Translate this page into:
N-Acetylcysteine reduces the neurotoxic effects of propionic acid in rat pups
*Tel.: +966 1 4769137; fax: +966 1 4646716 aldbass@ksu.edu.sa (Abeer M. Al-Dbass)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 11 September 2013
Peer review under responsibility of King Saud University.

Abstract
Propionic acid, a metabolite produced by intestinal bacteria, has been implicated in autism. N-Acetylcysteine is a well-known antioxidant. The present study investigated the protective effects of N-Acetylcysteine on propionic acid-induced neurotoxicity in rat pups. Male Western albino rats were divided into four groups. The first group served as the normal control group, the second was treated with propionic acid (PA group), the third group received propionic acid followed by N-Acetylcysteine (PANA group), and the fourth group received N-Acetylcysteine followed by propionic acid (NAPA group). In the PA group, there was a significant increase in lactate dehydrogenase activity, decrease in glutathione levels, and decrease in sodium levels (P < 0.05, all comparisons). Calcium and potassium levels did not significantly change. Additionally, urea and lipid peroxide levels were significantly elevated in propionic acid-intoxicated rats and significantly rescued in the N-Acetylcysteine-treated groups (P < 0.05, all comparisons). These results indicate that N-Acetylcysteine can both protect against and treat propionic acid intoxication.
Keywords
Propionic acid
N-Acetylcysteine
Lactate dehydrogenase
Lipid peroxides
Glutathione
1 Introduction
Propionic acid is a natural body metabolite that is utilised by most organs and tissues. It is produced as a result of fermentation of undigested carbohydrates by intestinal bacteria (Jan et al., 2002; Al-Lahham et al., 2010; Nyhan et al., 1999; Thompson et al., 1990; Zarate et al., 2004). Propionates also exist naturally in various foods, especially dairy products. In addition, studies have reported that several bacteria, such as clostridia, produce propionic acid during their natural metabolism (Finegold et al., 2010). Consumption of excessive propionate in children results in irritability, inattention, and restlessness associated with sleep disorders (Dengate and Ruben, 2002). Propionic acid crosses the blood–brain barrier and can cause mild, reversible intracellular acidification, which can affect the release of neurotransmitters including glutamate, dopamine, and serotonin (Gupta and Deshpande, 2008; Song et al., 2004). Propionic acid can increase N-methyl-D-aspartate (NMDA) receptor sensitivity, promote intracellular calcium release, elevate nitric oxide levels, and inhibit Na+/K+ATPase, all of which can influence synaptic transmission and/or neuronal activity (Bronstein et al., 1993; Wajner et al., 2004; DeMattos-Dutra et al., 2000). Mitochondrial fatty acid metabolism may be affected by propionic acid because of its interaction with propionyl coenzyme A and sequestration of carnitine (Wajner et al., 2004; Brass et al., 1986). This impaired fatty acid metabolism may be related to autism, which may be a mitochondrial disorder.
N-Acetylcysteine, a derivative of the amino acid l-Cysteine, is currently used as an antioxidant, nutritional supplement, and pharmaceutical drug. It is an excellent source of sulphydryl groups and can be converted into metabolites that can elevate glutathione synthesis in the body. N-Acetylcysteine is currently used as a mucolytic agent for respiratory diseases. It is also used to treat paracetamol overdoses (Tsai et al., 2005), in which case it acts by promoting detoxification by increasing free radical scavenger activity, thus maintaining normal glutathione levels in hepatocytes. N-Acetylcysteine is a precursor for glutathione, which acts as a free radical scavenger in neurons (Cooper and Kristal, 1997) and improves neuronal survival (Ratan et al., 1994). These functions are performed by preserving mitochondrial function and maintaining the mitochondrial oxidative metabolism by protecting the cytochrome oxidase complex I from NO-mediated damage (Moncada, 2000). N-Acetylcysteine can also act as a heavy metal chelating agent to clear the body of some toxic metals. Additionally, N-Acetylcysteine raises glutathione levels when taken as a supplement by immunocompromised patients.
The purpose of this study was to examine the toxic effect of propionic acid on rat pups and evaluate the potential neuroprotective effects of N-Acetylcysteine on propionic acid-induced neurotoxicity.
2 Materials and methods
2.1 Animals
Twenty Male Western albino rats (approximately 4 weeks old, weighing 45–60 g) were used in this study. They were separately housed at a controlled temperature of 21 ± 1 °C with ad libitum access to food and water.
2.2 Experimental design
The experimental design and treatment schedule was as follows:
Group 1: Control rats that did not receive any treatment (Normal healthy control; control group).
