Translate this page into:
Molecular mechanism of non-coding RNA targeting zinc finger binding protein 1 and cervical cancer cells suppression
⁎Corresponding author. zhaosufen0817@yahoo.com (Sufen Zhao)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The main aim of the study is to explore the mechanism of microrNA-145-3p targeting ZEB1 on migration and invasion in cervical cancer cells. In this study, cervical cancer cell line C33A was selected and transfected. After transfection of C33A cells, the expression level of Mir-145-3p was measured by QT-PCR. Human cervical cancer cell line (C33A) was cultured in RPMI 1640 medium and cell transfection experiment was performed. The related targets of Mir-145-3p, and a complementary binding site was found between the 3′UTR terminal of ZEB1 and Mir-145-3p was performed using a Targetscan biological software. The luciferase reporter system was used to determine the targeting relationship between Mir-145-3p and complementary binding site. The expression of e-cadherin and vimentin proteins was detected by western blot analysis. After 48 h, the expression level of Mir-145-3p in mimics group was significantly increased, the inhibitor group was significantly down-regulated and was statistically significant (P < 0.05). The clone formation, migration and invasion ability and vimentin expression in the MIMics group were significantly decreased than NC group. However, the apoptosis rate and e-cadherin protein expression were significantly increased than control group. In the inhibitor group, the cell cloning, cell migration, invasion and vimentin protein expression were significantly increased, while the cell apoptosis rate and e-cadherin protein expression were significantly decreased (P < 0.05). Analysis of luciferase expression genes revealed that mirNa-145-3p and ZEB1 have targeted regulatory relationship. Compared with Mir-NC, there was no significant difference between Mir-145-3p MUT and Mir-NC (P > 0.05), and luciferase activity of Mir-145-3p WT was significantly down-regulated (P > 0.05). Overexpression of microrNA-145-3p downregulated ZEB1 expression and inhibited proliferation, migration, invasion, and epithelial-mesenchymal transformation of cervical cancer cells, and promoted cell apoptosis.
Keywords
mirNa-145-3p
ZEB1
Cervical cancer
Proliferation
Apoptosis
1 Introduction
Cervical cancer is a malignant tumor of the cervix and is one of the most common gynecological malignant tumors. It occurs frequently in women over 50 years, and the clinical symptoms are associated with vaginal bleeding and contact bleeding (Johnson et al., 2019). Moreover, the cause may be associated with Human papillomavirus (HPV) infection, female sexual behavior and the number of childbirth (Vu et al., 2018). In the early stage, this disease can be cured by drugs, surgery and other methods, while patients in the later stages have poor prognosis and mortality within one year (Pimple and Mishra, 2019). At present, it is the main focus of medical research to explore the molecular mechanism of cervical cancer and to search the effective treatment methods. The occurrence and development of cervical cancer is closely related to abnormal gene expression, and targeted inhibition of related genes can be regarded as an effective way to treat cervical cancer (Li et al., 2016). MiRNA is a kind of non-coding RNA that exists widely in human tissues. A number of studies have confirmed that miRNA is involved in the occurrence and development of breast cancer, ovarian cancer, cervical cancer and other tumors (Yi et al., 2019; Li et al., 2020; Ghafouri-Fard et al., 2020). However, studies have confirmed that the down-regulated expression of mirNa-145-3p in cervical cancer, lung cancer and stomach cancer played significant role in tumor suppression (Shi et al., 2020). E-box zinc finger binding protein 1 (ZEB1) is a member of the Zeb gene family, and both ZEB2 and ZEB1 are important cellular transcription factors. In recent years, a large number of findings have confirmed the abnormal expression of ZEB1 in pancreatic cancer, lung cancer, liver cancer, colon cancer and breast cancer (Manshouri et al., 2019; Wang et al., 2019; Liu et al., 2020), however there is not much research on the mechanism of Mir-145-3p and ZEB1 in cervical cancer progression. In this study, cervical cancer cell line C33A was selected and lentiviral plasmid was constructed to overexpress and silence Mir-145-3p and ZEB1 to promote the progression of cervical cancer studies.
