Translate this page into:
Molecular study of geminiviruses: Complex biology, host-vector interactions, and increasing diversity
⁎Corresponding author. arif_1821uaf@yahoo.com (Muhammad Arif)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The Geminiviridae family has become the largest family of plant viruses, with >300 species and nine genera. This classification is based on genome organization, host range and insect-vectors. The capsid structure of geminiviruses is unique and constructed from twinned icosahedral with 110 duplicates of coat protein. The function of coat protein in geminiviruses is multidirectional which helps to cause the infection in wide range of host plants. The begomoviruses is one of the leading genera having ∼320 species of family geminiviridae. This review comprehensively describes viral pathogenesis, gene function, host-virus-vector interactions of geminiviruses and their increasing diversity. Several species of begomoviruses and their associated satellites are responsible to cause huge losses. Cotton leaf curl Multan virus (CLCuMuV) and Tomato yellow leaf curl China virus (TYLCCnV) are leading plant viruses to infect many alternate hosts. Modern mechanisms have been identified to disclose the hidden aspects of plant genomics. From these mechanisms, genome editing by “clustered regulatory interspaced short palindromic repeats” (CRISPR)/CRISPR associated nuclease 9 (Cas9) CRISPR/Cas9 has unfastened the fresh vistas for crop improvement and functional genomics. This review will be helpful for microbiologists and pathologists to understand the complex molecular biology of geminiviruses.
Keywords
Geminiviruses
Viral pathogenesis
Genome organization
Genome editing
CRISPR/Cas9
1 Introduction
In the 19th century, typical symptoms of certain viruses were observed in many tropical and sub-tropical regions of the world. In the 1970s, a group of single-stranded DNA (ssDNA) viruses were found associated with typical symptoms, and later this new group was distinguished as geminiviruses. The earliest record showed that geminiviruses symptoms were noticed in 752 CE in Japan as utilized by a Japanese poet (Empress Koken) in his poem. These plant viruses are insect-transmissible viruses that have become an economically vital group of plant viruses and cause significant losses to the production of crops and ornamental plants across the world. These viruses are responsible for infecting many plants including monocotyledonous and dicotyledonous plants in tropical and sub-tropical parts. During the last 20 years, geminiviruses have caused major epidemics in different regions including African Cassava mosaic virus (ACMV) in Africa, Bean golden mosaic virus (BGMV) in America, Cotton leaf curl virus (CLCuV) in Asia, and Tomato leaf curl virus (TLCV) in Asia, America and in Europe (Brown et al., 2015).
The genome of geminiviruses is reliant on host RNA polymerases for transcription. The ssDNA genome has been replicated into double-stranded DNA (dsDNA) form, which is determined to connect with host histone proteins as mini-chromosomes within the nucleus (Arif et al., 2020). It is quite clear that geminiviruses are not seed-transmissible, but some are mechanically and graft-transmissible. Typical symptoms induced by geminiviruses are leaf enations, leaf curling, distortion of growth, stunted growth, leaf crumpling, vein swelling, and leaf streaking (Brown et al., 2015).
2 Taxonomy of geminiviruses
The Geminiviridae family has become the biggest family of plant viruses, with >300 approved species. The family Geminiviridae consists of nine genera, i.e., Becurtovirus, Begomovirus, Capulavirus, Curtovirus, Eragrovirus, Grablovirus, Mastrevirus, Topocuvirus, and Turncurtovirus which are classified based on genome organization, host range, and the type of insect vector (Brown et al., 2015). International Committee on Taxonomy of Viruses (ICTV) has proposed a unique nomenclature criteria for classifying and naming of new geminiviruses (Zerbini et al., 2017). The new proposed guideline is that if full-length nucleotide sequence identity of freshly recognized geminivirus is <89% using clustral V algorithm (excluding Mastreviruses, which has <75% cut-off parameter) then it could be recognized as fresh specie while the sequence identity is >89% so it could be recognized as a member of same species. And for addition of new geminivirus isolate, if pairwise comparison is <93% it could be a fresh strain of that species and if it is >94% then it might be a variant of that strain of the same species.
3 Genome organization of geminiviruses
Begomovirus is the largest genus belonging to Geminiviridae family which has ∼320 species (Zerbini et al., 2017) and its transmission is mediated by an insect vector whitefly (Bemisia tabaci) (Gilbertson et al., 2015). For the last 30 years, begomoviruses have become a significant viral pathogen for food, fiber, and ornamental crops worldwide. Some begomoviruses have two ssDNA molecules, named as DNA-A and DNA-B, respectively, as their genome. Both DNA-A and DNA-B are ∼2.8 kb in length. DNA-A has 5–6 open reading frames (ORFs), one or two of which are in the virion-sense, and the other four in the viral complementary sense. Virion-sense ORFs are (AV1 and AV2) and complementary sense are (AC1, AC2, AC3, and AC4). Those having only DNA A component are monopartite begomoviruses while others having both DNA A and DNA B components are known as bipartite begomoviruses (Briddon et al., 2010). Monopartite begomoviruses contain only a single DNA molecule like genome which is similar to DNA A of bipartite viruses, and movement of the virus is being facilitated by coat protein (CP) or V2 open reading frame (ORF).
The DNA A typically harbors 6 ORFs including AV1: (recognized as AR1 and coat protein CP), AV2: (recognized as AR2, AV2 protein and movement protein MP) on the virion sense strand, AC1: (called as AL1, replication protein Rep (Nash et al., 2011)), AC2: (called as AL2, transcriptional activator TraP potential silencing suppressor (Yang et al., 2007)), AC3: (called as AL3, replication enhancer REn and act as cell cycle regulator protein (Pasumarthy et al., 2011)) and AC4: (called as AL4 and AC4 protein involved in the movement of monopartite begomoviruses) on the complementary sense strand. DNA B has only two ORFs encoding proteins including BV1: (BR1 and it interact with BC1 for cell–cell movement and called as nuclear shuttle protein on virion sense strand) and BC1: (BL1, pathogenicity determents and called as movement protein MPB on complementary sense strand) which are involved in movement functions.
Proteins encoded by DNA-A are associated with viral DNA replication, vector transmission, encapsidation, while those encoded by DNA-B are required in the intercellular and intracellular movement of viral particles. These proteins have multiple functions including host gene regulation, virus replication, vector transmission, viral assembly, and silencing suppression (Priyadarshini et al., 2011).
Genomic studies and Phylogenetic analysis has confirmed that begomoviruses are divided into two groups, New World (NW) and Old World (OW) begomoviruses (Brown et al., 2015). This might have occurred either by the association of betasatellite with monopartite begomoviruses or by displacement of DNA-B from OW bipartite begomovirus as demonstrated experimentally for Srilankan cassava mosaic virus (SLCMV). Although, few species of bipartite begomoviruses exists in the OW and their genome contains only a single component which is homologous to DNA A of bipartite viruses. A few monopartite begomoviruses can induce disease lonely in the whole area i.e. Tomato yellow leaf curl virus (TYLCV) (Scholthof et al., 2011).