Group 2: Rats treated with a neurotoxic dose of propionic acid (purchased from Sigma) (250 mg/kg body weight over three days) to induce autism (PA group).
Group 3: Rats treated with N-Acetylcysteine (purchased from Sigma) (50 mg/kg body weight over a period of one week), followed by propionic acid intoxication as described above (NAPA group).
Group 4: Rats treated with a toxic dose of propionic acid followed by N-Acetylcysteine (PANA group).
2.3 Ethics approval and consent
The Ethics committee of the College of Science Research Centre of King Saud University, Riyadh, Saudi Arabia approved this study. The approval number is 8/25/220358.
2.4 Blood collection
At the end of the experimental period, the rats were sacrificed using ether anaesthesia. Blood samples were collected from the inferior vena cava of each rat. The serum was separated from the blood cells and stored at −80 °C until analysis.
2.5 Biochemical analysis
2.5.1 Measurement of lactate dehydrogenase (LD)
The activity of LD5 was measured with the pyruvate to lactate kinetic method and the two-point colorimetric method for lactate dehydrogenase (LD). An increase in the absorbance at 340 nm indicated NADH formation, which was directly proportional to serum LD-L activity. The procedure was based on the modifications of the Amador and Wacker method (Amador et al., 1963; Snodgrass et al., 1959).
2.5.2 Determination of sodium levels
Sodium levels were assayed by enzymatic determination of sodium, i.e., measurement of sodium-dependent galactosidase activity using ONPG as a substrate (Tiez, 1986).
2.5.3 Determination of glutathione levels
Glutathione levels were measured by the development of a relatively stable yellow colour (read at 412 nm) upon addition of 5, 5-dithiobis-2-nitrobenzoic acid (Beutler et al., 1963).
2.5.4 Determination of calcium levels
Calcium levels were measured using a procedure involving a chromogenic complex that forms upon reaction of o-cresolphthalein complexone (o-CPC) with calcium. The chromogenic complex absorbs light and was therefore measured photometrically between 570 nm and 580 nm (Faulker and Meites, 1982).
2.5.5 Determination of potassium levels
Potassium levels were measured in a protein-free alkaline medium by reaction with sodium tetraphenyl boron, which produced a colloidal suspension. The turbidity of such a suspension is proportional to the potassium concentrations in the range of 2–7 mmol/L (Terri and Sesin, 1958).
2.5.6 Determination of urea levels
Urea levels were measured using the enzymatic, colorimetric, endpoint-Berthelot method (Kits from Crescent Diagnostics Co., Saudi Arabia), in which the conversion of urea to ammonia was catalysed by urease.
2.5.7 Determination of lipid peroxide levels
Endogenous lipid peroxidation was evaluated by measuring the malondialdehyde concentration at 532 nm and calculating the concentration using an extinction coefficient value (€) of 156 ∗ 105 M−1 cm−1 (Buege and Aust, 1978).
2.6 Statistical analysis
The one-way analysis of variance (ANOVA) was used for statistical analysis. The values were expressed as means ± standard deviation. A P value of <0.05 was considered statistically significant. The Receiver Operating Characteristic (ROC) curve is a fundamental tool for evaluating biomarkers. On the ROC curves, the true positive rate was plotted against the false positive rate (100-Specificity) for various cut-off values.
3 Results
Table 1 and Fig. 1 present the neurotoxic effects of propionic acid along with the protective and therapeutic effects of N-Acetylcysteine. Propionic acid induced significant increases in serum LD activity and oxidative stress through the induction of glutathione depletion and a significant increase in lipid peroxidation (P < 0.05). Propionic acid did not affect calcium or potassium levels but did induce sodium depletion. Urea levels were dramatically elevated in the serum of propionic acid-treated rats. Table 1 shows the protective effect of N-Acetylcysteine on propionic acid neurotoxicity. N-Acetylcysteine-pre-treated rats had much lower LD activity and lipid peroxidation and higher levels of glutathione. N-Acetylcysteine treatment did not affect sodium levels. Significant levels between the three groups are illustrated as superscript letters when P < 0.05.
Parameters
Groups
Min.
Max.
Percent change
Mean ± S.D.