2 Materials and methods
2.1 Materials
Human cervical cancer cell line C33A (Shanghai Yaji Biology, China), conventional cell culture reagents and equipment, Cell transfection Kit, Annexin V-FITC/PI Apoptosis Kit and Transwell Kit (Shanghai Varan Biology, China); Dual Luciferase reporting Kit (Shanghai leaf source, China); Fluorescence Quantitative PCR Kit (Hangzhou Zhonuo Biology, China); and Chemiluminescence immunoassay system (Shanghai Jumu, China) were used in this study. All plasmids were constructed by Shanghai Medisi Biomedical Co., LTD (Shanghai, China). Mouse anti-human e-cadherin and vimentin monoclonal antibodies were purchased from Abcam, China.
2.2 Cell culture
Human cervical cancer cell line (C33A) was cultured in RPMI 1640 medium containing 10% calf serum. It was incubated at 37 °C with 5% CO2 and saturated humidity.
2.3 Cell transfection
Cell transfection experiment was performed according to the manufactures instructions. Lipofectamine 2000 transfection reagent was used to transfect Mir-145-3p MIMC, Mir-145-3p inhibitor and Mir-NC into C33A cells at a dose of 20 nnmol/L, and the transfection efficiency was measured after 48 h. In this study, Mir-145-3p MIMC was labeled as the MIMics group, Mir-145-3p inhibitor was labeled as the inhibitor group, and Mir-NC was labeled as the NC group. Mammalian expression vector (pcDNA3.1) was transfected using Lipofectamine 2000 transfection reagent (Ishii et al., 2001).
2.4 Cell cloning assay to determine cell proliferation
The clone formation ability of control and experimental groups of cells was tested. Cells in logarithmic growth stage were taken and digested and digested with 0.25% trypsin. Approximately, 500 cells/well were inoculated into 6-well plates, and stained with Giemsa staining solution for 15 min. It was rinsed with running water, and cell count was performed with a computerized camera (Fedr et al., 2013). Clone formation rate was calculated using the following formula.
2.5 Annexin V-FITC/PI double staining
The logarithmic growth phase of the experimental and control groups of cells were washed with phosphate buffered saline. The cell density was adjusted to 1 × 106 cells/ml, and the cells were collected by centrifugation at 2000g. Then, 400 µl binding buffer, 5 µl PI and Annexin V-FITC solution were added. It was incubated at room temperature for 15 min. Apoptosis was measured by flow cytometry.
2.6 Cell migration assay
All the apparatus and instruments used were completely sterilized. About 5 × 105 cells of the experimental groups were added into the sterilized well and incubated for overnight. The cells were washed with PBS for 3 times, the delimited cells were removed, and serum-free culture medium was added. It was incubated at 37 °C in an incubator with 5% CO2. After 24 h, the mean distance between cells was determined (Yarrow et al., 2004).
2.7 Cell invasion and transwell assay
The cell culture reagents and transwell chamber were incubated at 37 °C, and 30 µg matrix glue was spread on Transwell chamber to form matrix membrane. The cells were cultured to logarithmic growth stage, digested, washed successively with PBS and serum-free medium. It was suspended with serum-free medium and the concentration was adjusted to 2 × 105/mL. About 600–800 μl of culture medium containing 10% serum was added in the lower chamber (the bottom of the 24-well plate) and 200 μl of serum-free culture medium containing 1 × 105 cells in the upper chamber and incubated for 24 h. After 24 h, the cells were treated with 800μLGiemsa dye solution and fixed. The final results were observed under microscope and analyzed.
2.8 Dual luciferin reporting system
The related targets of Mir-145-3p, and a complementary binding site was found between the 3′UTR terminal of ZEB1 and Mir-145-3p was performed using a Targetscan biological software. The luciferase reporter system was used to determine the targeting relationship between Mir-145-3p and complementary binding site. The luciferase reporter vectors containing the 3′UTR binding site of ZEB1 and the 3′UTR binding site of mutated ZEB1 were established. These two vectors were transfected into C33A cells with Mir-145-3p and NC, respectively. After 48 h, the luciferase activity was measured by luciferase assay kit (Jathoul et al., 2014).
