Translate this page into:
Multivariate diversity analysis and systematics of hemipteran insects of family Reduviidae
⁎Corresponding authors. zuhaibali357@yahoo.com (Zuhaib Ahmad), rashidazad@uoh.edu.pk (Rashid Azad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In current study, extensive field surveys are carried out to explore the fauna of assassin bugs of family Reduviidae, along with their habitats and prey records from different localities in the Pothohar region of Pakistan. The quantitative web structure is constructed for better understanding species habitat linkage and is represents a total of twelve different assassin bug species, of which five were identified as new country records i.e., Tribelocephala indica (Stal, 1853), Coranus spiniscutis (Reuter, 1881), Oncocephalus schioedtei (Reuter, 1882), Scadra annulipes (Reuter, 1881) and Ectrychotes dispar (Reuter, 1881). The result of diversity indices, Shannon-Weiner’s and Simpson’s index showed that Trail-5 (Islamabad), Taxilla (Rawalpindi), Sohawa (Jehlum) have maximum values (1.092, 0.662; 1.75, 0.82 and 1.69, 0.78) respectively. The assassin bug species i.e., Rhynocoris marginatus and R. fuscipes are found to predate maximum number of economically important pests of various crops. The diversity indexes of assassin bugs from the Pothohar region indicated that the species have uneven distribution in the five districts. The variation in richness of assassin bug population was recorded in the selected three districts of the Pothohar region. The species richness showed almost same pattern in Islamabad and Jehlum demonstrated by minute difference in Shannon’s equitability index (0.65–0.929 and 0.77–0.87) respectively.
Keywords
New species country records
Prey records
Quantitative web
Reduviidae
Temporal distribution
1 Introduction
Diversity and abundance of insects varies according to geographic and temporal variability (Bashir et al. 2018; Khan et al. 2021). Reduviidae Latreille, 1802 (Cimicomorpha) is the most diversified family of order Hemiptera which represents a large number of terrestrial predators commonly known as assassin bugs (Schuh and Slater, 1995). Members of this group comprises of 25 sub-families and almost 7000 described species worldwide (Weirauch et al., 2014). Assassin bugs have adapted to a wide array of terrestrial habitats stretching from burrows of mammal in the Sonoran Desert to the decomposed logs, in the Bornean rainforest in addition, they have broadened their prey preferences along with the development of a wide range of improved prey capturing strategies (Forero et al., 2011; Soley et al., 2011; Zhang and Weirauch, 2011). There are a large number of reduviid species that are either found in association by the bark of trees or the foliage of herbs, shrubs, and trees however, kissing bug (Triatominae) have adopted to live besides human habitat and feed on their blood (Readio, 1927; Miller, 1953; Louis, 1974) (Fig. 1).
Dorsal habitus of species 1: Tribelocephala indica 2: Coranus spiniscutis 3: Rhynocoris marginatus 4: Rhynocoris fuscipes 5: Sphedanolestes faterculus 6: Sinea diadema.
Assassin bugs are larger in size than other predaceous species of true bugs and they consume not only more prey but also a wider array of prey (Schaefer, 1988). The predation behaviour of assassin bugs is used in biological control of many insect pests and there are almost 182 reduviid species in 69 genera and 10 subfamilies that can prey upon around 261 known forest and agriculture insect pests (Ambrose, 2006). There are several species that are used as the biological control agent, most importantly Pristhesancus plagipennis (Harpactorinae) as predator of stink bug and cotton bollworm (Grundy and Maelzer, 2000). Other species that are being explored for integrated pest management (IPM) includes species of Zelus, Rhynocoris and Sinea that feed, among others, on Lygus bugs, caterpillars and boll weevils (Cogni et al. 2002) (Fig. 2).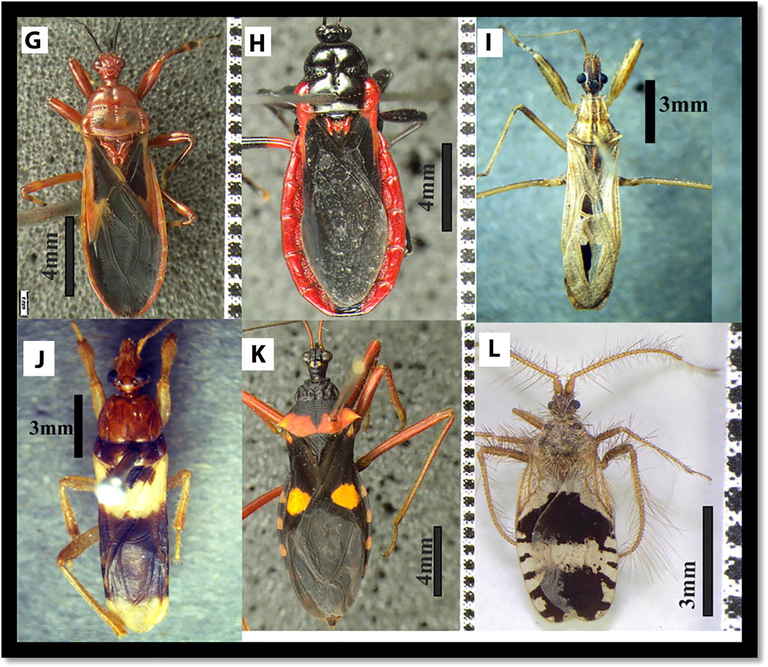
Dorsal habitus of species 7: Scadra annulipes 8: Ectrychotes dispar 9: Oncocephalus schioedtei 10: Sirthenea flavipes 11: Acanthaspis quinquespinosa 12: Holoptilus fasciatus.