P value
LD enzyme (U/L)
Control
102.18
253.79
100.00
160.89 ± 50.76
0.001
PPA
112.06
372.45
160.962
258.96 ± 85.94 a
N-acetyl protective
102.18
288.41
108.920
175.24 ± 84.71
N-acetyl therapeutic
29.66
125.25
40.563
65.26 ± 36.70 a
Sodium (mmol/l)
Control
48.99
95.00
100.00
66.94 ± 17.37
0.001
PPA
22.00
62.99
61.01
40.84 ± 13.80 a
N-acetyl protective
18.20
58.80
54.70
36.62 ± 15.00 a
N-acetyl therapeutic
9.70
26.60
24.90
16.67 ± 7.75 a
Glutathione (ug/ml)
Control
34.48
37.06
100.00
35.57 ± 0.96
0.001
PPA
23.27
31.03
78.25
27.83 ± 3.20 a
N-acetyl protective
32.32
36.20
96.10
34.18 ± 1.52
N-acetyl therapeutic
20.68
28.01
68.46
24.35 ± 2.54 a
Calcium (mmol/L)
Control
1.75
3.45
100.00
2.42 ± 0.54
0.169
PPA
1.84
2.38
84.37
2.04 ± 0.17
N-acetyl protective
2.15
2.62
96.87
2.34 ± 0.19
N-acetyl therapeutic
2.07
2.39
91.82
2.22 ± 0.13
Potassium (mmol/l)
Control
4.70
8.32
100.00
6.40 ± 1.16
0.166
PPA
4.46
6.87
88.89
5.69 ± 0.97
N-acetyl protective
6.09
7.49
104.12
6.67 ± 0.59
N-acetyl therapeutic
5.18
7.92
107.48
6.88 ± 1.02
Urea (mmol/L)
Control
4.97
8.59
100.00
6.49 ± 1.23
0.012
PPA
7.08
10.06
128.54
8.34 ± 1.20 a
N-acetyl protective
6.73
8.24
113.66
7.38 ± 0.58
N-acetyl therapeutic
5.96
7.72
107.49
6.98 ± 0.64
Lipid peroxides (nmole/ml)
Control
1.02
1.70
100.00
1.40 ± 0.24
0.001
PPA
2.33
6.54
295.30
4.13 ± 1.37 a
N-acetyl protective
1.28
2.09
115.86
1.62 ± 0.37
N-acetyl therapeutic
1.50
1.81
118.22
1.65 ± 0.10
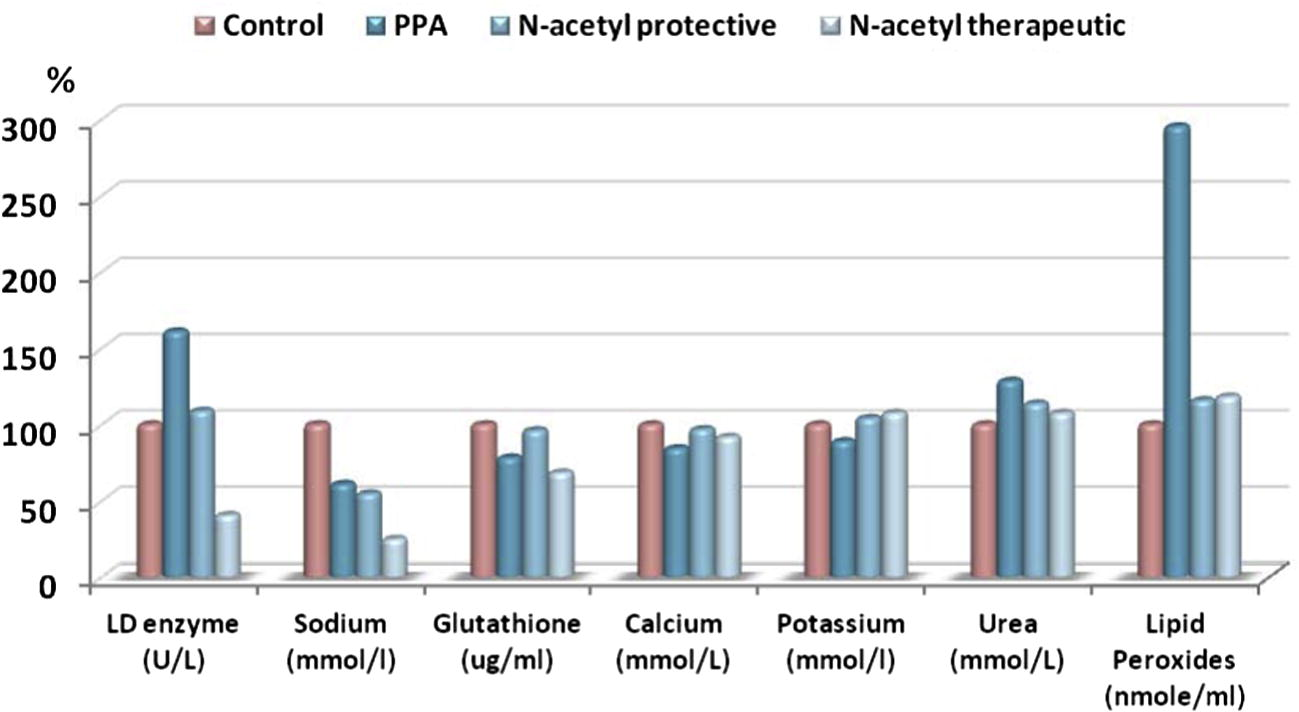
Percentage change of control, PPA, N-acetyl protective and N-acetyl therapeutic groups in LD enzyme (U/L), Sodium (mmol/l), Glutathione (ug/ml), Calcium (mmol/L), Potassium (mmol/l), Urea (mmol/L) and Lipid peroxide (nmole/ml) groups compared to control.