2.9 Qt-PCR
TRIzol reagent was used to extract total RNA from the cells and the amount of RNA was determined using UV–visible spectrophotometry. The reverse transcription kit was used to convert RNA into cDNA. The real-time fluorescence quantitative PCR reaction was performed using cDNA as the template under standard experimental condition using a PCR machine. The upstream and downstream primers were described in Table 1. The relative quantitative analysis was performed using the 2-△△CT method, and GAPDH was used as the internal reference.
Primers,
upstream
downstream
miR-145-3p
GCGTCCAGTTTTCCCAGGA
TGGTGTCGTGGAGTCG
ZEB1
TCCAGTGGTAATCGAAAATTCA
GAACCAGAATGGGAAAAACG
GAPDH
GAGTCAACGGATTTGGTCGT
TTGATTTTGGAGGGATCTCG
2.10 Western blot method
The expression of e-cadherin and Vimentin proteins were detected. The samples were prepared as described previously and the supernatant was used for the assay (Zhu et al., 2011). The prepared sample was loaded on 11% sodium dodecyl sulfate polyacrylamide gel electrophoresis and separated with constant power supply (50 v). The target proteins were transferred to PVDF membrane by wet membrane transfer device, and 5% defatted milk powder solution was incorporated to seal the membrane at 4 °C for overnight. Then the membrane was washed with TNST buffer solution for 2 min and treated with primary and secondary antibody. The relative expression of protein is the ratio of absorbance value of target band to absorbance value of internal reference protein.
2.11 Statistical methods
SPSS 20.0 statistical software was used for data processing. All data were expressed as standard deviation ± mean. A t-test was used for mean comparison between experimental and control groups. One-way analysis of variance was used for multi-group comparison, and snK-Q test was used for inter-group comparison. The “P” value <0.05 was considered as statistically significant.
3 Results
3.1 Transfection efficiency of mirNA-145-3p
After 48 h transfection of C33A cells, the expression level of Mir-145-3p was measured by QT-PCR. The results showed that compared with the NC group the expression level of Mir-145-3p in the MIMics group was significantly increased. Moreover in the inhibitor group the expression was significantly down-regulated. The difference of transfection efficiency was statistically significant (P < 0.05), as shown in Fig. 1.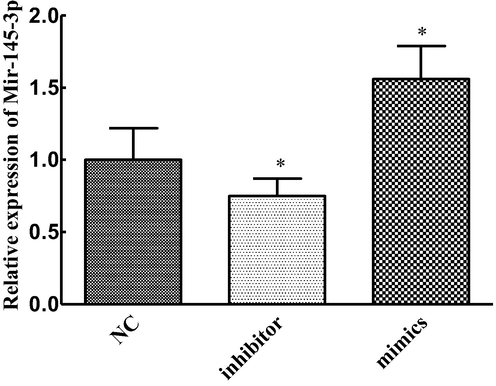
Mirna-145-3p transfection efficiency (compared with group NC, *P < 0.05).
3.2 Effects of overexpression and knockdown of mirNA-145-3p on proliferation and apoptosis of cervical cancer cells
Compared with the NC group, the clone formation ability of cells in the MIMics group was significantly decreased and the apoptosis rate was significantly increased. Moreover, the clone formation ability of cells in the inhibitor group was significantly increased and the apoptosis rate was significantly decreased (P < 0.05), (Fig. 2). Up-regulation of mirNA-145-3P inhibited proliferation and promoted apoptosis of cervical cancer cells.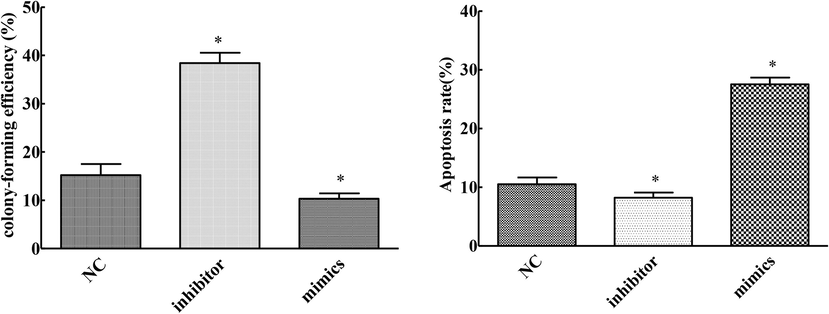
Effects of overexpression and knockdown of mirNA-145-3p on proliferation and apoptosis of cervical cancer cells (compared with NC group, *P < 0.05).
