Translate this page into:
Morphological changes in myocardium of Wistar rat caused by hyperthermia
⁎Corresponding author at: Department of anatomy, Faculty of Medicine, University of Sarajevo, Čekaluša 90, Bosnia and Herzegovina. zurifa.ajanovic@mf.unsa.ba (Zurifa Ajanović),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Aim of our study was to examine whether the intensity of myocardial changes in Wistar rats exposed to hyperthermia was related to the temperature and to classify the degree and type of cardiomyocyte damage.
Methods
The study was conducted on 40 adult Wistar rats that were methodologically divided into groups, depending on water temperature exposure of 37 °C (n = 8), 41 °C (n = 16), and 44 °C (n = 16). Depending on the length of the water temperature exposure time of 41 °C and 44 °C, rats were divided into antemortem (water temperature exposure 20 min) and postmortem groups (water temperature exposure to death). Myocardial sample for pathohistological analysis was taken after the death of the animal. Microscopic examination with semiquantitative analysis of myocardial changes was performed.
Results
Pathohistological analysis determined the normal myocardial structure of control group (animals exposed to water temperature of 37 °C, with mild, degenerative changes in cardiomyocytes nonspecific for hypertemia in rats exposed to water temperatures of 41 °C and 44 °C. Type of myocardial changes in rats in groups exposed to water temperatures of 41 °C and 44 °C was classified as minimal and multifocal degenerative changes (45.25 %; 51.6 %, respectively), which was significantly more pronounced compared to the myocardial control group (p < 0.0005).
Conclusion
The dynamics of the development of morphological changes in cardiomyocytes did not show high significance, which is attributed to the short manifestation time, and does not make it impossible to visualize changes at the subcellular level in tissuestained by hematoxylin-eosin method.
1 Introduction
Exposure to high temperatures and extreme heat over a long period of time can cause the body to overheat. This condition is also known as hyperthermia, and it means that the body can no longer cool itself and return the body temperature to the optimal one. (Bouchama and Knochel, 2002., Mahant, 2015.). There is not enough data in the literature about the role of hyperthermia and unexpected heart-related fatal case. Changes in body temperature beyond normal, physiological limits can be fatal. An increase in internal body temperature even by a few degrees in the body will trigger numerous processes for regulating body temperature (Becker et al., 2018). Previous studies showed that heat can induce tissue injury by disruption of cellular mechanisms, which can result in the breakdown of proteins, the melting of cell membranes, and all of which ultimately leads to their weakening (Bouchama and Knochel, 2002).
When a healthy person is exposed to elevated ambient temperature, numerous physical reactions occur as the body's physiological response to hyperthermia, which play a key role in regulating body temperature (Cui et al., 2005). Previous studies have shown unfavorable heart reactions that occur more often when the ambient temperature is higher (Depasquale and Burch, 1961, Semenza, 1996).
In conditions when the body temperature is above the physiological limit, the cardiac flow rate drops sharply and is associated with increased oxygen usage by myocytes.
Also, signs of heart failure and blood extravasation have been reported as a result of rise in heart rate and vasodilation in response to hyperthermia (Singh et al., 2015).
In order for the blood pressure to remain within physiological limits, the heart rate must increase, even up to 13 L/min in healthy individuals in cases of overheating of the entire body. When the inner rats' body temperature increases from 37 °C to 42 °C, it results in accelerated heart rate, rapid blood flow and increase in vascular friction of 13 % (Nakazawa et al., 1999).
Previous studies of heat-exposed rat myocardial samples have shown focal necrosis as well as myocyte fragmentation, leading to empty intercellular spaces (Singh et al., 2015, Marchand and Gin, 2022). Hypoxia results in a number of lesions in the heart muscle: hemorrhage, cardiomyocyte necrosis, and fibrin fiber rupture. A study by Quin et al. (Quinn et al., 2014) found severe cardiac muscle edema with thinning muscle fibers, with the appearance of hemorrhagic tissue infiltration. Microscopic analysis of the heart after exposure to high temperature showed the occurrence of moderate interstitial sclero-lipomatosis and coronary atherosclerosis, with necrotic foci and myocardial edema (Ceausu et al., 2010).