Table 2 and Figs. 2–4 display ROC curves for the parameters measured, including the area under the curve, cut-off, and specificity and sensitivity values.
Group parameter
Area under
The curve
Cutoff
Value (%)
Sensitivity (%) Specificity (%)
PPA
LD enzyme (U/L)
0.821
201.880
85.7
87.5
Sodium (mmol/l)
0.929
47.595
85.7
100.0
Glutathione (ug/ml)
1.000
32.755
100.0
100.0
Calcium (mmol/L)
0.750
2.394
100.0
62.5
Potassium (mmol/l)
0.661
6.510
85.7
50.0
Urea (mmol/L)
0.893
7.035
100.0
75.0
Lipid peroxides (nmole/ml)
1.000
2.016
100.0
100.0
N-acetyl protective
LD enzyme (U/L)
0.479
262.031
33.3
100.0
Sodium (mmol/l)
0.917
47.595
83.3
100.0
Glutathione (ug/ml)
0.781
34.265
66.7
100.0
Calcium (mmol/L)
0.521
2.346
66.7
62.5
Potassium (mmol/l)
0.563
5.866
100.0
37.5
Urea (mmol/L)
0.750
6.602
100.0
62.5
Lipid peroxides (nmole/ml)
0.667
1.276
100.0
37.5
N-acetyl therapeutic
LD enzyme (U/L)
0.958
96.408
83.3
100.0
Sodium (mmol/l)
1.000
37.795
100.0
100.0
Glutathione (ug/ml)
1.000
31.245
100.0
100.0
Calcium (mmol/L)
0.667
2.396
100.0
62.5
Potassium (mmol/l)
0.625
6.846
66.7
75.0
Urea (mmol/L)
0.677
6.506
83.3
62.5
Lipid peroxides (nmole/ml)
0.802
1.478
100.0
62.5
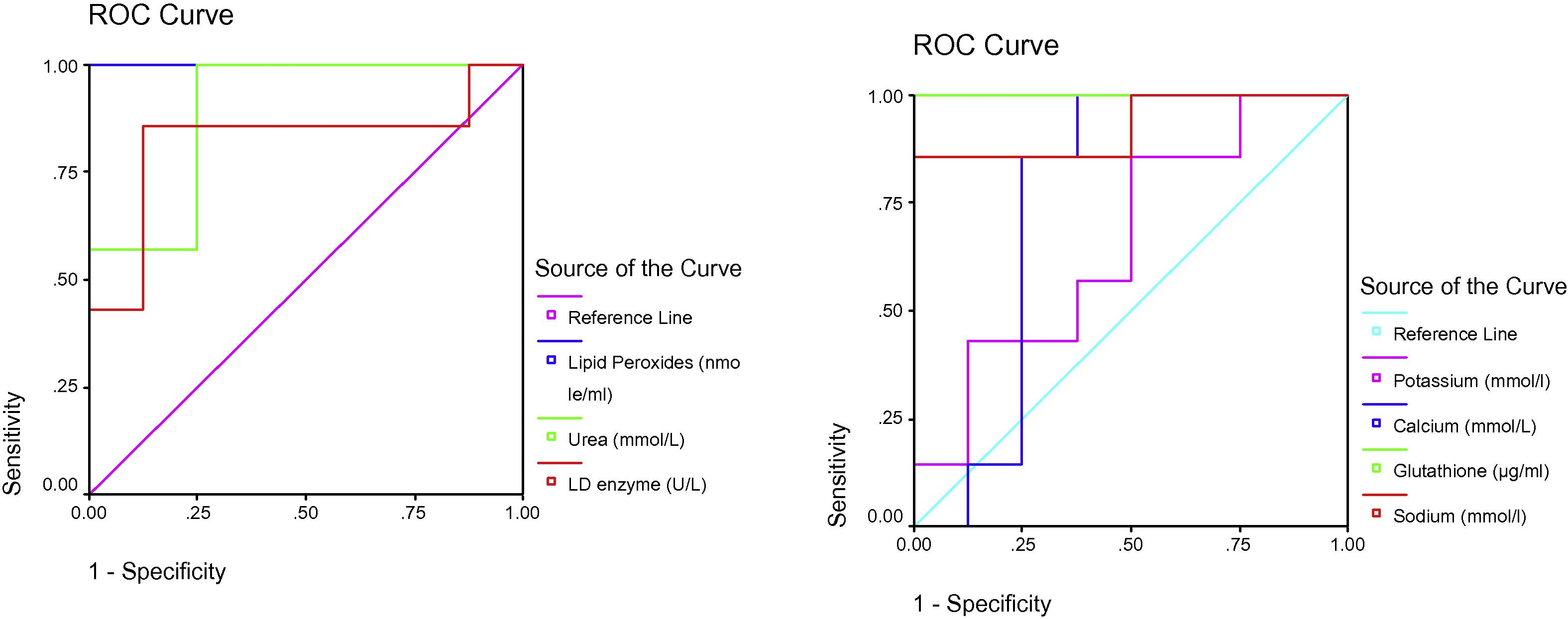
ROC Curve of LD enzyme (U/L), Sodium (mmol/l), Glutathione (ug/ml), Calcium (mmol/L), Potassium (mmol/l), Urea (mmol/L) and Lipid peroxides (nmole/ml) in the PPA group.