3.3 Effects of overexpression and knockdown of mirNA-145-3p on invasion and metastasis of cervical cancer cells
Compared with the NC group, the migration and invasion ability of cells in the MIMics group was significantly decreased, while that of cells in the inhibitor group was significantly increased (P < 0.05). Up-regulation of mirNA-145-3P inhibited migration and invasion of cervical cancer cells and the result was described in Fig. 3.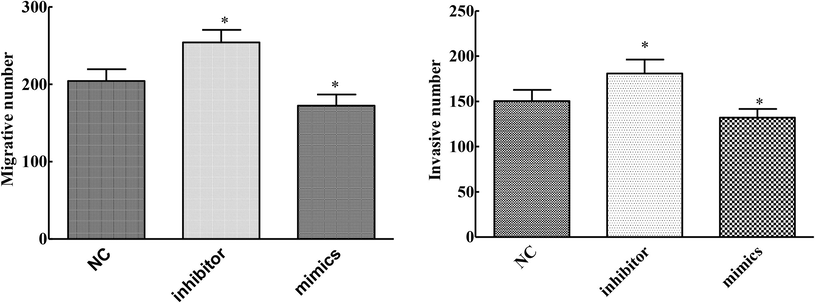
Effects of overexpression and knockdown of mirNA-145-3p on invasion and metastasis of cervical cancer cells (compared with NC group, *P < 0.05).
3.4 Effects of overexpression and knockdown of mirNA-145-3p on EMT protein expression in cervical cancer cells
Compared with the NC group, the expression of e-cadherin protein was increased and the expression of Vimentin was decreased in the MIMics group. Moreover, the expression of e-cadherin protein was decreased and the expression of Vimentin was increased in the inhibitor group. The expression was statistically significant (P < 0.05), and the result was described in Table 2. Up-regulation of mirNA-145-3P inhibited epithelial-mesenchymal transformation of cervical cancer cells.
Group
Mimics group
Inhibitor group
NC group
T-value
P - value
E-cadherin
1.63 ± 0.18
0.72 ± 0.15
1.33 ± 0.19
10.532
0.001
Vimentin
1.76 ± 0.11
2.48 ± 0.42
2.05 ± 0.46
9.145
0.002
3.5 Dual fluorescein determination of mirNA-145-3p targeted binding to ZEB1
Dual luciferase expression genes results showed that mirNa-145-3p and ZEB1 were in a targeted regulatory relationship. Compared with Mir-NC, there was no significant difference between Mir-145-3p MUT and Mir-NC (P > 0.05), and the luciferase activity of Mir-145-3p WT was significantly decreased. The difference was statistically significant (P < 0.05), as shown in Fig. 4.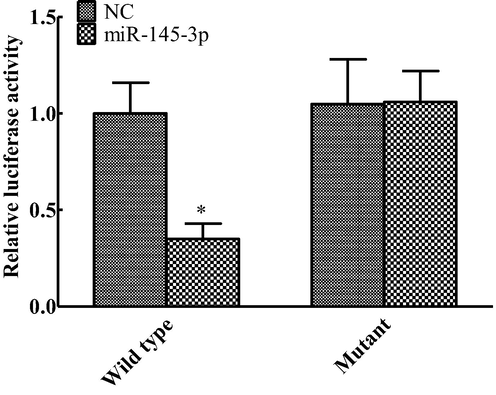
Change of dual luciferase activity (compared with NC group, *P < 0.05).