Numerous deaths during a heat wave are not only the result of overheating of the body but also due to heat stress, which can accelerate the manifestation of a pre-existing medical condition in a person and result in a fatal outcome (Naughton et al.,2002, Wolfe et al., 2001, Vandentorren et al., 2006).
In the view of above, the objective of this research was to examine the effect of temperature and length of exposure to the target temperature on the occurrence of changes in rat cardiomyocytes in the animal model of Wistar rat hyperthermia.
2 Material and methods
The research has been carried out at the Medical Faculty of the University of Sarajevo, Bosnia and Herzegovina, after obtaining approval of the Ethical Committee of Medical Faculty University of Sarajevo under registration number 02–3-4–1253/20, Bosnia and Herzegovina. Animal handling complied with the Principles of Care and Preservation of Laboratory animals. It is as a foresight, randomized control trial done on a Wistar rat hyperthermia induction. As material for this study 40 adult male and female Wistar rats and body mass from 250 g to 300 g were used.
The animals were randomized to one of the following groups, depending on water temperature (WT) exposure: WT 37 °C (n = 8); WT 41 °C, antemortem (n = 8), WT 41 °C, postmortem (n = 8); WT 44 °C, antemortem (n = 8), WT 44 °C, postmortem (n = 8). Antemortem groups were exposed to the target temperature for 20 min, and postmortem groups were exposed to death to the target temperature. Myocardial samples for pathohistological analysis were taken after animal sacrifice. Morphologically descriptive (qualitative) and semiquantitative analysis of the myocardium on preparations stained with the standard hematoxylin-eosin method (HE) were performed.
2.1 Experimental protocol and cardiac sampling for pathohistological analysis of myocardium
Water was poured into the water bath (Memmert GmbH + Co. KG, model size WPE 22), which was heated until the desired temperature was reached. The temperature of the water was constantly followed on the screen integrated in the water bath, and it was verified by immersing the thermometer (Physitemp Thermalert Model TH-8, Physitemp Instruments Clifton, USA) in the water and reading the results.
A pre-anesthetized rat, tied to a panel made of wood, was immersed in water heated to the desired temperature, with its head above the water level. The survival time was noted, which was defined as the time from the immersion of the rat in the water (41 °C and 44 °C) until the moment of fatal outcome. Hyperthermia was defined as an elevated inner body temperature more than 0.5 °C, and heat stroke when the inner body's temperature was above 40 °C (Kidane and Peters, 2020).
Twenty-four hours before the start of the experiment, all animals were weighed to determine the amount of anesthetic. Before the start of the experiment, the anesthetic ketanol (Ketamine Hydrochloride Injection USP Rotexmedica-Germany) was applied to the thigh muscle in a dose calculated according to the formula: 1.2 ml/1 kg body weight +/- 10 % (Flecknell, 2015). The extremities of already anesthetized animals were fixed on a wooden board and tied with rubber bands for easier manipulation. An esophageal probe (RET-4 Probe for mice and rats, Physitemp Instruments Clifton, USA), 5 cm long, was placed along the length of the esophagus and the internal temperature was noted: before exposure to the set water temperature, at the time of immersion of the animal in water and in the 20th minute and after death.
After a period of 20 min, all animals were sacrificed by applying anesthetics in excess, and the procedure of opening the anterior abdominal wall was started. The skin in the symphysis area was lifted with surgical tweezers and cut with scissors. The blunt end of the scissors was inserted into this opening and the skin was cut obliquely, on both sides, to the height of the collarbones. The abdominal muscles were cut in the same way, followed by the diaphragm which was together with the skin, transferred upwards, and the abdominal cavity was accessed. The chest was also opened, which enabled the dissection of the heart for pathohistological analysis (Marinković and Nešić, 2013, Mitchell et al., 2016).