Members of subfamily Emesinae, commonly known as the thread-legged bugs, are known to prey on spider, also few species, are able to lure spiders using aggressive mimicry (Soley et al., 2011). The tribes of subfamily Harpactorinae i.e. Apiomerini, Ectinoderini and Diaspidiini have adapted to adhere plant resins on their fore legs with for capturing prey, whereas some members of the tribe Harpactorini have developed their own sticky secretions to serve same purpose (Forero et al., 2011; Zhang and Weirauch, 2011). The subfamily, Holoptilinae that includes the feather-leg bugs can attract ant species to feed on the secretions which results in paralysation (Jacobson, 1911). The most notorious species of Reduviidae belongs to subfamily Triatominae i.e. the kissing bugs, which feeds on vertebrate blood including humans which enables them to vector i.e. Trypanosoma cruzi Chagas, the causal agent of Chagas disease (Lent and Wygodzinsky, 1979).
The family, Reduviidae is distributed worldwide however, level of diversity among species, and are clearly highest in the tropics of the Old and New Worlds. There are several subfamilies that are confined to specific biogeographic regions (Froeschner and Kormilev, 1989; Maldonado, 1990; Cassis and Gross, 1995). So far, the Indian assassin bug fauna is represented by 466 species in 15 families (Ambrose, 2007) and if compared with Pakistan, the number of assassin bugs is represented by only 41 species, which is far less and requires extensive exploration work (Afzal, 2005; Afzal and Ahmad, 2019; Shah et al., 2019). Therefore, an extensive field survey is carried out in different ecosystems from the Pothohar region (i.e. Islamabad, Chakwal, Jhelum, Attock and Rawalpindi) during 2017–2019. The present studies aimed to explore and identify reduviid species and to find out their biodiversity and relationship of habitats in various ecosystems.
2 Materials and methods
The study was conducted in the Pothohar region i.e. Rawalpindi, Jehlum and Chakwal Districts with Capital territory Islamabad of Pakistan. The data was collected from different habitats like forest, agricultural zones, hilly areas and grass lands in the peak months of pest pressure i.e. May to October during 2017–2019 by transect line methodology. The species distribution of family Reduviidae in each district was assessed by dividing the territory into a number of areas from where collection was done fortnightly (before and afternoon) with the help of sweeping net. These locations were selected in different latitudes of Islamabad, Rawalpindi and Jehlum as shown in Table 1.
Districts
Sites selected
Latitude
Longitude
Islamabad
Said pur Village
33° 42′ 9.72′'
73° 7′ 33.96′'
Phulgran Village
33° 45.0′'
73° 13.0′'
F-9 Park
33° 42′ 6.48′'
73° 1′ 22.08′'
Kachnar Park
33° 40′ 12.72′'
73° 4′ 54.12′'
Trail # 1
33° 43′ 57′'
73° 02′ 10′'
Trail # 5
33° 45′ 246′'
73° 05′ 147′'
Rawalpindi
Taxilla
33° 44′ 46.68′'
72° 50′ 22.92′'
Kallar Syedan
33° 24′ 45.72′'
73° 29′ 19.32′'
Nawaz-Sharif Park
33°38′54.96″
73°4′36.84″
Ayyub National Park
33° 34′ 18.84′'
73° 4′ 59.16′'
Kuldana Forest
33° 55′ 28.2′'
73° 23′ 58.56′'
Phagwari Forest
33° 58′ 55.2′'
73° 29′ 51.36′'
Jhelum
Sohawa
33° 6′ 46.44′'
73° 24′ 43.56′'
Dina
33° 1′ 40.44′'
73° 36′ 4.32′'
Tainpura Dam
33° 03′ 22′'
73° 31′ 15′'
Domeli Dam
33° 01′ 28′'
73° 21′ 07′'
Lehri Park Forest
33° 8′ 21.12′'
73° 35′ 9.24′'
Bela Forest
32° 56. 0′'
73° 44.0′'
In each location ten places were selected (10 replicated per locations) with three replicates per place. The ten places in each location were at least twenty kilometer apart from each other (Zhao et al., 2020).
In order to remove the confounding effects of insect species habitat heterogeneity of the insect families the first hand sweep collected sample was at least 2 m apart from one another and in total, thirty (10 locations × 3 replicates) sampling points were covered. All the specimens were preserved as standard procedure, identified via recently published literature (Shah et al., 2019). The identified reduviid fauna is deposited in the Biosystematics laboratory of Pir Mehr Ali Shah- Arid Agriculture University, Rawalpindi, Pakistan. The morphological characters of specimens were observed with Olympus SZX7 stereo-microscope that has camera with Olympus Soft Imaging Solutions, GMBH (Model Olympus LC30).
The collected data was analyzed by paleontological statistics software package (PAST), which calculates the diversity indices including richness and evenness of the collected species (Hammer et al. 2001). Shannon-Weiner’s and Simpson’s index, Margalef’s and Menhenick’s index of different insect species were also calculated.
3 Results
3.1 Subfamily tribelocephalinae
3.1.1 Tribelocephala indica (Walker, 1873)
Opistoplatys indica Walker, 1873: 20; Tribelocephala indica Ambrose, 2006: 27.
Diagnostic characters: The body color ranges from chocolate-brown to piceous; tomentose heavily; legs and labium paler than dorsum; head and pronotum provided with granules; antennae pilose; pronotum narrower behind mid, transverse; forewings bear membrane with darker veins; connexivum well exposed dorsally and legs pilose.