3.6 Effects of ZEB1 overexpression on proliferation and apoptosis of cervical cancer cells
Compared with the Vector group, the clone formation ability of ZEB1 group was significantly enhanced, and the apoptosis rate was decreased (P < 0.05) (Fig. 5). Overexpression of ZEB1 reversed the inhibition of proliferation and promotion of apoptosis by Mir-145-3p in CA33 cells.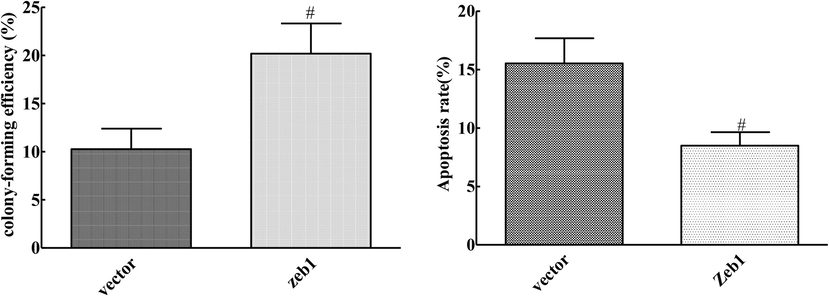
Effect of ZEB1 overexpression on proliferation and apoptosis of cervical cancer cells (compared with Vector group, #P < 0.05).
3.7 Invasion and metastasis of cervical cancer cells induced by overexpression of ZEB1
Compared with the vector group, the number of cell migration and invasion in ZEB1 group were significantly up-regulated, and the difference was statistically significant (P < 0.05), as shown in Fig. 6. Overexpression of ZEB1 reversed the inhibition of Mir-145-3p on migration and invasion of CA33 cells.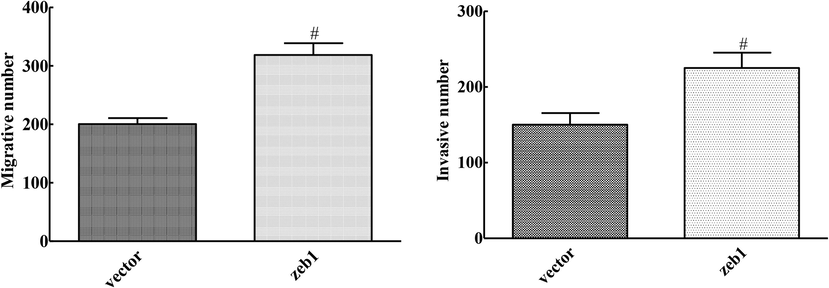
Effects of ZEB1 overexpression on invasion and metastasis of cervical cancer cells (compared with Vector group, #P < 0.05).
3.8 Effect of ZEB1 overexpression on EMT protein expression in cervical cancer cells
The expression of e-cadherin decreased and the expression of Vimentin increased in ZEB1 group compared with the vector group. The result was statistically significant as shown in Table 3 (P < 0.05). Overexpression of ZEB1 reversed the inhibition of Mir-145-3p on the epithelial-mesmal transformation of CA33 cells.
Group
Zeb1 group
Vector group
T - value
P-value
E-cadherin
0.65 ± 0.13
1.54 ± 0.38
6.745
0.001
Vimentin
2.52 ± 0.14
1.65 ± 0.31
8.413
0.002
4 Discussion
Cervical cancer is the second leading cause of death among women worldwide and is one of the most common female malignancies. The cause of cervix is not very clear, however most researchers report that it may be related to premature sex, sexual disorder, preterm birth, increased fecundity, ethnic and geographical reasons, and HPV infection (Hu and Ma, 2018). MiRNA is a kind of non-coding RNA with a variety of biological functions, involved in cell growth, energy metabolism, embryonic development and other processes. It is an important regulatory factor in human cellular processes (Sun et al., 2018). In recent times, it has been revealed that miRNA is expressed in tumor tissues and participates in the process of tumor genesis and development (Khan et al., 2019). miRNA plays the role of oncogene or oncosuppressor gene in tumor progression, thus affecting the malignant proliferation of tumor cells (Ali-Syeda et al., 2020). ZEB1 is a transcriptional regulatory factor containing multiple functional domains, and most findings indicate that ZEB1 is involved in the physiological process of tumorgenesis and development. In addition, the positive or negative target gene regulation is affected by the cellular environment (Caramel et al., 2018). In this study, the targeted regulation of ZEB1 by Mir-145-3p to promote the progress of cervical cancer was expressed and determined.