The dissection-obtained heart sample was fixed in 10 % buffered, neutral formalin. Paraffin molding was performed according to standard procedures. Histological incisions 4–5 µm thick were made from paraffin blocks. The obtained paraffin sections were stained by the standard HE method. The anterior wall of the left ventricle was microscopically analyzed (Rampy and Glassy, 2016).
Semiquantitative analysis of the myocardium included two parameters. For the first type of myocardial changes, division and gradation were taken from Obrien et al. (O’Brien et al., 2006). All incisions were examined without prior information to the pathologist about the treatment of the animal.
2.2 Statistical analysis
The Statistical Package for Social Sciences (SPSS) program, version 25.0 was utilized for statistical analysis. The Shapiro Wilk test was utilized for the determination of the normal distribution of the tested variants. To test the significance of the difference between the observed parameters between several groups, ANOVA (analysis of variance) was used, ie the Kruskal-Wallis test if the variables did not follow the normal distribution. A statistically significant difference when comparing three more groups with variables having a normal distribution was accompanied by post hoc tests — Bonferroni or Dunett depending on whether the same variant was satisfied or not. If a significant difference of the observed variants in three or more groups was found for the variables without a normal distribution, the comparison of the two groups was performed using the Mann-Whitney test. Accepted statistical significance was at the level of p < 0.05.
3 Results
No statistically significant difference was found (p = 0.125) in basal body temperatures of rats in the observed groups (Table 1). Reaction in the change in body temperature of rats was the largest in the group exposed to water heated to 44˚C (p = 0.007). Measurement of body temperature after 20 min of exposure to different water temperatures showed the highest mean value in the group exposed to a temperature of 44˚C (p < 0.0005). Significant changes in rat body temperature were observed in animals with fatal outcome (p < 0.0005) (Fig. 1). KG37-control group of rats exposed to water temperatures of 37˚C; G41-group of rats exposed to water temperatures of 41˚C; G44- group of rats exposed to water temperatures of 44˚C; N- number of rats; p-probability; 1-no degenerative changes; 2-minimal and multifocal changes.
Type of myocardial changes
Group
In totalp
KG37
G41
G44
1
N°
6
1
0
7
<0,0005
% in change group 1
85.7 %
14.3 %
0.0 %
100.0 %
2
N°
1
14
16
31
% percentage in change group 2
3.2 %
45.2 %
51.6 %
100.0 %
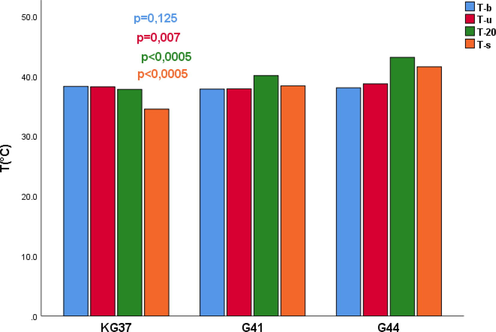
Influence of water temperature of 37˚C, 41˚C and 44˚C on the body temperature of rats measured at four time points in the examined groups. T (° C) - Water temperature in degrees Celsius; T-b- basal temperature of rats;T-u Immersion temperature; T-temperature at 20 min; T-s- death temperature; p-probability; KG37-control group of rats exposed to water temperatures of 37˚C; G41 group exposed to water temperature 41˚C; G44-group of rats exposed to water temperatures of 44˚C;
