Translate this page into:
Morphological and molecular analyses Protolamellodiscus senilobatus (Monogenea: Diplectanidae), a gill parasite infecting the soldier bream Argyrops filamentosus (Sparidae)
⁎Corresponding author at: Zoology Department, College of Science, King Saud University, Riyadh, Saudi Arabia. rabdelgaber.c@ksu.edu.sa (Rewaida Abdel-Gaber)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Monopisthocotyleans belonging to the family Diplectanidae infect marine fish and are distinguished by a posterior complex haptor. The solider bream Argyrops filamentosus Valenciennes (Perciformes: Sparidae), is one such species that is under consideration caught from the Red Sea (Saudi Arabia). This study is the first description of a diplectanid species from the gills of the soldier bream fish, morphologically by light as well as by molecular analysis of the parasite partial 28S rRNA sequences through multiple alignments and phylogeny by maximum likelihood (ML) analysis which is provided for the first time for the described parasite species. Thirty soldier bream fish were collected from marine water off Saudi Arabia; gills were detached and further analyzed for parasitic infection. A monogenean parasite with a complicated haptor made up of two comparable lamellodiscs, three bilateral pairs of lobes, two pairs of anchors, three bars, and seven pairs of marginal hooks was discovered to naturally infect 21 samples of the examined fish. The molecular analysis of the parasite 28S rRNA and phylogeny revealed a percentage of identities 98.41–77.97 % for Diplectanidae species within a monophyletic clade of Dactylogyridea where a maximum percentage of 98.41 % were obtained for morphologically different sister taxon Lamellodiscus acanthopagri. The results of phylogeny are in line with those obtained through morphological classification, which showed that the parasite identified shared morphological characteristics with Protolamellodiscus senilobatus, a species that had not before been identified by DNA analysis. Under accession number OP419541.1, the obtained parasite sequences were added to the GenBank database.
Keywords
Monogenea
Diplectanidae
Lamellodiscinae
Protolamellodiscus
Morphology
Phylogeny
1 Introduction
The Red Sea’s sparid fish fauna consists of 16 species that fall into nine genera (Machkewskyi et al., 2013). One of these is the soldier bream Argyrops filamentosus (Valenciennes) (Sparidae), which is one of the most widely consumed commercial fish in the area and an attractive proposition for aquaculture (Basurco et al., 2011). It is widely known that numerous eukaryotic species may parasitize fish (M’Rabet et al., 2016). Most monogeneans are ectoparasites that parasitize fish’s skin, fins, and gills. They are highly host-specific and have a simple life cycle (Bakke et al., 2007). A significant taxonomic diversification, with 3500 identified species found in marine fish, distinguishes this group of parasites (Rohde, 2005). They severely damage the gills and produce significant issues with clear pathogenicity (Intamong et al., 2016).
About 250 species of the monogenean monopisthocotyleans Diplectanidae Monticelli, 1903 are found largely on the gills of marine perciform fish (Domingues and Boeger, 2008). Five subfamilies are recognized primarily based on the morphology and presence/absence of the accessory adhesive organs of haptor, as follows: Diplectaninae Monticelli, 1903, Lamellodiscinae Oliver, 1969, Murraytrematoidinae Oliver, 1982, Rhabdosynochinae Oliver, 1987, and Rhamnocercinae Monaco, Wood & Mizelle, 1954. According to Sánchez-García et al. (2011), this family is distinguished by the presence of a complex haptor with numerous different attachment elements, including two pairs of main hooks connected by medial bars, 14 peripheral marginal hooks, and one or two clusters of sclerotized rodlets or lamellae known as ‘squamodiscs’ or ‘lamellodiscs’ (Sánchez-García et al., 2011).
According to World Register of Marine Species (WoRMS, 2022), the genus Protolamellodiscus Oliver, 1969 (Diplectanidae, Lamellodiscinae) currently consists of 4 described species. These species include P. convolutes (Yamaguti, 1953) Oliver, 1987, P. raibauti Oliver & Radujkovic, 1987, P. senilobatus Kritsky, Jiménez-Ruiz & Sey, 2000, and P. serranelli (Euzet & Oliver, 1965) Oliver, 1969, that have been mainly parasites of perciform fish of Nemipteridae, Serranidae and Sparidae families.
Genetic analysis has recently been combined with morphological descriptions, enabling researchers to precisely identify monogenean species (Jovelin and Justine, 2001; Desdevises et al., 2002; Verma et al., 2018; Lablack et al., 2022). While, mitochondrial cytochrome-b DNA sequences were used to investigate the evolutionary relationship between these parasites and their hosts (Desdevises et al., 2000; Choudhary and Agrawal 2017), partial sequences of the ribosomal DNA coding regions were frequently used to estimate the level of divergence at the intra- and interspecific levels among species of Lamellodiscinae (Domingues and Boeger, 2008; El-Nabati et al., 2021; Kaci-Chaouch et al., 2008; Mallatt and Winchell, 2002; Mendoza-Franco et al., 2018; Poisot et al., 2011; Šimkova et al., 2006; El-Nabati et al., 2021).