The present finding indicated that the expression level of Mir-145-3p in mimics group was significantly increased and down-regulated in inhibitor group compared with NC group. Compared with the NC group, the clone formation ability, migration and invasion ability of cells, and vimentin protein expression were significantly decreased in the MIMics group, while the apoptosis rate and e-cadherin protein expression were significantly increased. In the inhibitor group, the cell clonogenic ability, cell migration and invasion ability, and vimentin protein expression were significantly increased. Moreover, the cell apoptosis rate and e-cadherin protein expression were significantly decreased, suggesting that mir-145-3p overexpression and knockdown plasmid transfection were successful in this study. Overexpression of Mir-145-3p inhibited proliferation, invasion, metastasis, and epithelial-mesenchymal transformation of tumor cells, and promoted cell apoptosis. Wu et al. (2018) showed that mir-145-3p in osteosarcoma was significantly reduced compared with normal bone tissue. The overexpression of Mir-145-3p significantly weakened the proliferation of osteosarcoma cells and induced apoptosis and autophagy, demonstrating that Mir-145-3p inhibited the malignant behavior of osteosarcoma by downregulating HDAC4 expression. Pan et al. (2019) showed that MTDH acts as an oncogene in PCa, and the inhibition of Mir-145-5p or Mir-145-3p on MTDH inhibits the growth and metastasis of prostate cancer cells. Therefore, Mir-145-5p /MTDH and Mir-145-3p /MTDH pathways may be new therapeutic targets for prostate cancer. The above results were consistent with the present results, confirming that the overexpression of Mir-145-3p can inhibit the proliferation, metastasis and invasion of tumor cells.
In order to explore the specific mechanism of Mir-145-3p in cervical cancer, targeted fluorescein assay and ZEB1 overexpression assay were performed. The dual luciferase expression gene analysis indicated that mirNa-145-3p and ZEB1 were in a targeted regulatory relationship. Compared with Mir-NC, there was no significant difference between Mir-145-3p MUT and Mir-145-3p and WT luciferase activity was down-regulated. Compared with the vector group, the clone formation ability, the number of cell migrations and invasions, and the expression of vimentin in ZEB1 group were significantly increased. However, the apoptosis rate and the expression of e-cadherin protein were down-regulated. Wu et al. (2019) reported that M6a induced lncRNA RP11 could trigger CRC cell metastasis through post-translation upregulation of Zeb1. Mir-101 silenation promotes cell migration, and mir-101 overexpression inhibits EMT and cell migration in OvCa cell lines by regulating ZEB1 (Liang et al., 2018). Wang et al. (2020) showed that the overexpression of circ_KIAA1429 could promote the migration, invasion and EMT process of liver cancer cells, while the knockout of circ_KIAA1429 would lead to the reverse result, and proved that Zeb1 was the downstream target of circ_KIAA1429. Upregulation of Zeb1 led to cirC_KiAA1429-induced metastasis of HCC cells, while YTHDF3 enhanced the stability of Zeb1 mrna in a m6A dependent manner. The above previous findings were consistent with the results of this study, confirming that the up-regulation or overexpression of ZEB1 can promote the proliferation and metastasis of tumor cells. The anti-tumor effect of Mir-145-3p in cervical cancer may be related to the mechanism of overexpression of Mir-145-3p and down-regulation of ZEB1.
5 Conclusions
Analysis of luciferase expression genes revealed that mirNa-145-3p and ZEB1 have targeted regulatory relationship. Compared with Mir-NC, there was no significant difference between Mir-145-3p MUT and Mir-NC, and luciferase activity of Mir-145-3p WT was significantly down-regulated. In conclusion, overexpressed microRNA targeted regulation of ZEB1 inhibits proliferation, migration, invasion, and epithelial-mesenchymal transformation of cervical cancer cells, and promotes cell apoptosis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Regulatory mechanism of microRNA expression in cancer. Int. J. Mol. Sci.. 2020;21(5):1723.