The basal temperatures of the animals in the examined groups did not significantly differ (p = 0.325). After immersion in water, the difference in body temperature in the groups of rats was significantly different (p = 0.022) (Fig. 2). The greatest changes in body temperature were noticed between the groups at the temperature after 20 min of exposure to the target water temperature and at the time of death p < 0.0005 (Fig. 2).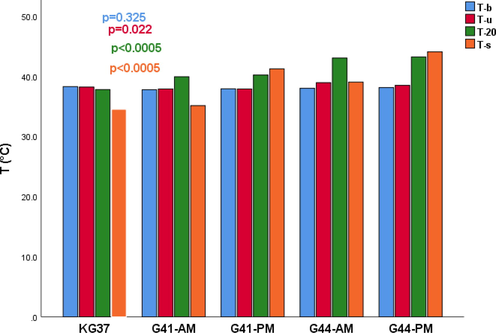
Influence of the length of water temperature exposure on the body temperature of Wistar rats in the examined groups. T (°C) - Water temperature in degrees Celsius; T-b- basal temperature of rats;T-u Immersion temperature; T-20-temperature at 20 min; T-s- death temperature; p-probability; KG37-control group of rats exposed to water temperatures of 37˚C; G41-AM-antemortem group exposed to water temperature 41˚C (exposure length 20 min); G41-PM- postmortem group exposed to water temperature 41˚C (length of exposure to death); G44-AM-antemortem group of rats exposed to water temperatures of 44˚C (exposure length 20 min); G44-PM-postmortem group of rats exposed to water temperatures of 44˚C (length of exposure to death);
No morphological changes were noticed in the left ventricular myocardium of a group of rats exposed to a water heated to 37˚C (Fig. 3). Most cardiomyocytes are longitudinally oriented with large oval to rectangular nuclei. Between the myocytes, a fine interstitial space with fibroblasts and capillaries lined up next to the endothelial cells was noted. Erythrocytes are present within the capillaries.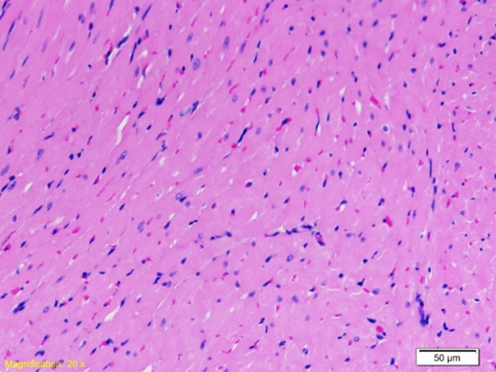
Myocardium of the left ventricle of rat exposed to a water temperature of 37˚C. First degree of change: no degenerative changes; uniform-looking myocardial cells (HE 20X).
In the myocardium of rats exposed to WT of 41˚C and 44˚C, the type and extent of lesions were analyzed depending on the desired WT and duration of exposure The group of animals exposed to a temperature of 37˚C was without degenerative changes in the myocardium, while in other groups exposed to WT of 41˚C and 44˚C, minimal and multifocal degenerative changes developed, with the occurrence of mineralization in some cases. Analyzing the extent of the changes, damage to individual cardiomyocytes was noticed in terms of loss of transverse striation and homogenization of the cytoplasm, which was the type of mild damage to individual cardiomyocytes in the group exposed to 41˚C and 44˚C, with sometimes shown greater focal damage to cardiomyocytes in the postmortem group exposed to a temperature of 44˚C (Fig. 4).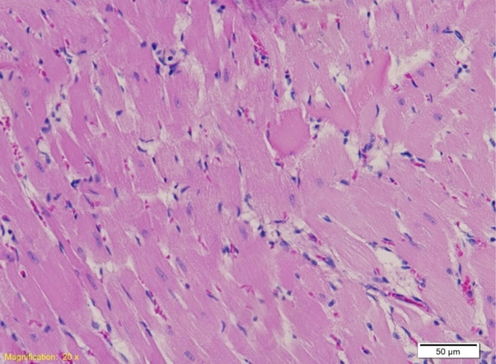
Left ventricle myocardium of rat exposed to a temperature of 41˚C. Second degree of change: minimal damage to individual cardiomyocytes, most cells of regular appearance (HE, 20X).
