Translate this page into:
Molecular characteristics of volatile components from willow bark
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Willow (Salix matsudana) bark is the bark of willow trees, it is rich in resources. Extracting these resources becomes difficult due to the relative lack of systematic and in-depth analysis of the total chemical composition. Analysis was performed using TDS-GC/MS, TG, FTIR and PY-GC/MS techniques. Results and discussion: the extracts form volatile organic compounds are mainly included in the analysis of TDS-GC/MS, such as, organic acids, exters, alcohols, etc. Extracts are rich in VOCs:1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester, its function is mainly as plasticizer. FTIR analysis are further confirmed, the ingredients of total extracts include ethers, The treatment of total heat loss is divided into two distinct phases: the first phase of 50 °C to 100 °C, the second phase of 150 °C to 250 °C, the sequence of quality loss is the second and the first stage, important turning point temperature is 245 °C, with significant changes in quality, for example, high polymer pyrolysis into small volatile molecules. In addition, various pyrolysis VOCs have been analyzed by PY-GC/MS, And have good development space, such as, Carbamic acid, monoammonium salt (4.80%), Glycolaldehyde dimer (4.04%), Catechol (0.44%). etc. Both of them have good development prospects, including many new chemical components produced by the pyrolysis of total extracts and residues, which is a new method to provide high quality applications.
Keywords
Willow bark
Volatile organic component (VOC)
Extraction of organic solvents
Component characteristics
1 Introduction
Salix matsudana koidz leaves large trees, Willow slender, soft sagging, sexual hi wetlands, growing rapidly; bark tissue thick, longitudinal, old trunk center rotten and hollow, branchlets slender, brown or green, branches glabrous; winter buds linear, dense in branches, leaves alternate, linear-lanceolate; margin glandular serrate, dark green on the surface, greenish white on both sides, smooth and glabrous on both sides, with stipules; flowers in the leaves, and the male inflorescence for the catkin, the fruit for the capsule. About the introduction of the Ming Dynasty, so far more than three hundred years of history. It is highly resistant to air pollution and dust, and is suitable for growing in city gardens. Especially in pools or streams (Chen et al., 2018; Zhao, 2015), willow trees rely on its unparalleled adaptability to make it one of the most popular species of land in ancient China.
Willow bark is the name of the bark of willow, Willow bark is used as a medicinal material, which has been recorded in both ancient China and western countries. Because willow bark contains natural salicylin, it is absorbed by enzymes into salicylic acid, a natural painkiller (Cao, 1995). Before the Western modern pharmaceutical technology is not popular, “Willow Bark Extract” is the only pain medication. Because in ancient times, willow bark was used in China to treat pain, fever and ventilation. So it is still a common ingredient in over-the-counter drugs and belongs to an herb. But the analysis of this paper has not been studied, willow bark in the volatile organic compounds (VOC) of the total extract of organic solvents after extraction of residues. The test willow bark was analyzed, studies were carried out by TG, Py-GC/MS, TDS-GC/MS and FTIR.
2 Materials and methods
2.1 Materials and reagents
Willow bark is manufactured by Jiangsu Nantong forging equipment Co., Ltd. After drying under natural conditions, the willow peel was crushed into powder with a fz102 grinder for use in the factory (Tanjingtai Ins). Unless otherwise stated, the AS200 screening instrument (USA) is used for treatment, and all reagents used are purchased from Sigma Chemical (USA).
2.2 Methods
2.2.1 Analysis of VOCs by TDS-GC/MS
The TDS: transmission line temperature is 230 °C. The initial temperature is 30 °C and maintained for 1 min, 10 °C/min to 100 °C for 5 min, and then 10 °C/min to 200 °C without retention.
GC: uses silica capillary column of 30 mm × 0.25 μm, incubated at 50 °C. It then heats up to 250 °C at a rate of 8 °C/min and then to 300 °C at a rate of 5 °C/min. The flow rate of the column is 1.0 ml, the shunt ratio is 20:1, and the carrier gas is high helium.
MS: qualitative, first set the ionization mode to EI, electron energy 70 eV. Next, set the ion temperature to 230 °C and the quadrupole temperature to 150 °C. The final quality range is 30–600 M/Z, computer search WILY7n.1 (Jiang et al., 2017).
2.2.2 Analysis of residues extracted from willow bark by FTIR
First, the willow bark sample and its extract were dried at 100 °C for 4 h, and then placed in a drying container containing desiccant to prevent moisture absorption and avoid influence. Use the AS200 screening instrument (USA) to grind and screen a certain amount of potassium bromide and place it in a dry pan. It is then placed in a muffle furnace at 150 °C (with the SX-2.5–10 box controlled resistance furnace control box) for 5 h, and then the lid is removed. 200 mg potassium bromide and surface smooth agate mortar, were used to mix potassium bromide with 0.5–2 mg samples and mortar quickly and completely, and then put them on a pressing machine for pressing. The samples were measured in Fourier transform infrared spectra from 400 cm−1 to 500 cm−1 (Shimadzu, IRAffinity-1) (Li et al., 2018).