This study aims to study the occurrence of monogeneans infecting Argyrops filamentosus fish that inhabits the Red Sea (Saudi Arabia) and the taxonomic status of the parasites was determined through morphological features and confirmed by molecular tools.
2 Materials and methods
2.1 Fish samples collection
During the period of this study, thirty specimens of the soldier bream Argyrops filamentosus (Family: Sparidae) were collected via local fishermen in Jeddah province (along the coast of the Red Sea), Saudi Arabia. Fish were put on ice before being moved to the Lab, where they are identified using the external morphological standards set out by Abu Shusha et al. (2010).
2.2 Parasitological examination
Fish skin, fins, and gills were examined macro- and microscopically to check for parasitic infections. The fish’s gills were separated, submerged in 0.9 % saline solution to eliminate any extra gill mucus, and then inspected for monogeneans under a stereomicroscope (Nikon SMZ18, NIS ELEMENTS software). Using delicate dissection needles, monogeneans were extracted from the gills. According to Bush et al. (1997), the prevalence and mean intensity of infection in terms of parasitology were determined. Worms were preserved in 4 % formalin for microscopic examinations or 96 % ethanol for molecular analysis. To remove excess fixatives, the fixed specimens (n = 10) were rinsed in distilled water (Hassan et al., 2015). According to Malmberg (1957), worms were first mounted as semi-permanent preparations in a glycerin ammonium-picrate mixture before being mounted in Canada balsam. The parasites were then examined using light microscopy (aNTI-MOULD, MICROS, Austria) to determine their identities using Oliver’s description (1969) for the morphology of the haptor and male copulatory organ. Illustrations were made using photos taken by Leica DM 2500 microscope (NIS ELEMENTS software, ver. 3.8). Using the program ImageJ 1.53e software, measurements were collected and expressed in micrometers (µm).
2.3 Molecular analysis
Ten parasites, previously fixed in 96 % ethanol, were digested overnight at 55 °C in the DNA buffer containing 100 µg/ml proteinase K. Following the instructions, genomic DNA was extracted using a DNeasy tissue kit© (Qiagen). Using the LSU5/LSU1200R primer combination, which was designed by Littlewood (1994) and Littlewood et al. (2000), a partial 28S rRNA gene was amplified. The thermocycling profile for the PCR reaction was as follows: 4 min at 95 °C, followed by 40 cycles of 1 min at 92 °C, 1 min for 54 °C, 1 min 30 sec at 72 °C, and 10 min at 72 °C. The final volume of the PCR reaction was 25 µl. PCR products were checked in a 1 % agarose gel in 1 × Tris-acetate–EDTA (TAE) containing ethidium bromide A BigDyeTM Terminator v3.1 chemistry (Applied Biosystems) and a 310 × DNA analyzer (Applied Biosystems) were used for the sequencing process. Sequence identities were determined with the BLAST analysis on the NCBI nucleotide database. ClustalW multiple alignments (Thompson et al., 1997) were used for the initial sequence alignment, and regions of ambiguous sequence alignments were manually edited using BioEdit sequence alignment editor v.4.8.9 (Hall, 1999). With the general time-reversible (GTR) substitution model, phylogenetic analyses were carried out in MEGA7 (Kumar et al., 2016) using the maximum likelihood (ML) approach. Using 1.000 replicates, a bootstrap approach was used to estimate branch support.
3 Results
A monogenetic parasite from the Diplectanidae was naturally present in the gill region of twenty-one (70 %) of the specimens of the studied Argyrops filamentosus. This parasitic species was identified morphologically as Protolamellodiscus senilobatus Kritsky, Jiménez-Ruiz & Sey, 2000. Each parasitized fish has a mean parasite intensity of no more than eleven (Figs. 1 and 2).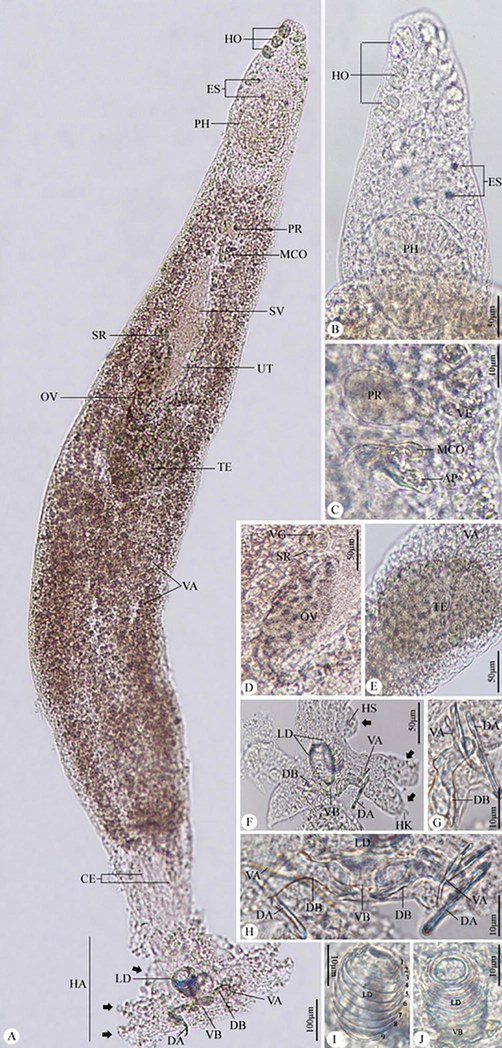
Photomicrographs of Protolamellodiscus senilobatus infecting Argyrops filamentosus. (A) whole mount preparation. (B) anterior region of the body. (C) male copulatory complex and prostatic reservoir. (D) ovary. (E) testis. (F-H) haptor with related structures. (I,J) Lamellodiscs with 9 lamellae. Note: HO, head organs; ES, eye spots; PH, pharynx; PR, prostatic reservoir; MCO, male copulatory organ; SV, seminal vesicle; OV, ovary; TE, testis; VA, vitellaria; CE, Ceca; HA, haptor; LD, lamellodiscs; DA, dorsal anchor; VA, ventral anchor; VB, ventral bar, DB, dorsal bar, Black arrows, bilateral lobes of haptor; HS, hook(s); AP, accessory piece; SR, seminal receptacle.