Morphological measurements (mm): Body length 10.725 mm, head length 2.46 mm, head width 1.24 mm, pronotal length 1.68 mm, pronotal width 2.48, scutellum length 0.64, scutellum width 1.26 mm, labium length 2.523 mm and antennae length 1.26 mm.
Distribution: India and Pakistan (Jehlum, new country record).
3.2 Subfamily Harpactorinae
3.2.1 Coranus spiniscutis (Reuter, 1881)
Coranus spiniscutis Reuter, 1881:275; Coranus spiniscutis Distant, 1904: 381.
Diagnostic characters: The body color is testaceous-fuscous to grayish; head brownish black, post-ocular region provided with central yellow brownish line; antennae 1st joint longest, 1st and 2nd joint base luteous; labial 1st joint less in length than 2nd, not reaching the pro-coxae; legs ochraceous, femoral annulations, tibial base and apex and apices of tarsi black, tibiae extreme base provided with pale annulation; forewing membrane bronzy-fuscous, slightly passing the abdominal apex; connexivum exposed and yellowish brown with black spots.
Measurements (mm): Body length 9.225; head length 1.95; head width 1.273; pronotal length 1.575; pronotal width 2.68; scutellum length 0.804; scutellum width 1.072; labium length: 2.485 and antennae length 4.55.
Distribution: India, Nepal, Myanmar and Sri Lanka.
3.2.2 Sphedanolestes faterculus (Bergroth, 1908)
Sphedanolestes faterculus Bergroth, 1908: 591; Distant, 1904: 264
Diagnostic characters: The head, pro-lobe of pronotum, labium, antennae, mid and hind sternal disks and the legs blackish in hue color; body ventrally and hind lobe of pronotum pale luteous, 2 black marks on hind pronotal lobes; post-ocular area little shorter than ante-ocular area; scutellum black with its apex luteous; corium dull or brownish ochraceous; antennal basal segment shorter than pro femora length; labial 1st joint reaching eyes; connexivum exposed considerably from dorsal view.
Measurements (mm): Total body length 9.63; head length 2.67; head width 1.53; pronotal length 2.18; pronotal width 2.71; scutellum length 0.77; scutellum width 1.12; labium length 2.35; antennae length 5.01.
Distribution: India and Pakistan (Jehlum, new country record).
3.2.3 Rhynocoris marginatus (Fabricius, 1794)
Reduvius marginatus Fabricius, 1794: 196; Rhynocoris marginatus Biswas and Bal, 2010: 260.
Diagnostic characters: The body color is sanguineous, setose; scutellum, eyes, antennae, inner region of corium, membrane and tibiae apical two-third violaceous black; antennae base and lateral abdominal margins blood-red in hue; corium wrinkled; sternal disk, trochanters, coxae and anterior pronotal lobe reddish-ochraceous; 1st labium joint apex reaching eyes; basal antennal joint somewhat equal in length to the anterior femora; pronotum posterior lobe and corium rugulose; membrane crossing the apical point of abdomen; connexivum exposed.
Measurements (mm): Total length 16.65 mm.
Distribution: China, India, Pakistan and Sri Lanka.
3.2.4 Rhynocoris fuscipes (Fabricius, 1787)
Reduvius fuscipes Fabricius, 1787: 312; Rhynocoris fuscipes: Ambrose, 2006: 11.
Diagnostic characters: The body color generally coral-red in hue; antennae, hind pronotal lobe interior region, legs, scutellum base, oblong mark between the antennae, pronotal 2 marks, black; labium with its basal joint coral-red while remaining joint blackish in hue; fore-lobe of pronotum sculptured, hind lobe medially little longitudinally impressed, hind margin of posterior lobe facing scutellum truncate.
Measurements (mm): Total length 17.05 mm.
Distribution: China India, Japan, Pakistan, Sri Lanka and Taiwan.
3.2.5 Sinea diadema (Fabricius, 1776)
Cimex multispinosus De-Geer, 1773: 348; Sinea diadema Stal, 1872b: 70.
Diagnostic characters: The body color is brownish ochraceous; head elongate supported with spines, ocelli yellowish, antennal basal joint longest; labial basal joint longest; pronotum more broad than its length, anterior pronotal lobe not sculptured, humeral angles sub-acute, lateral and postero-lateral margins sinuate; connexivum considerably exposed, membrane longer than the abdomen.
Measurements (mm): Total length 10.75 mm.
Distribution: England, Pakistan and North Mexico.
3.3 Subfamily stenopodainae
3.3.1 Oncocephalus schioedtei (Reuter, 1881)
Oncocephalus schioedtei Reuter, 1882: 702; Oncocephalus schioedtei Ambrose, 2006: 25.
Diagnostic characters: The body color is pale brownish; antennae with 1st segment fuscously biannulated, little longer than antero-ocular region; head disk with conspicuous mark behind the eyes, lateral regions fuscous; ante-ocular length almost 2 times the length of post-ocular area; pronotum bearing 2 middle longitudinal lines, merged at anterior lobe; scutellum black with middle pale reddish fascia; corium with sub-quadrate mark close to inner angle, elongate hinder subclaval mark, large elongate mark on membrane disk, dark brownish-black ; labium 2nd segment except base, its apex, sub-basal, middle and apical annulation to pro-tibiae, meso and meta femur apices, sub-basal annulation to meso and meta tibiae, brownish-black; membrane little shorter than the abdominal apex.
