolutionary conserved by all cysteine proteases and are involved in hydrolysis of insect basement membrane, skeleton and midgut (Silva et al., 2016).
5 Conclusions
The results of this study reveal that nanoencapsulation of ApCP could be a promising biological tools of insect pest control. ApCP could be used at much less concentration and can give favourable results than similarly studied cysteine proteases against the stored grain insect pests. This research would be commercialized for bio-pesticide synthesis by multiplying its experimentation on different insect pests in laboratory and field conditions.
Conflict of interest
On behalf of all authors, the corresponding author state that there is no conflict of interest.
Acknowledgements
We acknowledge the Higher Education Commission (HEC), Pakistan and Bahauddin Zakariya University Multan, Pakistan for financial support in execution of this work.
References
- Insecticidal efficacy of Cleistopholis patens (Benth) against Sitotroga cerealella Olivier (Lepidoptera: Gelechiidae) infesting rice grains in Nigeria. J. Crop. Prot.. 2016;5:1-10.
- [Google Scholar]
- Occupational pesticide exposures and cancer risk: a review. J. Toxicol. Environ. Health. B.. 2012;15:238-263.
- [Google Scholar]
- Graphene quantum dots as cysteine protease nanocarriers against stored grain insect pests. Sci. Rep.. 2020;10
- [Google Scholar]
- Citrus peel essential oil nanoformulations to control the tomato borer, Tuta absoluta: chemical properties and biological activity. Sci. Rep.. 2017;7:1-10.
- [Google Scholar]
- Targeted Delivery of Pesticides using Biodegradable Polymeric Nanoparticles. Springer; 2014.
- How helpful is nanotechnology in agriculture? Adv. Nat. Sci-Nanosci.. 2012;3:033002
- [Google Scholar]
- The use of phosphine as curative treatment against date palm borers. Outlooks. Pest. Manag.. 2019;30:204-207.
- [Google Scholar]
- Functional characterization of recombinant bromelain of Ananas comosus expressed in a prokaryotic system. Mol. Biotechnol.. 2014;56:166-174.
- [Google Scholar]
- Nanoengineered Systems for Biopesticides. Handbook of Nanomaterials for Industrial Applications: Elsevier; 2018. p. :243-259.
- Biological activity and binding site characteristics of the PA1b entomotoxin on insects from different orders. J. Insect. Sci.. 2007;7(12):1-10.
- [Google Scholar]
- Pesticides pollution: classifications, human health impact, extraction and treatment techniques. Egypt. J. Aquat. Res.. 2020;46:207-220.
- [Google Scholar]
- New insights into peritrophic matrix synthesis, architecture, and function. Annu. Rev. Entomol.. 2009;54:285-302.
- [Google Scholar]
- Novel and environmental friendly approach; Impact of Neem (Azadirachta indica) gum nano formulation (NGNF) on Helicoverpa armigera (Hub.) and Spodoptera litura (Fab.) Int. J. Biol. Macromol.. 2018;107:59-69.
- [Google Scholar]
- Wildlife ecotoxicology of pesticides: can we track effects to the population level and beyond? Science. 2013;341:759-765.
- [Google Scholar]
- Papain protects papaya trees from herbivorous insects: role of cysteine proteases in latex. Plant J.. 2004;37:370-378.
- [Google Scholar]
- Nano-based smart pesticide formulations: emerging opportunities for agriculture. J. Control. Release.. 2019;294:131-153.
- [Google Scholar]
- A garlic substance disrupts odorant-binding protein recognition of insect pheromones released from adults of the angoumois grain moth, Sitotroga cerealella (Lepidoptera: Gelechiidae) Insect. Mol. Biol.. 2016;25:530-540.
- [Google Scholar]
- Chemical analysis of Carica papaya L. Crude latex. Am. J. Plant. Sci.. 2013;4:1941.
- [Google Scholar]
- Biological control of the Pulse beetle, Callosobruchus maculatus in stored grains using the entomopathogenic bacteria, Bacillus thuringiensis. Microb. Pathog.. 2018;114:139-146.
- [Google Scholar]
- Losses in the grain supply chain: causes and solutions. Sustainability. 2020;12:23-42.
- [Google Scholar]
- Degradation of the S. frugiperda peritrophic matrix by an inducible maize cysteine protease. J. Insect. Physiol.. 2006;52:21-28.
- [Google Scholar]
- Moore III, S., Petty, H., Luckmann, W., Byers, J., 1966. Losses caused by the Angoumois grain moth in dent corn. J. Econ. Entomol. 59, 880-882.
- Influence of the Soybean Seed Coat upon Seed Infestation and Development of the Insect Callosobruchus maculatus. Soybean and Wheat Crops: Growth, Fertilization and Yield. Nove Publishers; 2009. p. :1-21.
- A unique 33-kD cysteine proteinase accumulates in response to larval feeding in maize genotypes resistant to fall armyworm and other Lepidoptera. Plant. Cell.. 2000;12:1031-1040.
- [Google Scholar]
- Nanotechnology in sustainable agriculture: present concerns and future aspects. Afr. J. Biotechnol.. 2014;13:705-713.
- [Google Scholar]
- Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol.. 2012;94:287-293.
- [Google Scholar]
- A core metabolic enzyme mediates resistance to phosphine gas. Science. 2012;338:807-810.
- [Google Scholar]
- Albizia lebbeck seed coat proteins bind to chitin and act as a defense against cowpea weevil Callosobruchus maculatus. J. Agric. Food. Chem.. 2016;64:3514-3522.
- [Google Scholar]
- Adult dispersal of Sitotroga cerealella in a conventional small-farm in Southern Italy. Bull. Insectol.. 2015;68:111-118.
- [Google Scholar]