- [Google Scholar]
- Automatic cell cloning assay for determining the clonogenic capacity of cancer and cancer stem-like cells. Cytometry Part A. 2013;83A(5):472-482.
- [Google Scholar]
- The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications. Cancer Med.. 2018;7(10):5217-5236.
- [Google Scholar]
- Mechanism of cell transfection with plasmid/chitosan complexes. Biochim. Biophy. Acta-Biomem.. 2001;1514(1):51-64.
- [Google Scholar]
- A dual-color far-red to near-infrared firefly luciferin analogue designed for multiparametric bioluminescence imaging. Angew. Chem. Int. Ed.. 2014;53(48):13059-13063.
- [Google Scholar]
- Cervical cancer: an overview of pathophysiology and management. Semin. Oncol. Nurs.. 2019;35(2):166-174.
- [Google Scholar]
- Role of miRNA-regulated cancer stem cells in the pathogenesis of human malignancies. Cells. 2019;8(8):840.
- [Google Scholar]
- Advances in diagnosis and treatment of metastatic cervical cancer. J. Gynecol. Oncol.. 2016;27(4):e43
- [Google Scholar]
- LncRNA PTAR promotes EMT and invasion-metastasis in serous ovarian cancer by competitively binding miR-101-3p to regulate ZEB1 expression. Mol Cancer.. 2018;17(1):1-13.
- [Google Scholar]
- ZIP4 increases expression of transcription factor ZEB1 to promote integrin α3β1 signaling and inhibit expression of the gemcitabine transporter ENT1 in pancreatic cancer cells. Gastroenterology. 2020;158(3):679-692.e1.
- [Google Scholar]
- ZEB1/NuRD complex suppresses TBC1D2b to stimulate E-cadherin internalization and promote metastasis in lung cancer. Nat. Commun.. 2019;10(1):5125.
- [Google Scholar]
- The targeting of MTDH by miR–145–5p or miR–145–3p is associated with prognosis and regulates the growth and metastasis of prostate cancer cells. Int. J. Oncol.. 2019;54(6):1955-1968.
- [Google Scholar]
- Global strategies for cervical cancer prevention and screening. Minerva. Ginecol.. 2019;71(4):313-320.
- [Google Scholar]
- Long noncoding RNA HOXA-AS2 acts as an oncogene by targeting miR-145-3p in human non-small cell lung cancer. Eur. Rev. Med. Pharmacol. Sci.. 2020;24(3):1243-1249.
- [Google Scholar]
- Effect of exosomal miRNA on cancer biology and clinical applications. Mol. Cancer. 2018;17(1):147.
- [Google Scholar]
- circ_KIAA1429 accelerates hepatocellular carcinoma advancement through the mechanism of m6A-YTHDF3-Zeb1. Life Sci.. 2020;257:118082.
- [Google Scholar]
- ZEB1 causes the production of hsa-microRNA-99b/let-7e/microRNA-125a cluster and promotes invasion of liver cancer cells. Eur. Rev. Med. Pharmacol. Sci.. 2019;23:1468-1475.
- [Google Scholar]
- MicroRNA-145-3p suppresses proliferation and promotes apotosis and autophagy of osteosarcoma cell by targeting HDAC4. Artif. Cells Nanomed. Biotechnol.. 2018;46(sup2):579-586.
- [Google Scholar]
- m6 A-induced lncRNA RP11 triggers the dissemination of colorectal cancer cells via upregulation of Zeb1. Mol. Cancer. 2019;18(1):1-16.
- [Google Scholar]
- A high-throughput cell migration assay using scratch wound healing, a comparison of image-based readout methods. BMC Biotechnol.. 2004;4(1):1-9.
- [Google Scholar]
- Reconstruction and analysis of circRNA–miRNA–mRNA network in the pathology of cervical cancer. Oncol. Rep.. 2019;41(4):2209-2225.
- [Google Scholar]
- Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis. 2011;215(2):286-293.
- [Google Scholar]