Second degree changes were manifested through minimal and multifocal changes, with minimal damage to individual cardiomyocytes. The usual structure of the sarcoplasm was noticed, in which the network connection of individual cardiomyocytes is presented. Blood vessels are lined with endothelial cells whose nuclei are large and darker in color. The development of more extensive myocardial changes included the appearance of multiple cardiomyocyte damage with noticeable lighter zones of the sarcoplasm (Fig. 5).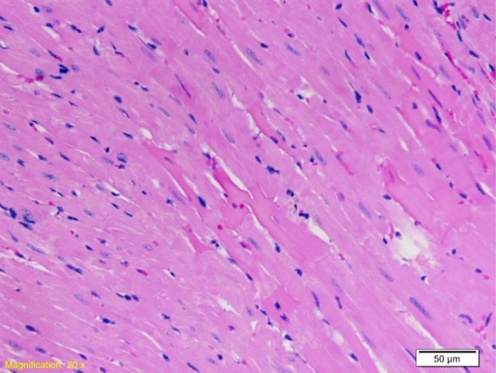
Left ventricle myocardium of rats in the postmortem group exposed to a water temperature of 41˚C. Second degree of change with multiple damage to myocardial cells (in the central part of the microphotograph) (HE 20X).
In the myocardium of rats of the examined group that was exposed to water temperature from 44˚C to death, more frequent focal, extensive damage to cardiomyocytes was noticed, which is also change with the greatest degree of extensiveness. We monitored the occurrence of degenerative changes, which imply the loss of the usual structure of the sarcoplasm, in which the network connection of individual cardiomyocytes is not presented. Areas of myofibrillar degeneration are presented as lighter zones of the myocardium in which cardiomyocytes have lower affinity for color. The nuclei are altered in shape and position, pycnotic, and the nucleoli stand out only in individual cardiomyocytes. Blood vessels are lined with endothelial cells whose nuclei are large and darker in color (Fig. 6).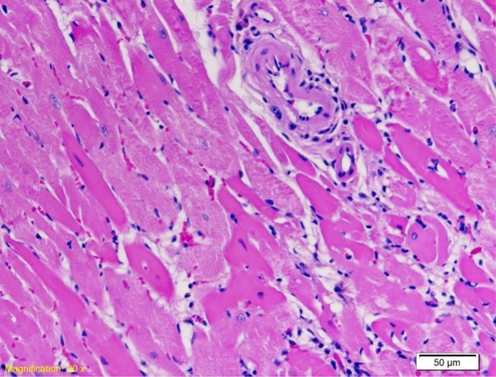
Left ventricle myocardium of rat exposed to water temperature of 44˚C to death. Second degree changes with greater focal damage to myocardial cells (HE 20X).
No degenerative changes were observed in 7 rats, which corresponds to the first degree based on the classification of the type of myocardial change. Minimal and multifocal changes corresponding to the second degree type of changes occurred in one rat of the control group, in 14 rats in the group exposed to WT of 41˚C and in 16 rats in the group exposed to WT of 44˚C. The type of third, fourth and fifth degree changes was not determined on the myocardium of rats of the experimental groups (Table 1).
No damage to individual cardiomyocytes was noticed in 18 rats, which corresponds to the first degree based on the classification of the extent of myocardial changes. Multiple cardiomyocyte lesions, corresponding to the second degree of myocardial change, developed in 1 rat in control group, 9 rats in the 41˚C exposed W group, and 8 rats in the 44˚C exposed WT group. Greater focal to locally extensive cardiomyocyte damage corresponding to the third degree of myocardial changes was found in 2 rats in the group exposed to a water temperature of 44 °C. The extent of fourth-degree changes did not develop in any of the myocardium of rats of the experimental groups (Table 2). KG37-control group of rats exposed to water temperatures of 37˚C; G41-group of rats exposed to water temperatures of 41˚C; G44- group of rats exposed to water temperatures of 44˚C; N- number of rats; p-probability; 1-minimal (damage to individual cardiomyocytes); 2-gentle (multiple cardiomyocyte damage); 3-moderate (major focal to local extensive cardiomyocyte damage);
Extent of myocardial changes
Group
In total
p
KG37
G41
G44
1
N °° 6
6
6
18
0.109
% in change group 1
33.3 %
33.3 %
33.3 %
100.0 %
2
N°
1
9
8
18
% in change group 2
5.6 %
50.0 %
44.4 %
100.0 %
3
N°
0
0
2
2
% in change group 3
0 %
0 %
100 %
100 %
4 Discussion
Recognizing hyperthermia as a cause of fatal outcomes is a great challenge in forensic medicine, due to non-specific, inaccurate findings as well as the absence of macroscopic and microscopic lesions.Although it is known that the cardiovascular system is the place where the negative effects of heat stress are first manifested, the mechanisms by which heat stress leads to the damage of heart muscle are still unknown (Shahzad et al., 2020).