The ssDNA 1.3 kb satellites have an association with several OW monopartite begomoviruses and NW bipartite begomoviruses. Betasatellites earlier called DNA β have single ORF (βC1), which was utilized as a suppressor of host gene silencing (Cui et al., 2005). It has a dramatic effect on enhancing the virulence of partner begomovirus. The homolog sequence of a begomovirus determined in betasatellite is stem-loop and TAATATTAC sequences. The other remaining satellite sequences are not explicitly associated with helper begomoviruses. Betasatellites require assistance from helper begomoviruses for encapsidation and replication, and in some instances, play a vital role in the establishment of the pathogen in the field. Betasatellites are assumed to be unrestrained because they might function and associate with more than one helper begomoviruses.
Alphasatellites (∼1380 bp) belong to circular ssDNA molecules which acts as an assistant virus for transmission and encapsidation. Alphasatellites are also known as DNA 1. Alphasatellites are still perceived as a mystery in plant virology. Their exact function and occurrence with begomovirus complexes still need to be studied. Few studies suggest that Alphasatellites have a decisive role in the suppression of Post-transcriptional gene silencing (PTGS) and symptoms reduction (Wu and Zhou, 2005; Nawaz-ul-Rehman et al., 2010; Idris et al., 2011). Alphasatellites supposed to be from nano viruses because they have 3 conserved regions: (1) a stem-loop structure with a non-nucleotide (TAGTATT/AC) sequence which is assimilated with the Nano viridae, this part has ori on which Rep cleaves DNA to initiate rolling circle replication RCR, (2) an ORF encoding a Rep protein which has a size of about 36.6 kDa and also has 315 amino acids, (3) a rich region of ∼200 nucleotides which are known as stuffer sequence.
Independently replicating nano virus-like satellites are commonly linked with begomoviruses and their betasatellite complexes, which are known as alphasatellites (formerly accredited as DNA-1). Till now, three kinds of alphasatellites have been identified (Rosario et al., 2013). Type one has been known to enrich the symptoms during co-infection of plants with helper virus and its betasatellite, proposing that it downregulates the virulence to some extent (Idris et al., 2011). Type two and three of alphasatellite are associated with bipartite begomoviruses in the new world (Brazil, Cuba, and Venezuela) (Paprotka et al., 2010; Romay et al., 2010).
Betasatellites were firstly discovered in 2000 and after that >260 full-length betasatellites sequences are available in sequence databases (Briddon et al., 2008). Analysis of these betasatellite sequences showed a conserved single complementary sense ORF (βC1), an adenine-rich region and conserved satellite region which have sequence similarity with TYLCV of Australia satellite DNA (Briddon et al., 2003). The βC1 ORF of all the betasatellite molecules is conserved in size and position (Saeed et al., 2005).
4 Gene function and host-virus-vector interactions of geminiviruses
The geminiviruses not only cooperate regarding synergism and interference but also interrelate with their transmission vector and the host plant from where they endure their life cycle. It has been reported that there are 10–100 viruses for single host species of plants and animals. It has been reported in many studies that approximately 1000 different viruses have significant potential to infect humans (Norrby, 2008). The ∼47% viruses require a certain causal agent for causing a serious epidemic in emerging plants. Apart from a small sequence of ∼200 nucleotides with high sequence identity that is called “common region” (CR), and their size, the DNA-A and DNA-B components of bipartite begomoviruses are completely different from each other.
The replication of CLCuMuB occurs in tomato, tobacco and datura plant with the existence of helper viruses, which are TYLCV of Australia, TYLCV of Iran isolate, TYLCV Karnataka and BSTV. The CLCuMuB infectious recombinant constructs were prepared in which 35S, or petunia and ChsA promoter segments haves changed the CLCuMuB βC1 ORF and were labeled as pBinbD C1-35S and pBin bDC1-ChsA. Normal petunia plants having pBin bD C1-ChsA with the occurrence of helper virus can cause silencing of GUS, ChsA has a function in transgenic tobacco and non-transgenic petunia plants (Kharazmi et al., 2012).
The probability of CLCuMuB encapsidation in TYLCV-Ab coat protein was tested by an immunocapture Polymerase chain reaction and with whitefly mediated transmission of CLCuMuB. But immunocapture PCR data validated that the CLCuMuB DNA and TYLCV-Ab coat protein are connected in vivo, so this data was not satisfactory to confirm that CLCuMuB DNA can be encapsidated in viral coat protein (Tabein et al., 2013).
A lot of failed efforts to reproduce yellow vein symptoms in Ageratum conyzoides through re-inoculating the Ageratum yellow vein virus (AYVV) has confirmed the existence of a supplementary component that has a responsibility to induce the symptoms. Many recombinant components were characterized from that diseased host plant A. conyzoides. After discovering the recombinant component, a unique ssDNA component (having half size of helper begomovirus) was identified from A. conyzoides host plant which could reproduce the symptoms in AYVV. Later this component was recognized as DNA β and afterward named as Betasatellite. These betasatellites are unique in nature and linked with helper begomoviruses (Sattar et al., 2013).
There is a vector specificity under the specie level with different biotypes of whitefly, they confirmed vector specialty of 15 begomoviruses linked with B biotype of whitefly rather than other tested biotypes. This study proved that certain begomoviruses could be transmitted effectively via the exact biotype of whitefly. Transmission of plant viruses by insect vector depends on the length of time on which they associate with the vector. Foregut and stylet-borne viruses associate quickly with cuticle lining and their transmission occur within hours or days after acquisition as shown in Fig. 1. Circulative viruses have acquired into vector hemolymph, from where they reach to salivary tissues. Once circulative viruses’ infection happens, they remain attached till the remaining life of the insect vector. Stylet and foregut-borne viruses require an extended feeding period of insect vectors for the acquisition of the virus from an infected plant to a healthy plant (Gray et al., 2014).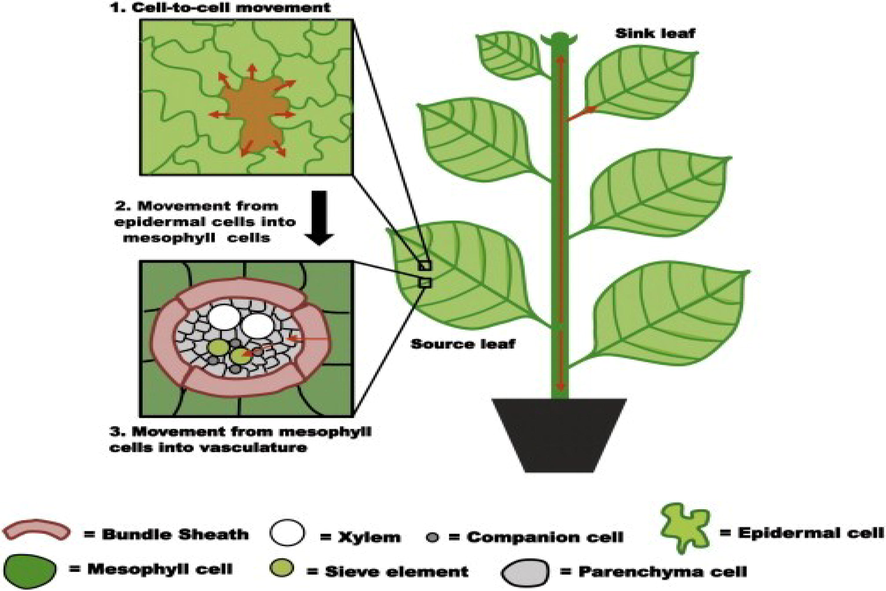
The demonstration of virus movement within the plant cells by semi-persistent manner. Firstly virus enters into plant cell from insect midgut and later on move to different physiological sections of plants.
