Translate this page into:
Myrtus communis and its bioactive phytoconstituent, linalool, interferes with Quorum sensing regulated virulence functions and biofilm of uropathogenic bacteria: In vitro and in silico insights
⁎Corresponding author.
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Ever increasing spread of drug resistance among bacterial pathogens have rendered the current antibiotic therapy ineffective. Drug-resistant urinary tract infections caused by Gram negative bacterial pathogens such as Pseudomonas aeruginosa, Escherichia coli, Acinetobacter baumannii, and Serratia marcescens are considered more severe and life threatening because of strong biofilm dependent growth characteristics among majority of them. Moreover, most uropathogens coordinate their complex virulence responses via a highly structured network of cell to cell communication known as Quorum sensing (QS). Phytochemicals/bioactive extracts obtained from established ethnomedicinal plants have previously been demonstrated as effective anti-QS agent against numerous pathogenic bacteria including uropathogens. Myrtus communis (L.) (Myrtaceae) is one such plant of immense ethnomedicinal importance and has been used in traditional medicine. In the present investigation, methanolic extract of Myrtus communis (MCME) demonstrated 65% inhibition in the QS regulated violacein production in C. violaceum without affecting the growth of the bacteria. Further, the MCME interfered significantly with QS controlled virulence production in P. aeruginosa (elastase, protease, pyocyanin and chitinase) and S. marcescens (prodigiosin and protease). The leaf extract exhibited 16–74%, 31–84%, 12–66% and 19–71% inhibition of biofilm biomass of P. aeruginosa, E. coli, A. baumannii and S. marcescens respectively at the increasing concentration corresponding to MIC/16-MIC/2. GC–MS analysis revealed Linalool as one of the major bioactive compounds present in the MCME. Further, in vitro assays demonstrated QS and biofilm inhibition by sub-MICs of linalool. In silico docking and simulation studies showed that the possible mechanisms responsible for the interference of QS were AHL synthesis inhibition, antagonization of QS-regulatory proteins, and blocking of the receptor proteins. Thus, from the obtained results it is envisaged that M. communis and its bioactive compound linalool may have medicinal implications and could prove as effective therapeutic agent in combating the threat of biofilm based persistent infections caused by uropathogenic bacteria.
Keywords
Myrtus communis
Quorum sensing
Biofilm
Virulence
Linalool
Docking
- CV
-
Chromobacterium violaceum ATCC 12,472
- EC
-
E. coli ATCC 35,218
- PA
-
Pseudomonas aeruginosa PAO1
- SM
-
S. marcescens ATCC 13,880
- AB
-
A. baumanii ATCC BAA747
Abbreviations
1 Introduction
Increasing spread of antibiotic resistance among bacterial pathogens have placed the conventional therapeutic management regimens at the risk of sinking effectivity. Numerous reports have indicated that infectious mortality and morbidity is rising rapidly due to emergence of multidrug resistance among bacterial pathogens (Friedman et al., 2016). Although, the ominous rise of antibiotic resistance is frequent in hospital acquired infections, recent reports have also shown that this trend is becoming more common in urinary pathogens. Gram negative bacterial pathogens such as Pseudomonas aeruginosa, Escherichia coli, Acinetobacter baumannii, and Serratia marcescens are often implicated in urinary tract infections (UTIs) (Ronald, 2003). Urinary tract infections are considered more severe and life threatening because of strong biofilm dependent growth characteristics among majority of urinary pathogens (Flores-Mireles et al., 2015). Biofilm structural organization and altered metabolic state of bacterial cells enclosed in biofilms supplement the overall drug resistance efficacy of the bacterial pathogens (Mah, 2012). Majority of the uropathogens coordinate their complex virulence responses via a highly structured network of cell to cell communication known as “Quorum sensing”. Quorum sensing (QS) is a bacterial ability to synchronize their individual response with respect to changes in overall population density in a given environment to act as single functional unit. The coordination among bacterial cells in a population is mediated via specialized secreted signaling molecules known as autoinducers. Several virulence factors such as exoproteases production, biosurfactant production, exopolysaccharides (EPS) production and motility which are associated with biofilm formation are entirely or partially under the control of QS. Further along, these QS linked virulence determinants are also involve in facilitating the successful establishment of UTIs (Jones et al., 2004). For instance, bacterial motility viz. swimming and swarming play crucial role in colonization and subsequent upward movement of uropathogens along the urinary tract (Lane et al., 2005). Likewise, production of biosurfactants by the uropathogens such as rhamnolipids production by Pseudomonas aeruginosa facilitate the bacterial motility due its tensioactive properties which in turns enhanced the biofilm formation by the bacteria (Caiazza et al., 2005). Exopolysaccharides provides basic skeletal support to biofilm structure and sustain its integrity, and also act as a physical barrier for residing bacterial cells against outside interventions such as by weakening the penetrations of chemotherapeutic molecules, phagocytosis etc. (Algburi et al., 2017). Thus, sessile uropathogenic bacterial community residing inside the biofilm are better equipped with resistance mechanism against inbuilt host defense responses and external antimicrobial chemotherapeutic pressures. Therefore, alternative therapeutic strategies are urgently required to overcome the challenge. Among numerous novel anti-infective strategies, targeting QS linked virulence factors including biofilm formation by means of anti-QS agents offers an effective option. Agents interrupting QS system may act via multiple mechanism including directly interfering with signal productions or by blocking signal transduction system (Khan et al., 2018).
Phytochemicals/active-extracts obtained from established ethnomedicinal plants have previously been demonstrated as effective anti-QS agent against numerous pathogenic bacteria including uropathogens. Owing to their long traditional usage history, these plants are generally considered as safe for therapeutic applications in humans (Pan et al., 2014). One such plant of immense ethnomedicinal importance, Myrtus communis (L.) belongs to family Myrtaceae, a native of Mediterranean basin and Arabian Peninsula was selected in the present study. Among other traditional uses, leaves and fruit of Myrtus communis is been extensively used as general antiseptic and treatment of many types of infectious diseases including bacterial dysentery, diarrhea and in treatment of urinary infections (Sumbul et al., 2011). Essential oil components of the leaves constitute the major group of active phytochemicals, other bioactive compounds such as flavonoids and their derivatives have also been reported in different part of the plant (Sisay and Gashaw, 2017). Therefore, in the present study, in vitro anti-QS and anti-biofilm activity of the leaf extract of M. communis was performed. Moreover, the antibiofilm activity of activity was also tested against E. coli, P. aeruginosa, S. marcescens and A. baumannii. GC/MS analysis revealed the presence of major phytocompounds of the extract. The effect of one of the major components, i.e., linalool, was also tested to identify the bioactive compound. To obtain an enhanced vision into mechanistic action of the active extracts, an in-silico modelling approach was also adopted.
2 Materials and methods
2.1 Preparation of methanolic extract
Myrtus communis plants were procured in October 2019 from Alfath nursery (Al-Qassim region, Saudi Arabia). Leaves were brought in the lab in an airtight zip line sterile bag. Leaves were washed with running tap water to remove dust particles and then with 70% alcohol. Air dried under dark shade for 2–3 days. Dried leaves were subjected to traditional grinding using electrical grinder into fine powder.
Leaf powder (100 gm) was suspended in 500 ml methanol in a conical flask with intermittent shaking every 1 hr for 3 days at room temperature. The resultant supernatant was filtered through Whatman filter paper and collected in a round bottom flask and further subjected to concentration on a rotary evaporator under reduced pressure.
2.2 Bacterial strains
Chromobacterium violaceum ATCC 12472, E. coli ATCC 35218, Pseudomonas aeruginosa PAO1, S. marcescens ATCC 13,880 and A. baumanii ATCC BAA747 strains were used in this study. Bacterial strains from cryofreezer were routinely inoculated and sub-cultured in Luria Bertani (LB) broth for growth and incubation for 24 h at 37 0C according to manufacturer instructions, except Chromobacterium violaceum which was maintained at 30 0C.
