Translate this page into:
Mosquito repellent activity of essential oil of Ethiopian ethnomedicinal plant against Afro-tropical malarial vector Anopheles arabiensis
*Corresponding author. Tel.: +251 913 547 847, mobile: +91 9600 918 524; fax: +251 471 111 450 karunamoorthi@gmail.com (Kaliyaperumal Karunamoorthi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 18 January 2014
Peer review under responsibility of King Saud University.

Abstract
In Ethiopia, malaria remains to be one of the major public health issues that causes significant impediment to socio-economic development too. A laboratory-based study has been conducted to evaluate the repellency of Ethiopian ethnomedicinal plant Tedh [vernacular name (local native language, Amharic); Juniperus procera (Cupressaceae)], against Afro-tropical malarial vector, Anopheles arabiensis Patton at four different concentrations viz., 1.0, 1.5, 2.5, and 5.0 mg/cm2. Experimentation on the percentage of protection in relation to the dosage has been performed. The tested concentrations of the essential oil of J. procera exhibited various degrees of repellency in terms of percentage of repellency and complete protection time against female An. arabiensis viz., 1.0, 1.5, 2.5 and 5.0 mg/cm2 [64.10% (92 min)], [68.10% (125 min)], [72.20% (190 min)], and [80.60% (311 min)], respectively. Student’s t-test results show statistically significant (P < 0.001) [0.1 mg/cm2 (t = 82.7; df = 4); 0.15 mg/cm2 (t = 124.8; df = 4); 2.5 mg/cm2 (t = 25.3; df = 4); 5.0 mg/cm2 (t = 175.3; df = 4)] difference between treated and control groups. The examined essential oil exhibited significant repellent properties and it has been identified that it could serve as a potent repellent against insect vectors of disease. In Africa, Tedh is well-known as a therapeutic agent to treat various illness and insects’ repellent plant to drive-away insect vector of diseases. As the essential oil of Tedh is exceptionally safe and economical it could serve as a potent personal protective tool to minimize the burden of insect-transmitted diseases particularly malaria in the future.
Keywords
Anopheles arabiensis
Juniperus procera
Repellent activity
Ethiopia
1 Introduction
Over the centuries, the vector-borne diseases are imposing a serious public health threat to humankind in terms of illness and deaths worldwide. Besides their negative public health impact, these diseases are also posing serious obstacle to socio-economic development in countries wherever they are endemic in nature (Karunamoorthi and Sabesan, 2010). Malaria continues to be a major global public health problem with 3.3 billion people at risk in 106 endemic countries (Karunamoorthi et al., 2013). It imposes not only severe public health impact but also negative socio-economic development in the resource-poor settings of Africa, Asia, Latin America and beyond (Karunamoorthi and Bekele, 2009). It remains as one of the important causes of maternal and childhood morbidity and mortality in sub-Saharan Africa (SSA) particularly in Ethiopia (Karunamoorthi et al., 2010a). It also contributes to stillbirths, low birth weight, and early infant mortality (Menendez, 1995).
WHO Malaria Report (2012) estimates that the malaria related illness and mortality have been dramatically declined by the combined efforts of distribution of long lasting insecticide treated mosquito nets (LLINs), effective case management with potent antimalarials, source reduction and selective intradomicillary spraying. However, the heavy reliance on pyrethroids has led to the emergence of resistance in a wide variety of malaria-endemic settings of Africa and the rest of the world. It is considered to be a potential threat to the global public (Karunamoorthi and Sabesan, 2013). Generally, plant-based insecticides are target-specific and relatively non-toxic. Consequently, there is a revived interest observed by several researchers to develop green pesticides/reduced-risk of pesticides. As a result, over thousands of plant species were evaluated as potential insecticidal agents in terms of larvicidal, antifeedant, repellent, oviposition deterrent, growth regulatory and anti-vector activities (Karunamoorthi, 2012a).
An. arabiensis is one of the important malarial vectors in sub-Saharan Africa (Onyabe and Conn, 2001; Mutero et al., 2004) particularly in Ethiopia, while An. funestus Giles, An. pharoensis Theobald, and An. nili Theobald are secondary vectors (Karunamoorthi and Yirgalem, 2013). It adapts to endophagic and endophilic patterns, where hosts are domestic and indoor, but are of exophagic and exophilic nature, where the hosts are mainly outdoors (Coluzzi et al., 1979; Ameneshewa and Service, 1996; Mendis et al., 2000). It has the ability to adapt to all types of climatic features, and it can avoid insecticide sprayed surfaces and can adjust into exophagic. In this context, repellents could play a pivotal role whenever and wherever other vector control interventions are impossible and impracticable (Karunamoorthi and Sabesan, 2010).