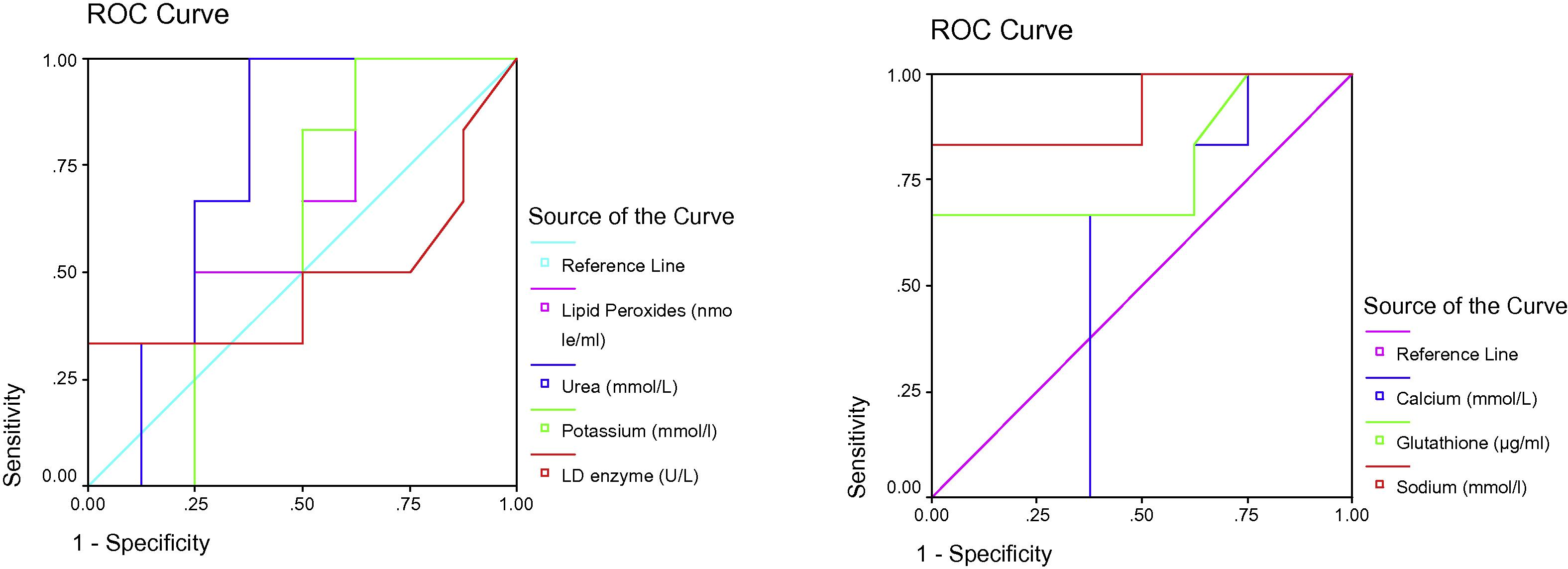
ROC Curve of LD enzyme (U/L), Sodium (mmol/l), Glutathione (ug/ml), Calcium (mmol/L), Potassium (mmol/l), Urea (mmol/L) and Lipid peroxides (nmole/ml) in the N-acetyl protective group.