2.3 Determination of MIC
Minimum inhibitory concentration is determined as the lowest concentration at which pant extract inhibits the growth of test pathogen. MIC was evaluated using TTC as color indicator. Plant extract with varying concentration (mg/ml) were inoculated in a microtiter plate along with test bacterial strain. Well without plant extract and only test pathogen was treated as positive control, whereas negative control had plant extract with no test pathogen. Plate was incubated for 24 h at 37 0C. 40 µl TTC (2 mg/ml) per well was added, after 20 min of incubation period intensity of the pink color determined the presence of active cells. The lowest concentration at which there’s no color change was observed as MIC of plant extract (Qais et al., 2019).
2.4 Quantification of violacein
Extraction and Quantification of violacein inhibition was carried out using C. violaceum ATCC 12472. This bacterial strain was grown overnight in the presence and absence of sub inhibitory concentration (ug/ml) of test agents in LB medium. 1 ml culture from overnight grown wells were centrifuged at 10,000 rpm for 10 mins to pellet out insoluble violacein with bacterial cells. Pellet was dissolved in 1 ml of Dimethyl sulphoxide (DMSO) and vortexed for 30 secs to extract violacein. Suspension was centrifuged at 10,000 rpm for 10 mins to extract violacein as a supernatant and pellet out bacterial cells. Absorbance of supernatant containing violacein was quantified spectrophotometrically at 585 nm (Qais et al., 2019).
2.5 Quantification of pyocyanin
P. aeruginosa overnight grown culture was incubated with varying concentration (µgmL−1) of test extract in LB medium for 24 hr. The incubated culture suspension was centrifuged at 9500 rpm for 15 min. Resultant supernatant was used to extract pyocyanin pigment using Chloroform and 0.2 N HCl. The intensity of the pink or deep red color was recorded at 520 nm (Qais et al., 2019).
2.6 Proteolytic activity
Proteolytic activity of P. aeruginosa was determined using azocasein degradation assay. 100 µl cell free supernatant was incubated with 1.5 % azocasein solution mixture containing 0.5 mM CaCl2 in 0.05 M Tris HCl for 30 mins at 37 °C. To terminate the reaction, 500 µl of TCAA was added and further centrifuged at 12000 rpm for 10 min. Optical density of the supernatant proteolytic activity was measured at 400 nm (Qais et al., 2019).
2.7 Elastase activity
Elastin Congo Red was used to determine elastolytic activity. 100 µl of treated and untreated P. aeruginosa culture supernatant free from cells was mixed with 900 µl of ECR buffer (5 mg/ml ECR in 100 ml Tris, 1 mM CaCl2, pH 7.5) and incubated in incubator shaker at 37 0C for 3 hr. 1 ml if sodium phosphate buffer was added to terminate the reaction and placed on ice for 30 min. The insoluble ECR was separated using centrifugation at 10,000 rpm for 10 min, OD was recorded at 495 nm to determine the activity (Qais et al., 2019).
2.8 Prodigiosin assay
Test Strains were cultured in LB medium with varying concentration of MCME for 24 h at 37 0C. Incubated cells were subjected to centrifugation at 12000 rpm for 10 min. resultant supernatant was discarded and prodigiosin pigment was extracted from the harvested cell pellet using 1 ml of acidified ethanol solution. Quantification of extracted prodigiosin was measured using UV–visible spectrophotometer at 534 nm (Qais et al., 2019).
2.9 EPS extraction and estimation
Effect of sub-MICs of MCME on EPS formation was determined as described previously (Maheshwari et al., 2019). Test pathogens were grown on a cover slip immersed in a microtitre plate seeded with and without MCME. Plates were incubated at 37 °C for 16 h. after incubation, glass coverslips were removed and washed with 0.5 ml of NaCl solution with 0.9% concentration. Equal volume of 5% phenol and concentrated 5 ml sulphuric acid was added to the suspension and incubated in dark for 1 h and later centrifuged at 10,000 rpm for 10 mins to measure the absorbance of supernatant at 490 nm. EPS was assayed by estimating sugars and quantified using standard curve of glucose.
2.10 Swarming motility assay
Overnight grown cultures of test pathogens were point inoculated on LB plates (0.5 % agar) supplemented with varying concentrations of MCME. Plates were incubated at appropriate temperature of 37 0C for 24 h in an upright position. Plates without MCME was considered as a control. Migration or Swarm was recorded using swarm fronts of bacterial cells (Qais et al., 2019).
2.11 Inhibition of biofilm formation
Assay was performed in a 96 well microtitre plate. Test organisms were grown overnight and seeded in 200 µl LB broth in microtitre plate along with varying concentration of MCME. Wells without extract was termed control. Plates were incubated at 37 °C for 18 h in a static condition. Hereafter incubation, excess of broth was washed thrice with sterile phosphate buffer and air dried at room temperature for 30 min. 0.3 % w/v of crystal violet dye was added to wells for 15–20 min to stain preformed biofilm. Excess unbound stain was washed off gently with distilled water. Attached dye to the biofilm was solubilized with ethanol/acetic acid. Absorbance was recorded at 585 nm (Qais et al., 2019).
2.12 Confocal laser scanning microscopy (CLSM) analysis of biofilm inhibition
For CLSM, overnight grown culture were adjusted to McFarland units using LB medium and seeded in a 6 well microtitre plate containing 150 µl LB broth. Cover slips of 1 cm2 were placed in the wells along with respective 1/2xMICs of MCME for 18 h for incubation at respective temperatures. Thereafter, cover slips were washed thrice with distilled water to remove excess of unbound cells and media. Biofilm formed on coverslip was stained with 0.1% acridine orange in dark for 10 min. excess stain was washed twice with distill water and air dried and stained cover slips were visualized under CLSM.
2.13 GC/Ms
MCME was analyzed for phytoconstituents using Perkin Elmer GC Autosystem XL and Turbomass as described previously (Husain et al., 2015). Quantitative data were obtained by the peak normalisation technique using integrated FID response.
2.14 In silico studies
2.14.1 Molecular docking
To obtain the detailed insight of the interaction of linalool with the proteins and enzymes involved in QS and biofilms development, molecular docking studies were conducted using AutoDock Vina (Trott and Olson, 2009). Different target proteins were selected for this study. CviR' is receptor protein of C. violaceum 12472, LasI is AHL Synthase of P. aeruginosa, and LasR is transcriptional activator of P. aeruginosa virulence factors. The crystal structure of the receptor molecules was downloaded from Protein Data Bank. Only monomers form of receptors were used for molecular docking. All water surrounding the crystal structure of receptors were deleted and non-polar hydrogen molecules were added. The Kollman charges were added to the receptor using MGL Tools-1.5.6 and coordinates were saved in pdbqt format. The structure of linalool was obtained from PubChem [CID: 6549]. The ligand was made flexible by detecting the rotatable bonds using MGL Tools-1.5.6. The grid size was made to occupy the entire receptor molecule. The analysis docking results were performed using PyMol 2.4, Discovery Studio, and LigPlot+.
2.14.2 Molecular dynamics simulation
Molecular dynamics simulation of the complexes with lowest binding energy was performed using gromacs 2018.1 with amber99sb-ildn force field. The topology of linalool was generated using AmberTools19. Proteins and their complexes were solvated in triclinic box using TIP3P water model. Overall charge of the structures were neutralized by adding the sodium or chlorine ions (Cui et al., 2015). Molecular dynamics simulation was carried out for 50 ns. For analysis of molecular dynamics simulation, root-mean-square fluctuation (RMSF), root mean square deviation (RMSD), and radius of gyration (Rg) were calculated their initial backbone structure. Secondary structure of protein and solvent accessible surface area (SASA) analysis was performed using gromacs utility. MM-PBSA analysis was used to calculate binding energy of linalool with proteins (Kumari et al., 2014).