The TYLCV ssDNA molecules and its coat protein have the ability with each other invitro but non-availability of virion related particles. For confirmation of this encapsidation of CLCuMuB in TYLCV-[Ab] coat protein, whitefly-mediated transmission of TYLCV-[Ab] and CLCuMuB was tested in tomato plants. Analysis of Southern blot and PCR reaction showed the unavailability of both betasatellite DNA and TYLCV-[Ab] in the body of whitefly and not in test plants which were inoculated with viruliferous Bemisia tabaci. It is commonly assumed that geminiviruses transmission through insect vectors includes complete virus particles (Caciagli et al., 2009). So best description for betasatellite transmission through whitefly is its encapsidation in the virus particle. TYLCV-Ab has strong potential to encapsidate and trans-replicate CLCuMuB, but no cognate betasatellite identified in nature having monopartite begomovirus (Pakniat et al., 2010).
It is quite deceptive that begomoviruses' genome displays exciting plasticity leading to the capacity for developing the quick response to change the cropping systems. A plant host in a certain area can directly or indirectly affect by the vector population of that area. The density of the whitefly population on the horizontal leaved cotton variety can be different from hairy leaved cultivars and eventually affects the selection of a particular biotype of whitefly (Amrao et al., 2010).
Techniques which are commonly used to detect begomoviruses are Enzyme-linked immunosorbent assays (ELISA) and immunoblotting, molecular hybridization, conventional (end-point) Polymerase chain reaction (PCR) techniques by using specific primers or restriction length fragment polymorphism (RFLP) (Martinez-culebras et al., 2001; Davino et al., 2008), quantitative real-time Polymerase chain reaction (qRT-PCR) for quantification of viruses (Mason et al., 2008), loop-mediated isothermal amplification (LAMP) (Fukuta et al., 2003). The conventional polymerase chain reaction is extensively used for the detection of begomoviruses. A lot of studies showed drawbacks of this technique because of low accuracy to determine the number of viruses. Some plants have a few virus titers which are below the detection limit by conventional PCR. So, it is necessary to quantify the viruses from symptomatic and non-symptomatic plants. The quantification and identification of DNA/RNA viruses by qPCR have removed a lot of problems, and it is better to use rather than conventional PCR because of more speedy and accuracy (Mason et al., 2008; Papayiannis et al., 2010).
Virus-induced gene silencing has become a powerful tool to study the plant functional genomics, particularly for those plants that pose complications with genetic engineering. cotton leaf curl crumple virus based virus-induced gene silencing vector (CLCrV-VIGS) provides an effective gene silencing tool that can help in reverse genetics and genomics study of candidate gene of cotton (Fu et al., 2015; Liu et al., 2015).
Next-generation or deep sequencing can show some comprehensions in virus-induced plant defense mechanisms. The VsiRNA characterization through deep sequencing has been investigated in some host plants which are, maize plants which showed infection with Sugarcane Mosaic virus (Xia et al., 2014), apple with Apple stem grooving virus (Visser et al., 2014), tomato plants through Tomato yellow leaf curl virus (Yang et al., 2011), rice crop with Rice stripe virus (Xu et al., 2012). Tomato plants showing infection of tomato yellow leaf curl Sardinia virus have vsiRNAs accounted 21nt (56%) and (31%) prevailed on the basis of length (Miozzi et al., 2013). The Cabbage leaf curl virus (CaLCuV) have about 21 and 24 nucleotide vsiRNAs which characterize as 2nd largest segments of about 20–25 nucleotide reads (Aregger et al., 2012), but this mechanism has a difference in infections of tomato yellow leaf curl China virus (TYLCNV) in which 22 nucleotide vsiRNAs added especially in infected plants of tomato and tobacco. That difference in distribution size of vsiRNAs recommended a difference of biosynthetic pathways of small interfering RNA (siRNA) in inoculated virus plants.
5 Viral pathogenesis of geminiviruses
The ecology of certain virus emphasizes the virus populations interrelating with host populations inside the variable environment, while epidemiology emphasizes the complex association between virus, host plant and the factors that influence the spread within hosts. Plant viruses directly or indirectly affect insect vectors by altering the insect’s life cycle, behavior, and fitness (Jones, 2014).
During the last 20 years, molecular ecological methods contributed significantly to assess the factors allied with viral diseases of economical crops, i.e., cassava (cassava mosaic virus) and maize (maize streak virus). These tactics were applied to check the epidemic factors associated with viral emerging diseases. These strategies were employed in Reunion Island to evaluate the epidemiology of TYLCV, which was infecting potato crops in the island since the 1990s. Wild hybrids between indigenous and invasive species of whitefly B. indica were subsequently categorized over multiple generations (Péréfarres et al., 2012).
The environmental factors including temperature, relative humidity, and rainfall play a significant role in the spread of viral diseases. The intensity of viral disease is regulated by environmental factors in a certain area. Begomoviruses entirely depend on insect vectors for successful transmission. That’s why controlling the insect vector is necessary to manage the virus infection properly. The rate of rainfall has affected the egg-laying capacity of insects especially the development of eggs in whitefly is severely dependent on rainfall. To handle these environmental factors and its association with insect vectors, a disease predictive model is necessary to measure the intensity of viral pathogens (Khan et al., 2015).