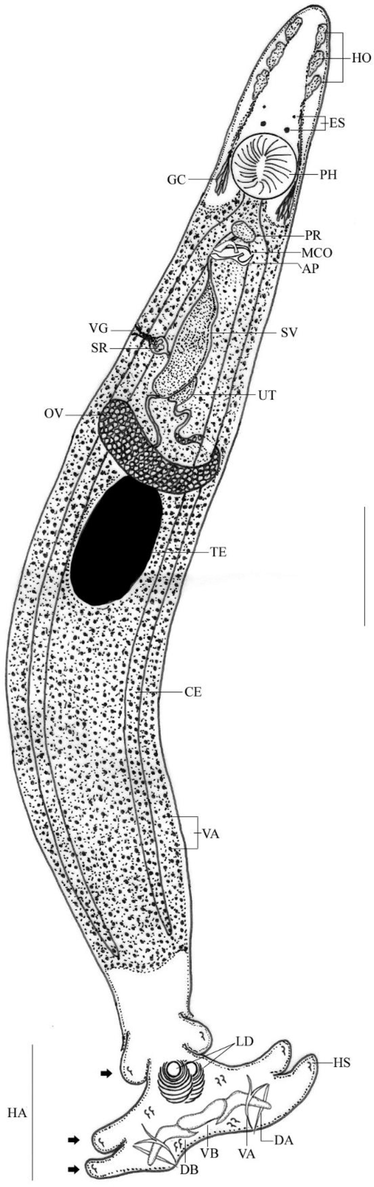
Line drawing of Protolamellodiscus senilobatus infecting Argyrops filamentosus. Note: HO, head organs; ES, eye spots; PH, pharynx; PR, prostatic reservoir; MCO, male copulatory organ; SV, seminal vesicle; OV, ovary; TE, testis; VA, vitellaria; CE, Ceca; HA, haptor; LD, lamellodiscs; DA, dorsal anchor; VA, ventral anchor; VB, ventral bar; DB, dorsal bar; Black arrows, bilateral lobes of haptor; AP, accessory piece; SR, seminal receptacle; GC, glandular cells; HS, hook(s). Scale bar = 100 µm.
3.1 Microscopic examination
Body slender, fusiform with parallel lateral margins. Tegument smooth. The anterior region with 3 pairs of head organs, 2 pairs of eye spots, accessory granules, and two groups of glandular cells lateral to the pharynx. Mouth subterminal, opening ventrally. Pharynx oval. The esophagus very short. Ceca terminate blindly. Haptor with 2 similar lamellodiscs and 3 bilateral pairs of lobes, 3 bars, and 14 marginal hooks.
Nine concentric tubular lamellae make up lamellodiscs. Details of lamellae from center to periphery: 1 complete circle (ring 1), 8 incomplete circles (rings 2–9). The two types of anchors are different; ventral anchor with an elongated deep root and point that is acutely recurved, and dorsal anchor with a straight shaft. Ventral bar plate-like with small knob-like ends; paired dorsal bar with the medial bend, proximal end with spinous protrusion. Hooks 7 pairs; hook pair 1 located close to the ventral anchor, followed by hook pairs 2, 3, and 4 at the apices of the haptoral lobes; hook pair 5 behind the ventral bar; hook pair 6 close to the dorsal anchor’s point; and hook pair 7 close to the dorsal anchor’s base.
Testis oval, intercaecal; vas deferens arise from the antero-sinistral portion of the testis, enlarge into broad seminal vesicle in the midline of the body, and then simple dilate to form a saccate prostatic reservoir that is located anterior to the copulatory complex. The male copulatory organ (MCO) had a curved tube with a broad-ended distal loop and a subterminal recurved spine. The accessory piece consists of a flattened proximal portion that bifurcates at the midpoint.
The ovary is pyriform, intercaecal, pre-testicular, and loops around the left intestinal caecum; the oviduct is lengthy and forms an ootype; the uterus extends anteriorly along the midline of the body; the vagina funnel-shaped, opening into the medial seminal receptacle; vitellaria densely packed throughout the trunk (Table 1).
Comparable items
Kritsky, Jiménez-Ruiz & Sey (2000)
Present study
Fish host
Argyrops spinifer, Argyrops filamentosus
Argyrops filamentosus
Body (L)
0.720–1.318 (1.065)
0.710–1.298 (1.002)
Body (W)
0.120–0.240 (0.185)
0.116–0.235 (0.179)
Haptor (L)
0.104–0.117 (0.111)
0.100–0.113 (0.109)
Haptor (W)
0.169–0.235 (0.155)
0.163–0.230 (0.201)
Lamellodisc (L)
0.037–0.053 (0.044)
0.035–0.050 (0.041)
Lamellodisc (W)
0.029–0.038 (0.032)
0.026–0.035 (0.030)
No. of lamellae
9 (1 complete + 8 incomplete)
9 (1 complete + 8 incomplete)
Pharynx (W)
0.061–0.089 (0.073)
0.059–0.085 (0.070)
Copulator organ (L)
0.038–0.053 (0.045)
0.035–0.048 (0.042)
Accessory piece (L)
0.018–0.034 (0.028)
0.015–0.030 (0.024)
Dorsal anchor (L)
0.037–0.044 (0.041)
0.034–0.040 (0.038)
Ventral anchor (L)
0.038–0.049 (0.045)
0.035–0.044 (0.042)
Dorsal bar (L)
0.035–0.046 (0.040)
0.032–0.043 (0.038)
Ventral bar (L)
0.034–0.047 (0.041)
0.031–0.044 (0.039)
Hook (L)
0.009–0.011 (0.010)
0.009–0.011 (0.010)
No. of hooks
7 pairs
7 pairs
Ovary (W)
0.044–0.056 (0.047)
0.040–0.052 (0.044)
Testis (L)
0.101–0.113 (0.107)
0.100–0.109 (0.106)
Testis (W)
0.048–0.055 (0.052)
0.046–0.052 (0.049)
3.2 Measurements
Total length 0.710–1.298 (1.002), maximum width 0.116–0.235 (0.179); pharynx width 0.059–0.085 (0.070); haptor 0.100–0.113 (0.109) × 0.163–0.230 (0.201); lamellodisc 0.035–0.050 (0.041) × 0.026–0.035 (0.030); ventral anchor 0.035–0.044 (0.042); dorsal anchor 0.034–0.040 (0.038); ventral bar 0.031–0.044 (0.039); dorsal bar 0.032–0.043 (0.038); hooks 0.009–0.011 (0.010); testis 0.100–0.109 (0.106) × 0.046–0.052 (0.049); male copulatory organ 0.035–0.048 (0.042); accessory piece 0.015–0.030 (0.024); and ovary 0.040–0.052 (0.044).
3.3 Molecular analysis
The partial 28S rRNA sequence was 630 bp with 46.7 % GC content (A(22.7 % 143) | C(18.41 % 116) | G(28.25 % 178) | T(30.63 % 193)) and assigned in GenBank (acc. no. OP419541.1). A phylogenetic dendrogram was created by aligning the nucleotide sequence of 31 taxa over 619 positions using the ML approach (Table 2). Between each specimen sequence, there was an overall mean distance of 0.254. Comparable species have different identity ranges, as 98.41–77.97 % for Diplectanidae, 85.82–84.41 % for Ancylodiscoididae, and 75.95–73.99 % for Dactylogyridae. The current dendrogram is split into two major clades (Fig. 3), the first of which was strongly supported by species belonging to the Diplectanidae and received a value of 100, and the latter of which was represented by taxa belonging to the Dactylogyridae and Ancylodiscoididae. High sequence identity (98.41–84.62 %) is shown by the examined species for Lamellodiscus species, and this is highly confirmed by a value of 98. With a high bootstrap value of 100, the current species was robustly grouped in the same clade as Lamellodiscus acanthopagri (DQ054822.1).
Parasite species
Family
Source
% Identity
GC content
DQ054822.1 Lamellodiscus acanthopagri
Diplectanidae
GenBank
98.41
47.1
LC565451.1 Lamellodiscus takitai
Diplectanidae
GenBank
93.33
47
FJ767865.1 Lamellodiscus japonicus
Diplectanidae
GenBank
93.32
47.1
LC565449.1 Lamellodiscus chin
Diplectanidae
GenBank
88.92
49.4
LC565450.1 Lamellodiscus spari
Diplectanidae
GenBank
88.77
49.2
EF100556.1 Lobotrema sciaenae
Diplectanidae
GenBank
86.87
48.3
MK937581.1 Dolicirroplectanum lacustre
Diplectanidae
GenBank
86.22
53
JN254760.1 Diplectanocotyla gracilis
Diplectanidae
GenBank
84.86
53.4
KY640620.1 Lamellodiscus pagrosomi
Diplectanidae
GenBank
84.62
47.7
FJ882609.1 Echinoplectanum leopardi
Diplectanidae
GenBank
84.06
53.7
KY008490.1 Teraplectanum angustitubus
Diplectanidae
GenBank
83.05
54.5
EF100563.1 Lepidotrema longipenis
Diplectanidae
GenBank
81.38
55.2
OK104115.1 Calydiscoides flexuosus
Diplectanidae
GenBank
80.79
47.8
MK203833.1 Diplectanum aequans
Diplectanidae
GenBank
80.42
48.1
DQ054824.1 Laticola latesi
Diplectanidae
GenBank
79.85
50.6
AY553621.1 Pseudorhabdosynochus latesis
Diplectanidae
GenBank
79.85
51.1
LC494521.1 Latiphagum setosum
Diplectanidae
GenBank
79.36
49.7
MF784090.1 Nasobranchitrema sp.
Diplectanidae
GenBank
79.14
53.8
DQ157672.1 Murraytrema pricei
Diplectanidae
GenBank
78.02
49.6
GU573891.1 Sinodiplectanotrema malayanum
Diplectanidae
GenBank
77.97
49.3
DQ157657.1 Euryhaliotrema johnii
Ancylodiscoididae
GenBank
85.82
50.1
EF100545.1 Quadriacanthus kobiensis
Ancylodiscoididae
GenBank
85.66
48.3
MK282177.1 Cornudiscoides sp.
Ancylodiscoididae
GenBank
85.38
46.5
KP056241.1 Chauhanellus boegeri
Ancylodiscoididae
GenBank
85.38
44.1
EF100553.1 Thaparocleidus sp.
Ancylodiscoididae
GenBank
85.26
48.6
MT994792.1 Bychowskyella pseudobagri
Ancylodiscoididae
GenBank
84.95
47.5
MT023785.1 Microncocotyle bicoccae
Ancylodiscoididae
GenBank
84.41
51.2
MZ408907.1 Characithecium paranapanemense
Dactylogyridae
GenBank
75.95
49.8
LC494515.1 Gobioecetes biwaensis
Dactylogyridae
GenBank
73.99
53.4
MT556797.1 Urocleidoides tenuis
Dactylogyridae
GenBank
73.99
51.1
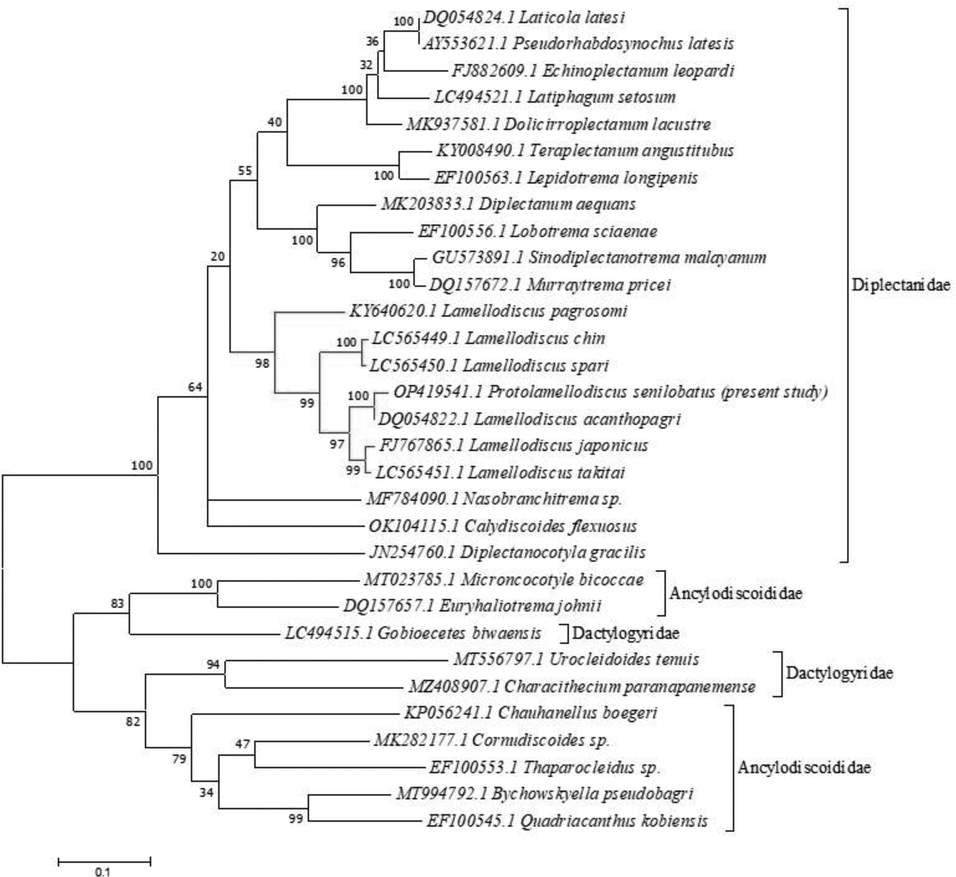
Molecular Phylogenetic analysis of the partial 28S rRNA sequence by Maximum Likelihood (ML) method based on the Tamura-Nei model. The tree with the highest log likelihood (-10117.20) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site.
4 Discussion
Although there have been many studies about marine fish parasites from the Red Sea, little is known about Lamellodiscinae, especially Protolamellodiscus (Kritsky et al., 2000). In the present study, only 21 specimens of the total 30 Argyrops filamentosus fish examined had an infection rate of 70 % for the recovered parasite that inhibit the gill region. This rate is quite similar to Boudaya et al. (2009) who stated that 77 % of Lithognathus mormyrus were infected with Lamellodiscus flagellatus.
The current species is compatible with other diplectanids, Protolamellodiscus species, that have inhabited four host families, by sharing all the species’ distinguishing features. Our results corroborated with Kritsky et al. (2000) that the primary key feature for differentiating Protolamellodiscus species is the existence of lamellodiscs formed of concentric lamellae. The recovered species has all morphological and morphometric features with P. senilobatus, which was isolated previously from A. spinifer and A. filamentosus (Sparidae). Oliver and Radujkovic (1987) claim that while this species and P. raibauti (from Diplodus annularis, Sparidae) share similarities in the comparative morphology of the copulatory complex, they differ from this species by possessing a subterminal spine that arises from the male copulatory organ, a medial vaginal opening in the seminal receptacle (sublaterally on the left side of the body in P. raibauti), presence of three bilateral pairs of haptoral lobes (lobes lacking in P. raibauti), a flattened subrectangular ventral bar (bar rod-shaped in P. raibauti), and each dorsal bar with a proximal spine. The comparative anatomy of the copulatory complex and intercecal vas deferens in Oliver’s (1969) description of P. serranelli (from Serranus hepatus and S. scriba, Serranidae) highlights the difference between the recovered species. P. senilobatus differs from P. convolutus (from Nemipterus hexodon, Nemipteridae) in that it has three bilateral pairs of haptoral lobes, the vaginal aperture is submarginal on the sinistrodorsal body surface that is situated midway between the ovary and copulatory complex, and the vas deferens loops the left intestinal cecum. Yamaguti (1953) did not include details of P. convolutus for the sclerotized structures of the haptor and copulatory complex.
For the classification of this species, molecular studies should support the degree of morphological variation between the current parasite and other monogenean species. The phylogenetic position of Protolamellodiscus species identified from Saudi Arabia was validated in the current investigation using the partial genetic sequences of the 28S rRNA gene. The family Diplectanidae is unambiguous and located in a distinct clade, as demonstrated by the sequence alignment and phylogenetic trees in the current study. Similar findings relating to the partial 28S rRNA of parasitic monogeneans have been reported in earlier research (Nitta, 2021). Following Desdevises et al. (2000), Chotnipat et al. (2015), van Steenberge et al. (2015), Tambireddy et al. (2016), and Villar-Torres et al. (2019), the current sequencing analysis demonstrated that the partial 28S rRNA gene contains enough phylogenetic signal. The recorded parasite cannot be assigned to any of the aligned sequences since the percentage of sequence identities between the present parasite and the aligned sequences exhibited a maximum identity of 98.41 % with Lamellodiscus acanthopagri (DQ054822.1), which is morphologically distinct. A high bootstrap value of 98 further substantiated the link between the Protolamellodiscus and Lamellodiscus species. The claims made by Kritsky et al. (2000) and Domingues and Boeger (2008) that Protolamellodiscus is closely related to Calydiscoides considering the existence of ventral and dorsal lamellodiscs (each with several concentric unpaired lamellae, with the most anterior lamella forming a complete circle) conflict with this study. The only possible explanation for that issue is Oliver’s (1987) differential diagnosis of these two genera based on lamellodisc structure is unclear because it relates to lamellodiscs observed from different orientations. Oliver (1987) also noted that the morphology of the eggs, with Calydiscoides having elongate eggs and Protolamellodiscus having tetrahedral eggs, may be utilized as a significant feature for distinction. In contrast to Protolamellodiscus, which hosts serranids, sparids, and nemipterids, Justine (2007) noted that Calydiscoides is consistent with a list of species that hosts lethrinids and nemipterids. The present reported parasite has most similarities with the previously described P. senilobatus which was not phylogenetically analyzed before.
5 Conclusion
This study, which is included in the establishment of data for the genus that will aid future studies and species circumscription, should be regarded as the first report combining the morphological description and molecular phylogenetic analysis of the partial 28S rRNA sequences of P. senilobatus isolated from the A. filamentosus (Sparidae) from the coasts of the Red Sea at Jeddah, Saudi Arabia. To further understand the taxonomic classification of these parasites, extensive phylogenetic studies should be carried out.
Acknowledgments
This study was supported by the Researchers Supporting Project (RSP-2021/25), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abu Shusha, T.L., Kalantan, M.Z., Al-Nazry, H.H., Al-Ghamdi, Y.A., 2010 Fishes from territorial Saudi Red Sea waters. Marine Research Centre-Jeddah, Deputy Ministry of fisheries affairs, Ministry of Agriculture, Saudi Arabia.
- The biology of gyrodactylid monogeneans: The Russian-doll killers. Adv. Parasitol.. 2007;64:161-218.
- [Google Scholar]
- Current status of Sparidae aquaculture. In: Parlidis M.A., Mylonas C.C., eds. Sparidae Biology and Aquaculture of Gilhead Sea Bream and Other Species. UK: Blackwell Publishing Ltd; 2011. p. :1-50.
- [Google Scholar]
- Diplectanid parasites of Lithognathus mormyrus (L.) (Teleostei: Sparidae) from the Mediterranean Sea, with the description of Lamellodiscus flagellates n. sp. (Monogenea: Diplectanidae) Sys. Parasitol.. 2009;74:149-159.
- [Google Scholar]
- Parasitology meets ecology on its own terms: margolis et al. revisited. J. Parasitol.. 1997;83:575-583.
- [Google Scholar]
- Molecular and morphological evidence for the widespread distribution of Laticola paralatesi infecting wild and farmed Lates calcarifer in Australia. Dis. Aquat. Org.. 2015;113(3):195-205.
- [Google Scholar]
- Ribosomal DNA analysis of isolates of the liver fluke Opisthorchis pedicellata (Verma, 1927) from two siluroid fish species in India. J. Helminthol.. 2017;9:302-311.
- [Google Scholar]
- Comaprison of ribosomal DNA sequences of Lamellodiscus spp. (Monogenea, Diplectanidae) parasiting Pagellus (Sparidae, Teleostei) in the North Mediterranean Sea: species divergence and coevoltutionary interactions. Int. J. Parasitol.. 2000;30(6):741-746.
- [Google Scholar]
- Evolution and determinants of host specificity in the genus Lamellodiscus (Monogenea) Biol. J. Linn. Soc.. 2002;77:431-443.
- [Google Scholar]
- Phylogeny and revision of Diplectanidae Monticelli, 1903 (Platyhelminthes: Monogenoidea) Zootaxa. 2008;1698(1):1.
- [Google Scholar]
- Heteromicrocotyla polyorchis Unnithan, 1961 (Monogenea: Heteromicrocotylidae), a gill parasite of the yellow-spotted trevally, Carangoides fulvoguttatus (Carangidae) from Saudi Arabia: Morphology and phylogeny. Microb. Pathog.. 2021;160:105165
- [Google Scholar]
- Lamellodiscus serranelli n. sp. (Monogenea) parasite de Téléostéens du genre Serranus. Ann. Parasit. Hum. Comp.. 1965;40:261-264.
- [Google Scholar]
- BioEdit: a user-friendly biological sequence alignment editor and anlaysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser.. 1999;41:95-98.
- [Google Scholar]
- Infestation of Cage-Cultured marine fish with Benedenia acanthopagri (Monogenea: Capsalidae) in Eastern Province of Saudi Arabia. Glob. Vet.. 2015;14(2):219-227.
- [Google Scholar]
- New locality record of Monaxinoides austrosinensis (Mazocrraeidea, Monaxinoididae) of finlet crevalle, Atule mate (Perciformes: Carangidae) from the Gulf of Thailand. Agric. Nat. Resour.. 2016;50:416-420.
- [Google Scholar]
- Phylogenetic relationships within the polyopisthocotylean monogeneans (Platyhelminthes) inferred from partial 28S rDNA sequences. Int. J. Parasitol.. 2001;31:393-401.
- [Google Scholar]
- Justine, J.L., 2007. Species of Calydiscoides Young, 1969 (Monogenea: Diplectanidae) from lethrinid fishes, with the redescription of all of the type-specimens and the description of C. euzeti n. sp. from Lethrinus rubrioperculatus and L. xanthochilus off New Caledonia. Sys. Parasitol. 67 (3), 187-209.
- Host specificity is linked to intraspecific variability in the genus Lamellodiscus (Monogenea) Parasitology. 2008;135(5):607-616.
- [Google Scholar]
- Diplectanids (Monogenoidea: Dactylogyridea) from the gills of marine fishes of the Persian Gulf off Kuwait. Comp. Parasitol.. 2000;67(2):145-164.
- [Google Scholar]
- MEGA7: molecular evolutionary genetics analysis version 7/0 for bigger datasets. Mol. Biol. Evol.. 2016;33:1870-1874.
- [Google Scholar]
- Novel molecular data for monogenean parasites of sparid fishes in the Mediterranean and a molecular phylogeny of the Microcotylidae Taschenberg, 1879. CRPVBD. 2022;2:100069
- [Google Scholar]
- Molecular phylogenetics of cupped oysters based on partial 28S rRNA gene sequences. Mol. Phylogenet. Evol.. 1994;3:221-229.
- [Google Scholar]
- The interrelationships of Proseriata (Platyhelminthes: Seriata) tested with molecules and morphology. Mol. Phylogenet. Evol.. 2000;16:449-466.
- [Google Scholar]
- A preliminary study on gill parasites of gilthead sea bream Sparus aurata (Linnaeus 1758) (Pisces: Teleostei) from the eastern Tunisian sea-cage aquaculture. GERF Bull. Biosci. 2016;7(1):1-5.