The repellent plant usage an integral part of Ethiopian tradition and culture (Karunamoorthi and Husen, 2012) is a common practice to drive-away insects/mosquitoes. Interestingly, the East African plant called Golden flower/pyrethrum (Chrysanthemum cinerariaefolium) is a precursor to all the existing commercial insect repellents (Karunamoorthi, 2012b). Essential oils are the best-known sources tested against insects (Pitasawat et al., 2007). Even today, in the rural parts of SSA countries, people do still use numerous plants as repellents blindly or hardly knowing their efficiency, safety and mode of action through trial-and-error or word-of-mouth communications from elders (Karunamoorthi, 2012b). In this perspective, this study is a spinoff research work aimed to determine the indigenous Ethiopian ethnomedicinal plant Juniperus procera essential oil as a repellent against the Afro-tropical vector An. arabiensis.
2 Materials and methods
2.1 Selection of the plant
The Tedh was selected from the secondary data i.e. based on some reports in the literature or some bio-ethnological knowledge from the farmers, traditional healers, key informants and local residents. In Ethiopia, the local rural residents have been using this indigenous plant for various curative and miscellaneous purposes. The collected voucher specimen has been pressed, numbered, dried, identified and deposited in the Jimma University Regional Herbarium, Ethiopia.
2.2 Taxonomy of J. procera Hochst. ex Endl
Kingdom
Plantae
Division
Pinophyta
Class
Pinopsida
Order
Pinales
Family
Cupressaceae
Genus
Juniperus
Species
procera
2.3 Description and traditional usage of J. procera
The Tedh is commonly known in English as African Juniper or East African Juniper. It is a coniferous tree native to the mountains of eastern Africa from eastern Sudan south to Zimbabwe, and the southwest of the Arabian Peninsula. In traditional African medicine, the smoke of fruiting branches of Tedh is used to get relieved from rheumatic pains and an infusion of ground twigs and buds is consumed against intestinal worms. Stem is used as a tooth brush and leaves are used to treat or cure tonsillitis. The vapour from a leaf decoction has been inhaled several times a day for the treatment of flu like illness.
2.4 Selection of mosquito species
Nearly all members of An. gambiae complex, that are potent vectors of malaria in tropical Africa, have shown various degrees of resistance to commonly applied insecticides like DDT (dichlorodiphenyltrichloroethane) and pyrethroids. An. arabiensis, and An. gambiae s.s. are the most important vectors of human malaria in sub-Saharan Africa particularly Ethiopia (Coetzee et al., 2000). Thus, the Adama Malaria Research Centre, Ethiopia laboratory reared An. arabiensis was chosen for the evaluation of repellent activity. It was maintained at 27 ± 2 °C, 75–85% RH, under 14 L:10 D photoperiod cycles.
2.5 Preparation of mosquitoes
The colony was maintained in the laboratory at 27 ± 1 °C and 85% relative humidity. The larvae were fed with dog biscuits and yeast powder in the ratio of 3:1. Adults were provided with 10% sucrose solution and 1-week-old chick for blood meal. The mosquitoes were starved for 3–4 days before the commencement of each experiment. Conditions during the test followed a standard diel cycle, with air temperature 27 ± 0.2 °C, 47 ± 3% RH, and light intensity of 290 ± 45 lux.
2.6 Preparation of plant extract
The leaves of J. procera were collected from the outskirts of Jimma town, Oromiya Region, Ethiopia, and brought to the laboratory. The leaves were dried under shade at room temperature (29 ± 1 °C) for about 20 days. The completely dried leaves were powdered and sieved to get the fine powder of leaf. The methanol-leaf extracts from the sieved fine leaf powder were obtained by using Soxhlet apparatus. Two hundred and fifty grams of leaf powder was dissolved in 200 ml of methanol (as a solvent) and extracted using the Soxhlet apparatus (Vogel, 1978), for 8 h over a mantle heater at 55 °C. The methanol extracts were concentrated using a vacuum evaporator at 45 °C under low pressure. After complete evaporation of the solvents, the concentrated extracts were collected and stored in the refrigerator for later use.