Measurements (mm): Total body length 12.135; head length 2.25; head width 1.50; pronotal length 2.475; pronotal width 2.775; scutellum length 1.2; scutellum width 1.172; labium length 3.17; antennae length 6.448.
Distribution: India and Pakistan (Jhelum, new country record).
3.4 Subfamily ectrichodiinae
3.4.1 Scadra annulipes (Reuter, 1881)
Scadra annulipes (Reuter, 1881):309; Scadra annulipes: Ambrose, 2006: 4.
Diagnostic characters: The body color is reddish-brown; antennae strongly pilose, 2nd joint longest; labium with 1st joint longest, 3rd shortest; antennae, eyes, a mark on hind pronotal lobe each side, abdomen, triangular mark on anterior corial zone, hemelytral membrane, posterior-lateral rounded marks of venter, terminal ventral joint, tibial annulations, and tarsi apices, black; connexivum exposed and unspotted; hemelytral membrane little shorter in length than the abdominal apex.
Measurements (mm): Total body length 10.275; head length 1.34; head width 1.541; pronotal length, fore-lobe, 1.005 and hind lobe, 1.306; pronotal width 1.365; scutellum length 1.273; scutellum width 1.675; labium length 6.715; antennae length 6.633.
Distribution: Pakistan and India.
3.4.2 Ectrychotes dispar (Reuter, 1881)
Ectrychotes dispar (Reuter, 1881): 304; Ectrychotes dispar: Ambrose, 2006: 2.
Diagnostic characters: The body color is black and reddish; antennae shiny black and moderately pilose; pronotum with posterior lobe lateral regions reddish, scutellum except base, coxae apices, trochanter, hind femur base, claval and corial bases, connexivum, tarsi bases, and abdomen blackish; abdominal apex ventrally piceous.
Measurements (mm): Total body length12.85; head length 1.809; head width 1.809; pronotal length: fore-lobe, 1.608; hind lobe, 2.01; pronotal width (Pro lobe) 3.015; (hind lobe) 3.953; scutellum length 0.871; scutellum width 1.407; labium length 4.623; antennae length 5.259.
Distribution: India and Pakistan (Islamabad, Rawalpindi, new country record).
3.5 Subfamily Peiratinae
3.5.1 Sirthenea flavipes (Stal, 1855)
Rasahus flavipes Stal, 1855: 187; Sirthenea flavipes: Distant, 1904: 303; Sirthenea flavipes Ambrose, 2006: 18.
Diagnostic characters: Head, pronotum with anterior lobe, abdominal disk beneath is castaneous; 1st antennae segment, legs, claval base and apical area, above and below marks to connexivum, apex of hemelytral membrane, abdominal spots, luteous; scutellum black; sub-basal mark to clavus and a mark in the middle of inner corial margins are piceous; head, antennae, legs, pronotum, abdomen beneath are pilose; 1st antennae joint little in length than the head apex, 2nd almost equal in length to the pre-ocular region; tarsi 3-segmented, 1st joint shortest, 2nd and 3rd sub-equal in length.
Measurements (mm): Total body length 15.75 mm.
Distribution: China, India, Japan, Sri Lanka and Philippines.
3.6 Subfamily reduviinae
3.6.1 Acanthaspis quinquespinosa (Fabricius, 1781)
Reduvius quinquespinosa Fabricius, 1781: 382; Acanthaspis quinquespinosa: Distant, 1904: 257.
Diagnostic characters: The body is generally black; head oblong, eyes well developed, furrow like longitudinal depression present between the eyes and ocelli; antennae 4 jointed, basal joint considerably crossing the head apex, and sparsely setose, others finely setose; labium reddish-brown, 3 segmented, terminal segment the shortest, 1st and 2nd joint sub-equal; pronotal anterior lobe sculptured, pronotum with 2 discal and 2 lateral spines on posterior lobe, 2 sub-rounded marks on corium, and marks on and beneath connexivum, luteous; abdomen exposed; legs unicolorous and pilose; hinder tibiae much longer than the pro and meso tibiae.
Measurements (mm): Total body length 17.65 mm.
Distribution: Bangladesh, China, India, Myanmar and Pakistan.
3.7 Subfamily Holoptilinae
3.7.1 Holoptilus fasciatus (Reuter, 1881)
Holoptilus fasciatus (Reuter, 1881): 272; Holoptilus fasciatus Lethierry & Severin, 1896: 94.
Diagnostic characters: The body is covered with elongate golden yellowish setae; head, antennae, pronotal margins, corial veins, legs and connexivum golden yellowish, antennae with long golden yellowish and piceously hued setae, head from beneath, coxae dark mustard in hue; pronotal pro lobe brownish, hind lobe mustard; scutellum base piceous; corial and claval cells hyaline; hind femur robust, hind tibia long; abdomen oval round, covered with elongated setae, hemelytra passing abdominal apex.
Measurements (mm): Total body length 6.65 mm.
Distribution: India and Pakistan.
4 Discussion
Seasonal collection of various species of assassin bugs from the Pothohar region of Pakistan revealed (Table 2) that most of these were collected from May to October, particularly R. marginatus and R. fuscipes. Species T. indica has very narrow range of habitats and were only collected from the forest area while species of subfamily Harpactorinae, R. marginatus and R. fuscipes, were found from all the selected habitats. Moreover, species R. marginatus of subfamily Harpactorinae showed highest relative abundance (35.85%), whereas the species T. indica showed the least abundance i.e. 1.26% (Fig. 3). Among 7 assassin bugs subfamilies, Harpactorinae showed highest relative abundance (80.81) with maximum five species, while the Peiratinae recorded least relative abundance (0.63%) with only one species (Fig. 4).