2.2.3 Analysis of willow bark by TG
The thermogravimetric analysis was carried out by using thermogravimetric analyzer (SDTQ600V20.5 Build 15) and empty crucible clamp as a reference. The sensitivity of the thermogravimetric analysis was 0.1 μg. And the temperature range of the control furnace is 30 °C to 1000 °C. The accurate quality willow bark sample (3.975 mg) was put into the TG crucible. The initial temperature of TA is set to 30 °C and the temperature of 250 °C is 5 °C/min, carrier gas is high purity nitrogen of 100 ml/min.
2.2.4 The extracts and residues were analyzed by Py-GC/MS
Using an ISQ140420 gas chromatography-mass spectrometer (GC/MS), the pyrolysis apparatus pyrolyzed up to 0.2 μL of the extracted sample or 0.1 mg of the residue to PY-2020iS (Frontier Co., Ltd., Japan), and then passed the GC/MS analysis of pyrolysis products. GC/MS analysis was performed on an Agilent 5975C/6890N (Agilent Corp., USA) coupled to a mass spectrometric selective detector. An elastic quartz capillary column (Agilent Corp., USA) with a column of DB-5MS (30 m × 0.25 mm × 0.25 μm) was used, the carrier gas was helium, the flow rate of helium was 1.0 ml/min, and the inlet temperature was 250 °C. Column lift conditions: from 50 °C to 300 °C at a rate of 10 °C/min, then split injection at a ratio of 30:1. Mass spectrometry conditions: Scanning range of 35–550 AMU (m/z) scanning MS program, ionization voltage of 70 eV, ionization current of 150 μA electron ionization (EI) (Peng et al., 2017; Mi et al., 2019).
3 Results and analysis
3.1 Analysis of VOC components in extracts of willow bark
The TDS-GC/MS chromatogram of the extract of “Willow bark” VOC in the range of 50 °C–250 °C is shown in Fig. 1. It can be seen that a total of 12 compounds were detected, and the extract had two distinct peaks between 19.585 min and 25.344 min. In 19.585 min in essence is 1,2-Benzenedicarboxylic acid, bis (2-methylpropyl) ester (content 59.04%), referred to as DIBP, chemical formula for c16h22o4, it can be used as a plasticizer for cellulose resin, vinyl resin, nitrile rubber and chlorinated rubber. (Wang, 2008) Plasticity and DBP similar, but the volatile and water pumping than DBP, DBP can be used as a substitute, the goods have toxic effects on crops, not suitable for agricultural PVC film. The goods low toxicity, rat oral LD 50 20–25 g/kg (Sedha et al., 2015). Britain, the Netherlands, the United States to allow the goods used in food packaging materials, the United Kingdom provides in the PVC and vinyl chloride copolymer food packaging products, the maximum amount of the goods should not exceed 40%, DIBP for the main plasticizer one. it can be used as a plasticizer for cellulose resin, vinyl resin, nitrile rubber and chlorinated rubber. The plasticizer is similar to DBP, and has excellent solubility, dispersibility and adhesion. This product and the pigment can be good compatibility, can be used for coloring film, leather and plastic products, this product can also be used as natural rubber and synthetic rubber softener, can improve the resilience of the products can be used to replace the DBP, but did not measure the material at 25.344 min (Sun et al., 2013; Wang, 2008).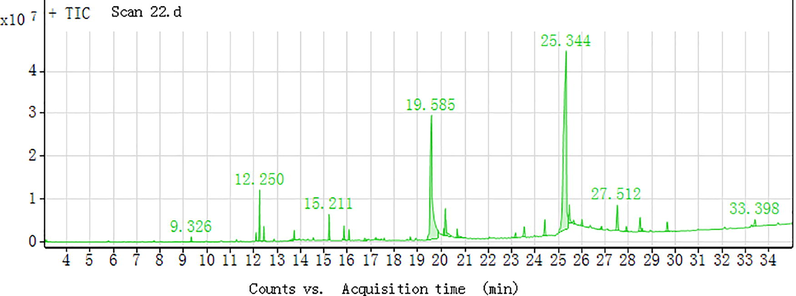
Shows the extraction of VOC ion chromatograms from willow bark by TDS-GC/MS.