- [Google Scholar]
- Microcotyle omanae n. sp. (Monogenea: Microcotylidae), a parasite of Cheimerius nufar (Valenciennes) (Sparidae) from the Arabian Sea. Sys. Parasitol.. 2013;86:153-163.
- [Google Scholar]
- Testing the new animal phylogeny: first use of combined large-subunit and small-subunit rRNA gene sequences to classify the protostomes. Mol. Biol. Evol.. 2002;19(3):289-301.
- [Google Scholar]
- On a Gyrodactylus species from Northern Sweden and the subgeneric position of G. hrabei Ergens, Trematoda, Monogenea. Zool Scr.. 1957;1973(2):39-42.
- [Google Scholar]
- Morphological and molecular (28S rRNA) data of monogeneans (Platyhelminthes) infecting the gill lamellae of marine fishes in the Campeche Bank, southwest Gulf of Mexico. ZooKeys. 2018;783:125-161.
- [Google Scholar]
- Studies on monogenetic trematodes. XVI. Rhamnocercinae new subfamily of Dactylogyridae. Am. Midl. Nat.. 1954;52:129-132.
- [Google Scholar]
- Per una nuova classificazione degli “Heterocotylea”. Monit. Zool. Ital.. 1903;14:334-336.
- [Google Scholar]
- Lamellodiscus (Monogenea: Diplectanidae) species parasitic on Japanese Acanthopagrus Peters, with proposals of L. chin n. sp. infecting A. sivicolus Akazaki and L. egusai nom. nov. for L. japonicus Ogawa & Egusa, 1978, a junior homonym of L. japonicus Pillai & Pillai, 1974. Sys. Parasitol.. 2021;98:177-188.
- [Google Scholar]
- Recherches sur les Diplectanidae (Monogenea) parasites de Téléstéens du Golfe du Lion. II. Lamellodiscinae nov. sub. fam. Vie et Milieu, Serie A, Biologie Marine. 1969;20:43-72.
- [Google Scholar]
- Quelques aspects de la spécifiticité parasitaire chez les Diplectanidae Bychowsky, 1957 (Monogenea, Monopisthocotylea) Bull. Mus. Natl. Hist. Nat., Zool., Paris, Sér. A. 1982;123:295-301.
- [Google Scholar]
- Oliver, G., 1987. Les Diplectanidae Bychowsky 1957 (Monogenea, Monopisthocotylea, Dactylogyridea). Systématique, Biologie, Ontogénie, Écologie, Essai de Phylogénèse. Thèse d’état. Université des Sciences et Techniques du Languedoc, 433 pp.
- Protolamellodiscus raibauti n. sp., une nouvelle espèce de Diplectanidae Bychowsky, 1957 (Monogenea, Monopisthocotylea) parasite de Diplodus annularis (Linnaeus, 1758) (Sparidae) Ann. Parasit. Hum. Comp.. 1987;62:209-213.
- [Google Scholar]
- Oliver, G., 1987. Les Diplectanidae Bychowsky, 1957 (Monogenea, Monopisthocotylea, Dactylogyridae) Systématique: biologie: ontogénie: ecologie: essaide phylogenèse. Doctor of Science Thesis, I’Université des Sciences et Techniques du Languedoc, Académie de Montpellier, France, 433 pp.
- Morphological and molecular evolution are not linked in Lamellodiscus (Platyhelminthes, Monogenea) PLoS ONE. 2011;6(10):e26252.
- [Google Scholar]
- Marine Parasitology. Melbourne: CSIRO Publishing; 2005.
- Morphological and attachment changes of Lamellodiscus theroni (Monogenea: Diplectanidae) during its post-larval development on fish. Folia Parasitol.. 2011;62(1):26.
- [Google Scholar]
- Specificity and specialization of congeneric monogeneans parasitizing cyprinid fish. Evolution. 2006;60:1023-1037.
- [Google Scholar]
- Molecular characterization and phylogeny of some mazocraeidean monogeneans from carangid fish. Acta Parasitol.. 2016;61:360-368.
- [Google Scholar]
- The CLUSTAL-X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res.. 1997;24:4876-4882.
- [Google Scholar]
- Morphology, molecules, and monogenean parasites: an example of an intergrative approach to cichlid biodiversity. PLoS ONE. 2015;10(4):e0124474.
- [Google Scholar]
- Redescription and new host record of Diplostamenides sciaenae (Monogenea, Microcotylidae) and its phylogenetic status using molecular markers. Vestnik Zoologii. 2018;52:37-46.
- [Google Scholar]
- Neither Diplectanum nor specific: a dramatic twist to the taxonomic framework of Diplectanum (Monogenea: Diplectanidae) Int. J. Parasitol.. 2019;49(5):365-374.
- [Google Scholar]
- WoRMS, 2022. Protolamellodiscus Oliver, 1969. Accessed at: https://www.marinespecies.org/ aphia.php? p=taxdetails&id=119294 on 2022-08-16
- Parasitic worms mainly from Celebes. Part 2. Monogenetic trematodes of fishes. Acta Med.. 1953;8:203-256.
- [Google Scholar]