6 Diversity of geminiviruses
Several plant species including major crops, horticultural crops and weeds have been infected by begomoviruses in China. These plant species and their associated begomoviruses are: on tomato as Chinese tomato yellow leaf curl virus (TYLCnV) (He et al., 2004), tobacco as tobacco curly shoot virus (TbCSV)) (Li et al., 2005), squash as squash leaf curl Yunnan virus (SLCYNV) (Xie and Zhou 2003), Euphorbia pulcherrima as Euphorbia leaf curl virus (EuLCGxV) (Ma et al., 2004), and Ageratum conyzoides as Ageratum yellow vein China virus (AYVCnV) (Xiong et al., 2007). Until now, approximately 16 or more begomoviruses with their associated satellites have been identified in China which are Cotton leaf curl Multan virus (CLCuMuV), Tomato yellow leaf curl China virus (TYLCCnV), Squash leaf curl China virus (SLCCV), Tomato leaf curl Taiwan virus (ToLCTwV), Squash leaf curl Yunnan virus (SLCYNV), Tomato leaf curl Thailand virus (TYLCTHV), Tobacco curly shoot virus (TbCSV), Tobacco leaf curl Yunnan virus (TbCLYnV), Euphorbia leaf curl Guangxi virus (EuLCGxV), Ageratum yellow vein China virus (AYVCNV), Malvastrum yellow vein virus (MYVV), Stachytarpheta leaf curl virus (StaLCuV), Ageratum yellow vein China virus (AYVCNV), Papaya leaf curl China virus (PaLCuCnV), Corchorus yellow vein virus (CoYVV) (https://www.ncbi.nlm.nih.gov/ICTVdb/) Papaya leaf curl Guangdong virus (PaLCuGdV), Tomato leaf curl Guangdong virus (ToLCGuV), (He et al., 2008) Tomato yellow leaf curl Guangdong virus (TYLCGuV) (He et al., 2005) Malvastrum leaf curl Fujian virus (MaLCFuV) (Yang et al., 2008).
Cotton (genus Gossypium, family Malvaceae) is a major cash crop in China. The CLCuD has prominent disease symptoms including curling of leaves, thickening of large veins, leaf enations and stunting the growth of plant (Nawaz-ul-Rehman et al., 2009). The Chinese CLCuMuV isolates show pairwise nucleotide sequence identities higher than 99% and are >94% identical to their closest relatives from Pakistan. This suggests that the CLCuMuV-CLCuMuB has been introduced to China from Pakistan by a single introduction event (Du et al., 2015) as shown in Fig. 2. CLCuMuV (Fai [CN: GZ: G6: Hib: 06], EF465535) was found in Guangzhou, China first time in 2006 by infecting H. rosa sinensis and Hibiscus sabdariffa in Fujian province of China. The high genetic homogeneousness of CLCuMuV in China reveals that its establishment was by a single founder event. (Mao et al., 2008; Du et al., 2015; Arif et al., 2018).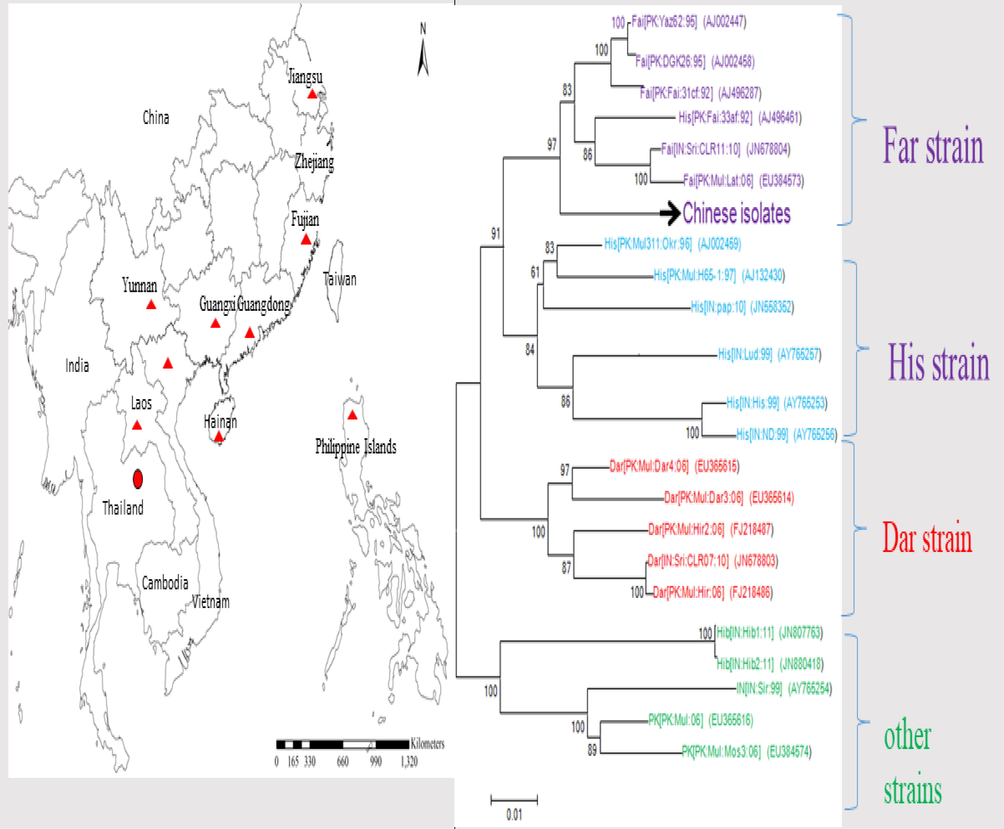
The prevalence of begomoviruses in south East Asia is spreading alarmingly. The dispersion of Chinese isolates shows higher identity with begomoviruses associated from Pakistan. The Chinese CLCuMuV isolates of Far-strain show pairwise nucleotide sequence identities 99% and >94% to their closest relatives from Pakistan.
During October 2008 and July 2009, few cotton plants were observed in Nanning city of Guangxi province; these plants showed upward leaf curling symptoms and dark thickening of green veins of leaves, leave enations and stunning the growth of plants (Cai et al., 2010). Three plants were screened to identify the existence of geminivirus/begomovirus. DNA was extracted from symptomatic plants by using CTAB. Universal begomovirus (Liu et al., 1998) (these primers can amplify the partial intergenic region and V2 gene) were used in PCR. The PCR products have amplified a 500 bp which were then cloned and sequenced. Additional primers were designed to obtain the whole genome of DNA A on the basis of gained sequence. All the data was submitted to gene bank to obtain accession number (GenBank Accession No. GQ924756). The complete sequence of DNA β 1346 bp of the GX1 isolate was obtained from GenBank. The accession number for this sequence betasatellite was GQ906588 (Cai et al., 2010). Symptoms and sequence information has confirmed the presence of CLCuD in China, and it has a connection with CLCuMuV and CLCuMuB.
The Plant-to-plant transmission of CLCuMuV-CLCuMuB in China is supported by the detection of this virus complex in an increasing number of malvaceous plants propagated by seeds. To date, CLCuMuV-CLCuMuB has been detected on H. esculentus in Guangdong in 2008 (Di et al., 2012; Tang et al., 2013), Gossypium hirsutum in Guangxi in 2010 (Cai et al., 2010) and H. cannabinus in Hainan province in 2014 (Tang et al., 2015). This plant-to-plant transmission indicated a biologically meaningful establishment of CLCuMuV-CLCuMuB in these territories. Unfortunately, historical details of these events are missing or inconclusive due to the lack of close surveillance. In addition, whether or not CLCuMuV-CLCuMuB can establish in other provinces is an epidemiologically important event which deserves the close attention (Arif et al., 2021).