2.7 Repellent activity
The percentage of protection in relation to dose method was used (WHO, 1996). Three–four-day-old blood-starved hundred female An. arabiensis were kept in a net cage (45 × 30 × 45 cm2). Before each test, the readiness of the mosquitoes to bite was confirmed by having subjects inserted their untreated forearm (control) into the test chamber. Once the subjects are observed to have five mosquito landings on the untreated arm, they were allowed to remove their arm from the chamber. The arms of the test person were cleaned with ethanol, and ethanol served as a control. After air drying the arms of the test person, are once again exposed to the mosquitoes. Only 25 cm2 dorsal side of the skin on the each arm was exposed and the remaining area was covered with rubber gloves. The leaf extract of J. procera was applied at 1.0, 1.5, 2.5, and 5.0 mg/cm2 concentrations separately (Venkatachalam and Jebanesan, 2001). The control and treated arms were introduced simultaneously into the cage. The number of bites was counted over 3 min to every 30 min from 18:00 h to 06:00 h. The experiment was replicated five times in each concentration. All experiments were carried out at a temperature of 28 ± 2 °C and RH 75 ± 5% under laboratory conditions. It was observed that there was no skin irritation due to the leaf extract tested. The percentage of protection was calculated by using the formula:
3 Results
3.1 Repellent activity under laboratory condition
The essential oil of the leaves of J. procera exhibited various degrees of repellent efficacy against female An. arabiensis. The results of mean protection time and total percentage of protection in relation to dose of J. procera essential oil are given in Table 1. Fig. 1 clearly suggests that the J. procera essential oil has excellent repellent properties against An. arabiensis. The results are quite promising and remarkable against An. arabiensis. Furthermore, the Table 1 and Fig. 1 clearly show that the repellent efficacy of J. procera is strongly dose dependent.
Concentrations (mg/cm2)
Mean number of bites received
Mean complete protection time (h)a
Total protection for 12 h (%)
t-Value
(Treated)a
(Control)a
0.10
62.7 ± 1.20
173.0 ± 0.58
1.32 ± 0.03
64.10
82.7*; df = 4
0.15
58.7 ± 0.88
176.3 ± 0.33
2.05 ± 0.05
68.10
124.8*; df = 4
0.25
57.3 ± 3.18
171.3 ± 3.18
3.10 ± 0.03
72.20
25.3*; df = 4
0.50
27.3 ± 0.33
173.7 ± 1.20
5.11 ± 0.02
80.60
117.3*; df = 4
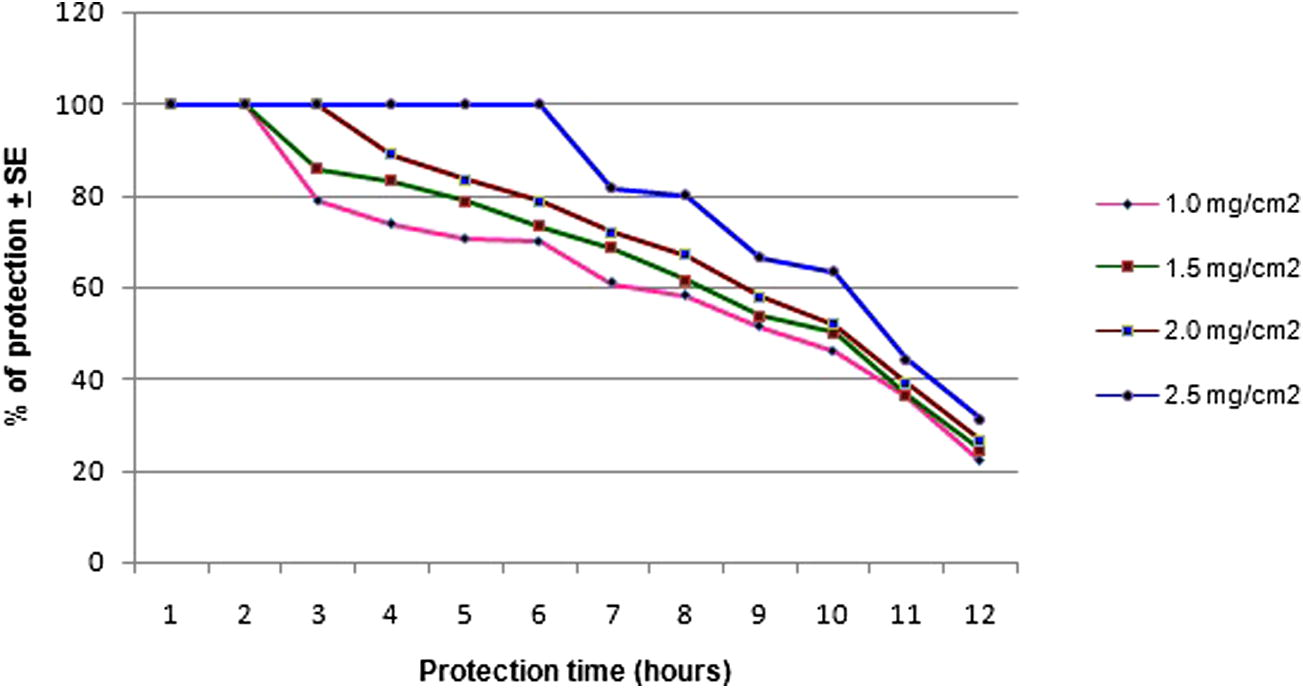
The repellency efficacy of Juniperus procera essential oil leaf extract against An. arabiensis at various concentrations.