The objective of this research was to evolve and use animal model of rat hyperthermia, and to investigate the influence of temperature and length of exposure to target temperature on rat cardiomyocytes. Our results are in accordance with the literature data which indicate that the minimum time required for the occurrence of heat stroke as a consequence of hyperthermia, with the compensatory mechanisms of the organism, is 20 min from the moment of action of the target temperature (Suzuki and Hori, 2014).
In our investigation, we focused on the search for pathohistological changes of the myocardium, which were subacute in nature, and reflected in the occurrence of damage such as minimal and multifocal changes with mild, ie. multiple cardiomyocyte damage. Pathohistological changes were not specific for hyperthermic damage. Similar changes were found by Chen et al. (Chen et al., 2015), such as karyopycnosis and degenerative changes in heart fibers. The intensity of the changes was related length of time of exposure to high temperatures. In a study by Ceaus et al. (Ceausu et al., 2010) the presence of myocardial fibrosis on human, autopsy material is stated.
Pathohistological analysis of myocardial tissue showed that myocardial fiber lesions were not specific for hyperthermia. Pathohistological analysis of the myocardium showed that in 45.25 % of rats in the group exposed to WT of 41˚C, and 51.6 % of rats in the group exposed to WT of 44˚C had changes in the myocardium of the type of minimal and multifocal changes, which is statistically significant in relation to rats exposed to a WT of 37˚C, p < 0.0005. Analysis of the intensity of pathohistological changes showed that type 2 change (multiple cardiomyocyte damage) dominated in groups of rats exposed to water temperatures of 41˚C (50 %) and 44˚C (44.4 %), while type 1 changes (damage to individual cardiomyocytes) were equally represented in all three groups (33.3 %), which was not statistically significant (p = 109). Results from Zhang et al. (Zhang et al., 2013) are in agreement with our results, where they recorded myocardium cells. They found enlarged and swollen myocytes, with dilated and blocked blood vessels inside the muscles, swelling of interstitial tissue as well as a large number of inflammatory cell elements after rats were exposed to hyperthermia in a simulated climate cabin.
A study on Sprague-Dawley rats exposed to heat stress were used until the internal temperature reached above 39 °C showed pathohistological changes of cardiomyocytes as branched anastomotic cells with acidophilic sarcoplasm and central elongated vesicular nuclei were noticed. Edematous areas between cells were observed in the group of rats whose internal temperature was above 40 °C, while cardiac muscle fibers with focal erythrocyte extravasation were present in the group of rats whose internal temperature was measured above 41 °C (Bouchama and Knochel, 2002). The severity of pathohistological changes in the heart increased with increasing temperature and length of exposure, which is in agreement with our results.
The results of previous research have shown that the final acceleration of the function of the sympathetic system, which includes the medulla of the adrenal gland, may be one of the causes of a strong reaction to physical stress during death caused by hyperthermia and consequent histological changes in internal organs (Ishikawa et al., 2008, Ishikawa et al, 2010), as found on heart muscle in our study. Obvious histopathological lesions, such as degeneration and necrosis of myocardium were observed in acute heat stressed broilers (Endong et al., 2004).