The TYLCV has become a destructive pathogen by causing a severe threat to tomato production. Earlier it was identified in the Middle East during the 1960s. TYLCV was first time identified in the industrial city Shanghai, China in March 2006 (Wu et al., 2006). After that, this pathogen started to report in other provinces of China (Zhang et al., 2009). Transmission of TYLCV is through an insect vector whitefly (Bemisia tabaci) (Gennadius) (order Hemiptera: family Aleyrodidae) in a circulative manner. In past studies, it has been reported that Mediterranean B. tabaci (MED) and indigenous Asia II 1 B. tabaci could retain TYLCV DNA in their whole life. However, MED an aggressive B. tabaci can transfer TYLCV more effectively rather than indigenous Asia II 1 B. tabaci (Li et al., 2010). The study of (Yang et al., 2017) has compared the TYLCV complete genome sequences gained from TYLCV infected tomato, as well as from aggressive MED B. tabaci and Asia II 1 indigenous B. tabaci. By assessing the frequency of nucleotide change and the spreading form of mutations, they reported that the genetic unpredictability of TYLCV was changed in both species of whiteflies. The prevalence of TYLCV has been confirmed in 6 provinces of China in the last 5 years (Wu et al., 2006). Continuous deep monitoring displayed that TYLCV also had a presence in Zhejiang province in 2006. After that, this virus starts to move towards the northern territories of China including Shandong, Jiangsu, Hubei and Beijing, where this virus has caused significant losses to tomato crops (Ji et al., 2008; Tao et al., 2010).
Cultivated and non-cultivated plants are major hosts of begomoviruses. The Eclipta prostrata is a widely spread annual weed in China. During 2005, yellow vein symptoms were seen on E. prostrata plants in Guangzhou, Guangdong province of China. Preliminary analysis of infected plants was carried out by PCR (He et al., 2005), and PCR fragment of coat protein (CP) proposed the connection of monopartite begomovirus from these infected plants. G8 virus isolate was cloned from these plants carrying viral symptoms. The whole nucleotide sequence of G8 DNA A was 2745 nucleotides. This complete nucleotide sequence revealed typical features of the begomovirus genome group. By comparing the full-length nucleotide sequence of DNA A, it has been confirmed that G8 and Hn51 isolates have maximum sequence identity with Alternanthera yellow vein virus (AlYVV) at 95.9% and 94.3% respectively. This was the first report of AIYVV infecting E. prostrata in Guangzhou, Guangdong province of China (Tang et al., 2013).
Natural incidence of Sweet potato leaf curl virus (SPLCV) was reported in sweet potato (Ipomoea batatas, family Convolvulaceae) in China and the United States of America (Briddon et al., 2006). In 2007, tall morningglory (I. purpurea L.), also known as Pharbitis purpurea L.), plants displayed begomovirus-like symptoms in the Fujian province of China. Isolation of total DNA from symptomatic leaves was carried out by rolling circle amplification (RCA), and then these samples were cloned and sequenced. Obtained sequence (GenBank Accession No. FJ515896) compared with other DNA sequences from NCBI by using BLAST. The whole sequence displayed the highest nucleotide identity of 92.1% with Jiangsu SPLCV isolate (GenBank Accession No. FJ176701). These analyses confirmed the natural incidence of SPLCV in tall morningglory in China (Yang et al., 2009).
7 Genome editing mechanisms against geminiviruses
Due to fast and unbalanced increase in world population, it is much necessary to apply the advanced crop improvement tactics like genome editing mechanism for climate smart sustainable agriculture to provide the balanced food having proper nutritional value, enhanced biotic and abiotic stress tolerance, superior disease resistance. The identification of fastidiousness genome editing methods has progressed the plant genetic engineering to innovative statures (Kumar et al., 2020).
In last decade, many hidden aspects of plant genomics have been unfolded to improve the quality and quantity of crops. Many fresh and modern mechanisms have been identified to reveal the hidden aspects of plant genomics. From these mechanisms, genome editing by “clustered regulatory interspaced short palindromic repeats” (CRISPR)/CRISPR associated nuclease 9 (Cas9) CRISPR/ Cas9 has unfastened the fresh vistas for crop improvement and functional genomics. The CRISPR/Cas9 was implemented from a naturally occurring genome editing mechanism in bacteria. In contrasting to earlier generation genome editing tactics like transcription activator like effector nucleases (TALENs) and zinc-finger nucleases (ZFNs), CRISPR/Cas9 has brought the much easiness in cloning, flexibility in arraying the gRNAs and cost effectiveness. Many commercial crops have been imperiled to genome editing via CRISPR/Cas9 and this methods has significant potential in providing the global food security (Kumar et al., 2020).
For avoiding the multiplication of viruses, several strategies starting from conventional breeding to molecular tactics have been used. These methods functioned very well for a short period, and then viruses degenerate because of numerous reasons including the multiple infections, from where these viruses synergistically make interaction with each other, virus propagation and evolution. One of the significant shortcomings till now is that all molecular biology techniques are developed to manage only the helper begomoviruses but not for its satellites. Although, these satellites might enhance extra functions to helper begomoviruses but were ignored. This situation necessitates in establishing a broad approach which not only helps to control helper begomoviruses but also associated DNA satellites. For this problem, one comprehensive technique CRISPR/Cas9 has been successfully used to manage several geminiviruses, but it targets only a single virus-like earlier techniques which are not applied to check the begomoviruses associated complexes with DNA satellites (Iqbal et al., 2016). They proposed a unique, inimitable, and comprehensive program established on multiplexed CRISPR)/Cas9 system, in which cassette of single guide RNA (sgRNA) is designed to control complexes of CLCuD and also associated DNA satellites.
The study (Sattar et al., 2019) of has described the significance of CRISPR/Cas9 via genome editing in cotton. In cotton, the application of CRISPR/Cas9 has significant potential to regulate the gene expression having superior quality traits, to load unique molecular traits of preferred locus and to confine the plant pathogens. The application of gene stacking via site specific endonucleases, the preferred genes can be deployed in close vicinity to precise locus in cotton genome with less segregation risk. Moreover, these implementations are monotonous to attain via modern breeding tactics. But with the CRISPR/Cas based techniques, transgenic cotton can be formed via simply selfing or backcrossing method to encounter the cotemporary GMOs strategies (Sattar et al., 2019).
The application of genome editing mechanism has transformed the whole fields of life sciences. The CRISPR Cas/9, a modern genome editing mechanism has revolutionized the study of hidden aspects of plants, devasting and beneficial microorganisms and animals. This method has become an interesting and useful for microbiologists and pathologists because of its uncomplicated cloning and tranquil designing. It has become a useful method for improving the quality and quantity of commercial crops and most importantly in incorporating the disease resistance in many plants (Khan et al., 2021). Now a days, a unique technology is applied to mapp the 5′ termini of different begomoviruses with the help of a heteroviruses. It was concluded from the study of (Arif et al., 2020) that cap-snatching mechanism was employed to mapp the 5′ termini of viral mRNAs of CLCuMuV, CoYVV, and RamV by using a Rice stripe virus (RSV) as shown in Fig. 3. They obtained ∼53, 30 and 2 unique sequences at 5′ ends of begomoviruses.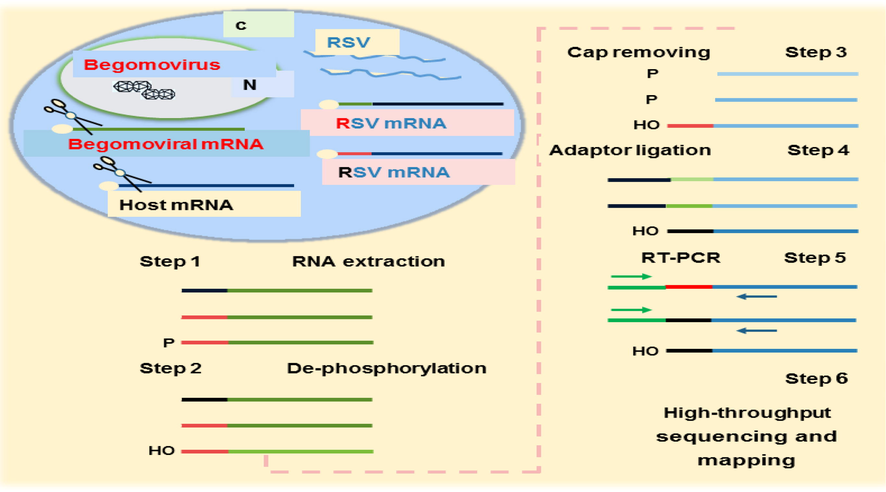
Experimental framework to identify 5′ends of begomoviral mRNAs utilized by cap-Snatching with RSV. In a co-infected host, RSV snatches the capped RNA leaders from host mRNAs and co-infected begomovirus.
The CRISPR Cas/9 mechanism is a widely used method to manage the different species of geminiviruses throughout world particularly in Pakistan. But the main drawback of this method is that it has potential to target only single species of virus and not helpful against the complexes of begomoviruses originated from DNA satellites. Moreover, a cassette of segregated RNA (sgRNA) is developed to mark not only CLCuD allied begomoviruses nucleases (Mishra et al., 2017; Sattar et al., 2019). The application of CRISPR Cas/9 has accomplished the editing of genome in simple and quite effective way. It have significant ability in nonspecific editing because of divergence in guide RNA (gRNA) sequence but this technology is still in exploration because of its many precincts like: effectiveness, less mutagenesis, risk of variability in edited genome, persisted CRISPR Cas/9 activity in upcoming generations, dependency on invitro regeneration protocols for recovery of stable plant lines and inadequacy of indorsed targets (Ahmad et al., 2020). The study of (Agrahari et al., 2020; Sahu et al., 2020) has described the significance of plant microbe interactions and its employment in crop protection. They described the post-genomic period tactics like CRISPR Cas/9 GWAS, NGS in collaboration with marker assisted selection, recombination techniques and cloning. Different models including spatial immunity model, invasion model and zig-zag model are being executed to apprehend the plant defense responses against the emerging phytopathogens.
8 Conclusions and future perspective
In this review, we have summarized the complex molecular biology with host-virus-vector interactions of geminiviruses. Several published studies indicated that geminiviruses are responsible to cause huge significant losses in plants. The plant pathogenic viruses of family geminiviridae are insect-borne having genomes entailing of ssDNA fragments encapsidated in distinctive twinned icosahedral specks. The geminiviruses are currently divided into nine genera based on their sequence similarity, genome arrangement and insect-vector. These nine genera includes Becurtovirus, Begomovirus, Capulavirus, Curtovirus, Eragrovirus, Grablovirus, Mastrevirus, Topocuvirus, and Turncurtovirus. The Genus Begomovirus is the largest genus of family Geminiviridae, which has almost 320 species and its transmission is mediated by an insect vector whitefly (Bemisia tabaci).
It has been reported in many studies that diversity of begomoviruses is increasing in China. There are many factors responsible for the development of these begomoviruses and their associated satellites. Several cultivated and non-cultivated plant species including crops, horticultural crops and weeds have been infected by begomoviruses in China. Majority of plant species including major crops, vegetables and ornamental plants have significant infection of CLCuMuV and CLCuMuB and TYLCV. It has been estimated that almost 16 or more begomoviruses have been detected from different provinces of China.
For avoiding the multiplication of viruses, several strategies starting from conventional breeding to molecular tactics have been used. These methods functioned very well for a short period, and then viruses degenerate and cause significant losses. There are several mechanisms which are recognized to disclose the concealed aspects of plant genomics for crop improvement. From these mechanisms, genome editing by CRISPR/ Cas9 has unfastened the fresh vistas for boosting the quality and quantity of crops and reduce the biotic and abiotic stresses. In contradiction to earlier genome editing tactics including TALENs and zinc-finger nucleases (ZFNs), the CRISPR/Cas9 has enhanced the easiness in cloning, flexibility in arraying the gRNAs and cost effectiveness. Many commercial crops have been imperiled to genome editing via CRISPR/Cas9 and this method has significant potential in providing the global food security. From a pathologist’s point of view, safety feature of biotech products needs supreme importance, and these biotech products require developed regulatory system to manage the speedy changes occurred in biotechnology fields.
It has been observed that phenotypes of several plant virus infection can cause a significant complex by encompassing interactions between host-viral dynamics which are boosted by favorable environmental conditions. It is very exigence of time to design the novel experimental methods to understand the complex molecular biology and pathology of geminiviruses.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research, King Khalid University for funding this work through research groups program under grant number R.G.P. 2/17/43.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Plant-microbe interactions for sustainable agriculture in the postgenomic era. Curr. Genomics. 2020;21(3):168-178.
- [Google Scholar]
- A critical look on CRISPR-based genome editing in plants. J. Cell. Physiol.. 2020;235(2):666-682.
- [Google Scholar]
- Cotton leaf curl disease in resistant cotton is associated with a single begomovirus that lacks an intact transcriptional activator protein. Virus Res.. 2010;152(1-2):153-163.
- [Google Scholar]
- Primary and secondary siRNAs in geminivirus-induced gene silencing. PLoS Pathogens. 2012;8(9)
- [Google Scholar]
- Molecular characterization and RSV Co-infection of Nicotiana benthamiana with three distinct begomoviruses. Methods. 2020;183:43-49.
- [Google Scholar]
- Infectious clone construction and pathogenicity confirmation of Cotton leaf curl Multan virus (CLCuMuV), Ramie mosaic virus (RamV) and Corchorus yellow vein Vietnam virus (CoYVV) by southern blot analysis. PloS One. 2021;16(5)
- [Google Scholar]
- Cotton leaf curl Multan virus infecting Hibiscus sabdariffa in China. Can. J. Plant Pathol.. 2018;40(1):128-131.
- [Google Scholar]
- Recommendations for the classification and nomenclature of the DNA-β satellites of begomoviruses. Arch. Virol.. 2008;153(4):763-781.
- [Google Scholar]
- Briddon, R., S. Bull and I. Bedford, 2006. Occurrence of Sweet potato leaf curl virus in Sicily. Plant Pathology. 55 (2) 286-286.
- Diversity of DNA β, a satellite molecule associated with some monopartite begomoviruses. Virology. 2003;312(1):106-121.
- [Google Scholar]
- Distinct evolutionary histories of the DNA-A and DNA-B components of bipartite begomoviruses. BMC Evolution. Biol.. 2010;10(1)
- [Google Scholar]
- Revision of Begomovirus taxonomy based on pairwise sequence comparisons. Arch. Virol.. 2015;160(6):1593-1619.
- [Google Scholar]
- Virion stability is important for the circulative transmission of Tomato yellow leaf curl Sardinia virus by Bemisia tabaci, but virion access to salivary glands does not guarantee transmissibility. J. Virol.. 2009;83(11):5784-5795.
- [Google Scholar]
- Cotton leaf curl Multan virus newly reported to be associated with cotton leaf curl disease in China. Plant Pathol.. 2010;59(4):794-795.
- [Google Scholar]
- A begomovirus DNAβ-encoded protein binds DNA, functions as a suppressor of RNA silencing, and targets the cell nucleus. J. Virol.. 2005;79(16):10764-10775.
- [Google Scholar]
- A single-tube PCR assay for detecting viruses and their recombinants that cause tomato yellow leaf curl disease in the Mediterranean basin. J. Virol. Methods. 2008;147(1):93-98.
- [Google Scholar]
- Molecular characterization of Cotton leaf curl Multan virus and the associated satellite DNA infecting okra in Guangdong. J. South China Agric. Univ.. 2012;33(1):33-39.
- [Google Scholar]
- High genetic homogeneity points to a single introduction event responsible for invasion of Cotton leaf curl Multan virus and its associated betasatellite into China. Virol. J.. 2015;12(1):1-5.
- [Google Scholar]
- Acyl-CoA N-acyltransferase influences fertility by regulating lipid metabolism and jasmonic acid biogenesis in cotton. Sci. Rep.. 2015;5(1)
- [Google Scholar]
- Detection of tomato yellow leaf curl virus by loop-mediated isothermal amplification reaction. J. Virol. Methods. 2003;112(1-2):35-40.
- [Google Scholar]
- Role of the insect supervectors Bemisia tabaci and Frankliniella occidentalis in the emergence and global spread of plant viruses. Ann. Rev. Virol.. 2015;2(1):67-93.
- [Google Scholar]
- Circulative, “nonpropagative” virus transmission: an orchestra of virus-, insect-, and plant-derived instruments. Adv Virus Res.. 2014;89:141-199.
- [Google Scholar]
- Eclipta prostrata yellow vein disease caused by whitefly-transmitted geminiviruses. J. Northwest Sci.-Tech. Univ. Agric. Forest.. 2005;33:137-139.
- [Google Scholar]
- First report of a strain of Alternanthera yellow vein virus infecting Eclipta prostrate (L.) L. (Compositae) in China. J. Phytopathol.. 2008;156(7-8):496-498.
- [Google Scholar]
- Detection of whitefly-transmitted geminiviruses from tomato by PCR. Virol. Sin.. 2004;19(1):67-69.
- [Google Scholar]
- The molecular characteristics of DNA-A of tomato leaf curl Guangdong virus isolate G3. Acta Phytopathol. Sin.. 2005;3:003.
- [Google Scholar]
- An unusual alphasatellite associated with monopartite begomoviruses attenuates symptoms and reduces betasatellite accumulation. J. Gen. Virol.. 2011;92(3):706-717.
- [Google Scholar]
- CRISPR/Cas9: a tool to circumscribe cotton leaf curl disease. Front. Plant Sci.. 2016;7
- [Google Scholar]
- Molecular diagnosis of Tomato yellow leaf curl disease in Jiangsu Province. Acta Horticult. Sin.. 2008;35:1815-1818.
- [Google Scholar]
- Plant virus ecology and epidemiology: historical perspectives, recent progress and future prospects. Ann. Appl. Biol.. 2014;164(3):320-347.
- [Google Scholar]
- Mathematical modeling of cotton leaf curl virus with respect to environmental factors. Eur. J. Microbiol. Immunol.. 2015;5(2):172-176.
- [Google Scholar]
- Disease Resistance in Crops Through CRISPR/Cas. CRISPR Crops, Springer; 2021. p. :151-175.
- Cotton leaf curl Multan betasatellite as a plant gene delivery vector trans-activated by taxonomically diverse geminiviruses. Arch. Virol.. 2012;157(7):1269-1279.
- [Google Scholar]
- Use of CRISPR in Climate Smart/Resilient Agriculture. CRISPR/Cas Genome Editing, Springer; 2020. p. :131-164.
- Transmission of Tomato Yellow Leaf Curl Virus by two invasive biotypes and a Chinese indigenous biotype of the whitefly Bemisia tabaci. Int. J. Pest Manage.. 2010;56(3):275-280.
- [Google Scholar]
- Tobacco curly shoot virus DNAβ is not necessary for infection but intensifies symptoms in a host-dependent manner. Phytopathology. 2005;95(8):902-908.
- [Google Scholar]
- Targeted lipidomics studies reveal that linolenic acid promotes cotton fiber elongation by activating phosphatidylinositol and phosphatidylinositol monophosphate biosynthesis. Mol. Plant.. 2015;8(6):911-921.
- [Google Scholar]
- Chinese tomato yelIow Ieaf curl virus—a new species of geminivirus. Sci. China Series C: Life Sci.. 1998;41(4):337-343.
- [Google Scholar]
- Molecular characterization of a distinct begomovirus infecting Euphorbia pulcherrima in China. J. Phytopathol.. 2004;152(4):215-218.
- [Google Scholar]
- Mao, M., Z. He, H. Yu, et al., 2008. [Molecular characterization of cotton leaf Curl Multan virus and its satellite DNA that infects Hibiscus rosa-sinensis]. Bing du xue bao= Chinese journal of virology/[bian ji, Bing du xue bao bian ji wei yuan hui]. 24 (1) 64-68.