The tested concentrations of the essential oil of J. procera exhibited various degrees of repellency against female An. arabiensis viz., 1.0, 1.5, 2.5 and 5.0 mg/cm2 [64.10% (92 min)], [68.10% (125 min)], [72.20% (190 min)], and [80.60% (311 min)], respectively. The student’s t test results show statistically significant (P < 0.001) [0.1 mg/cm2 (t = 82.7; df = 4); 0.15 mg/cm2 (t = 124.8; df = 4); 2.5 mg/cm2 (t = 25.3; df = 4); 2.5 mg/cm2 (t = 175.3; df = 4)] difference between treated and control groups. The highest complete protection and % of repellency were observed with 5.0 mg/cm2 of J. procera.
4 Discussion
Over centuries, malaria is considered to be one of the long-standing public health issues in many of the tropical and subtropical regions of the world particularly in sub-Saharan Africa. The long-term combined efforts like distribution of LLINs, selective intradomicillary spraying, source reduction by attacking mosquito breeding sites and effective case management with Artemisinin Combination Therapies (ACTs) have reduced the number of malaria related illness dramatically. Indeed, it has changed the entire world malaria landscape and has shrunken the world malaria map considerably. However, WHO Malaria Report (2012) states that nearly 91% of world malaria cases and 60% of deaths have been reported from developing economies specifically from the sub-Saharan African (SSA) countries.
Therefore, in the resource-poor settings, people are using various repellent plants to drive-away blood-sucking insects mainly mosquitoes to minimize the nuisance and disease transmission significantly. The following factors have largely enabled them to apply repellent plants; (1) use of plant-based insect repellents is strongly blended with their tradition/culture, (2) lack of accessibility and affordability to procure modern personal protective devices, (3) easy accessibility and affordability of local repellent plants, (4) non-availability of simple and cheap method of personal protection measures, when other vector control interventions are impossible and impracticable.
It is important to note that since time immemorial Ethiopia has a rich tradition and practice of insect repellent plant usage to drive away insects particularly mosquitoes from their houses in the early evening hours. It is chiefly done by smoldering various repellant plants on the traditional charcoal stove (Karunamoorthi and Husen, 2012), unwittingly or knowingly little on their efficacy and safety through trial-and-error or word-of-mouth from elderly people. Therefore, this study is an attempt to evaluate the repellency efficacy of such a candidate plant called Tedh against An. arabiensis.
The present investigation results suggest that the essential oil of J. procera leaves has displayed various degree of repellency at various concentrations against An. arabiensis. The skin repellent test at 1.0, 1.5, 2.5, and 5.0 mg/cm2 of J. procera offered 100% protection up to 1.32 ± 0.03 h, 2.05 ± 0.05 h, 3.10 ± 0.03 and 5.11 ± 0.02, respectively (Table 1). The results are consistent with the previous study conducted in Ethiopia and the skin repellent test at 1.0, 1.5, 2.0, and 2.5 mg/cm2 concentrations of Cymbopogon citrates, which offered 100% protection up to 3.21 ± 0.06 h, 4.40 ± 0.13 h, 5.30 ± 0.07 h, and 6.37 ± 0.14 h, respectively. Though, the repellency effects of J. procera are relatively inferior with the lower concentrations, at higher concentrations the J. procera has offered similar protection like C. citratus (Karunamoorthi et al., 2010b).