Among them, including Ethanol, 2- (2-butoxyethoxy) - (content of 0.72%), can be used as diluent, stabilizer, and base inhibitor (Bing et al., 2015); 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate (content of 1.24%), he can also be used as plasticizer (Schwarzbauer et al., 2010); Ethanol, 2- (2-butoxyethoxy) -, acetate (content of 6.28%), for high boiling solvent and latex paint (Harutyunyan et al., 2005); Propanoic acid, 2-methyl-, 3-hydroxy-2,2,4-trimethylpentyl ester (content of 1.98%), mainly used as a coating agent, but also for gold, coal and other flotation agent, but also available For plasticizer (Xian et al., 2006); Benzene, 1,2,3-trimethoxy-5- (2-propenyl) - (content of 4.17%) was measured at 13.636 min and 15.211 min and it was mainly used for anesthesia (Naher et al., 2013); Dimethyl phthalate (content 1.35%), used as a cellulose acetate plasticizer, insect repellent and polyvinyl fluoride paint solvent, it is an intermediate of the rodenticide and is also an important solvent. It also has good film formation and water resistance. Cellulose acetate film, varnish, transparent paper and molding powder can be used in conjunction with diethyl phthalate. The product can also be used as plasticizer nitrile rubber and a small amount in the production of nitrocellulose. Mixed with other plasticizers, it can overcome the shortcomings of volatility and low temperature crystallization. can also be used as a mosquito oil (crude oil) and DDT solvent and gas chromatography fixative, toughening agent, high boiling solvent, plasticizer, gas chromatographic fixation (the maximum temperature of 122 °C, solvent ether), separation and analysis Hydrocarbons and esters (Gooch, 2011); Phthalic acid, hex-3-yl isobutyl ester (content of 1.01%) and 1,2-Benzenedicarboxylic acid, butyl 2-methylpropyl ester (content of 1.13%), but only the chemical formula and chemical structure are now measured, and its functional characteristics are worth We will carry out in-depth study.
3.2 Changes of chemical tissue and residual characteristics of willow bark
Fourier transform infrared spectroscopy (FTIR) is a fast detection technique. It has high sensitivity and is suitable for the identification of chemical bonds and functional groups of various compounds (Li et al., 2017). In this experiment, the changes of the COSC and extracted residues’ chemical groups were shown in Fig. 2, and the overall trend of the absorption peak of COSC and the extracted residues in the range from 4000 cm−1 to 400 cm−1 did not change significantly except the minority, and the absorption peak mainly occurred from 1742 cm−1 to 1058 cm−1. As shown in Fig. 2 in this experiment, the extraction of other residual chemical organic matter, the change of total absorption peak and extracted residue from 4000 cm−1 to 500 cm−1 was not obvious, except for a few range, and the absorption peak mainly appeared between 1740 cm−1 to 1056 cm−1 (Lui et al., 2013).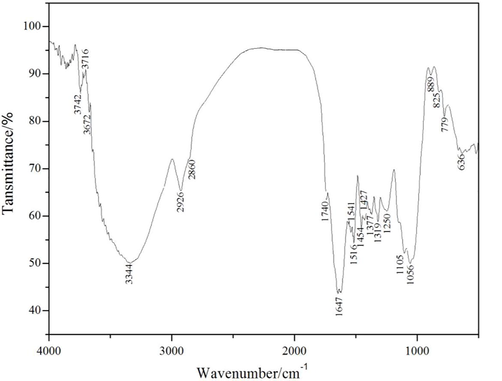
Infrared spectra of VOC extractives from Wllow Bark.
As can be seen from Fig. 2, it shows a total of 21 absorption peaks. First of all, there is a strong absorption peak at 636 cm−1, for the C—H bond, which is consistent with the results of TDS-GC/MS. However, the structure of aromatic compounds is complex, and a large number of absorption peaks overlap in the range of 900 cm−1, which will affect the determination of chemical structure reliability. In the 1740 cm−1 peak corresponding to the C⚌O bond ester compounds, at the same time there is C-O stretching vibration between 1056 cm−1 and 1250 cm−1 peaks to verify the production of ester compounds at one time; the 1647 cm−1 peak corresponds to the bending vibration of the N—H bond, thereby obtaining an amide compound. 3672 cm−1 and 3344 cm−1 two peaks with O—H-based stretching vibration, alcohol and phenolic compounds produced; 2926 cm−1 and 2860 cm−1 have two peaks with saturated C—H stretching vibration; 1516 cm−1 and 1541 cm−1 peaks proved that the C⚌C stretching vibration of mononuclear aromatic hydrocarbons, so there are aromatic compounds; the peaks at 1454 cm-to 1377 cm−1 demonstrate the bending vibration of —CH2 and —CH3; in the 1056 cm−1 peak corresponds to the C—O—C bond asymmetric stretching vibration, the generation of ether compounds; 889 cm−1 peak corresponds to the R2C⚌CH2 bond and the 825 cm−1 peak corresponds to the R2C⚌CHR bond. The C⚌O stretching vibration of the aldehyde compound corresponds to a peak at 2860 cm−1 and the latter two are overlapping peaks. The peak corresponding to the nitrogen-containing heterocyclic compound is 779 cm−1 (Mathi et al., 2016).