2.15 Statistical analysis
All studies were performed in triplicate and the data obtained from experiments were presented as mean values and the difference between control and test were analyzed using Student’s t-test.
3 Results and discussion
3.1 Determination of MIC
Minimum inhibitory concentration (MIC) of the test extract was determined against all the uropathagens incorporated in the study with varying concentration (25 – 1600 µgmL−1). The MIC of the methanolic leaf extract of Myrtus communis (MCME) was observed to be 400 µgmL−1 for C. violaceum, E. coli and S. marcescens, 800 µgmL−1 for PAO1 and 1600 µgmL−1 for A. baumannii. Sub-MICs (MIC/2-MIC/16) of the plant extract were used for further biofilm inhibition and QSI experiments.
3.2 Violacein inhibition assay in C. Violaceum
In qualitative analysis of QSI potential, the extract exhibited profound inhibition of QS regulated production of violacein pigment in C. violaceum as evident from halo zone. Further, quantitative violacein inhibition assay, the extract at its sub-MIC (MIC/16- MIC/2) showed dose dependent decrease in the pigment production (Table 1). A maximum of 65% inhibition in the violacein production was recorded at maximum tested concentration of the extract (MIC/2, 200 µgmL−1). In the present study, for the first time we demonstrated the QS suppressive potential of methanolic leave extract of M. communis, an important ethnomedicinal plant of Arabian Peninsula for its ability to inhibit the QS controlled virulence expression in important uropathogens. Even though, QSI activity of M. communis essential oil has been reported earlier (Kerekes et al., 2013), the precise mechanism is not completely understood and moreover its virulence suppressive activity in uropathogens is not reported yet. Preliminary QSI potential of methanolic leave extract of the plant was studied using widely used QS biomarker strain C. violaceum 12472. The production of violacein pigment by CV 12,472 is under direct control of bacterial CviR QS system via C6-HSL signaling molecule, thus any reduction in the pigment production is direct indicative of impaired QS architecture. The quantitative and qualitative assessment of violacein pigment production in C. violaceum 12,472 clearly indicated that the QS inhibitory effect of the methanolic leave extract of M. communis is concentration dependent. A significant decrease of about 65% in violacein production by the M. communis extract recorded in the present study is comparable to the findings published by Husain et al., (Husain et al., 2017), who reported the inhibition in violacein pigment production by 83.6% by methanol fraction of Mangifera indica.
Extract concentration (µgmL−1)
Violacein (OD @ 585 nm)
Log CFUmL−1 at 10-5
Control
0.437 ± 0.028
6.44 ± 0.4
25
0.359 ± 0.036*
6.44 ± 0.6
50
0.301 ± 0.029*
6.41 ± 0.2
100
0.221 ± 0.031**
6.39 ± 0.5
200
0.149 ± 0.039**
6.36 ± 0.6
3.3 Inhibition of QS controlled virulence in PAO1
Four virulence factors in PAO1 (Elastase, total protease, pyocyanin and chitinase) which are under direct or indirect control of QS system were assed to analyze the effect of M. communis extract on the virulence. Concentration dependent significant reduction in the production of virulence factors of PAO1 was recorded when treated with sub-MICs of MCME. As shown in the Fig. 1A, the extract clearly decreased the elastase activity at sub-MICs ranging from 50 to 400 µgmL−1 (MIC/16-MIC/2), with highest inhibition 74% was recorded at 400 µg/ml when compare to untreated PAO1 supernatant. The extract significantly reduced the proteolytic lytic (total protease) activity at the tested concentrations (Fig. 1A). At 50 µgmL−1, 28% inhibition of was observed while approximately 77% inhibition of total proteolytic activity was evident at 400 µgmL−1. Pyocyanin production by PAO1 was also found to be inhibited significantly with increasing sub-MICs of MCME (Fig. 1A). Suppression in pyocyanin production was found to be almost 78% when treated with 400 µgmL−1 of the extract when compare to untreated set. Chitinase production was also evaluated in the presence of M. communis extract and was found to be inhibited significantly at higher sub-MICs (Fig. 1A). This present study also revealed remarkable reduction in QS controlled virulence determinants of Pseudomonas aeruginosa PAO1 such as elastase, total protease, chitinase and pyocyanin pigment production when treated with sub-MIC of M. communis extract. It was observed that elastolytic activity of the bacterium was reduced in a dose dependent manner (Fig. 1A). Since elastase is believed to cause tissue damage to host during infection, inhibition of elastase would possibly decrease the severity of UTIs. Previously, Green tea extract have been shown to reduce the elastase activity of PAO1 at sub-MIC levels (Qais et al., 2019). As evident from Fig. 1A, another secretory virulence factor namely protease was also found to be reduced significantly with M. communis extract treatment. Total protease is not only involved in proteolytic activity facilitating overall pathogenesis but also has been found to support bacterial motility that is crucial for urinary tract colonization. Our findings corroborated well with that of Alyousef et al. (Al-Yousef et al., 2017), who observed significant reduction in total protease production in P. aeruginosa when treated with onion peel extracts, without affecting the growth of bacterium. Present study also demonstrates the efficacy of M. communis extract against chitinase secretion by PAO1 (Fig. 1A), another density dependent virulence phenotype that has been often implicated with pathogenicity of the bacterium. Previously, extract of Mangifera indica was shown to inhibit chitinase production in PAO1 (Husain et al., 2017). Pyocyanin pigment production by P. aeruginosa has been found to be reduce urothelial cell viability and which in turn may hamper normal wound healing during chronic UTIs (Newman et al., 2017). Here in, we found significant inhibition in pyocyanin pigment production upon the treatment with sub-lethal doses of the M. communis extract (Fig. 1A) which may be crucial in treating UTIs. Compared with previous studies, methanol fraction of Psoralea corylifolia decreased the production of pyocyanin upto 58% displaying anti QS potential of the active fraction (Husain et al., 2018).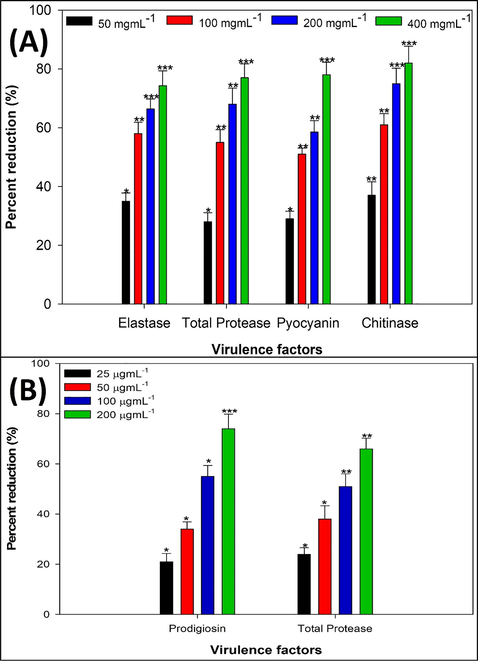
(A) Effect of sub-MICs of MCME on QS regulated virulence functions P. aeruginosa PAO1. * denotes significance at p ≤ 0.05, ** denotes significance at p ≤ 0.005, and *** denotes significance at p ≤ 0.001. (B) Effect of sub-MICs of MCME on prodigiosin and protease production in S. marcescens ATCC 13880. * denotes significance at p ≤ 0.05, ** denotes significance at p ≤ 0.005, and *** denotes significance at p ≤ 0.001.