The lower concentrations like 1.0 and 1.5 2.5 mg/cm2 offered the mean complete and total percent of protection as 1.32 ± 0.03 h (64.1%) and 2.05 ± 0.05 h (68.1%), respectively (Table 1). It could be possibly explained that the speedy loss of repellent activity is due to the significantly faster volatilization rate of bio-active compounds of the essential oil rather than the lack of efficacy. Tawatsin et al. (2001) confirmed that the repellency of volatile oils can be improved significantly if they are formulated with vanillin. In this perspective, the formulation technology plays a vital role for synthesizing long-lasting repellents. In China, by using appropriate formulation the protection time of Eucalyptus globulus against Aedes albopictus was dramatically prolonged from 3 to 5 h by adding 5% vanillin under laboratory conditions (Yang and Ma, 2005). Therefore, it provides an idyllic prospect to enhance the repellency of J. procera essential oil even at the lower concentrations against important vector of diseases, eventually to minimize the burden of vector-borne disease burden considerably.
The present investigation results demonstrated that the concentration and the percent of protection time are positively correlated. It can be seen that the degree of repellency could be improved significantly when the J. procera oil concentration increased rather than being used with lower concentrations (Fig. 1). This finding is in line with a study conducted in Ethiopia by Wano (2006) reporting that when the essential oil of Ocimum suave was mixed with Lippia adoensis at 10% concentration, the mixture offered protection against mosquito only for 2 h with 92–100% of repellency. However, while increasing the concentration of this blend to 20% the complete protection was increased dramatically to 3 h with nearly 88–100% of repellency. Many factors such as differences in plant species, different mosquito densities in the cages and cage size (Barnard et al., 1998) and different test mosquito species with different sensitivity to repellent oils (Robert et al., 1991) may contribute to these variations in the repellent activity of the essential oils.
The percentage of protection in relation to the dose method was performed and found that the J. procera essential oil has shown various degrees of repellency impact against An. arabiensis. It has provided the maximum total percentage of protection of about 80.6% at 5.0 mg/cm2 and followed 72.2% at 2.5 mg/cm2 for 12 h (Table 1 and Fig. 1). The results are quite comparable with the previous study conducted by Karunamoorthi et al. (2010b) reporting that the methanol-leaf extract of Ethiopian traditional insect repellent plant viz., Cymbopogon citratus against An. arabiensis provided the maximum total percentage protection of 78.83% at 2.5 mg/cm2 and followed 68.06% at 2.0 mg/cm2 for 12 h.
All four tested concentrations of J. procera essential oil offered significant protection and Student’s t test results show statistically significant (P < 0.001) difference between treated and control groups. The result suggests that the percentage of repellency was dose dependent and it could serve as a potent insect repellent against insect vectors of disease (Table 1). The present results are in accordance with very recent findings on the methanolic extracts of leaves and seeds from, Tribulus terrestris, tested against An. arabiensis under laboratory conditions. The repellency of T. terrestris extracts varied depending on the plant parts and the dose of extract. The seed extract was more effective in exhibiting the repellent action (100%) against the mosquito tested as compared with the leaves extract (79.5%) at the dose 1.0 and 2.0 mg/cm2, respectively (El-Sheikh et al., 2012). In Kenya, essential oils of Ocimum forskolei and O. fischeri were evaluated for repellency on forearms of human volunteers against An. gambiae s.s. and were found to be more effective than DEET (Odalo et al., 2005).
Essential oil based repellents are safe for human and domestic animals. However, the continuous topical application of DEET causes in folding of the epidermis with fewer hairs and a thickened dermis with more vascularity (Al-Sagaff et al., 2001). Nevertheless, J. procera essential oil did not cause any adverse effect in terms of discomfort or skin irritations on the human volunteers during and after the study period. Plant-based products are generally regarded to be safe, compared to the synthetic repellents (Novak and Gerberg, 2005).