Findings on an electron microscope of myocardium showed vascular stasis in the blood vessels between the fascicles, with partly unorganized myocardiocytes, more often damage to mitochondria and dilated endoplasmic reticulum. In the myocardiocytes nuclei, nuclear material was condensed. Large areas of damage with mitochondrial disorganization of atrial natriuretic peptide were found in the myocardium and enlarged interfascicular space by the interstitial edema (Vlad et al., 2013).
Data on dilated right heart ventricle, subendocardial bleeding of the left ventricle, visceral congestion and petechial hemorrhages have been found in the literature in people who died of heat stress (Bunai et al., 2008, Zhou et al., 2006). Ultrastructurally, a territory with excessively stretched myofibrils and contraction bands were associated with disturbance of the sarcolemma and extensive areas of myofibrolvsis throughout the heart is described in patients wiht malignant hyperthermia (Fenoglio and Irey, 1977).
An investigation by Singh et al. (Singh et al., 2015) found the separation of muscle fibers in the presence of edematous spaces between them when the body temperature raised up to 39˚C, while 40˚C body temperature caused, focal extravasation of erythrocytes was observed. In the group in which the analysis was performed postmortem, large areas of hemorrhage in the heart muscle were noticed and edema with thin muscle fibers was noticed. The lesions were in the form of focal areas of necrotic fibers with loss of normal cellular structure with the fragmentation of the microfilament with changes in the nucleus, which led to the development of so-called “empty spaces”, which is also proven in other studies (Walter and Carraretto, 2016, Costache and Stoian, 2007).
Significant changes in the myocardium occur due to the increased capacity of the cardiovascular system due to the greater work required to adapt to heat stress by increasing cardiac output, vasodilation, and increased blood flow. The intensity of changes is consistent with the intensity of heat stress (Randall et al., 1995, Ananya et al., 2005).
Magda A et al stated that histological and ultrastructural changes in the myocardium after exposure to hyperthermia could be caused by mast cells. The study showed a localized area of myocardial necrosis, an area with the formation of a vacuole and the disappearance of a specific striated appearance with the accumulation of inflammatory mononuclear cells, as well as occlusion of blood vessels and signs of extravasation, and the accumulation of edematous fluid between the hearts muscle fibers, which is in agreement with our results (Magda et al., 2008). In our study, on the myocardium of rats exposed to WT of 41˚C and 44˚C, zones of multiple cardiomyocyte damage with lighter sarcoplasmic zones were observed. These changes were greater in extent and diameter in rats exposed to WT of 44˚C.
Investigations after the occurrence of death are important to rule out other possible causes of fatal outcomes, not to establish the actual cause of the fatal outcomes. Many questions need to be clarified and answered, and much more comprehensive research needs to be conducted to establish the exact mode of influence of temperature and length of exposure to target temperature on cardiomyocyte changes. In the future, additional knowledge could lead to improvements in the therapeutic sense and application in daily clinical work.
5 Conclusion
The severity of myocardial pathohistological changes increased with increasing temperature and the duration of exposure of the animal to elevated WT. The dynamics of the development of morphological changes in cardiomyocytes did not show high significance, which is attributed to the short manifestation time, which does not make it impossible to visualize changes at the subcellular level in tissue stained by hematoxylin-eosin method.
Funding: None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Oxidative stress and histopathological changes in the heart following orallindane (gamma hexachlorohexane) administration in rats. Med. Sci. Monit.. 2005;11:325-329.
- [Google Scholar]
- Fatal hyperthermia associated with excited delirium during an arrest. Led. Med. (Tokyo). 2008;10:306-309.
- [Google Scholar]
- Morphological diagnosis of hyperthermia-related deaths. Rom. J. Leg. Med.. 2010;18:239-246.
- [Google Scholar]
- Localization and expression of heat shock protein 70 with rat myocardial cell damage induced by heat stress in vitro and in vivo. Mol. Med. Rep.. 2015;11:2276-2284.
- [Google Scholar]
- Costache, E., Stoian, M.G., 2007. Connections made by forensic investigation between judiciary toxicology and forensic medicine. RJLM. 15, 219-228.