Sr. #.
Species
Host field
Prey record
Month
References
1
Tribelocephala indica
Dried leaves, Maize.
S. litura
June-Aug
Ambrose (1999)
2
Coranus spiniscutis
Brinjal, Okra, Sunflower
H. armigera, S. litura, E. ventralis
April-Oct
Claver and Reegan (2010)
3
Rhynocoris marginatus
Cabbage, Lady finger, Cucumber, Chilies.
D. indicus, S. litura, D. cingulatus, E. vitella, O. laetus, H. armigera, G. servus, S. hospes, S. pandurus, R. linearis, N. inconspicuous
May- July
Ambrose (2003, 2007)
4
Rhynocoris fuscipes
Cabbage, Lady finger, Cuccumber, Chilies.
D. indicus, S. litura, D. cingulatus, E. vitella, O. laetus, H. armigera, S. hospes, S. pandurus, R. linearis
May- July
Ambrose and Claver (2001a), Nagarajan (2010)
5
Sphedanolestes faterculus
Parthenium, Brinjal
C. obscura, caterpillars
Mar-June
Rao et al., (1981)
6
Sinea diadema
Cabbage, Mungbean
Caterpillars in general, C. biguttata, N. viridula
May- Oct
Ambrose (1999)
7
Oncocephalus schioedtei
Peanut, Sunflower, fallen leaves
N. inconspicuous, H. armigera, M. uniguttatus
May- Oct
8
Scadra annulipes
Cabbage, Lady finger, pumpkin
A. ipsilon, Pieris rapae
April-Oct
Singh and Gangrade (1975)
9
Ectrychotes dispar
Wild plants, millipede feeder.
Millipedes, H. armigera
Aug-Oct
Pawar et al. (1986)
10
Sirthenea flavipes
Maize
H. armigera
May-July
Ambrose (2007)
11
Acanthaspis quinquespinosa
Ladyfinger
D. koengii, H. armigera
May-Aug
Sahayaraj (1991)
12
Holoptilus fasciatus
Peanut crop
Cletus trigonus
April-July
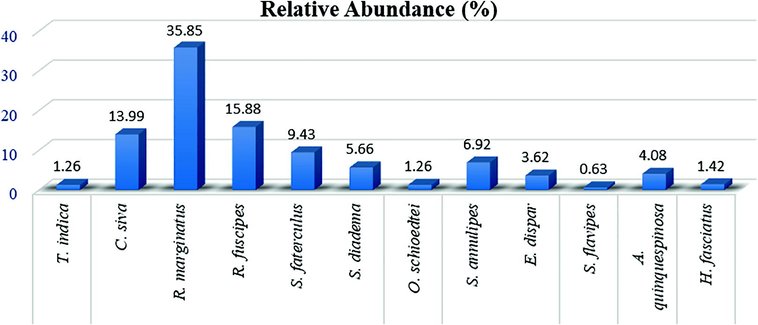
Relative abundance of all the collected species of Reduviidae from the Pothohar areas.
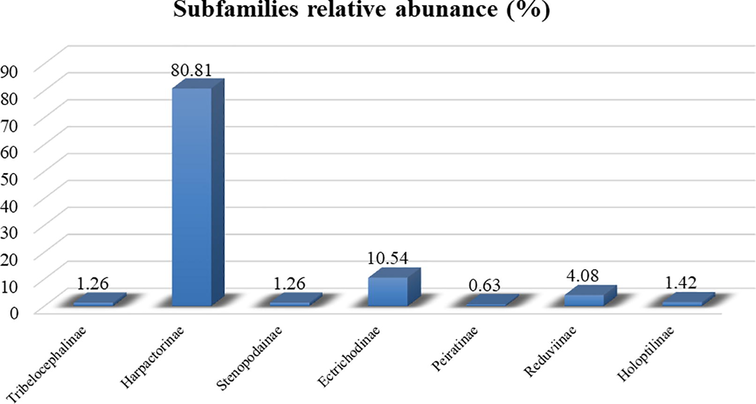
Relative abundance of assassin bug subfamilies collected from 2017 to 2019.
From the selected sites of the Pothohar region of Punjab the site Sohawa of district Jehlum showed maximum species richness i.e. 9 species (Table 3). It has been noted that forest area of Islamabad territory exhibits maximum species richness while agricultural areas of District Rawalpindi and Jehlum are the most species rich sites with maximum abundance.