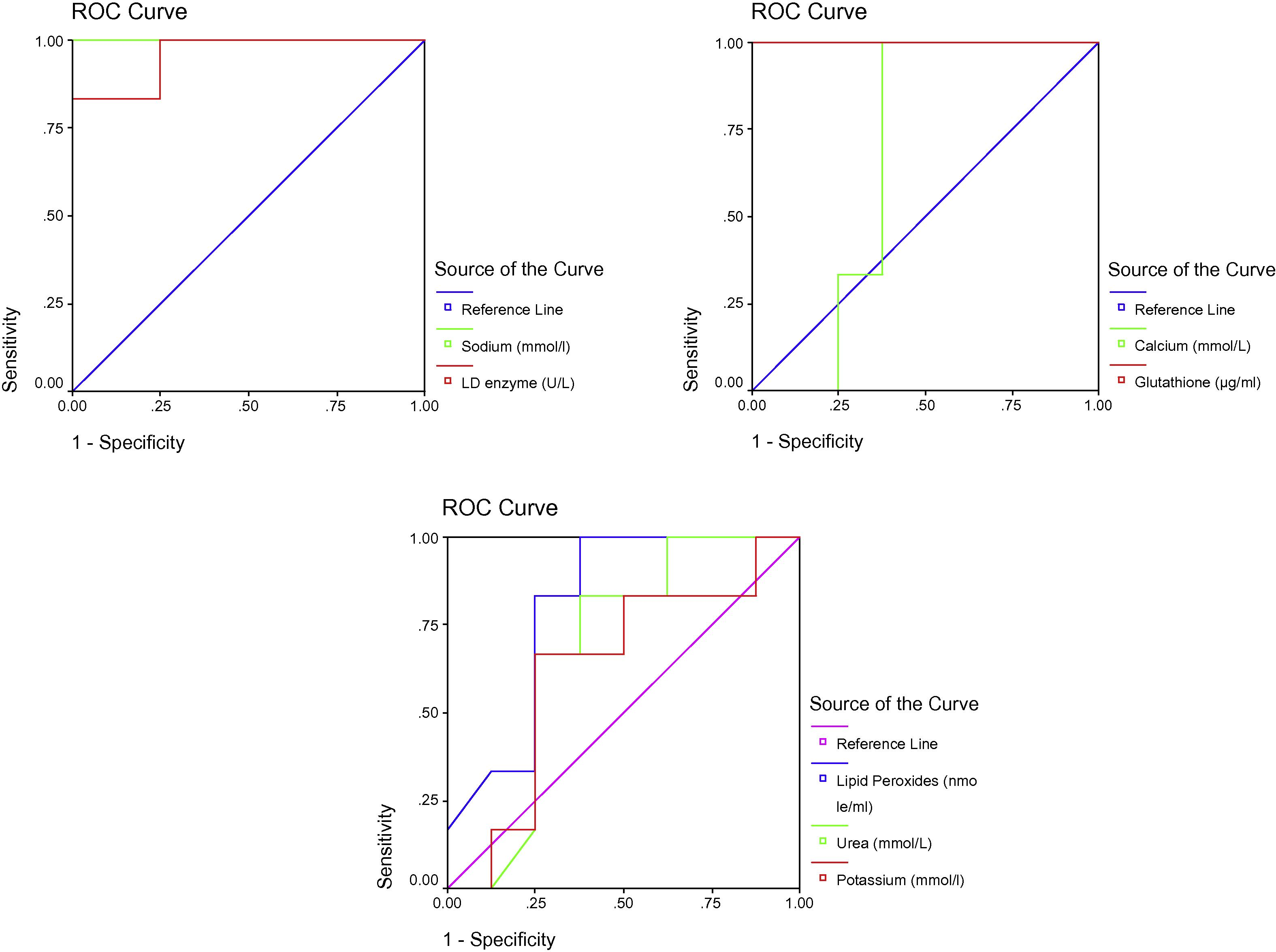
ROC Curve of LD enzyme (U/L), Sodium (mmol/l), Glutathione (ug/ml), Calcium (mmol/L), Potassium (mmol/l), Urea (mmol/L) and Lipid peroxides (nmole/ml) in the N-acetyl therapeutic group.