- Effects of Heat Stress on Thermoregulatory Responses in Congestive Heart Failure Patients. Circulation.. 2005;112:2286-2292.
- [Google Scholar]
- The seasonal incidence of myocardial infarction in New Orleans. Am. J. Med. Sci.. 1961;242:468-474.
- [Google Scholar]
- Endong, B., Yuanying, G., Hartung, J., et al. 2004. Relation Between Pathologic Damages and HSP70 Changes in Acute Heat Stressed Broilers Zhongguo Nong ye ke xue = Zhongguo Nongye Kexue. 37, 301-305.
- Laboratory animal anesthesia (4th Edition). San Diego: Academic Press Limited; 2015.
- Postmortem biochemistry and immunohistochemistry of adrenocorticotropic hormone with special regard to fatal hypothermia. Forensic. Sci. Int.. 2008;179:147-151.
- [Google Scholar]
- Immunohistochemistry of catecholamines in the hypothalamic-pituitary-adrenal system with special regard to fatal hypothermia and hyperthermia. Leg. Med. (Tokyo). 2010;12:121-127.
- [Google Scholar]
- Histological study on the effect of heat stress on cardiac muscle of adult male albino rats and the possible role of mast cells. Egypt. J.. 2008;31:220-232.
- [Google Scholar]
- The evaluation and management of heat injuries in an intensive care unit. Indian J. Crit. Care. Med.. 2015;19:479-483.
- [Google Scholar]
- Pocket Companion to Robbins and Cortan Pathologic Basis of Disease (9th Edition). Elsevier Science; 2016.
- Effects of Environmental Hyperthermia on Cardiovascular Function in the Rat Embryo. Ped. Res.. 1999;30:6.
- [Google Scholar]
- Heat-related mortality during a 1999 heat wave in Chicago. Am. J. Prev. Med.. 2002;22:221-227.
- [Google Scholar]
- Cardiovascular and thermoregulatory biomarkers of heat stroke severity in a conscious rat model. J. Appl. Physiol. 2014:1978-1988.
- [Google Scholar]
- Pathology gross photography: the beginning of digital pathology. Clin. Lab. Med.. 2016;36:67-87.
- [Google Scholar]
- Heat-related deaths during the July 1995 heat wave in Chicago. N. Engl. J. Med.. 1996;335:84-90.
- [Google Scholar]
- Shahzad, A., Khan, A.A., Awais Arif, M., Yousaf, Z. Acute Myocarditis in a Patient with Exertional Heat Illness: A Rare Association. EJCRIM 2020;7.
- Histological changes in mammalian liver and heart in response to graded hyperthermia. IJCPCR.. 2015;5:184-218.
- [Google Scholar]
- Experimental investigation in rats to identify the cause of sudden death during bathing in Japan. AMS.. 2014;1:101-104.
- [Google Scholar]
- August 2003 heat wave in France: risk factors for death of elderly people living at home. Eur. J. Public. Health.. 2006;16:583-591.
- [Google Scholar]
- Electron microscopy of the morphological changes in rat viscera during experimental hyperthermic shock. Journal of Medicine and Life.. 2013;6:55-60.
- [Google Scholar]
- The neurological and cognitive consequences of hyperthermia. Crit. Care.. 2016;20:199.
- [Google Scholar]
- Heat-related mortality in selected United States cities, summer 1999. Am. J. Forensic. Med. Pathol.. 2001;22:352-357.
- [Google Scholar]
- Expression change of TNF-α in myocardium and hepatic tissue of rats with compound stress of hyperthermia and lipopolysaccharide. Asian Pacific Journal of Tropical Medicine. 2013:300-304.
- [Google Scholar]
- Heat stroke deaths caused by electric blanket: case report and review of the literature. Am. J. Forensic. Med. Pathol.. 2006;27:324-327.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102473.
Appendix A
Supplementary material
The following are the Supplementary data to this article:






