Translate this page into:
Morphological and molecular phylogenetic analysis of Bivesicula claviformis Yamaguti, 1934 infecting the tomato hind Cephalopholis sonnerati (Serranidae) in Saudi Arabia
⁎Corresponding author. rabdelgaber.c@ksu.edu.sa (Rewaida Abdel-Gaber)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Groupers are large predators in the Serranidae family that feed on fish and small invertebrates. Identification of parasitic taxa of groupers was done concerning their morphological and biological characteristics. A total of 30 Cephalopholis sonnerati (Serranidae) specimens were investigated for digenetic trematodes in the Red Sea, Saudi Arabia. Only one bivesiculid species, belonging to the Bivesiculidae family, has been identified, with a prevalence rate of 60% (18/30) among infected fish. The Bivesicula species obtained from the fish host’s intestine is morphologically and morphometric compatible with Bivesicula claviformis, which was previously identified from Epinephelus fasciatus (Serranidae) in North Borneo. In addition, maximum parsimony based on Tamura-Nei model was utilized to determine the phylogeny of the recovered Bivesicula species using partial small subunit ribosomal RNA gene (18S rRNA) and large subunit ribosomal RNA gene (28S rRNA) sequences. The query sequences showed the identities of 99.39% and 94.02% for 18S (AJ287485.1) and 28S rRNA (AY222182.1) of the previously described Bivesicula claviformis, respectively. The present study demonstrated that Bivesicula claviformis is the first reported of this genus as endoparasites from C. sonnerati, as well as providing novel DNA data for this species.
Keywords
Host-specificity
Serranidae
Bivesiculata
Phylogeny
1 Introduction
The Red Sea and the Arabian Gulf are both accessible to Saudi Arabia. The Red Sea provides more than half of the country’s marine fish (Shellem et al., 2021). The family Serranidae Swainson, 1839 sometimes known as groupers, is one of Saudi Arabia’s most important food fish (Hariri et al., 2000). Groupers have a lot of biodiversities, and they are known to have various parasite fauna (Rohde and Heap, 1998). Identification for parasitic taxa of groupers associated with their morphological and biological peculiarities (Justine et al., 2010).
The Bivesiculidae Yamaguti, 1934 is a small family of trematodes that mostly infects the intestines of marine fish (Cribb, 2002; Trieu et al., 2015). Yamaguti (1934) established the genus Bivesicula for a single species, Bivesicula claviformis, as the first representative of Bivesiculidae. There are currently 27 accepted species from five recognized genera, Bivesicula Yamaguti, 1934, Bivesiculoides Yamaguti, 1938, Treptodemus Manter, 1961, Paucivitellosus Coil et al., 1965, and Treptodemoides Shen, 1995 (WoRMS, 2021). Bivesiculids have just two hosts in their life cycle, and the cercariae emerge from the snail and are consumed without encysting by the final fish host (Holocentridae, Muraenidae, and Serranidae) (Le Zotte, 1954; Pearson, 1968; Mani, 1989). Cribb et al. (1998) hypothesized that bivesiculids may have three host life cycles, due to the isolation of metacercaria of Bivesicula claviformis from the intestine of Thalassoma lunare from Lizard Island. The three host life cycles enable bivesiculids to infect large carnivorous fish (Trieu et al., 2015).
The genus Bivesicula is the most complicated genus within Bivesiculidae, with 16 recognized species and several undefined “types” represented by a single morphological specimen (WoRMS, 2021). The type-species of the genus, Bivesicula claviformis, is the most problematic. Because of the significant morphological resemblance across Bivesicula species, genetic evidence is needed to supplement traditional techniques of species classification. Recent research has shown that the ribosomal DNA (rDNA), which is made up of coding regions (18S, 5.8S, and 28S) as well as a non-coding region of two internal transcribed spacers (ITS-1, ITS-2) and one non-transcribed spacer (NTS), may be used to identify parasites (Wei et al., 2006).
There is no sufficient information about the infection with Cephalopholis sonnerati. Therefore, the study was designed to investigate more about the morphology and phylogeny of one bivesiculid in this fish species from the Red Sea in Saudi Arabia.
2 Materials and methods
2.1 Fish collection and parasitological study
During the period of January-September 2021, 30 fish specimens of the tomato hind Cephalopholis sonnerati (F: Serranidae) were bought from fish markets of the Red Sea coastlines in Jeddah, Saudi Arabia. A comprehensive examination of each fish was conducted to discover metazoan parasites using the gut-wash method (Cribb and Bray, 2010). Flukes were removed, washed in saline, and then preserved in a buffered formalin solution (10%) for morphological study, or kept immediately in 100% ethanol for DNA extraction. Worms were stained with Semichon’s acetocarmine, cleared with clove oil, and mounted with Canada balsam for entire mounts. Using Leica DM 2500 microscope (NIS ELEMENTS software, ver. 3.8), photographs of each parasite species (along with comprehensive photos of diagnostic morphological features) were taken at different magnifications. Drawing of the recovered parasite specimens was done with the help of camera Lucida. According to Bush et al. (1997), the prevalence and mean intensity of parasite species were calculated. The range of measurements was given in millimeters (mean in parentheses).
2.2 Molecular analysis
The genomic (g) DNA was extracted using the Qiagen DNeasy Tissue Kit© (Hilden, Germany) according to the steps of the manufacturer. For identification of the recovered parasite, partial 18S rRNA and 28S rRNA regions were amplified using polymerase chain reaction (PCR). Indaryanto et al. (2015) designed primers for 18S rRNA PCR amplification that used in this study of 18SU467_F: 5′–ATC CAA GGA AGG CAG CAG GC–3′ and 18SL1170_R: 5′–GTG CCC TTC CGT CAA TTC CT–3′. Furthermore, Lee et al. (2007) designed primers that were used here for 28S rRNA of JB10_F: 5′–GAT TAC CCG CTG AAC TTA AGC ATA–3′ and JB9_R: 5′–GCT GCA TTC ACA AAC ACC CCG ACT C–3′. The following thermocycling profile was used: 1 cycle of 94 °C for 3 min, 35 cycles at 94 °C for 30 s, 62 and 66 °C (for 18S rRNA and 28S rRNA, respectively) for 30 s, 72 °C for 2 min, and 1 cycle of final extension at 72 °C for 7 min. QIAquick™ PCR Purification Kit (Qiagen, Hilden, Germany) was used to purified the PCR amplicons. The PCR products were sequenced (see details in Abdel-Gaber et al., 2020). Under accession numbers OK181883.1 and OK353794.1, nucleotide sequences were submitted in GenBank (NCBI, Bethesda, Maryland). To find the closest sequences, BLASTn compared the sequences in the NCBI database. The sequences were aligned using the BioEdit (Hall, 1999) and ClustalW multiple alignment software (Thompson et al., 1994). MEGA 7.0 was used to construct a phylogenetic tree using the Maximum Likelihood (ML) approach (Kumar et al., 2016) with bootstrap analysis (heuristic search of 1000 replicates) by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances and all positions containing gaps and missing data were eliminated.
3 Results
The investigated tomato hind, Cephalopholis sonnerati, was naturally infected with a digenean parasite with a particular location for the intestine in 18 out of thirty (60%) fish specimens. This parasitic species was identified as Bivesicula claviformis Yamaguti, 1934. The parasite’s mean intensity in each parasitized fish does not exceed six.
3.1 Description
The body was spindle-shaped, oculate, and covered with minute spines. There were no oral or ventral suckers. Pharynx had oval-shaped. Oesophagus was narrow. Ceca were simple and ended at the level of the posterior end of the testis. Location of a single large testis was middle and post-equatorial. Cirrus sac was oval and situated in the middle portion of the body. External vesicula seminalis was located immediately in front of the cirrus sac. Internal vesicula seminalis was found near the cirrus sac’s anterior end. The space inside the cirrus sac was being filled by pars prostatica. Genital atrium was small and inconspicuous. Genital pore was equatorial. Ovary was sub-median, spherical, and on the right side of the body. Shell gland was posteromedial to the ovary. Uterus extends beyond the posterior end of the testis and then ascends. About 25 eggs were collapsed. Numerous vitelline follicles extending from the oesophageal level to the level of the posterior end of the testis make up vitellaria. The vitelline reservoir was dorsal, between ovary and testis. Excretory vesicle was V-shaped with arms that reached the level of the oesophagus. Excretory pore was located near the body’s posterior extremity (Figs. 1 and 2).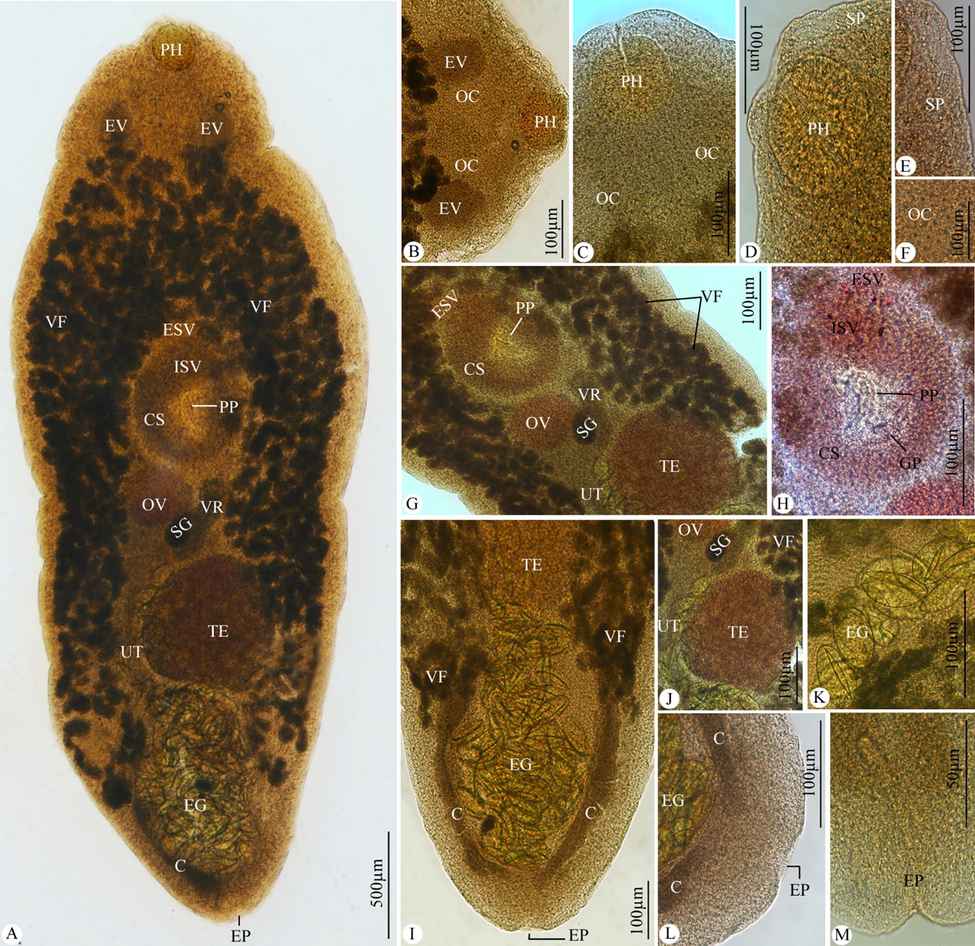
Photomicrographs of Bivesicula claviformis infecting Cephalopholis sonnerati. (A) Whole-mount preparation. (B-F) Anterior portion of the body. (G, H) Middle portion of the body. (I-M) Posterior portion of the body. Note: C, ceca; CS, cirrus sac; EG, eggs; EP, excretory pore; ESV; external seminal vesicle; EV, excretory vesicle; GP, genital pore; ISV, internal seminal vesicle; OC, ocular region; OV, ovary; PH, pharynx; PP, pars prostatica; SG, shell gland; SP, spinules; TE, testis; UT, uterus; VF, vitelline follicles; VR, vitelline reservoir.
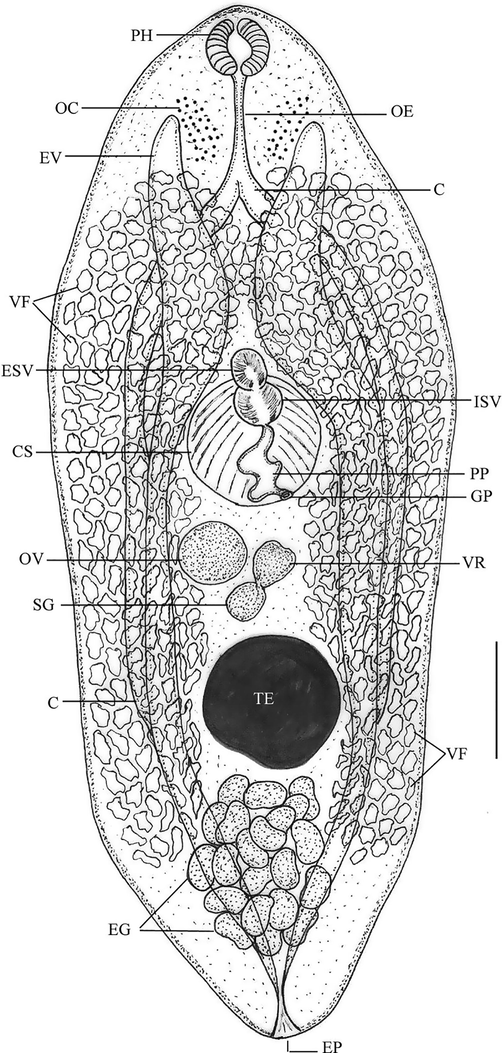
Line drawing for the whole mount of the recovered Bivesicula claviformis. Scale bar = 200 µm. Note: C, ceca; CS, cirrus sac; EG, eggs; EP, excretory pore; ESV; external seminal vesicle; EV, excretory vesicle; GP, genital pore; ISV, internal seminal vesicle; OC, ocular region; OE, oesophagus; OV, ovary; PH, pharynx; PP, pars prostatica; SG, shell gland; SP, spinules; TE, testis; UT, uterus; VF, vitelline follicles; VR, vitelline reservoir.
3.2 Measurements
Body 1.043–1.546 (1.223) long × 0.651–0.692 (0.670) wide, pharynx 0.143–0.221 (0.168) long × 0.173–0.232 (0.183) wide, testis 0.185–0.256 (0.221) long × 0.183–0.264 (0.242) wide, cirrus sac 0.258–0.340 (0.291) long × 0.215–0.272 (0.252) wide, ovary 0.139–0.146 (0.142) long × 0.127–0.198 (0.183) wide, eggs 0.078–0.089 (0.085) long × 0.038–0.054 (0.047) wide (Table 1).