4 Discussion
Propionic acid toxicity is an organic acidemia (Xu et al., 2012) that is often present during the neonatal period along with lethargy, poor feeding, and vomiting. Progression may lead to coma if not recognised and appropriately treated. Table 1 and Fig. 1 show a dramatic increase in LD activity in the plasma of propionic acid-treated rats compared to the control group. The increase in LD in the serum could be due to the leakage of enzyme from the brain into the plasma, which may indicate cellular and membrane damage. El-Ansary et al.’s (2011) study supports this observation. They found a remarkable decrease in LD activity in the brain homogenates of propionic acid-treated rats compared to untreated controls (El-Ansary et al., 2011). The outcomes of our study indicate that N-Acetylcysteine is effective against propionic acid-induced neurotoxicity (Table 1). N-Acetylcysteine has both protective and therapeutic effects, as indicated by the rescued levels of LD activity in the serum. Hsu et al. (2006) showed that pre- versus post-treatment with N-Acetylcysteine decreases plasma LD as a marker of organ injury induced during sepsis. Additionally, the protective effects shown in our study agree with Abd El-Fattah and El-Sheikh (2012), who reported that N-Acetylcysteine partially corrects propionic acid-induced changes in enzyme activities and protects the kidney from tubular damage.
Increased oxidative damage due to neuroinflammation, mitochondrial dysfunction, and impaired GSH metabolism may be related to the development of autism (James et al., 2004; Al-Gadani et al., 2009). Oxidative stress has been implicated in synaptic plasticity. MacFabe et al. (2007) and El-Ansary et al. (2012) hypothesised that similar processes could arise in animals given an oral or intraventricular neurotoxic dose of propionic acid. We have found that propionic acid treatment induces a substantial increase in lipid oxidation, indicating increased oxidative stress. We found that N-Acetylcysteine exerted a protective effect on GSH depletion induced by propionic acid and a smaller effect when given after propionic. Increased lipid peroxide levels serve as a marker for the neurotoxic effects of propionic acid. The antioxidant effect of N-Acetylcysteine was confirmed by its ability to decrease the concentration of lipid peroxides. Hence, N-Acetylcysteine exerts both protective and therapeutic effects. The effectiveness of N-Acetylcysteine is due to increases in cysteine levels that increase the available pool of glutathione, compared to other antioxidants examined in autism. Previous studies support this suggested antioxidant effect. For example, Gibson et al. (2009) concluded that N-Acetylcysteine is a weak antioxidant that requires prior conversion into GSH to impart antioxidant and antithrombotic benefits at therapeutic levels. Additionally, N-Acetylcysteine acted as an antiplatelet agent only when the intra-platelet activity that converts it into GSH is functional (Gibson et al., 2011). The failure of N-Acetylcysteine to exert a therapeutic antioxidant effect in the present study may suggest that propionic acid irreversibly impaired the glutathione synthesis pathway.
The effectiveness of the low dose of N-Acetylcysteine (50 mg/kg/day) used in the present study concurs with a previous animal study by Sprong et al. (1998). In their study, high-dose N-Acetylcysteine treatment (550 and 950 mg/kg in 48 h) decreased lung glutathione (75 mg/kg/day) and resulted in a significantly lower survival rate compared to low-dose N-Acetylcysteine (Sprong et al., 1998).
Our results indicate that hyponatraemia could be one of the neurotoxic effects of propionic acid (Table 1). Although plasma calcium and potassium levels were not significantly altered by propionic acid, plasma sodium levels were dramatically decreased. We found that N-Acetylcysteine was not effective at ameliorating propionic acid-induced hyponatraemia (Table 1). This observation indicates that N-Acetylcysteine is likely to work through the proposed anti-oxidative mechanism. Additionally, the weak therapeutic effect of N-Acetylcysteine in the present study concurs with a study by El-Ansary et al. (2012) on the persistent neurotoxic effects of propionic acid and the induction of biochemical autistic features in rats.
Intraperitoneal administration was selected to test the role of the gut-brain axis in the neurotoxicity of propionic acid and the possibility of treating autistic patients through oral administration of N-Acetylcysteine.
5 Conclusion
The outcomes of this study indicate that N-Acetylcysteine could be used to both protect against and treat propionic acid intoxication.
Acknowledgement
This research project was supported by a grant from the research centre of the Centre for Female Scientific and Medical Colleges at King Saud University.
References
- Evaluation of chemoprotective role of N-Acetylcysteine and Vitamin E on gentamicin induced nephrotoxicity. Aust. J. Basic Appl. Sci.. 2012;6(3):263-270.
- [Google Scholar]
- Metabolic biomarkers related to oxidative stress and antioxidant status in Saudi autistic children. Clin. Biochem.. 2009;42:1032-1040.
- [Google Scholar]
- Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta. 2010;1801:1175-1183.
- [Google Scholar]
- Serum lactic dehydrogenase activity: an analytical assessment of current assays. Clin. Chem.. 1963;9:391.
- [Google Scholar]
- Improved method for determination of blood glutathione. J. Lab. Clin. Med.. 1963;61:882-888.
- [Google Scholar]
- Inhibition of oxidative metabolism by pro- pionic acid and its reversal by carnitine in isolated rat hepatocytes. Biochem. J.. 1986;236:131-136.
- [Google Scholar]
- Regulation of type-II calmodulin kinase: functional implications. Brain Res. Rev.. 1993;18:135-147.
- [Google Scholar]
- Multiple roles of glutathione in the central nervous system. Biol. Chem.. 1997;378:793-802.
- [Google Scholar]
- Methylmalonic and propionic acids increase the in vitro incorporation of 32P into cytoskeletal proteins from cerebral cortex of young rats through NMDA glutamate receptors. Brain Res.. 2000;856:111-118.
- [Google Scholar]
- Controlled trial of cumulative behavioural effects of a common bread preservative. J. Paediatr. Child Health. 2002;38(4):373-376.
- [Google Scholar]
- Effectiveness of creatine and cyclosporine to protect against propionic acid- induced neurotoxicity. Int. J. Pharmacol. Toxicol. Sci.. 2011;2:28-43.
- [Google Scholar]
- Etiology of autistic features: the persisting neurotoxic effects of propionic acid. J. Neuroinflammation. 2012;9:74.
- [Google Scholar]
- Faulker, W.R., Meites, S., 1982. Selected methods for the small clinical chemistry laboratory. American Association for Clinical Chemistry, Incorporated, Washington D.C., P-125.
- Pyrose- quencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16:444-453.
- [Google Scholar]
- Evaluation of the antioxidant properties of N-acetylcysteine in human platelets: prerequisite for bioconversion to glutathione for antioxidant and antiplatelet activity. J. Cardiovasc. Pharmacol.. 2009;54(4):319-326.
- [Google Scholar]
- Therapeutic potential of N-acetylcysteine as an antiplatelet agent in patients with type-2 Diabetes. Cardiovasc. Diabetol.. 2011;10:43.
- [Google Scholar]
- 3-Nitropropionic acid depresses spinal reflexes involving GABAergic and glycinergic transmission in neonatal rat spinal cord in vitro. Life Sci.. 2008;83(21–22):756-760.
- [Google Scholar]
- Post-treatment with N-acetylcysteine ameliorates endotoxin shock-induced organ damage in conscious rats. Life Sci.. 2006;79(21):2010-2016.
- [Google Scholar]
- Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am. J. Clin. Nutr.. 2004;80:1611-1617.
- [Google Scholar]
- Propionibacteria induce apoptosis of colorectal carcinoma cells via short chain fatty acids acting on mitochondria. Cell Death Differ.. 2002;9:179-188.
- [Google Scholar]
- Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav. Brain Res.. 2007;176:149-169.
- [Google Scholar]
- Nitric oxide and cell respiration physiology and pathology. Verh. K. Acad. Geneeskd. Belg.. 2000;62:171-179.
- [Google Scholar]
- Neurological nonmetabolic presentation of propionic acidemia. Arch. Neurol.. 1999;56:1143-1147.
- [Google Scholar]
- Oxidative stress induces apoptosis in embryonic cortical neurons. J. Neurochem.. 1994;62:376-379.
- [Google Scholar]
- Metalloenzymes and myocardial infarction. III. Lactic dehydrogenase activity of serum-a determinate diagnostic measure. N. Engl. J. Med.. 1959;261:1259-1266.
- [Google Scholar]
- Real-time PCR quantification of clostridia in feces of autistic children. Appl. Environ. Microbiol.. 2004;70:6459-6465.
- [Google Scholar]
- Low-dose N-acetylcysteine protects rats against endotoxin-mediated oxidative stress, but high-dose increases mortality. Am. J. Respir. Crit. Care Med.. 1998;157(4 Pt 1):1283-1293.
- [Google Scholar]
- Sources of propionate in inborn errors of propionate metabolism. Metabolism. 1990;39:1133-1137.
- [Google Scholar]
- Tiez N.W., ed. Textbook of Clinical Chemistry. Philadelphia: Saunders Company; 1986. p. :1845.
- A patient-tailored N-acetylcysteine protocol for acute acetaminophen intoxication. Clin. Ther.. 2005;27(3):336-341.
- [Google Scholar]
- The role of oxidative damage in the neuropathology of organic acidurias: insights from animal studies. J. Inherit. Metab. Dis.. 2004;27:427-448.
- [Google Scholar]
- Clinical analysis of organic acidemia in neonates from neonatal intensive care units. Zhongguo Dang Dai Er Ke Za Zhi. 2012;14(5):336-339.
- [Google Scholar]
- Assessing survival of dairy propionibacteria in gastrointestinal conditions and adherence to intestinal epithelia. Methods Mol. Biol.. 2004;268:423-432.
- [Google Scholar]