3.4 Inhibition of QS controlled virulence in S. Marcescens
Prodigiosin and proteases production by S. marcescens are considered as QS dependent characteristic of the bacterium. These virulence determinants were assessed in presence and absence of sub-MICs of the MCME. As expected, prodigiosin production was found to be suppressed by 21–73% in the supernatant treated with the different sub-MICs of the extract (25–200 µgmL−1) as depicted in Fig. 1B. Similar effect of the MCME was observed on the proteolytic activity of the bacterium (S. marcescens). At highest tested sub-MIC (MIC/2), a reduction of almost 66% in protease production was evident (Fig. 1B). Previous reports have already shown that QS mediated virulence factors in S. marcescens play important role in its pathogenesis (Rice et al., 2005). The present study, revealed inhibitory effect of M. communis extract on QS controlled prodigiosin pigment production in S. marcescens at sub-MICs (Fig. 1B). The finding goes well with the results of Srinivasan et al. (Srinivasan et al., 2016) who reported extract of Piper betel inhibited the prodigiosin production upto 85% by attenuating QS system of S. marcescens. In addition, S. marcescens produce various secreted virulence factors such as proteases, that contributes in initiation of host immune response and overall pathogenicity of the bacterium. Considerable reduction in proteases production was observed after treatment with sub-MICs of M. communis extract (Fig. 1B), and the observed results are in accordance with findings of Salini and Pandian (Salini and Pandian, 2015), who reported significant inhibition in protease production upon treatment with Anethum graveolens extract. Therefore, inhibition in these virulence factors after subsequent treatment with MCME may be considered as a direct indicative of the anti-uropathogenic potential of the extract via modulation in cell to cell communication system of the targeted bacteria.
3.5 Effect of MCME on EPS production
Biofilm architecture and strength is positively correlated with EPS production by uropathogens thus M. communis treated and untreated cultures were subjected to EPS quantification using spectrometric method. The extract with increasing concentration (MIC/16-MIC/2) decreased the production of EPS by the test uropathogens. The extract at highest tested concentration (MIC/2) exhibited 63%, 76%, 75% and 66% decrease in EPS production in P. aeruginosa, E. coli, A. baumannii and S. marcescens respectively when compared to untreated controls (Fig. 2A). Pathogenic biofilms have also been characterized with extensive EPS secretions, which provide overall structural integrity to biofilms enabling the uropathogens to become more persistent and also act as protective barriers against antibiotics obstructing its entry into sessile bacterial cells (Flemming et al., 2007). It has been well demonstrated that biofilm linked EPS production is under control of bacterial QS mechanism, if not entirely, at least up to the noticeable degree. Therefore, an impaired EPS secretion will lead to structurally fragile and antibiotic sensitive biofilm and that in turn facilitate the eradication of UTIs. Herein, in this present study we observed that the extract of M. communis significantly inhibited the production of EPS in the uropathogens at its sub-MIC (Fig. 2A). Srinivasan et al., (Srinivasan et al., 2016) observed significant decrease production (64%) of EPS by Vibro harveyi upon the treatment with Piper betle extract at the concentration of 400 µgmL−1. Thus, it can be apprehended that fragile biofilm formation with reduce EPS production under the effect of M. communis could possibly lead to increase in sensitivity of sessile uropathogenic bacterial cells towards antibiotics.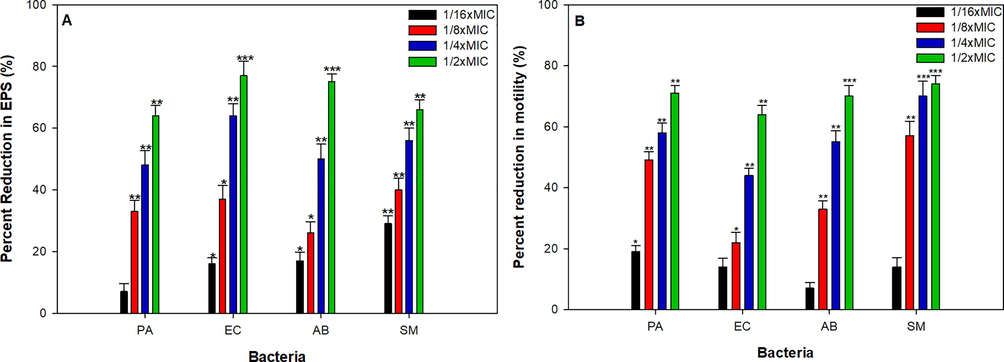
Effect of sub-MICs of MCME on (A). EPS production (B). swarming motility in test bacteria. * denotes significance at p ≤ 0.05, ** denotes significance at p ≤ 0.005, and *** denotes significance at p ≤ 0.001.
3.6 Effect of MCME on motility
Swarming motility patterns of the test uropathogens under the effect of increasing concentration of the plant extract revealed dose dependent decrease in overall swarming diameter (Fig. 2B). The maximum inhibition was observed at MIC/2 of the plant extract with swarming diameter value corresponding to 12 ± 1.4 mm (70%), 13 ± 2.9 mm (63%), 8 ± 1.2 mm (70%) and 13 ± 1.2 mm (74%) of P. aeruginosa, E. coli, A. baumannii and S. marcescens respectively (Fig. 2B). Flagellar mediated distinct upward movement (swarming motility) along the urinary tract by uropathogens may results in some very serious infections forms. This flagellar mediated swarming motility requires differentiation of normal bacterial cells into swarm cell morphology and is apparently controlled by QS mechanism in many of uropathogens (Köhler et al., 2000). From Fig. 2B it is evident that amendment of M. communis extract in swarm agar results in poor swarming motility in all the tested uropathogens. The reduced swarming motility could be explained perhaps by two possible underlying mechanism, either the phytoconstituent present in the extract of M. communis interfered with cell differentiation process, as proposed previously by Packivathy et al., (Packiavathy et al., 2014), for curcumin or by directly acting on the receptors involves in QS system, refraining the binding of signal molecules as in the case of quercetin rich extract of Allium cepa (Quecan et al., 2019). Swarming motility has also been positively correlated with biofilm formation, here in the present study our results goes well with findings of Overhage et al., (Overhage et al., 2007), who showed that mutant bacterial cells with deleted swarming genes showed impairment in biofilm formation. Moreover, along with reduction of overall swarm diameter, we also observed altered spacing of the radiating dendrites in the presence of higher concentration of M. communis extract, which may lead to formation structurally fragile biofilm and could have critical implications on treatment of urinary infections. Lakshmanan et al., (Lakshmanan et al., 2020) observed that treatment of compound isolated from Alpinia officinarium results in altered spacing and loosely packed dendrite cells of Pseudomonas aeruginosa when compared with untreated actively swarming cells.
3.7 Effect of MCME on biofilm formation
Biofilm quantification assay using microtiter plate method shown decrease in biofilm forming ability of test uropathogens in a concentration dependent manner when treated with the methanolic extract of M. communis. The extract exhibited 16–74%, 31–84%, 12–66% and 19–71% inhibition of biofilm biomass of P. aeruginosa, E. coli, A. baumannii and S. marcescens respectively at the increasing concentration corresponding to MIC/16-MIC/2 (Fig. 3A). Autoinducer based QS system influence the formation and architectural integrity of bacterial biofilms which in turn play important role in pathogenesis of UTI causing bacterial pathogens (Anderson, 2003). Therefore, an impaired QS system would possibly led to reduced biofilm formation and hence affect over all pathogenicity of the uropathogens. In the present study, the exposure to MCME at MIC/2 concentration resulted in significant reduction in biofilm biomass (Fig. 3A) of test bacterial uropathogens such as PAO1, E. coli, A. baumannii and S. marcescens. The untreated control of all test bacteria showed a dense mat like structure of biofilm on glass coverslip’s surface as visualized under light microscope (Fig. 3B). Treatment with respective sub-MIC remarkably decreased the formation aggerate like structure of bacterial cells. Consistent with present study, previously published report of (Packiavathy et al., 2012) demonstrated antibiofilm activity of crude extract of Cuminum cyminum against uropathogens including PAO1, Proteus mirabilis and S. marcescens without influencing the growth planktonic growth. In another study, methanolic extracts of three plants belonging to Myrtaceae family viz. Syzygium masukuense, Syzygium species A and Eugenia natalita (2 mg/ml) has been shown to inhibit the initial cell attachment and biofilm formation by gram negative nosocomial pathogens such as E. coli, P. aeruginosa and Salmonella typhimurium (Famuyide et al., 2019).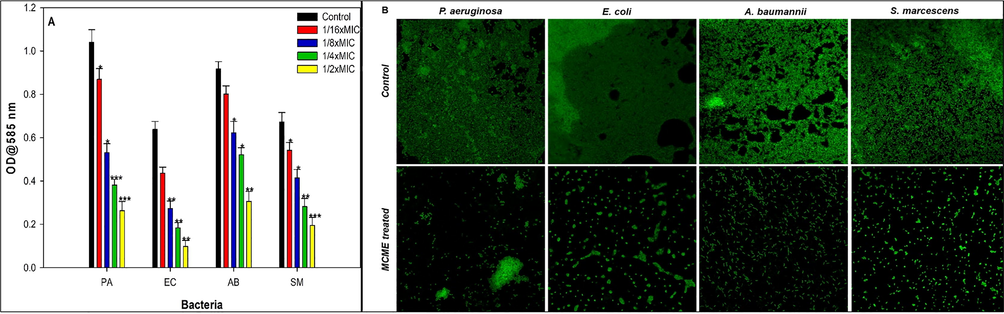
(A) Effect of sub-MICs of MCME on the biofilm formation in test bacteria. Data are represented as mean values of triplicate readings and bar is standard deviation. * denotes significance at p ≤ 0.05, ** denotes significance at p ≤ 0.005 and *** denotes significance at p ≤ 0.001. (B). Confocal laser scanning microscopic images of biofilm formation in the absence and presence of 1/2xMICs of MCME.
3.8 Gas chromatographic analysis
GC–MS analysis revealed presence of 23 different phytocompounds in the extract of M. communis as depicted in the Table 2. Comparing the percent peak area in the chromatogram, different major compounds was detected viz, linalool, 1,8-cineole, pyragallol etc (Table 2).
Peak No.
Components
Retention time
Percent Area (%)
1.
1,8-Cineole
12.13
15.420
2.
2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one
15.37
1.430
3.
Linalyl propionate
16.99
2.720
4.
2-Furancarboxaldehyde
17.74
1.210
5.
1,2,3-Propanetriol
18.19
1.590
6.
3,7-Dimethyl-1,6-octadien-3-ol/Linalool
18.51
6.520
7.
N,N'-Diethylidene-1,1-diaminoethane
18.78
2.920
8.
(-)-Myrtenyl acetate
20.53
4.980
9.
α.-Terpinenyl acetate
21.15
1.970
10.
1,2,3-Benzenetriol/pyrogallol
21.76
7.480
11.
1,6-Octadiene-3-ol,4,7-dimethyl-isovalerate
21.94
2.730
12.
α.-Humulene
24.01
1.310
13.
Diallyl ester of oxalic acid
26.49
11.470
14.
N-Acetyl-(S)-(-)-proline
30.91
2.160
15.
5,5-Dimethyloct-7-en-2-one
31.27
1.400
16.
Hexadecanoic acid
36.73
2.910
17.
1-(2,4,6-Trihydro)-1-Propanone4,4′-bis(methoxycarbonyl)
37.10
6.240
18.
Hydrobenzoin diacetate
38.87
1.09
19.
Linalyl formate
43.42
2.660
20.
Nerolidol
43.99
2.120
21.
Stigmast-7-en-6-one
52.58
2.600
22.
Vitamin E
54.74
1.230
23.
Clionasterol
57.12
2.480
3.9 QS inhibitory activity of linalool
Major phytocompounds linalool, 1,8-cineole and pyragallol were screened for their QS inhibitory activity. Linalool, 1,8-cineole and pyragallol demonstrated varying levels of pigment inhibition in the test biosensor strain but pigment inhibition by 1,8-cineole and pyragallol was accompanied by zone of growth inhibition while linalool inhibited violacein (pigment) only (data not shown). On the basis of the initial screening linalool was selected for further assays. Linalool, a naturally occurring terpene alcohol was found to be the major phytocompound of the MCME as revealed by GC–MS analysis, the compound was also evaluated for the violacein pigment inhibitory activity in C. violaceum 12472. Linalool at its sub-MICs, (6.25–50 µgmL−1) significantly inhibited the violacein production when compared untreated control (Fig. 4A), violacein inhibition was recorded to a maximum of 69% when treated with linalool at 50 µgmL−1 (Fig. 4A). Results obtained for inhibition in pigment production indicates the major role of linalool in the overall activity of the M. communis extract. The dose dependent QS inhibitory effect of linalool obtained in the present study (Fig. 4A) is consistent with the observation of Kerekes et al., (Kerekes et al., 2013), who reported similar dose dependent QS inhibitory activity of linalool in C. violaceum 6269. Our findings are also in agreement with those reported with the sub-MICs of eugenol in against two biosensor strains of C. violaceum (Al-Shabib et al., 2017). Furthermore, no significant reduction in growth pattern of bacterial population was observed when compared to untreated control (Fig. 4B).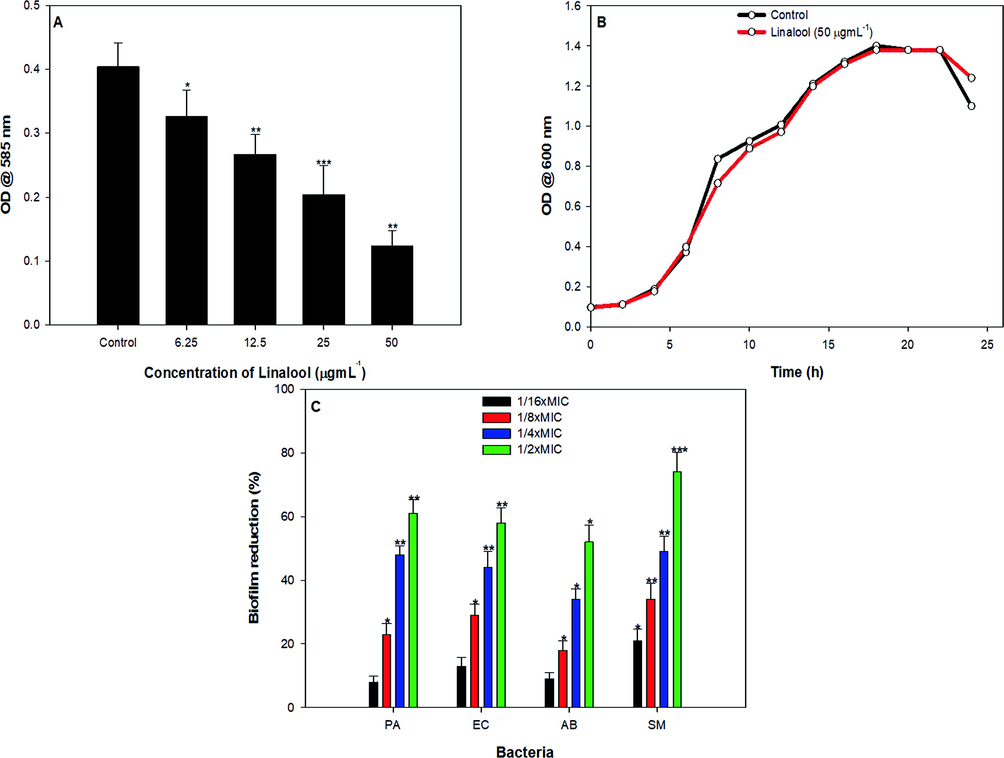
Effect of sub-MICs of linalool on (A). violacein production (B). growth and (C). biofilm formation. * denotes significance at p ≤ 0.05, ** denotes significance at p ≤ 0.005 and *** denotes significance at p ≤ 0.001.