Districts
Species
Abundance
Species
Saipur Village
Phulgran Village
Kachnar Park
Kachnar Park
Trail # 1
Trail # 5
Islamabad
C. spiniscutis
41
+
+
+
+
–
+
R. marginatus
41
+
+
+
–
+
+
E. dispar
15
–
–
–
+
+
+
Total abundance
97
29
21
12
6
8
21
Total # of Species
2
2
2
2
2
3
RAWALPINDI
Species
Abundanc eAbundanc e
Taxilla
Kallar Syedan
Nawaz Sharif Park
Ayub National Park
Kuldana Forest
Phagwari Forest
S. annulipes
39
+
+
+
–
+
+
R. marginatus
96
+
+
+
+
+
+
R. fuscipes
35
+
+
–
+
–
–
E. dispar
8
+
–
+
–
+
+
H. fasciatus
9
+
+
–
–
+
–
C. spiniscutis
27
+
+
+
–
+
–
S. faterculus
23
+
+
–
–
+
+
Total abundance
237
88
75
9
16
26
23
Total # of Species
7
6
4
2
5
44
JEHLUM
Species
Abundanc e
Sohawa site
Dina
Tainpura Dam
Domeli Dam
Lehri Park Forest
Bela Forest
R. marginatus
45
+
+
+
+
+
–
R. fuscipes
31
+
+
+
–
–
–
C. spiniscutis
21
+
+
+
+
–
+
S. faterculus
37
+
+
–
+
+
+
S. annulipes
5
+
–
–
–
+
–
S. diadema
36
+
+
+
+
+
+
A. quinquespinosa
26
+
+
–
–
–
+
T. indica
8
–
–
–
–
+
+
O. schioedtei
8
+
+
–
–
+
–
S. flavipes
4
+
–
–
–
+
–
Total abundance
221
69
50
16
14
32
40
Total # of Species
9
7
–
–
6
6
Diversity values, used for the evaluation of species richness and evenness of family Reduviidae from the selected sites of the Pothohar region (Figs. 5, 6 and 7) showed that Trail #5, Taxilla and Sohawa zones exhibited maximum values of Shannon wiener’s index (1.092, 1.75 and 1.69 respectively), Simpson’s index (0.662, 0.82 and 0.78 respectively) and Marglef’s index (0.656, 1.11 and 1.66 respectively).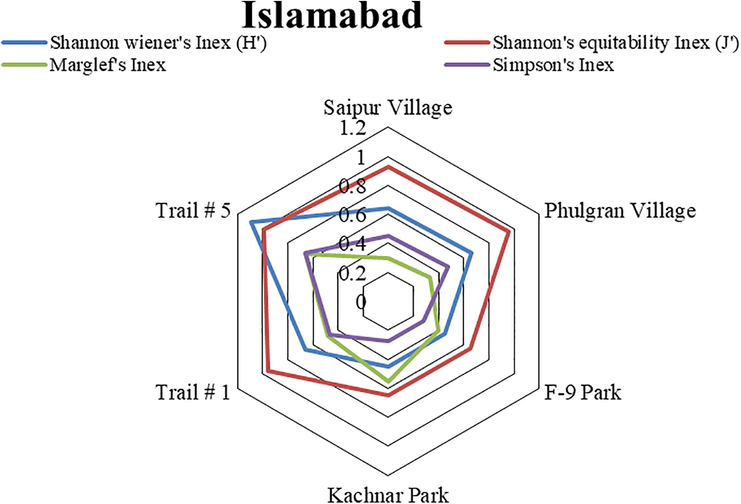
Diversity indices values of Assassin bug species collected from different sites of Islamabad.
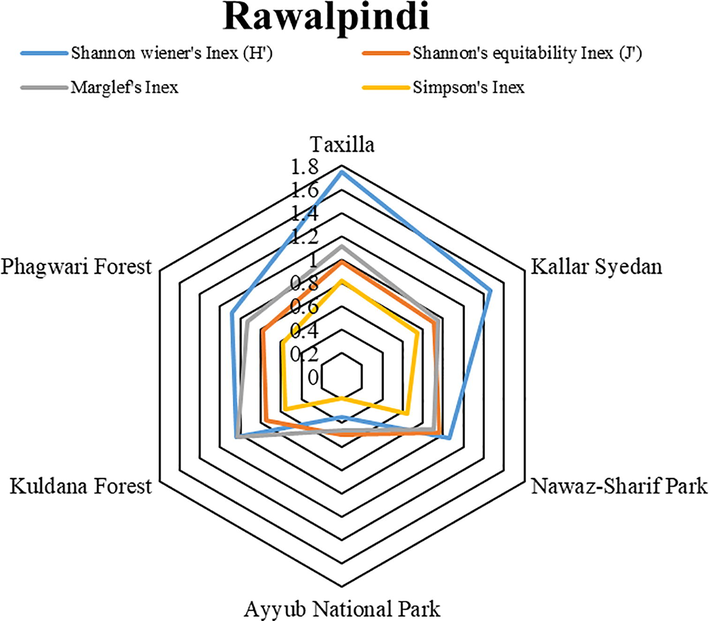
Diversity indices values of Assassin bug species collected from different sites of Rawalpindi.
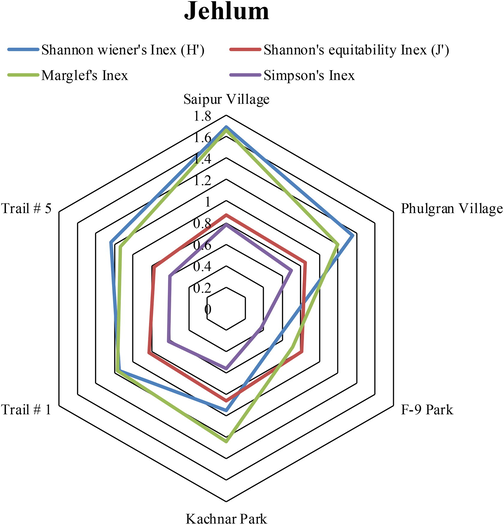
Diversity indices values of Assassin bug species collected from different sites of Jehlum.