An. gambiae complex consists of nearly nine morphologically indistinguishable species of mosquitoes. An. arabiensis is one of the major important members in An. gambiae complex with a highly anthropophilic nature (Sharp and Brian, 1991). It is important to note that An. arabiensis is one of the world efficient malarial vectors and it bites throughout night, although the peak biting activity occurs during the dusk and dawn particularly in the early evening hours, whenever and wherever people mostly spend their leisure time. Therefore, even if a person owns ITNs, An. arabiensis can still efficiently transmit the infection. Therefore supplementary personal protection interventions are extremely important to minimize the malaria burden. The present study results are quite encouraging and the essential oil of J. procera could serve as a potential mosquito repellent agent against An. arabiensis.
In these perspectives, plant-based insect repellents could be extremely helpful in avoiding mosquito bites and ultimately disease transmission, when other vector control interventions are impossible. Hence, the essential oil of Ethiopian Ethnomedicinal plant J. procera could serve as a repellent agent against An. arabiensis. The majority of the existing modern synthetic chemical repellents are relatively expensive. Besides their toxicity and adverse side effects, a few of them require electricity for their usage (Karunamoorthi et al., 2008). Plant-based repellents are extremely useful in the inaccessible rural areas, wherever there is a lack of electricity. Furthermore, plant-based repellent products are culturally acceptable, economical and locally known and available (Karunamoorthi et al., 2010b).
5 Conclusion
Since prehistoric era, Tedh has been well-known as an Ethnomedicinal and insect repellent plant in Ethiopia. In the rural area, people have been using this plant as a tooth-brush and as a phyto-therapeutic agent to treat tonsillitis. The results indicate that the essential oil of J. procera has strong repellent activity against An. arabiensis and it has been demonstrated that it is safe as it does not cause any adverse effect to human volunteers. Furthermore, it is economical and very easy to apply. Consequently, the topical application of Tedh essential oil could serve as a supplementary tool to minimize man-vector contact. It is one of the most feasible strategies, wherever the local vectors are exophagic and has the tendency to feed in the early evening hours before people get confined to bednets. However, the bio-active constituents and the mode of action of the essential oil remain unidentified. Therefore, this communication recommends for further studies to isolate and to identify the responsible bio-active molecules and their mode of action to employ Tedh as a potent commercial insect repellent in the near future.
Acknowledgements
The authors sincerely would like to acknowledge Jimma University Research & Publication division for pursuing this research work by providing financial assistance. We would like to thank Mrs. L. Melita for her sincere assistance in editing this manuscript. We are also grateful to the staff members of Adama Malaria Research Centre, Ethiopia for their technical assistance in terms of providing mosquito larvae, without their contribution, this study would have been impossible. Our last but not least heartfelt thanks go to our colleagues from the School of Environmental Health Science, Faculty of Public Health, Jimma University, Jimma, Ethiopia, for their kind support and cooperation.
References
- Toxic effects of diethyltoluamide and dimethylphthalate creams as mosquito repellents on rabbit’s skin. J. Anat. Soc. India. 2001;50(2):148-152.
- [Google Scholar]
- The relationship between female body size and survival rate of the malaria vector Anopheles arabiensis in Ethiopia. Med. Vet. Entomol.. 1996;10:170-172.
- [Google Scholar]
- Mosquito density, biting rate and cage size effects on repellent tests. Med. Vet. J.. 1998;12:39-45.
- [Google Scholar]
- Distribution of African malaria mosquitoes belonging to the Anopheles gambiae complex. Parasitol. Today. 2000;16:74-77.
- [Google Scholar]
- Chromosomal differentiation and adaptation to human environments in the An. gambiae complex. Trans. R. Soc. Trop. Med. Hyg.. 1979;73:483-497.
- [Google Scholar]
- Insecticidal and repellent activities of methanolic extract of Tribulus terrestris L. (Zygophyllaceae) against the malarial vector Anopheles arabiensis (Diptera: Culicidae) Egypt Acad. J. Biol. Sci.. 2012;5(2):13-22.
- [Google Scholar]
- Medicinal and aromatic plants: a major source of green pesticides/risk-reduced pesticides. J. Med. Aromat. Plants. 2012;1:e137.
- [CrossRef] [Google Scholar]
- Plant-based insect repellents: is that a sustainable option to curb the malaria burden in Africa? J. Med. Aromat. Plants. 2012;1:e106.
- [CrossRef] [Google Scholar]
- Prevalence of malaria from peripheral blood smears examination: a 1-year retrospective study from the Serbo Health Center, Kersa Woreda, Ethiopia. J. Infect. Pub. Health. 2009;2(4):171-176.