Comparable parameters
Yamaguti (1934)
Nagaty (1948)
Manter (1961)
Fischthal and Kuntz (1965)
Cribb et al. (1994)
The present study (2022)
Host
Seriola quinquoradiata, Parapristipoma trilineatum
Serranus fasciatus
Epinephelus merra
Epinephelus fasciatus
Epinephelus quoyanus
Cephalopholis sonnerati
Locality
Inland Sea
Abu Luli, Red Sea
Fiji
North Borneo
Heron Island
Saudi Arabia
Infection site
Small intestine
Intestine
Intestine
Small intestine
Intestine
Intestine
Body
L
0.84–1.25
1.3–1.7
1.373–1.600
1.040–1.510
0.611–1.665 (1.205)
1.043–1.546 (1.223)
W
0.36–0.55
0.519–0.572
0.603–0.710
0.645–0.680
0.203–0.443 (0.345)
0.651–0.692 (0.670)
Pharynx
L
0.095–0.13
0.167–0.180
–
0.140–0.201
0.078–0.158 (0.119)
0.143–0.221 (0.168)
W
0.1–0.16
0.153–0.239
–
0.172–0.218
0.084–0.142 (0.125)
0.173–0.232 (0.183)
Testis
L
0.16–0.18
0.203
–
0.182–0.235
0.039–0.150 (0.107)
0.185–0.256 (0.221)
W
0.13–0.21
0.261
–
0.181–0.290
0.036–0.159 (0.099)
0.183–0.264 (0.242)
Cirrus sac
L
0.15–0.25
–
–
0.242–0.336
0.094–0.303 (0.199)
0.258–0.340 (0.291)
W
0.11–0.14
–
–
0.201–0.266
0.066–0.123 (0.100)
0.215–0.272 (0.252)
Ovary
L
0.074–0.11
0.135
–
0.136–0.142
0.050–0.100 (0.083)
0.139–0.146 (0.142)
W
0.06–0.11
0.104
–
0.125–0.194
0.048–0.094 (0.078)
0.127–0.198 (0.183)
Eggs
L
0.087
0.081–0.09
0.082–0.096
0.076–0.082
0.075–0.083 (0.079)
0.078–0.089 (0.085)
W
0.047
0.050–0.086
0.037–0.048
0.037–0.055
0.035–0.052 (0.042)
0.038–0.054 (0.047)
3.3 Molecular analysis
3.3.1 18S rRNA gene
The examined digenean species’ partial 18S rRNA sequence was 980 bp with a GC content of 51.4% and deposited in GenBank under the accession number OK181883.1. The ML approach was used to align nucleotide sequence data from 32 taxa (including the outgroup) over 944 positions to produce a phylogenetic dendrogram that represented two Digenea and Aspidogastrea subclasses (Table 2). The genus Bivesicula was validated by pairwise comparison with the GenBank 18S rRNA gene data set, although non-species identification of previously deposited Bivesicula species was not (Fig. 3). The current phylogenetic dendrogram is divided into two main clades (Fig. 4), the first of which clustered taxa representing different orders of Digenea, and the second of which clustered two genera of Strigeidida (AB551567.1 Liolope copulans and AY829255.1 Spirorchid sp.), as well as two genera of Aspidogastrea (AY222083.1 Cotylaspis sp. and DQ482610.1 Multicalyx elegans), and the latter considered as out-group. The first major clade was split into two lineages, the first of which included Plagiorchiida and Opisthorchiida species with high support value (85). With a value of 100, the second lineage was highly supported by the Bivesiculidae family taxa. Cryptogonimidae appears as a sister group to Apocreadiidae. Haplosplanchnidae is also related to Bucephalidae + Diplodiscidae + Paramphistomidae. There was a strong relation (100) between Psilostomidae and Echinostomatidae + Philophthalmidae. There are sister relationships between Lepocreadiidae and Gyliauchenidae + Gorgocephalidae. A close relationship with strong support (98) was observed between Acanthocolpidae + Choanocotylidae and Deropristidae + Gorgoderidae + Haploporidae. Based on sequence comparisons, the sequence representing B. claviformis clustered with the sequence represented by AJ287485.1 for the previously deposited sequence for B. claviformis. These sequences resolved as a strongly supported monophyletic clade together with sequences representing B. unexpecta (AY222099.1), B. fusiformis (AY222100.1), and Paucivitellosus hirudinaceus (AJ287557.1).
Parasite species
Order, Family
Host species
% Identity
% GC content
Reference
AJ287485.1 Bivesicula claviformis
Azygiida, Bivesiculidae
Epinephelus quoyanus
99.39
50.9
Cribb et al. (2001)
AY222099.1 Bivesicula unexpecta
Azygiida, Bivesiculidae
Acanthochromis polyacanthus
96.73
51.3
Olson et al. (2003)
AY222100.1 Bivesicula fusiformis
Azygiida, Bivesiculidae
Atherinomorus lacunosus
94.30
49.9
Olson et al. (2003)
AJ287557.1 Paucivitellosus fragilis
Azygiida, Bivesiculidae
Crenimugil crenilabis
94.29
50.2
Cribb et al. (2001)
AY222110.1 Indosolenorchis hirudinaceus
Plagiorchiida, Paramphistomidae
Dugong dugon
91.50
51.1
Olson et al. (2003)
AJ287563.1 Preptetos caballeroi
Plagiorchiida, Lepocreadiidae
Naso vlamingii
91.34
49.9
Cribb et al. (2001)
MN700961.1 Skrjabinopsolus nudidorsalis
Plagiorchiida, Deropristidae
Acipenser ruthenus
91.18
50.4
Sokolov et al. (2020)
AY222135.1 Psilochasmus oxyurus
Plagiorchiida, Psilostomidae
Anas platyrhynchos
90.96
51.1
Olson et al. (2003)
AB551567.1 Liolope copulans
Strigeidida, Liolopidae
Semisulcospira libertina
90.92
51.9
Baba et al. (2011)
L06670.1 Tetracerasta blepta
Plagiorchiida, Lepocreadiidae
–
90.84
49.9
Blair and Barker (1993)
FJ550131.1 Paramphistomidae sp.
Plagiorchiida, Paramphistomidae
Physa acuta
90.81
51.8
Kraus et al. (2008)
AJ287497.1 Degeneria halosauri
Plagiorchiida, Gorgoderidae
Halosauropsis macrochir
90.60
50.9
Cribb et al. (2001)
AJ287481.1 Austroholorchis sprenti
Plagiorchiida, Lepocreadiidae
Sillago ciliata
90.54
49.7
Cribb et al. (2001)
AY222126.1 Gorgocephalus kyphosi
Plagiorchiida, Gorgocephalidae
Kyphosus vaigiensis
90.53
50.2
Olson et al. (2003)
L06567.1 Echinostoma caproni
Plagiorchiida, Echinostomatidae
–
90.37
50.6
Blair and Barker (1993)
DQ248207.1 Stephanostomum tantabiddii
Opisthorchiida, Acanthocolpidae
Carangoides fulviguttatus
90.37
50.6
Bray et al. (2005)
L06669.1 Gyliauchen sp.
Opisthorchiida, Gyliauchenidae
–
90.22
49.8
Blair and Barker (1993)
KY945918.1 Pegosomum saginatum
Plagiorchiida, Echinostomatidae
Ardea alba
90.16
52.4
Hirzmann et al. (2017)
AY829255.1 Spirorchid sp.
Strigeidida, Spirorchiidae
–
90.16
51.1
Brant et al. (2006)
KX506857.1 Diplodiscus mehrai
Plagiorchiida, Diplodiscidae
–
90.16
50.6
Besprozvannykh et al. (2016)
AY222119.1 Rhipidocotyle galeata
Strigeidida, Bucephalidae
Eutrigla gurnardus
90.07
49.8
Olson et al. (2003)
EU196357.1 Choanocotyle nematoides
Plagiorchiida, Choanocotylidae
Emydura kreftii
90.07
51.1
Tkach and Snyder (2007)
AY222123.1 Caecincola parvulus
Opisthorchiida, Cryptogonimidae
Micropterus salmoides
89.97
51.1
Olson et al. (2003)
FJ211228.1 Haploporus benedeni
Plagiorchiida, Haploporidae
Liza ramado
89.86
51.8
Blasco-Costa et al. (2008)
AY222129.1 Paraschistorchis zancli
Plagiorchiida, Apocreadiidae
Zanclus cornutus
89.84
50.8
Olson et al. (2003)
AY222122.1 Siphodera vinaledwardsii
Opisthorchiida, Cryptogonimidae
Sciaenops ocellatus
89.68
51
Olson et al. (2003)
MT280021.1 Hymenocotta mulli
Plagiorchiida, Haplosplanchnidae
–
89.57
50.3
Atopkin et al. (2020)
AY222083.1 Cotylaspis sp.