The web structure represented that eleven species of assassin bugs were present in Agricultural and forest zone while the grassland zone is represented a total of 7 species. However, agricultural zone showed maximum abundance of species compared to forest zone, however, both the zones were represented by eleven species. Among different collected species R. marginatus belonging to subfamily Harpactorinae, is the most abundant species in all the selected habitats, especially in agricultural zone (Fig. 8). The quantitative web structure of twelve species of assassin bugs belonging to different genera, comprising of 636 specimens was presented. Almost all the species, except T. indica (absent in Agriculture and Grassland zones), S. diadema, S. schioedtei, A. quinquespinosa (absent in grassland) and S. flavipes (not present in forest) were present in all the selected habitats (Fig. 9).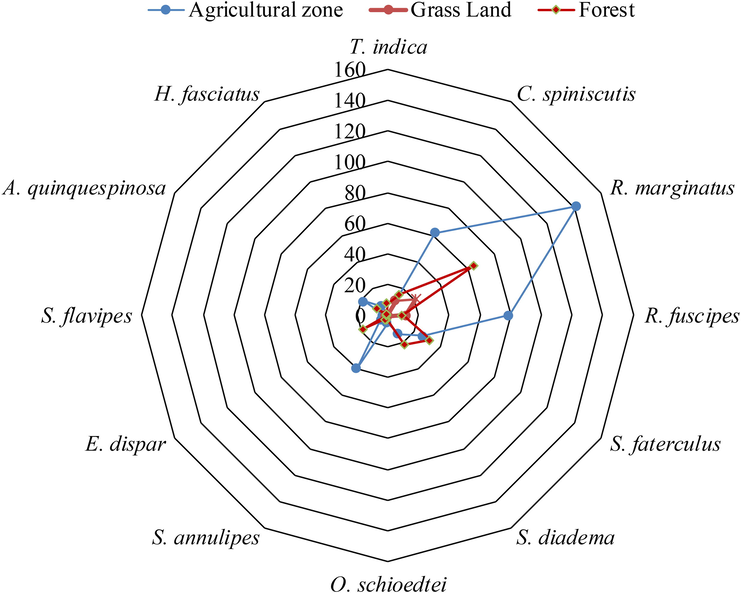
Web structure showing number of species present in all the habitats and habitat abundance.
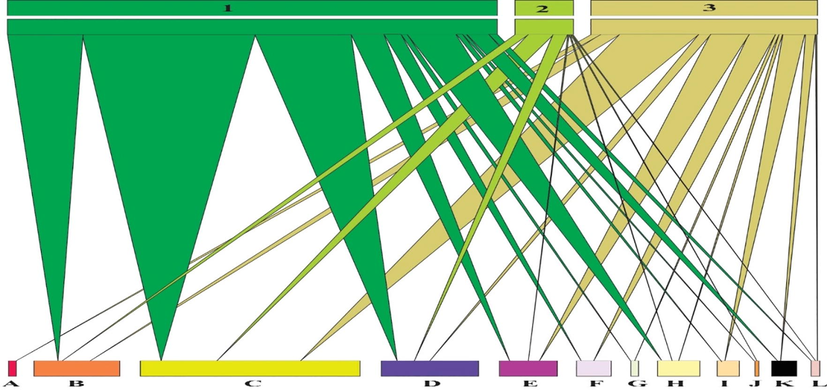
Quantitative web structure of Reduviidae species linked with selected habitats. In above picture: 1 (Agriculture zone), 2 (Grass land) and 3 (Forest zone) are three different habitats. A = (Tribelocephala indica), B = (Coranus spiniscutis), C: Rhynocoris marginatus, D: Rhynocoris fuscipes, E: Sphedanolestes faterculus, F: Sinea diadema, G: Oncocephalus schioedtei, H: Scadra annulipes, I: Ectrychotes dispar, J: Sirthenea flavipes, K: Acanthaspis quinquespinosa, L: Holoptilus fasciatus
5 Conclusion
A total of 12 species belonging to 11 genera under 7 subfamilies have been reported from the Pothohar region of Pakistan, which are quite less in number as compared to neighboring country; India 465 species. Species like R. marginatus and R. fuscipes were successfully utilized as bio control agents in economically important agricultural crops of India (Singh and Sing, 1987; Sahayaraj, 1995), Coranus spiniscutis (Jalali and Singh, 2002), Acanthaspis quinquespinosa (Das et al., 2010).
It can be concluded that the species of assassin bugs were not evenly scattered in all the selected sites of the Pothohar region. The variation in richness in the population of assassin bug was recorded in the selected three districts of the Pothohar region. The species richness recorded almost same pattern in Islamabad and Jehlum demonstrated by minute difference in Shannon’s equitability index (0.65–0.929 and 0.77–0.87) respectively. But species richness of selected sites of district Rawalpindi was not same as demonstrated by a significant difference between the values of Shannon’s equitability index (0.50–0.98) i.e. some sites were more species rich than the others. District wise maximum species have been found from Jehlum (10 species) followed by Rawalpindi and Islamabad (7 and 3 species, respectively). For the perspective researcher this study will provide a base line to study the assassin bugs ecology, biology and predatory potential against various insect pests. These findings will definitely prove handy in developing biocontrol strategies against various insect pests of different crops in plant protection programs.
Acknowledgement
The research was supported by Higher Education Commission of Pakistan (HEC) through Indigenous scholarship. The authors also appreciate the support of the Research Center for Advanced Materials Science (RCAMS) at King Khalid University Abha, Saudi Arabia through a grant KKU/RCAMS/G002-21
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The Insects: Beneficial and Harmful Aspects. New Delhi: Kalyani Publishers; 2006. p. :801.