- A rapid PCR method to discriminate between Tomato yellow leaf curl virus isolates. Ann. Appl. Biol.. 2001;139(2):251-257.
- [Google Scholar]
- Real-time PCR for the quantitation of Tomato yellow leaf curl Sardinia virus in tomato plants and in Bemisia tabaci. J. Virol. Methods. 2008;147(2):282-289.
- [Google Scholar]
- Analysis of small RNAs derived from tomato yellow leaf curl Sardinia virus reveals a cross reaction between the major viral hotspot and the plant host genome. Virus Res.. 2013;178(2):287-296.
- [Google Scholar]
- Mishra, G. P., B. Singh, T. Seth, et al., 2017. Biotechnological advancements and begomovirus management in okra (Abelmoschus esculentus L.): Status and perspectives. Front. Plant Sci. 8 360.
- Functional analysis of a novel motif conserved across geminivirus Rep proteins. J. Virol.. 2011;85(3):1182-1192.
- [Google Scholar]
- Maintenance of an Old World betasatellite by a New World helper begomovirus and possible rapid adaptation of the betasatellite. J. Virol.. 2009;83(18):9347-9355.
- [Google Scholar]
- Post-transcriptional gene silencing suppressor activity of two non-pathogenic alphasatellites associated with a begomovirus. Virology. 2010;405(2):300-308.
- [Google Scholar]
- Molecular characterization and construction of an infectious clone of a new strain of Tomato yellow leaf curl virus in southern Iran. Iran J. Plant Pathol.. 2010;46:101-115.
- [Google Scholar]
- Differentiation of Tomato yellow leaf curl virus and Tomato yellow leaf curl Sardinia virus using real-time TaqMan® PCR. J. Virolo. Methods. 2010;165(2):238-245.
- [Google Scholar]
- The first DNA 1-like α satellites in association with New World begomoviruses in natural infections. Virology. 2010;404(2):148-157.
- [Google Scholar]
- The presence of tomato leaf curl Kerala virus AC3 protein enhances viral DNA replication and modulates virus induced gene-silencing mechanism in tomato plants. Virol. J.. 2011;8(1):178.
- [Google Scholar]
- Biological invasions of geminiviruses: case study of TYLCV and Bemisia tabaci in Reunion Island. Viruses. 2012;4(12):3665-3688.
- [Google Scholar]
- Functional characterization of coat protein and V2 involved in cell to cell movement of Cotton leaf curl Kokhran virus-Dabawali. PLoS One. 2011;6(11)
- [Google Scholar]
- Association of an atypical alphasatellite with a bipartite New World begomovirus. Arch. Virol.. 2010;155(11):1843-1847.
- [Google Scholar]
- Discovery of a novel mastrevirus and alphasatellite-like circular DNA in dragonflies (Epiprocta) from Puerto Rico. Virus Res.. 2013;171(1):231-237.
- [Google Scholar]
- A single complementary-sense transcript of a geminiviral DNA β satellite is determinant of pathogenicity. Mol. Plant-Microbe Interact.. 2005;18(1):7-14.
- [Google Scholar]
- Insights in plant-microbe interaction through genomics approach (Part 1) Curr. Genom.. 2020;21(3):155-156.
- [Google Scholar]
- CRISPR/Cas9: a new genome editing tool to accelerate cotton (Gossypium spp.) breeding. In: Advances in Plant Breeding Strategies: Industrial and Food Crops. 61–84: Springer; 2019.
- [Google Scholar]
- Cotton leaf curl disease–an emerging threat to cotton production worldwide. J. Gen. Virol.. 2013;94(4):695-710.
- [Google Scholar]
- Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol.. 2011;12(9):938-954.
- [Google Scholar]
- Whitefly-mediated transmission of cotton leaf curl Multan betasatellite: evidence for betasatellite encapsidation in coat protein of helper begomoviruses. Arch. Virol.. 2013;158(1):19-26.
- [Google Scholar]
- Molecular characterization of a novel monopartite begomovirus isolated from Pouzolzia zeylanica in China. Arch. Virol.. 2013;158(7):1617-1620.
- [Google Scholar]
- Molecular characterization of the Cotton leaf curl Multan virus infecting Malvaiscus arboreus. Acta Phytopathol. Sin.. 2013;43(2):120-127.
- [Google Scholar]
- Detection and identification of the pathogen causing kenaf (Hibiscus cannabinus) leaf curl disease in Hainan Province of China. Acta Phytopathol. Sin.. 2015;45(6):561-568.
- [Google Scholar]
- Identification and control of Tomato yellow leaf curl virus disease in Beijing. Plant Protect. 2010
- [Google Scholar]
- High-throughput sequencing reveals small RNAs involved in ASGV infection. BMC Genomics. 2014;15(1):568.
- [Google Scholar]
- Wu, J., Dai, F., Zhou, X., 2006. First report of Tomato yellow leaf curl virus in China. Plant Dis. 90 (10) 1359–1359.
- Interaction between a nanovirus-like component and the tobacco curly shoot virus/satellite complex. Acta Biochim. Biophysi. Sin.. 2005;37(1):25-31.
- [Google Scholar]
- Characterization of small interfering RNAs derived from Sugarcane mosaic virus in infected maize plants by deep sequencing. PLoS ONE. 2014;9(5)
- [Google Scholar]
- Molecular characterization of squash leaf curl Yunnan virus, a new begomovirus and evidence for recombination. Arch. Virol.. 2003;148(10):2047-2054.
- [Google Scholar]
- Ageratum yellow vein China virus is a distinct begomovirus species associated with a DNAβ molecule. Phytopathology. 2007;97(4):405-411.
- [Google Scholar]
- Population diversity of rice stripe virus-derived siRNAs in three different hosts and RNAi-based antiviral immunity in Laodelphgax striatellus. PLoS ONE. 2012;7(9)
- [Google Scholar]
- Mixed infection of two begomoviruses in Malvastrum coromandelianum in Fujian, China. J. Phytopathol.. 2008;156(9):553-555.
- [Google Scholar]
- Yang, C., Wu, Z., Xie, L., 2009. First report of the occurrence of sweet potato leaf curl virus in tall morningglory (Ipomoea purpurea) in China. Plant Disease. 93 (7) 764-764.
- Functional modulation of the geminivirus AL2 transcription factor and silencing suppressor by self-interaction. J. Virol.. 2007;81(21):11972-11981.
- [Google Scholar]
- Molecular variation of tomato yellow leaf curl virus in the insect vector Bemisia tabaci. Sci. Rep.. 2017;7(1)
- [Google Scholar]
- Characterization of small interfering RNAs derived from the geminivirus/betasatellite complex using deep sequencing. PLoS ONE. 2011;6(2)
- [Google Scholar]
- Molecular characterization and pathogenicity of tomato yellow leaf curl virus in China. Virus Genes. 2009;39(2):249-255.
- [Google Scholar]