- [CrossRef] [Google Scholar]
- Knowledge and self-reported practice of the local inhabitants on traditional insect repellent plants in Western Hararghe zone. Ethiopia J. Ethnopharmacol.. 2012;141(1):212-219.
- [CrossRef] [Google Scholar]
- Laboratory evaluation of dimethyl phthalate treated wristbands against three predominant mosquito (Diptera: Culicidae) vectors of disease. Eur. Rev. Med. Pharmacol. Sci.. 2010;14(5):443-448.
- [Google Scholar]
- Insecticide resistance in insect vectors of disease with special reference to mosquitoes: a potential threat to global public health. Health Scope. 2013;2(1):4-18.
- [CrossRef] [Google Scholar]
- Insecticide risk indicators and occupational insecticidal poisoning in indoor residual spraying. Health Scope. 2013;1(4):166-173.
- [CrossRef] [Google Scholar]
- Laboratory evaluation of traditional insect/mosquito repellent plants against Anopheles arabiensis, the predominant malaria vector in Ethiopia. Parasitol. Res.. 2008;103(3):529-534.
- [Google Scholar]
- Knowledge and practice concerning malaria, insecticide-treated net (ITN) utilization and antimalarial treatment among pregnant women attending specialist antenatal clinics. J. Pub. Health. 2010;8(6):559-566.
- [Google Scholar]
- Laboratory evaluation of traditionally used plant-based insect repellent against the malaria vector Anopheles arabiensis Patton (Diptera: Culicidae) Parasitol. Res.. 2010;106(5):1217-1223.
- [CrossRef] [Google Scholar]
- The role of traditional anti-malarial plants in the battle against global malaria burden. Vect.-Borne Zoonot. Dis.. 2013;13(8):521-544.
- [CrossRef] [Google Scholar]
- Anopheles arabiensis and An. funestus are equally important vectors of malaria in Matola coastal suburb of Maputo, Southern Mozambique. Med. Vet./Entomol.. 2000;14:171-180.
- [Google Scholar]
- Malaria during pregnancy: a priority area of malaria research and control. Parasitol. Today. 1995;11:178-183.
- [Google Scholar]
- A transdisciplinary perspective on the links between malaria and agroecosystems in Kenya. Acta Trop.. 2004;89:171-186.
- [Google Scholar]
- Natural-based repellent products: efficacy for military and general public uses. J. Am. Mosq. Cont. Assoc.. 2005;21:7-11.
- [Google Scholar]
- Epellency of essential oils of some plants from the Kenyan coast against Anopheles gambiae. Acta Trop.. 2005;95(3):210-218.
- [Google Scholar]
- Population genetic structure of the malaria mosquito Anopheles arabiensis across Nigeria suggests range expansion. Mol. Ecol.. 2001;10:2577-2591.
- [Google Scholar]
- Aromatic plant-derived essential oil: an alternative larvicide for mosquito control. Fitoterapia. 2007;78:205-210.
- [Google Scholar]
- Comparative sensitivity of four Anopheles (Diptera: Culicidae) to five repellents. J. Med. Entomol.. 1991;28:417-420.
- [Google Scholar]
- Behavioural variation of Anopheles arabiensis (Diptera: Culicidae) populations in Natal, South Africa. Bull. Entomol. Res.. 1991;81:107-110.
- [Google Scholar]
- Repellency of volatile oils from plants against three mosquito vectors. J. Vect. Ecol.. 2001;26:76-82.
- [Google Scholar]
- Repellent activity of Ferronia elephantum Corr. (Rutaceae) leaf extracts against Aedes aegypti. Bioresour. Technol.. 2001;76:287-288.
- [Google Scholar]
- Text Book of Practical Organic Chemistry. London: The English Language Book Society and Longman; 1978. p. 1368
- Wano, M., 2006. Evaluation of Essential Oils of Some Local Plants for their Repellency against Anopheles arabiensis and Aedes aegypti. MSc. Thesis, School of graduate studies, Addis Ababa University, 14p.
- WHO, 1996. Report of the WHO Informal Consultation on the Evaluation and Testing of Insecticides, CTD/WHOPES/IC/96.1, p. 69.
- World Malaria Report, 2012. World Health Organization, Geneva, Switzerland.
- Repellent effect of essential oils against Aedes albopictus. J. Vect. Ecol.. 2005;30:231-234.
- [Google Scholar]