- Cassis, G., Gross, G.F., 1995. Hemiptera: Heteroptera (Coleorrhyncha to Cimicomorpha). In: Houston, W.W.K., Maynard, G.V. (Eds.), Zoological Catalogue of Australia, Houston, vol. 27. CSIRO, Melbourne, Australia, pp. 1–506.
- Biology and mating behaviour of Coranus spiniscutis Reuter (Hemiptera: Reduviidae), a key predator of rice gandhi bug Leptocorisa varicornis Fabricius. J. Biopest.. 2010;3(2):437-440.
- [Google Scholar]
- Influence of prey size on predation success by Zelus longipes L. (Het., Reduviidae) J. Appl. Entomol.. 2002;126(2-3):74-78.
- [Google Scholar]
- Diversity of arthropod natural enemies in the tea plantations of North Bengal with emphasis on their association with tea pests. Curr. Sci.. 2010;99(10):1457-1463.
- [Google Scholar]
- Resin gathering in neotropical resin bugs (Insecta: Hemiptera: Reduviidae): functional and comparative morphology. J. Morphol.. 2011;272(2):204-229.
- [Google Scholar]
- Phymatinae or ambush bugs of the world: a synonymic list with keys to species, except Lophoscutus and Phymata (Hemiptera) Entomography. 1989;6:1-76.
- [Google Scholar]
- Predation by the assassin bug Pristhesancus plagipennis (Walker) (Hemiptera: Reduviidae) of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) and Nezara viridula (L.) (Hemiptera: Pentatomidae) in the laboratory. Austr. J. Entomol.. 2000;39:280-282.
- [Google Scholar]
- Biological notes on the hemipteron Ptilocerus ochraceus. Tijdschrift voor Entomol.. 1911;54:175-179.
- [Google Scholar]
- Seasonal activity of stem borers and their natural enemies on fodder maize. Entomon. 2002;27(2):137-146.
- [Google Scholar]
- Foraging behavior of western honey bee (Apis mellifera) in different time intervals on Brassica campestris L. Fresenius. Environ. Bull.. 2021;30(3):2607-2612.
- [Google Scholar]
- Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas’ disease. Bull. Am. Museum Nat. History. 1979;163:123-520.
- [Google Scholar]
- Nagarajan, K., 2010 Mass multiplication, large scale release and bio control potential evaluation of a reduviid predator Rhynocoris fuscipes (fabricius) (insecta: heteroptera: reduviidae) against chosen insect pests. Ph. D. Thesis. Manonmaniam Sundaranar University, Tirunelveli, Tamil Nadu, India.
- Heliothis species and their natural enemies, with their potential in biological control. Proc. Indian Acad. Sci. (Animal Sci.). 1986;95:695-703.
- [Google Scholar]
- Notes on new addition to the natural enemies of Spodoptera litura F. and Myzus persicae Sulz. on fine cored tobacco in Andhra Pradesh. Sci. Cult.. 1981;47:98-99.
- [Google Scholar]
- Studies on the biology of the Reduviidae of America north of Mexico. Kansas Univ. Sci. Bull. 1927;17:1-291.
- [Google Scholar]
- Ad cognitionem Reduviidarum mundi antiqui. Acta Soc. Sci. Fennicae. 1881;12:269-339.
- [Google Scholar]
- Reduviidae (Hemiptera: Heteroptera) as agents of biological control. In: Ananthasubramanian K.S., ed. Bicovas I. Madras: Loyola College; 1988. p. :27-33.
- [Google Scholar]
- Schuh, R.T., Slater, J.A. 1995 True bugs of the world (Hemiptera: Heteroptera): classification and natural history. Cornell University Press, Ithaca, New York. xii + pp 336.
- First record of Holoptilinae from Pakistan, with the redescription of Holoptilus fasciatus (Hemiptera: Reduviidae) Zootaxa. 2019;4695:541-549.
- [Google Scholar]
- Record of Rhinocoris fuscipes Fabricius as a predator of green stink bug, Nezara viridula Linn. infesting soybean in India. Biol. Control. 1987;1:145-146.
- [Google Scholar]
- Parasites, predator and diseases of larvae of Diacrisia obliqua Walker (Lepidoptera: Arctiidae) on soybean. Curr. Sci.. 1975;44:481-482.
- [Google Scholar]
- Biology of Stenolemus giraffe (Hemiptera: Reduviidae), a web invading, araneophagic assassin bug from Australia. N. Z. J. Zool.. 2011;38:297-316.
- [Google Scholar]
- Weirauch, C., Bérenger, J.-M., Berniker, L., Forero, D., Forthman, M., Frankenberg, S., Freedman, A., Gordon, E., Hoey-Chamberlain, R., Hwang, W. S., Marshall, S. A., Michael, A., Paiero, S. M., Udah, O., Watson, C., Yeo, M., Zhang, G., Zhang, J. 2014. An Illustrated Identification Key to Assassin Bug Subfamilies and Tribes (Hemiptera: Reduviidae). Canad. J. Arthropod Identif., No. 26: December 10, 2014. 10.3752/cjai.2014.26.
- Zhang, G., Weirauch C., 2011. Sticky predators: a comparative study of sticky glands in harpactorine assassin bugs (Insecta: Hemiptera: Reduviidae). Acta Zoologica: doi: 10.1111/j.1463-6395.2011.00522.x.
- Release from below and aboveground natural enemies contributes to invasion success of a temperate invader. Plant Soil 2020:1-10.
- [Google Scholar]