3.10 Biofilm inhibition by linalool
Linalool was further assessed for its ability to interfere with the biofilm formation in test pathogens. Sub-MICs of linalool demonstrated varying levels of reduction in the biofilm formation in all the test bacteria in a concentration dependent manner (Fig. 4C). Linalool impaired biofilm formation by 8–61%, 13–58%, 9–52% and 21–74% in P. aeruginosa, E. coli, A. baumannii and S. marcescens, respectively. Thus, linalool exhibited broad-spectrum biofilm inhibitory activity at tested sub-MICs. Our findings are in accordance with the reports published on bakuchiol, menthol, phytol etc, wherein significant biofilm inhibition was reported in pathogenic bacteria upon treatment with sub-MICs of these phytocompounds (Husain et al., 2018, 2017; Srinivasan et al., 2016).
3.11 In silico analysis
3.11.1 Molecular docking studies
The possible interference of the antivirulence and antibiofilm potential of linalool was studied by molecular docking. First, the methodology was validated by extracting and redocking the natural ligand of LasR. The ligand was docked in same binding pocked as it was present in the crystal structure. Binding energy and binding constant of linalool with test proteins is enlisted in Table 3.
S. No.
PDB ID
Name
Binding energy (kcal mol−1)
Binding constant (M−1)
1.
1RO5
LasI
−5.8
1.79 × 104
2.
2UV0
LasR
−6.1
2.97 × 104
3.
3QP1
CviR′
−6.7
8.20 × 104
CviR' is a receptor of C. violaceum 12,472 which senses the 3-hydroxy-C10-AHL and the binding of this signal molecule to CviR' activates the expression of QS-controlled genes. The two domain (i.e. ligand-binding domain and DNA binding domain) of CviR' are joined by a short flexible random coil (Ahmed et al., 2013; Qais et al., 2019). CviR is a receptor of C. violaceum ATCC 31,532 that senses C6-AHL and CviR' exhibits 87% sequence identity with CviR. The acyl group and lactone carbonyl group of AHL makes hydrogen bonds with Asp97 and Trp84 respectively, while the carbonyl oxygen of C6-AHL interacts with Tyr80 and Ser155 of CviR via two hydrogen bonds (Ahmed et al., 2013). It was interesting to note that linalool interacted in same binding cavity where its antagonist (chlorolactone) binds with binding energy as −6.7 kcal mol−1. Linalool formed hydrogen bond with Tyr80 of CviR' with bond length of 1.95 Å as shown in Fig. 5A. Asp97 and Ser155 were involved in van der Waals interactions. The linalool-CviR' was also stabilized by hydrophobic interactions that included Leu57, Val75, Trp84, Leu85, Tyr87, Ile99, Leu100, Trp111, Phe115, Phe126, and Met135 of CviR'. The blocking of CviR or CviR' have been proposed as a novel strategy to antagonize the transcription factor and inhibit the expression of QS-mediated virulence genes. The results show that binding of linalool at the active site of CviR' may be responsible for the antagonization of expression of QS linked traits.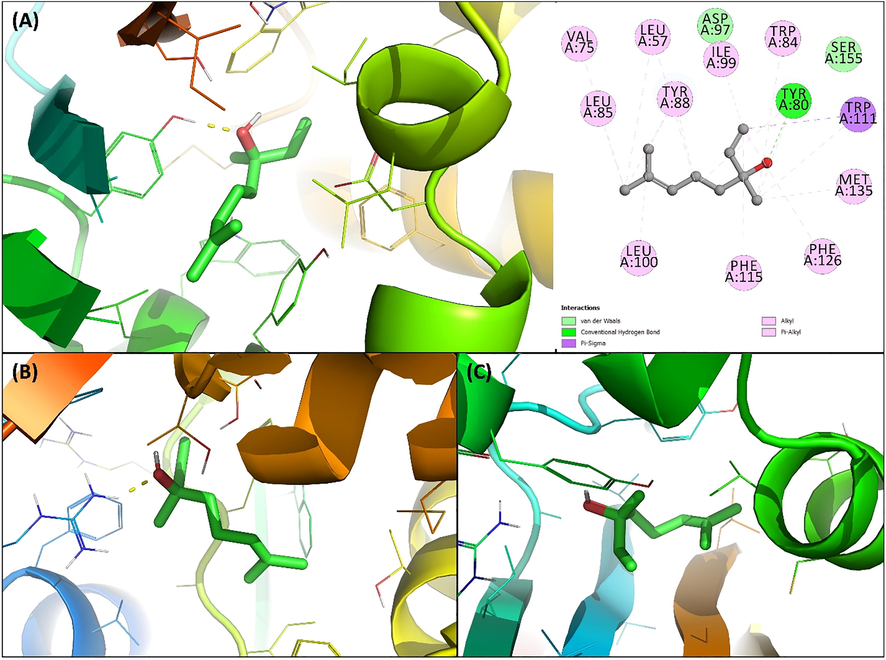
(A) Docked pose of coumarin to CviR′ complexed with linalool. (B) Docked pose of coumarin to LasI complexed with linalool. (D) Docked pose of coumarin to LasR complexed with linalool.
LasI is the signal molecule synthase protein that synthesizes 3-oxo-C12-HSL in P. aeruginosa. It has been found that N-terminal residues of LasI such as Phe27, Arg30, and Trp33 of LasI are important for S-adenosyl methionine (SAM) binding pocket formation and Phe105 of LasI is the conserved residue for binding of acyl-chain of the signal molecule [57]. The docked conformation with lowest binding energy (-5.8 kcal mol−1) is shown in Fig. 5B. Arg30 of LasI formed hydrogen bond with linalool while Thr121 and Thr145 interacted via van der Waals forces. Phe27, Trp33, Trp69, Phe105, Ile107, Phe117, and Val148 were involved in hydrophobic interactions. The positive interaction of linalool with AHL synthase of P. aeruginosa indicates that this interaction might be hindering the synthesis of the autoinducer and ultimately QS.
LasR is the transcriptional activator of the virulent genes of P. aeruginosa (Kiratisin et al., 2002). The binding energy for the interaction of linalool with LasR was found to be −6.1 kcal mol−1 and the docked pose is shown in Fig. 5C. Linalool was found to interact in the same binding cavity where the natural ligand (3-oxo-C12-acylhomoserine lactone) of LasR binds. Linalool interacted with Gly38, Ile52, Tyr64, and Thr80 of LasR via van der Waals forces. Moreover, many residues of LasR such as Leu36, Leu40, Tyr47, Ala50, Ala70, Cys79, Leu125, Ala127, and Val76 were involved in hydrophobic interactions. The binding of 3-oxo-C12-AHL to LasR activates the transcription of many virulent genes of P. aeruginosa (Packiavathy et al., 2012). The docking results suggested that binding of linalool might compete against 3-oxo-C12-AHL to interact with LasR and ultimately reduce the expression QS-controlled genes.
3.11.2 Molecular dynamics simulations
The molecular docked complexes with lowest binding energy were selected as initial conformations for molecular dynamics simulations. The RMSD the proteins and their complexes with linalool were calculated respect to their initial backbone structure and the results obtained is presented in Fig. 6A (Cui et al., 2015). The average RMSD of LasI and LasI-linalool complex was found to be 0.262 ± 064 and 0.265 ± 0.032 nm, respectively. Similarly, RMSD of LasR and CviR′ in absence and presence of linalool were found below 0.25 nm. For all simulations, the RMSD did not fluctuated much was below 0.3 nm showing the stability of the system. The RMSF of the proteins and their complexes with linalool were also calculated respect to their initial backbone structure as shown in Fig. 6B. A similar fluctuation in the restudies of the test proteins (LasI, LasR, and CviR′) was found in the presence of linalool. The RMSF of all atoms of linalool were calculated (Fig. 6C). The atoms of linalool showed some fluctuations indicating a dynamical shift from their respective initial positions. This shift may cause different interaction modes with nearby residues as simulation progressed. Such fluctuations shifts the between hydrogen bonds and hydrophobic interactions taking place between ligand and receptor (Siddiqui et al., 2019). The radius of gyration (Rg) of the proteins alone and their complex with linalool was calculated data is presented as function of time Fig. 6D. The Rg of test proteins and did not showed any significant change in presence of linalool suggesting that proteins did not undergo major conformational changes by the interaction (Khan et al., 2020). The stability of all the proteins was further assessed by calculating changes in solvent accessible surface area (SASA). The results showed that SASA of the proteins and their complexes was negligibly altered throughout simulation duration Fig. 6E. These calculations validate the stability of the complexes under solvent system.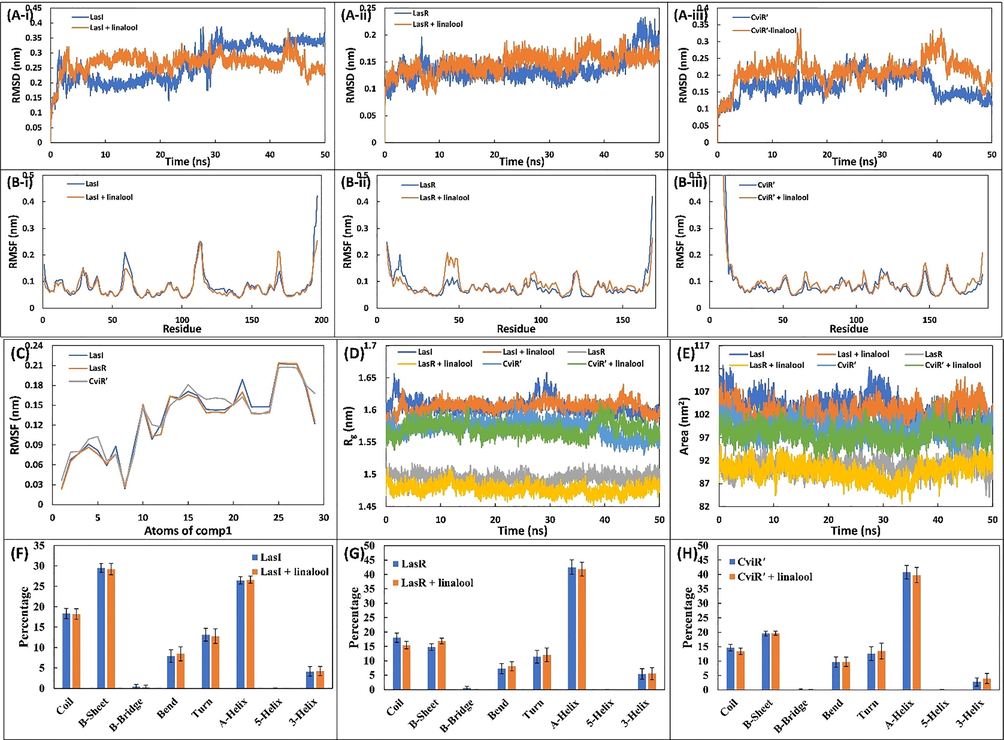
(A-i) The RMSD of backbone of LasI and LasI-linalool complex. (A-ii) The RMSD of backbone of LasR and LasR-linalool complex. (A-iii) The RMSD of backbone of CviR’ and CviR’-linalool complex. (B-i) The RMSF of backbone of LasI and LasI-linalool complex. (B-ii) The RMSF of backbone of LasR and LasR-linalool complex. (B-iii) The RMSF of backbone of CviR’ and CviR’-linalool complex. (C) The average RMSF value of each atom of linalool during the MD simulation. (D) Variation in the radius of gyration (Rg) of proteins in the absence and presence of linalool. (E) Solvent accessible surface area (SASA) of the proteins in the absence and presence of linalool. (F) Percentage of secondary structure in LasI in the absence and presence of linalool. (G) Percentage of secondary structure in LasR in the absence and presence of linalool. (H) Percentage of secondary structure in CviR’ in the absence and presence of linalool.
To binding energy for all complexes were calculated from 100 snapshots of the simulation using MM-PBSA methods and the results obtained are presented in Table 4. The non-covalent interactions responsible typical drug-protein interactions are hydrogen bond, electrostatic forces, hydrophobic interactions, polar interactions (Siddiqui et al., 2019). These interactions either favours or hinders the overall binding energy. In this study, mainly electrostatic and van der Waals forces contributed for positive interactions. A small contribution of SASA energy was found. However, polar solvation energy impaired the interaction of linalool with all test proteins. The average binding energy for the interaction of linalool with LasI, LasR, and CviR' were found to be −10.43 ± 4.18, −13.77 ± 2.71, and −13.02 ± 2.79 kcal mol−1. The effect of binding of linalool to test protein on their secondary structure were studied by calculating the secondary structure components and the data obtained is shown in Fig. 6(F-H). The amount of α-helix in LasI and LasI-linalool complex was found to be 26.46 and 26.62%, respectively. A similar negligible change on other secondary motifs of LasI was observed. Likewise, there was negligible alterations in the secondary structure of LasR, and CviR′ after interaction with linalool. The data validate that linalool did not altered the secondary structure of the test proteins. ΔEvdW: van der Waal energy, ΔEele: Electrostatic energy, ΔEPSE: Polar solvation energy, ΔESASA: Solvent accessible surface area energy, ΔEBE: Binding energy.
Energy
Proteins
LasI
LasR
CviR′
ΔEvdW
−14.89 ± 2.29
−21.99 ± 1.83
−20.64 ± 1.84
ΔEele
−4.36 ± 4.56
−3.56 ± 2.72
−11.94 ± 2.31
ΔEPSE
11.31 ± 4.28
14.77 ± 3.42
22.49 ± 2.70
ΔESSASA
−2.48 ± 0.25
−2.99 ± 0.14
−2.93 ± 0.15
ΔEBE
−10.43 ± 4.18
−13.77 ± 2.71
−13.02 ± 2.79
4 Conclusions
M. communis is a plant of immense ethnomedicinal importance and has been used in traditional medicine for curing various ailments. This investigation appends an additional note on its QS and biofilm inhibitory property against uropathogenic bacteria. Current study demonstrates that MCME could inhibit QS regulated virulence in C. violaceum (violacein), P. aeruginosa (elastase, protease, pyocyanin and chitinase) and S. marcescens (prodigiosin and protease). Further, sub-MICs of MCME could interfere with the formation of biofilm and inhibited EPS production and swarming motility in uropathogens (P. aeruginosa, S. marcescens, E. coli and A. baumanii). The results of GC–MS analysis showed linalool as one of the major phytoconstituents of MCME. Subsequently, linalool demonstrated inhibition of QS regulated violacein production in C. violaceum and broad-spectrum reduction in the biofilm forming capabilities of test uropathogens. Anti-QS and antibiofilm potential of linalool was also confirmed using in silico molecular docking and simulation studies. Thus, from the obtained results it is envisaged that M. communis and its bioactive compound linalool may have medicinal implications and could prove effective therapeutic agent against uropathogenic bacteria.
Acknowledgements
The authors extend their appreciation and acknowledge to the Deanship of Scientific Research, College of Applied Medical Sciences at King Saud University, Riyadh, Kingdom of Saudi Arabia for the financial support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- In Silico Investigation of Lactone and Thiolactone Inhibitors in Bacterial Quorum Sensing Using Molecular Modeling. Int. J. Chem.. 2013;5
- [CrossRef] [Google Scholar]
- Eugenol inhibits quorum sensing and biofilm of toxigenic MRSA strains isolated from food handlers employed in Saudi Arabia. Biotechnol. Biotechnol. Equip.. 2017;31(2):387-396.
- [CrossRef] [Google Scholar]
- Onion Peel Ethylacetate Fraction and Its Derived Constituent Quercetin 4′-O-β-D Glucopyranoside Attenuates Quorum Sensing Regulated Virulence and Biofilm Formation. Front. Microbiol.. 2017;8:1675.
- [CrossRef] [Google Scholar]
- Control of Biofilm Formation: Antibiotics and Beyond. Appl. Environ. Microbiol.. 2017;83(3)
- [CrossRef] [Google Scholar]
- Intracellular Bacterial Biofilm-Like Pods in Urinary Tract Infections. Science (80-.. 2003;301(5629):105-107.
- [CrossRef] [Google Scholar]
- Rhamnolipids Modulate Swarming Motility Patterns of Pseudomonas aeruginosa. J. Bacteriol.. 2005;187(21):7351-7361.
- [CrossRef] [Google Scholar]
- Investigate the Binding of Catechins to Trypsin Using Docking and Molecular Dynamics Simulation. PLoS One. 2015;10(5):e0125848.
- [CrossRef] [Google Scholar]
- Antibacterial and antibiofilm activity of acetone leaf extracts of nine under-investigated south African Eugenia and Syzygium (Myrtaceae) species and their selectivity indices. BMC Complement. Altern. Med.. 2019;19:141.
- [CrossRef] [Google Scholar]
- The EPS matrix: The “House of Biofilm Cells”. J. Bacteriol.. 2007;189(22):7945-7947.
- [CrossRef] [Google Scholar]
- Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol.. 2015;13(5):269-284.
- [CrossRef] [Google Scholar]
- The negative impact of antibiotic resistance. Clin. Microbiol. Infect.. 2016;22(5):416-422.
- [CrossRef] [Google Scholar]
- Leaf extracts of Mangifera indica L. inhibit quorum sensing - Regulated production of virulence factors and biofilm in test bacteria. Front. Microbiol.. 2017;8
- [CrossRef] [Google Scholar]
- Seed Extract of Psoralea corylifolia and Its Constituent Bakuchiol Impairs AHL-Based Quorum Sensing and Biofilm Formation in Food- and Human-Related Pathogens. Front. Cell. Infect. Microbiol.. 2018;8:351.
- [CrossRef] [Google Scholar]
- Trigonella foenum-graceum (Seed) Extract Interferes with Quorum Sensing Regulated Traits and Biofilm Formation in the Strains of Pseudomonas aeruginosa and Aeromonas hydrophila. Evidence-Based Complement. Altern. Med.. 2015;2015:1-10.
- [CrossRef] [Google Scholar]
- Ultrastructure of Proteus mirabilis Swarmer Cell Rafts and Role of Swarming in Catheter-Associated Urinary Tract Infection. Infect. Immun.. 2004;72(7):3941-3950.
- [CrossRef] [Google Scholar]
- Anti-biofilm forming and anti-quorum sensing activity of selected essential oils and their main components on food-related micro-organisms. J. Appl. Microbiol.. 2013;115(4):933-942.
- [CrossRef] [Google Scholar]
- Biotechnological Applications of Quorum Sensing Inhibitors. Singapore: Springer Singapore; 2018. p. :417-445.
- [CrossRef]
- Mechanistic inhibition of non-enzymatic glycation and aldose reductase activity by naringenin: Binding, enzyme kinetics and molecular docking analysis. Int. J. Biol. Macromol.. 2020;159:87-97.
- [CrossRef] [Google Scholar]
- LasR, a Transcriptional Activator of Pseudomonas aeruginosa Virulence Genes, Functions as a Multimer. J. Bacteriol.. 2002;184(17):4912-4919.
- [CrossRef] [Google Scholar]
- Swarming of Pseudomonas aeruginosa Is Dependent on Cell-to-Cell Signaling and Requires Flagella and Pili. J. Bacteriol.. 2000;182(21):5990-5996.
- [CrossRef] [Google Scholar]
- g_mmpbsa —A GROMACS Tool for High-Throughput MM-PBSA Calculations. J. Chem. Inf. Model.. 2014;54(7):1951-1962.
- [CrossRef] [Google Scholar]
- A compound isolated from Alpinia officinarum Hance. inhibits swarming motility of Pseudomonas aeruginosa and down regulates virulence genes. J. Appl. Microbiol.. 2020;128(5):1355-1365.
- [CrossRef] [Google Scholar]
- Role of Motility in the Colonization of Uropathogenic Escherichia coli in the Urinary Tract. Infect. Immun.. 2005;73(11):7644-7656.
- [CrossRef] [Google Scholar]
- Biofilm-specific antibiotic resistance. Future Microbiol.. 2012;7(9):1061-1072.
- [CrossRef] [Google Scholar]
- Bioactive extracts of Carum copticum and thymol inhibit biofilm development by multidrug-resistant extended spectrum β-lactamase producing enteric bacteria. Biofouling. 2019;35(9):1026-1039.
- [CrossRef] [Google Scholar]
- The contribution of Pseudomonas aeruginosa virulence factors and host factors in the establishment of urinary tract infections. FEMS Microbiol. Lett.. 2017;364
- [CrossRef] [Google Scholar]
- Identification of Genes Involved in Swarming Motility Using a Pseudomonas aeruginosa PAO1 Mini-Tn5-lux Mutant Library. J. Bacteriol.. 2007;189(5):2164-2169.
- [CrossRef] [Google Scholar]
- Antibiofilm and quorum sensing inhibitory potential of Cuminum cyminum and its secondary metabolite methyl eugenol against Gram negative bacterial pathogens. Food Res. Int.. 2012;45(1):85-92.
- [CrossRef] [Google Scholar]
- Inhibition of biofilm development of uropathogens by curcumin – An anti-quorum sensing agent from Curcuma longa. Food Chem.. 2014;148:453-460.
- [CrossRef] [Google Scholar]
- Historical Perspective of Traditional Indigenous Medical Practices: The Current Renaissance and Conservation of Herbal Resources. Evidence-Based Complement. Altern. Med.. 2014;2014:1-20.
- [CrossRef] [Google Scholar]
- Broad-spectrum quorum sensing and biofilm inhibition by green tea against gram-negative pathogenic bacteria: Deciphering the role of phytocompounds through molecular modelling. Microb. Pathog.. 2019;126:379-392.
- [CrossRef] [Google Scholar]
- Effect of Quercetin Rich Onion Extracts on Bacterial Quorum Sensing. Front. Microbiol.. 2019;10:867.
- [CrossRef] [Google Scholar]
- Biofilm Formation and Sloughing in Serratia marcescens Are Controlled by Quorum Sensing and Nutrient Cues. J. Bacteriol.. 2005;187(10):3477-3485.
- [CrossRef] [Google Scholar]
- The etiology of urinary tract infection: Traditional and emerging pathogens. Disease-a-Month. 2003;49(2):71-82.
- [CrossRef] [Google Scholar]
- Interference of quorum sensing in urinary pathogen Serratia marcescens by Anethum graveolens. Pathog. Dis.. 2015;73:ftv038.
- [CrossRef] [Google Scholar]
- A comprehensive spectroscopic and computational investigation on the binding of the anti-asthmatic drug triamcinolone with serum albumin. New J. Chem.. 2019;43(10):4137-4151.
- [CrossRef] [Google Scholar]
- Ethnobotanical, Ethnopharmacological, and Phytochemical Studies of Myrtus communis Linn: A Popular Herb in Unani System of Medicine. J. Evid. Based. Complementary Altern. Med.. 2017;22(4):1035-1043.
- [CrossRef] [Google Scholar]
- Piper betle and its bioactive metabolite phytol mitigates quorum sensing mediated virulence factors and biofilm of nosocomial pathogen Serratia marcescens in vitro. J. Ethnopharmacol.. 2016;193:592-603.
- [CrossRef] [Google Scholar]
- AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem.. 2009;31:455-461.
- [CrossRef] [Google Scholar]