Aspidogastrea, Aspidogastridae
Pelodiscus sinensis
89.54
50.2
Olson et al. (2003)
AJ287523.1 Homalometron synagris
Plagiorchiida, Apocreadiidae
Hemigymnus melapturus
89.44
50.2
Cribb et al. (2001)
JQ627832.1 Philophthalmus gralli
Plagiorchiida, Philophthalmidae
Tachuris rubrigastra
89.43
51.8
Literak et al. (2013)
DQ482610.1 Multicalyx elegans
Aspidogastrea, Multicalycidae
Callorhinchus milii
89.27
50.5
Gao et al. (2006)
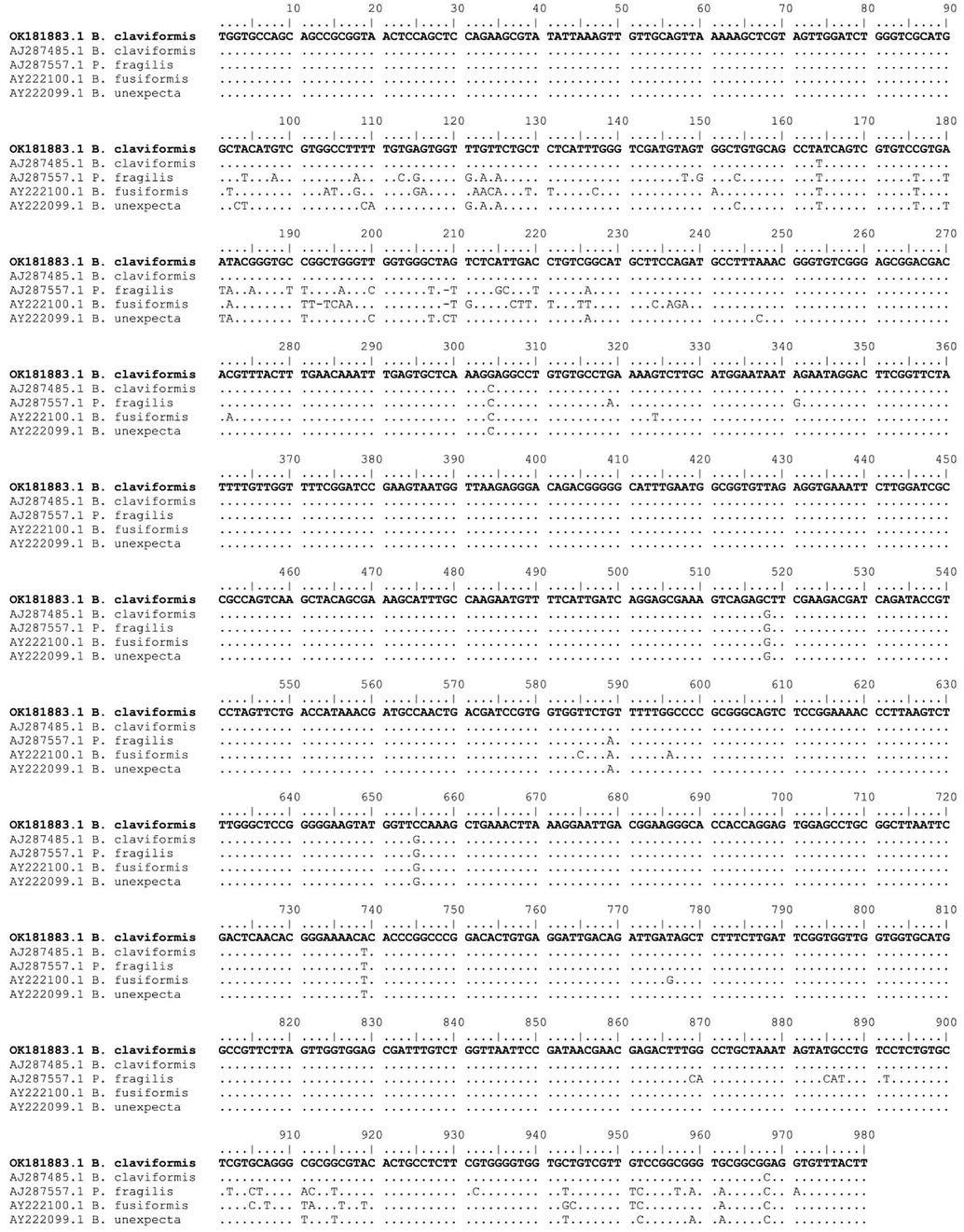
Sequence alignment of the partial 18S rRNA gene of Bivesicula claviformis with the most closely related species (Only variable sites are shown. Dots represent bases identical to those of the first sequences, and dashes indicate gaps).
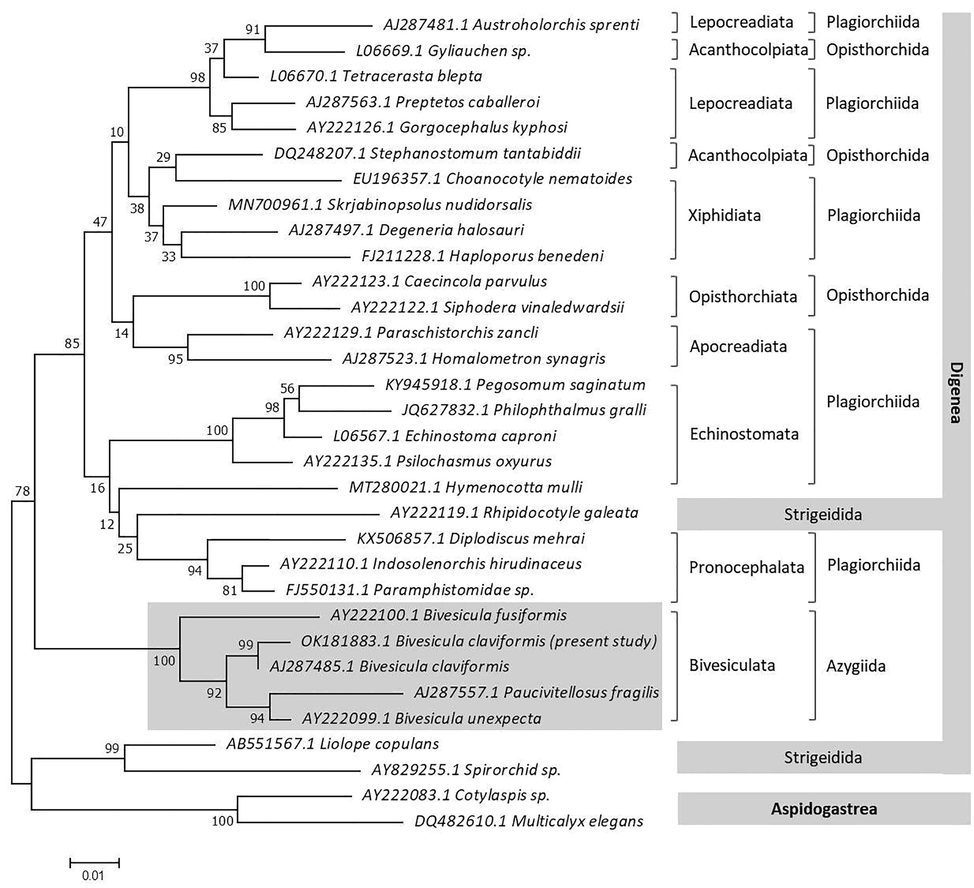
Molecular Phylogenetic analysis by Maximum Likelihood method (ML) based on the Tamura-Nei model. The tree with the highest log likelihood (−6175.42) is shown.
3.3.2 28S rRNA gene
The 28S rRNA PCR result had a band size of 262 bp in length with 52.7% GC content, according to sequencing analysis. With the accession number OK353794.1, the sequence was submitted to the NCBI database. The query sequence was compared to other comparable digenean 28S rRNA sequences in the GenBank database (Table 3, Fig. 5). The current phylogenetic tree was built using the ML method using 31 taxa (including the outgroup) and aligned over 226 positions (trimmed to match the shortest sequence length). The species examined formed two major clades (Fig. 6), the first of which includes 12 families from the subclass Digenea and five taxa from the Fellodistomidae family of Strigeidida. Whereas, the second clade contains taxa representing Cymnophalloidea and Monorchiidae (KJ648919.1 Pseudobacciger cheneyae and MN984478.1 Lasiotocus trachinoti, respectively) within Strigeidida and Plagiorchiida, in addition to the aspidogastrean outgroup taxa (AY222163.1 Multicalyx elegans and AY222165.1 Cotylaspis sp.). Fellodistomidae and Bivesiculidae are sibling families. The relationship between Troglotrematidae and Nanophyetidae has a well-supported clade (85). Opisthorchiidae + Liliatrematidae appears to be a sister group to Heterophyidae. This analysis demonstrated the basal position of Cymnophalloidea and Monorchiidae. There was a weak support (8) relationship between Cryptogonimidae and Lepocreadiidae. Among Bivesiculidae, the maximum identity (94.02%) with the lowest divergent value was recorded between the present digenean species and Bivesicula claviformis (AY222182.1) followed by B. unexpecta (91.60%, AY222181.1), B. fusiformis (87.95%, AY222183.1), and Paucivitellosus hirudinaceus (87.50%, MH257768.1). The monophyly of Bivesicula species was corroborated by the sister group Paucivitellosus in general. The query sequence B. claviformis is well-matched and placed into the Azygiida with special reference to Bivesiculidae, with a close relationship to the previously known B. claviformis (AY222182.1) in a well-supported taxon (85).
Parasite species
Order, Family
Host species
% Identity
% GC content
Reference
AY222182.1 Bivesicula claviformis
Azygiida, Bivesiculidae
Epinephelus quoyanus
94.02
53.2
Olson et al. (2003)
AY222181.1 Bivesicula unexpecta
Azygiida, Bivesiculidae
Acanthochromis polyacanthus
91.60
53.3
Olson et al. (2003)
AY222183.1 Bivesicula fusiformis
Azygiida, Bivesiculidae
Atherinomorus lacunosus
87.95
52.7
Olson et al. (2003)
MH257768.1 Paucivitellosus fragilis
Azygiida, Bivesiculidae
Clypeomorus batillariaeformis
87.50
52.3
Huston et al. (2018)
KC489791.1 Acanthostomum burminis
Opisthorchiida, Cryptogonimidae
Xenochrophis piscator
86.22
53.4
Jayawardena et al. (2013)
AJ405298.1 Steringophorus thulini
Strigeidida, Fellodistomidae
Coryphaenoides leptolepis
85.60
50.8
Bray et al. (1999)
AB521800.1 Euryhelmis costaricensis
Opisthorchiida, Heterophyidae
Martes melampus
85.26
51.8
Sato et al. (2010)
FJ788492.1 Postlepidapedon uberis
Plagiorchiida, Lepocreadiidae
Choerodon venustus
85.26
52.9
Bray et al. (2009)
MW000405.1 Metagonimoides sp.
Opisthorchiida, Heterophyidae
Juga sp.
84.74
52.5
Preston et al. (2020)
AY222255.1 Zalophotrema hepaticum
Plagiorchiida, Brachycladiidae
Zalophus californianus
84.74
55.2
Olson et al. (2003)
KR703279.1 Brachycladium goliath
Plagiorchiida, Brachycladiidae
Balaenoptera acutorostrata
84.80
51.7
Briscoe et al. (2016)
AY222217.1 Taprobanella bicaudata
Plagiorchiida, Rhabdiopoeidae
Dugong dugon
84.80
53.2
Olson et al. (2003)
MG806918.1 Apophallus zalophi
Opisthorchiida, Heterophyidae
Callorhinus ursinus
85.42
53.8
Kuzmina et al. (2018)
KT865201.1 Proctoeces cf. lintoni
Strigeidida, Fellodistomidae
Sicyases sanguineus
84.86
49.2
Oliva et al. (2015)
MN984478.1 Lasiotocus trachinoti
Plagiorchiida, Monorchiidae
Trachinotus carolinus
84.77
51.1
Panyi et al. (2020)
MF099790.1 Opisthorchis felineus
Opisthorchiida, Opisthorchiidae
Cat
84.86
54
Dao et al. (2017)
MT303945.1 Liliatrema skrjabini
Plagiorchiida, Liliatrematidae
–
84.86
53.5
Sokolov et al. (2020)
AY222283.1 Olssonium turneri
Strigeidida, Fellodistomidae
Alepocephalus agassizii
84.86
52.9
Olson et al. (2003)
MK482053.1 Metorchis orientalis
Opisthorchiida, Opisthorchiidae
Duck
84.46
51.5
Qiu et al. (2019)
EF654661.1 Clonorchis sinensis
Opisthorchiida, Opisthorchiidae
–
84.46
52.9
Lee et al. (2007)
AY222231.1 Caecincola parvulus
Opisthorchiida, Cryptogonimidae
Micropterus salmoides
84.46
53.9
Olson et al. (2003)
KJ648919.1 Pseudobacciger cheneyae
Strigeidida, Cymnophalloidea
Chromis weberi
84.92
50.2
Sun et al. (2014)
HM172630.1 Paragonimus westermani
Plagiorchiida, Troglotrematidae
–
84.43
54.5
Devi et al. (2010)
KX354834.1 Campula oblonga
Plagiorchiida, Brachycladiidae
East Asian finless porpoise
84.52
55.6
Wan et al. (2016)
AY222282.1 Fellodistomum fellis
Strigeidida, Fellodistomidae
Anarhichas lupus
84.40
52.8
Olson et al. (2003)
KJ425462.1 Coomera brayi
Strigeidida, Fellodistomidae
Monodactylus argenteus
84
52.5
Cribb et al. (2014)
MZ345681.1 Bianium plicitum
Plagiorchiida, Lepocreadiidae
Sphoeroides testudineus
84
52.9
Curran (2021)
MW000381.1 Nanophyetus salmincola
Plagiorchiida, Nanophyetidae
Juga sp.
84
53.2
Preston et al. (2020)
AY222163.1 Multicalyx elegans
Aspidogastrea, Multicalycidae
Callorhinchus milii
79
49.5
Olson et al. (2003)
AY222165.1 Cotylaspis sp.
Aspidogastrea, Aspidogastridae
Pelodiscus sinensis
79
52.1
Olson et al. (2003)

Sequence alignment of the partial 28S rRNA gene of Bivesicula claviformis with the most closely related species (Only variable sites are shown. Dots represent bases identical to those of the first sequences, and dashes indicate gaps).
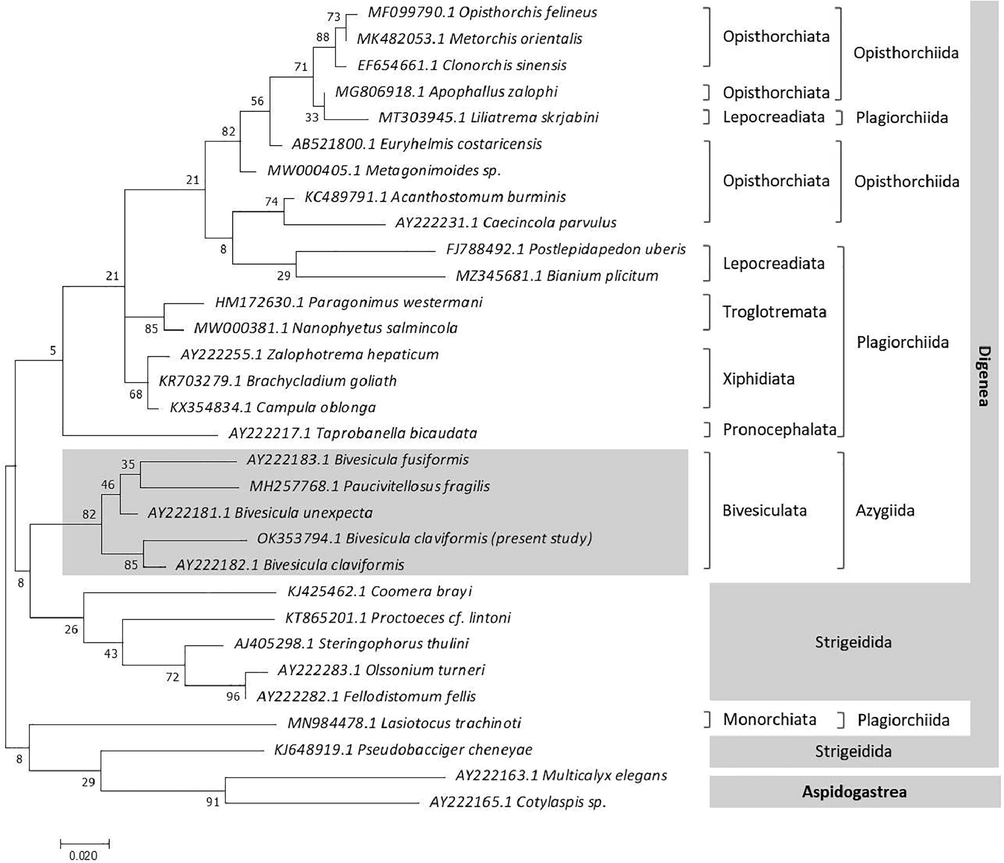
Molecular Phylogenetic analysis by Maximum Likelihood method (ML) based on the Tamura-Nei model. The tree with the highest log likelihood (−1839.73) is shown.
4 Discussion
Although several studies on marine fish parasites from the Red Sea have been conducted (Al-Nabati et al., 2021), the Digenea constitutes the largest group of internal metazoan parasites (Cribb et al., 2001). Only 18 of the total 30 fish evaluated in this study exhibited a 60% infection rate for B. claviformis in the intestinal region. This agreed with Yamaguti (1971), Shen (1982), Gu and Shen (1983) and Cribb et al. (1994) documented Bivesicula digenea as intestinal parasites in marine fish.
At the morphological and morphometric level, the current bivesculid species is compatible with other Bivesicula species that have occupied many hosts in several geographical areas by possessing all the species' characteristics exceptions. The newly discovered Bivesicula species is quite similar to the previously described Bivesicula claviformis Yamaguti, 1934. The identification key was emphasized that the absence of the oral and ventral sucker, the presence of muscular pharynx, the intestine and vitellaria extended backward beyond the testis, the uterus entirely post-testicular, the arms of excretory vesicle extend well anterior to caeca, and the cirrus sac overlapped the midlevel of the body and its level could easily vary depending on the extent of the body contraction, as the main distinguishing features for the differentiation of Bivesicula species. The present study agreed with the previous data of Yamaguti (1934), Le Zotte (1954), Cable and Nahhas (1962), Fischthal and Kuntz (1965), Cribb et al. (1994), and Trieu et al. (2015) indicated that the oral sucker was the pharynx in all bivesiculids, and that the so-called pharynx was a muscular enlargement of the oesophagus. Our specimens fall within the range suggested by Fischthal and Kuntz (1965) for those infecting the intestine of Epinephelus fasciatus (Serranidae) from North Borneo in East Malaysia.
Bivesicula species have a wide range of hosts, but only seven families have been identified so far: Congridae, Megalopidae, Scombridae, Serranidae, Lutjanidae (Yamaguti, 1938, 1939; Nagaty, 1948; Manter, 1961; Fischthal and Kuntz, 1965; Machida et al., 1970; Gu and Shen, 1983; Shen, 1995; Lester and Sewell, 1990; Cribb et al., 1994; Shimazu and Machida, 1995; Rigby et al., 1997; Cribb et al., 1998; Nahhas et al., 2004), Holocentridae (Koryakovtseva, 1984), Synbranchidae (Shimazu, 2013), and Apogonidae (Trieu et al., 2015). Although the Bivesicula species was described from a carangid (type-host) and a lutjanid by Yamaguti (1934), the overwhelming majority of records are from serranids. Our findings represent the first evidence of endoparasitic Bivesicula claviformis infection in the serranid fish Cephalopholis sonnerati, which inhabits the Red Sea (Saudi Arabia).
In the last three decades, the inclusion of molecular research into taxonomy has become an essential tool for species determination (Al-Hoshani et al., 2021; Al-Quraishy et al., 2021). Although morphological descriptions of species are still extremely successful, combining morphological and genetic approaches improves species identification, particularly when complexes of species are present (Cribb et al., 2016; Blasco-Costa et al., 2016). The 18S, ITS1-5.8S-ITS2, or 28S rDNA regions are used in the majority of taxonomic and systematic investigations that employ molecular analysis for species descriptions. We were able to offer phylogenetic resolution and a good estimate of the recovered digenean species by integrating data from two nuclear ribosomal RNA (18S and 28S) genes. This agreed with Cribb et al. (2001), who utilized complete ssrDNA sequences from 75 digenean species representing 55 families, as well as 56 adult and larval morphological characters for these families, to determine the interrelationships of constituent groupings.
The current phylogeny is divided into two subclasses: Aspidogastrea and Digenea, with the latter being the most abundant with 30 families. Because Aspidobothrea taxa have a larger and more loculate ventral sucker, they were included as an outgroup. While, Digenea species have an oral and ventral sucker, the latter being a modification of the posterior adhesive organ or being eliminated. The Bivesiculata is represented by the Bivesiculidae family, which is from the digenean tree’s basal lineage. It is agreed with Olson et al. (2003) that this position might be owing to the primitive absence of suckers being a derived state, or the development of suckers in the Diplostomida and the remainder of the Plagiorchiata occurring independently. In the present phylogeny, the Pronocephalata and Bivesiculata are sister taxa. This grouping is supported by the absence of the oral sucker or pharynx as described by Pearson (1992). Furthermore, there was a relationship with Diplodiscidae, which is compatible with Olson et al. (2003), who indicated that this family lacks the ventral sucker. According to Manter (1966), the Lepocreadiata includes two families: the Lepocreadiidae and the Gorgocephalidae, which are both characterized by unusual morphology, including an oral sucker with tentacles, a single caecum with a non-terminal ventral opening in the forebody, and a huge pocketed genital atrium opening dorsally. The current phylogeny revealed the Xiphidiata, which is regarded as the Digenea’s crown clade and consists of three families: Brachycladiidae, Deropristidae, and Gorgoderidae; the latter two of which are sister taxa. The existence of a penetrating stylet in their cercariae, which represented the term Xiphidiata, supported the union of these families, according to Olson et al. (2003).
5 Conclusion
The validity and position of Bivesicula claviformis within the Bivesiculidae are supported by morphological and molecular phylogenetic evidence. This is the first time a genetic sequence for Bivesicula species has been discovered. Furthermore, Saudi Arabia represents a novel geographic distribution for C. sonnerati’s digenean parasite species. More samples and genetic markers should be used in future studies to have a better understating of this group of digenean parasites.
Acknowledgements
Authors extend their appreciation to Zoology Department, College of Science, King Saud University (Saudi Arabia); for providing all facilities to complete this work.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Morphological and molecular analyses of Paropecoelus saudiae so. nov. (Plagiorchiida: Opecoelidae), a trematoda parasite of Parupeneus rubescens (Mullidae) from the Arabian Gulf. J. King Saud Univ. Sci.. 2020;32:2243-2253.
- [Google Scholar]
- Cucullanus bulbosus (Lane, 1916) Barreto, 1918 (Nematoda, Cucullanidae) from the common ponyfish Leiognathus equulus (Leiognathidae): Morphology and molecular study. Microb. Pathog.. 2021;154:104821
- [Google Scholar]
- Heteromicrocotyla polyorchis Unnithan, 1961 (Monogenea: Heteromicrocotylidae), a gill parasite of the yellow-spotted trevally, Carangoides fulvoguttatus (Carangidae) from Saudi Arabia: Morphology and phylogeny. Microb. Pathog.. 2021;160:105165
- [Google Scholar]
- First molecular data and morphological re-description of two copepod species, Hatschekia sargi and Hatschekia leptoscari, as parasites on Parupeneus rubescens in the Arabian Gulf. J. King Saud Univ. Sci.. 2021;33:101290
- [Google Scholar]
- Atopkin, D.M., Besprozvannykh, V.V., Ha, N.D., Nguyen, H.V., Nguyen, T.V., 2020. New species of Parasaccocoelium (Haploporidae) and new genus Pseudohaplosplanchnus (Haplosplanchnidae) from mullet fish of Russian Far East and Vietnam: morphological and molecular data. https://www.ncbi.nlm.nih.gov/nuccore/MT280021.1.
- Liolope copulans (Trematoda: Digenea: Liolopidae) parasitic in Andrias japonicus (Amphibia: Caudata: Cryptobranchidae) in Japan: life cycle and systematic position inferred from morphological and molecular evidence. Parasitol. Int.. 2011;60:181-192.
- [Google Scholar]
- Besprozvannykh, V.V., Rozhkovan, K.V., Ermolenko, A.V., 2016. Morphology, life cycles, and phylogenetic position of Diplodiscus mehrai Pande, 1937 and D. japonicus (Yamaguti, 1936) (Digenea: Diplodiscus). https://www.ncbi.nlm.nih.gov/nuccore/KX506857.1.
- Affinities of the Gyliauchenidae: utility of the 18S rRNA gene for phylogenetic inference in the Digenea (Platyhelminthes) Int. J. Parasitol.. 1993;23:527-532.
- [Google Scholar]
- Molecular approaches to trematode systematics: ‘Best practice’ and implications for future study. Syst. Parasitol.. 2016;93:295-306.
- [Google Scholar]
- A revision of the species of Saturnius Manter, 1969 (Digenea, Hemiuridae), parasites of mullets (Teleostei: Mugilidae) Syst. Parasitol.. 2008;71:53-74.
- [Google Scholar]
- An approach to revealing blood fluke life cycles, taxonomy, and diversity: provision of key reference data including DNA sequence from single life cycle stages. J. Parasitol.. 2006;92(1):77-88.
- [Google Scholar]
- Urotrematidae (Platyhelminthes: Digenea) in Chinese freshwater fishes. Syst. Parasitol.. 1999;44:193-200.
- [Google Scholar]
- The phylogeny of the Lepocreadioidea (Platyhelminthes: Digenea) inferred from nuclear and mitochondrial genes: Implications for their systematics and evolution. Acta Parasitol.. 2009;54:310-329.
- [Google Scholar]
- A molecular phylogenetic study of the Acanthocolpidae (Digenea) Acta Parasitol.. 2005;50:281-291.
- [Google Scholar]
- The mitochondrial genome and ribosomal operon of Brachycladium goliath (Digenea: Brachycladiidae) recovered from a stranded minke whale. Parasitol. Int.. 2016;65(3):271-275.
- [Google Scholar]
- Bush, A.O., Lafferty, K.D., Lotz, J.M., Shostak, A.W., 1997. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 83, 575–583.
- Bivesicula caribbensis sp. n. (Trematoda: Digenea) and its life history. J. Parasitol.. 1962;48:536-538.
- [Google Scholar]
- Paucivitellosus fragilis gen. et sp. nov. (Bivesiculidae: Digenea), a parasite of Chelon troscheli from Formosa. Trans. Am. Microsc. Soc.. 1965;84(3):365-368.
- [Google Scholar]
- Cribb, T.H., 2002. Superfamily Bivesiculoidea Yamaguti, 1934. In Gibson, D. I., A. Jones and R. A. Bray (eds.): Keys to the Trematoda, 1, pp. 25–29. CAB International and The Natural History Museum, Wallingford.
- A DNA-based demonstration of a three-host life-cycle for the Bivesiculidae (Platyhelminthes: Digenea) Int. J. Parasitol.. 1998;28:1791-1795.
- [Google Scholar]
- Trematodes of the Great Barrier Reef, Australia: emerging patterns of diversity and richness in coral reef fishes. Int. J. Parasitol.. 2014;44:929-939.
- [Google Scholar]
- Gut wash, body soak, blender, and heat-fixation: approaches to the effective collection, fixation and preservation of trematodes of fishes. Syst. Parasitol.. 2010;76:1-7.
- [Google Scholar]
- Bivesiculidae and Haplosplanchnidae (Digenea) from fishes of the southern Great Barrier Reef, Australia. Syst. Parasitol.. 1994;28:81-97.
- [Google Scholar]
- Trematodes of fishes of the Indo-west Pacific: Told and untold richness. Syst. Parasitol.. 2016;93:237-247.
- [Google Scholar]
- The Digenea. In: Littlewood D.T.J., Bray R.A., eds. Interrelationships of the Platyhelminthes. London: Taylor and Francis; 2001. p. :168-185.
- [Google Scholar]
- Opechona chloroscombri and Opechona corkumi n. sp. (Digenea: Lepocreadiidae) from the northern Gulf of Mexico with phylogenetic analysis based on 28S rDNA. J. Parasitol.. 2021;107(4):606-620.
- [Google Scholar]
- Dao, T.H.T., Nguyen, T.G.T., Gabriel, S., Bui, K.L., Dorny,P., Blair, D., Le, T.H., 2017. The recently discovered Opisthorchis sp. in ducks in Vietnam: Insights of the genomic analysis for phylotaxonomic position in the family Opisthorchiidae (Opisthorchioidea: Trematoda: Platyhelminthes). https://www.ncbi.nlm.nih.gov/nuccore/MF099790.1.
- Morphological and molecular characterization of Paragonimus westermani in northeastern India. Acta Trop.. 2010;116(1):31-38.
- [Google Scholar]
- Digenetic trematodes of fishes from North Borneo (Malaysia) PHSWA. 1965;32(1):63-71.
- [Google Scholar]
- Gao, Q., Chen, M., Nie, P., 2006. Phylogeny of the Subclass Aspidogastrea based on 18S rDNA Sequences. https://www.ncbi.nlm.nih.gov/nuccore/DQ482610.1.
- Digenetic trematodes of fishes from the Xisha Islands, Guangdong Province, China. I. Stud. Mar. Sinica. 1983;20:157-184.
- [Google Scholar]
- BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser.. 1999;41:95-98.
- [Google Scholar]
- Hariri, K.I., Nichols, P., Krupp, F., Mishrigi, S., Barrania, A., Ali, F.A., Kedidi, S.M., 2000. Status of the living marine resources in the Red Sea and Gulf of Aden region and their management. Regional Organization for the Conservation of the Environment of the Red Sea and Gulf of Aden (PERSGA), Jeddah, Saudi Arabia. Report number: PERSGA Technical Series No. 4, Affiliation: PERSGA/The World Bank.
- Hirzmann, J., Kley-Sonntag, A., Grossmann, E., Bauer, C., 2017. Molecular characterization of Pegosomum species (Trematoda, Echinostomatidae) from the gall bladder of a severe infected great egret in Germany. https://www.ncbi.nlm.nih.gov/nuccore/KY945918.1.
- Molecular systematics of the digenean community parasitizing the certhiid gastropod Clypeomorus batillariaeformis Habe & Kusage on the Great Barrier Reef. Parasitol. Int.. 2018;67:722-735.
- [Google Scholar]
- A Description of Lecithocladium angustiovum (Digenea: Hemiuridae) in Short Mackerel, Rastrelliger brachysoma (Scombridae), of Indonesia. Trop. Life Sci. Res.. 2015;26(1):31-40.
- [Google Scholar]
- Malformations and mortality in the Asian Common Toad induced by exposure to pleurolophocercous cercariae (Trematoda: Cryptogonimidae) Parasitol. Int.. 2013;62(3):246-252.
- [Google Scholar]
- An annotated list of parasites (Isopoda, Copepoda, Monogenea, Digenea, Cestoda and Nematoda) collected in groupers (Serranidae, Epinephelinae) in New Caledonia emphasizes parasite biodiversity in coral reef fish. Folia Parasitol.. 2010;57:237-262.
- [Google Scholar]
- Koryakovtseva, L.P., 1984. Morphological characteristics of trematodes from the family Bivesiculidae - parasites of marine fishes. Materialy Nauchnykh Konferentsii Vsesoyuznogo Obshchestva Germintologov 34, 29-34.
- Kraus, T.J., Brant, S.V., Adema, C.M., 2008. Trematode cercariae from physid snails from the Middle Rio Grande. https://www.ncbi.nlm.nih.gov/nuccore/FJ550131.1.
- MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol.. 2016;33:1870-1874.
- [Google Scholar]
- Digeneans of northern fur seals Callorhinus ursinus (Pinnipedia: Otariidae) from five subpopulations on St. Paul Island, Alaska. Parasitol. Res.. 2018;117:1079-1086.
- [Google Scholar]
- Studies on marine digenetic trematodes of Puerto Rico: The family Bivesiculidae, its biology and affinities. J. Parasit.. 1954;40:148-162. 4 pls
- [Google Scholar]
- Molecular phylogeny of parasitic Platyhelminthes based on sequences of partial 28S rDNA D1 and mitochondrial cytochrome c oxidase subunit I. Korean J. Parasitol.. 2007;45(3):181-189.
- [Google Scholar]
- Checklist of parasites from Heron Island, Great Barrier Reef. Aust. J. Zool.. 1990;37:101-128.
- [Google Scholar]
- Eye trematode infection in small passerines in Peru caused by Philophthalmus lucipetus, an agent with a zoonotic potential spread by an invasive freshwater snail. Parasitol. Int.. 2013;62(4):390-396.
- [Google Scholar]
- Digenetic trematodes collected from the fishes in the sea north of the Tsushima Islands. Mem. Natn. Sci. Mus. Tokyo. 1970;3:101-112.
- [Google Scholar]
- Morphology and life cycle of Paucivitellosus hanumanthai n. sp. (Trematoda: Bivesiculidae) Trans. Am. Microsc. Soc.. 1989;108:21-26.
- [Google Scholar]
- Studies on digenetic trematodes of fishes of Fiji. I. Families Haplosplanchnidae, Bivesiculidae, and Hemiuridae. PHSWA. 1961;28(1):67-74.
- [Google Scholar]
- A peculiar trematode, Gorgocephalus kyphosi gen. et sp. n. (Lepocreadiidae: Gorgocephalinae subfam. n.), from a marine fish of South Australia. J. Parasitol.. 1966;52:347-350.
- [Google Scholar]
- Trematodes of fishes from the Red Sea. Part 4. On some new and known forms with a single testis. J. Parasit.. 1948;34:355-363.
- [Google Scholar]
- Digenetic trematodes of marine fishes from Suva, Fiji: families: Acanthocolpidae, Lepocreadiidae, Bivesiculidae, Zoogonidae, Monorchiidae and description of a new species. Riv. Parassitol.. 2004;21:33-48.
- [Google Scholar]
- Oliva, M.E., Valdivia, I.M., Cardenas, L., Munoz, G., Geroge-Nascimento, M., 2015. A new species of Proctoeces and reinstatement of Proctoeces humboldti George-Nascimento and Quiroga 1983 (Digenea: Fellodistomidae) based on molecular and morphological evidence. https://www.ncbi.nlm.nih.gov/nuccore/KT865201.1.
- Phylogeny and classification of the Digenea (Platyhelminthes : Trematoda) Int. J. Parasitol.. 2003;33:733-755.
- [Google Scholar]
- Phylogenetic affinity of Genolopa (Digenea: Monorchiidae) with descriptions of two new species. Diversity. 2020;12(2):51.
- [Google Scholar]
- Observations on the morphology and life-cycle of Paucivitellosus fragilis Coil, Reid & Kuntz, 1965 (Trematoda: Bivesiculidae) Parasitology. 1968;58:769-788.
- [Google Scholar]
- On the position of the digenean family Heronimidae an inquiry into a cladistic classification of the Digenea. Syst. Parasitol.. 1992;21:81-166.
- [Google Scholar]
- Trematode parasites exceed aquatic insect biomass in Oregon stream food webs. J. Anim. Ecol.. 2020;90(3):766-775.
- [Google Scholar]
- Qiu, Y.Y., Su, X., Wang, C.R., 2019. Comparative analysis of Ribosomal DNA of Clonorchis sinensis and Metorchis orientalis about of human and animal health significance. https://www.ncbi.nlm.nih.gov/nuccore/MK482053.1.
- Patterns of species diversity in the gastrointestinal helminths of a coral reef fish, Epinephelus merra (Serranidae), from French Polynesia and the South Pacific Ocean. Can. J. Zool.. 1997;75:1818-1827.
- [Google Scholar]
- Latitudinal differences in species and community richness and in community structure of metazoan endo- and ectoparasites of marine teleost fish. Int. J. Parasitol.. 1998;28:461-474.
- [Google Scholar]
- Identification of Euryhelmis costaricensis metacercariae in the skin of Tohoku hynobiid salamanders (Hynobius lichenatus), northeastern Honshu, Japan. J. Wildl. Dis.. 2010;46(3):832-842.
- [Google Scholar]
- Red Sea fish market assessments indicate high species diversity and potential overexploitation. Fish. Res.. 2021;239:105922
- [Google Scholar]
- Three new species of genus Bivesicula Yamaguti, 1934 (Trematode [sic]: Bivesiculidae) from marine fishes of China. Oceanol. Limnol. Sin.. 1982;13:570-576.
- [Google Scholar]
- Notes on a new genus and species of Treptodemidae (Trematoda: Digenea) Stud. Mar. Sin.. 1995;36:233-236.
- [Google Scholar]
- Shimazu, T., 2013. Digeneans parasitic in freshwater fishes (Osteichthyes) of Japan. I. Aporocotylidae, Bivesiculidae and Haploporidae. Bull. Natl. Mus. Nat. Sci., Ser. A 39 (4), 167-184.
- Some species of the genus Bivesicula (Digenea: Bivesiculidae), including three new species, from marine fishes of Japan and Palau. Bull. Natl. Sci. Mus., Tokyo, Ser. A, Zool.. 1995;21:127-141.
- [Google Scholar]
- Phylogenetic affiliation of the lepocreadiid trematodes parasitizing some marine fishes in the North-western Pacific. Mar. Biol. Res.. 2020;16(5):1-10.
- [Google Scholar]
- Sun, D., Bray, R.A., Yong, R.Q., Cutmore, S.C., Cribb, T.H., 2014. Pseudobacciger cheneyae n. sp. (Digenea: Gymnophalloidea) from Weber's chromis (Chromis weberi Fowler & Bean) (Perciformes: Pomacentridae) at Lizard Island, Great Barrier Reef, Australia. Syst. Parasitol. 88 (2), 141-152.
- On the Natural History and Classification of Fishes, Amphibians, and Reptiles, or Monocardian Animals. 1839;Vol. 2:448.
- CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res.. 1994;22(22):4673-4680.
- [Google Scholar]
- Aptorchis megacetabulus n. sp. (Platyhelminthes: Digenea) from the northern long-necked turtle, Chelodina rugosa (Pleurodira: Chelidae), in Australia. J. Parasitol.. 2007;93(2):404-408.
- [Google Scholar]
- A species pair of Bivesicula Yamaguti, 1934 (Trematoda: Bivesiculidae) in unrelated Great Barrier Reef fishes: implications for the basis of speciation in coral reef fish trematodes. Syst. Parasitol.. 2015;91:231-239.
- [Google Scholar]
- Wan, X.L., Zheng, J.S., Li, W.X., Zeng, X.Y., Yang, J.W., Hao, Y.J., Wang, D., 2016. Survey on parasitic infections in the East Asian finless porpoise (Neophocaena asiaeorientalis sunameri) living off the Chinese Yellow/Bohai Sea coast. https://www.ncbi.nlm.nih.gov/nuccore/KX354834.1.
- Analyses of the ribosomal internal Transcribed spacers (ITS) and the 5.8S gene indicate that extremely high rDNA heterogeneity is a unique feature in the scleractinian coral genus Acropora (Scleractinia; Acroporidae) Zool. Stud.. 2006;45(3):404-418.
- [Google Scholar]
- WoRMS, 2021. Bivesiculidae Yamaguti, 1934. Accessed at: https://www.marinespecies.org/aphia.php?p=taxdetails&id=411241 on 2021-10-22.
- Studies on the helminth fauna of Japan. Part 2. Trematodes of fishes. I. Jpn. J. Zool.. 1934;5:249-541.
- [Google Scholar]
- Yamaguti, S., 1938. Studies on the Helminth Fauna of Japan. Part 21. Trematodes of Fishes, IV. Kyöto: Yamaguti, S, 139 pp.
- Studies on the helminth fauna of Japan. Part 26. Trematodes of fishes. VI. Jpn. J. Zool.. 1939;8(2):211-230.
- [Google Scholar]
- Synopsis of Digenetic Trematodes of Vertebrates. Tokyo: Keigaku Publishing Co; 1971.