In addition, the general strong exponential vibration C≡C is substituted between 2150 cm−1-2100 cm−1, however, it is observed from the known infrared absorption peaks in this range. Thus, it can be said that the instability of certain chemical bonds of VOC and condensation that occur under the high temperature conditions of the C≡C bond should not be included. In addition, the chemical structure of VOCs in gas chromatography/mass spectrometry is consistent with that of IR spectra, further demonstrating that the extracts are ethers, amides, esters, alcohols and the like (Min et al., 2018; Shen et al., 2017)
3.3 Analysis of fluctuation characteristics of willow bark by thermal stability
Willow bark quality changes from 30 °C to 600 °C as show in Fig. 3. The total heat loss is divided into two distinct phases: the first stage of the loss from 50 °C to 100 °C, the temperature at which the mass remains at 3.95 mg is 100 °C, which is due to the loss of a small amount of water and volatile matter released by evaporation. The second phase begins at 150 °C until the quality of 250 °C is reduced to 3.56 mg (10.31%). There is a slight loss of quality that should be further contraction caused by intermolecular. According to the DTG curve, the mass loss rate was observed directly, the order is: the second stage, the first stage, and there is a critical temperature of the turning point in the whole process of the total mass of these temperatures have undergone significant changes. This may lead to significant chemical changes in the compounds. For example, macromolecules break down to form volatile small molecules. Therefore, these data temperature points provide a theoretical basis for total thermogravimetric measurement (Lourençon et al., 2015).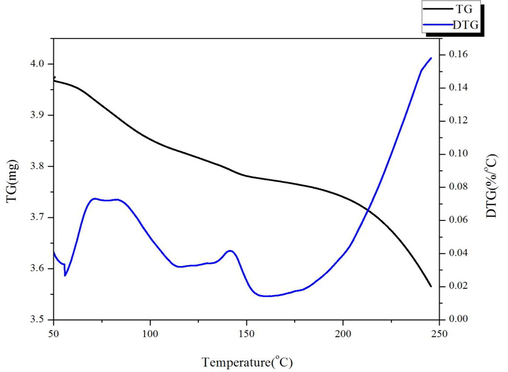
heat curve of Wllow Bark.
3.4 The characterization of the pyrolyzed matter of the residue
As shown in Fig. 4, a total of 50 compounds were detected by the PY-GC/MS method for the total pyrolysis of the total extract at 250 °C. For example, the peak at 4.09 min corresponds to Carbamic acid, monoammonium salt, used as an intermediate for phosphide, and also for pharmaceuticals and aminating agents (Wang et al., 2016); the corresponding peaks at 19.72 min is Phenol, 2-methoxy-(Lee et al., 2014), used in pharmaceuticals, dyes and perfume intermediates, and can also be used for dye synthesis and analysis reagents, As well as in medicine for the manufacture of calcium guaiacosulfonate and perfume industry used to make vanillin and artificial musk, test copper, hydrocyanic acid and nitrite. 8.73 min corresponding to the peak Furfural, mainly used as industrial solvents and the preparation of furfuryl alcohol, furoic acid, tetrahydrofuran, γ-valerolactone, pyrrole, tetrahydropyrrole and so on, can also be used as analytical reagents, organic synthesis of raw materials for tanning leather and so on; another 17.12 min corresponds to the peak 2-Cyclopenten-1-one, 2-hydroxy-3-methyl-9 (Ren et al, 2016), used as a fragrance and sweetness enhancer; 19.17 min corresponding p-Cresol, the central nervous system has a toxic effect, severe death, LD501800 mg/kg (rat, oral). But as a spice can be safely used in food (FDA, § 172.515, 2000), GB 2760--1996 provides for the use of edible spices and fungicides, fungicides, organic synthesis; 25.97 min corresponds to the peak of the compound is Catechol, it is an important chemical intermediate, which can be used in the manufacture of rubber curing agent, electroplating additive, skin preservative and analytical reagent, etc. It is also an intermediate of fungicides, methicillin, insecticides and carbofuran, not only that can also be used as an important pharmaceutical intermediate, used to make berberine and isoproterenol and so on. But there is a kind of extract Glycolaldehyde dimer, accounting for a higher proportion but has not yet found any effect, to be we continue to study.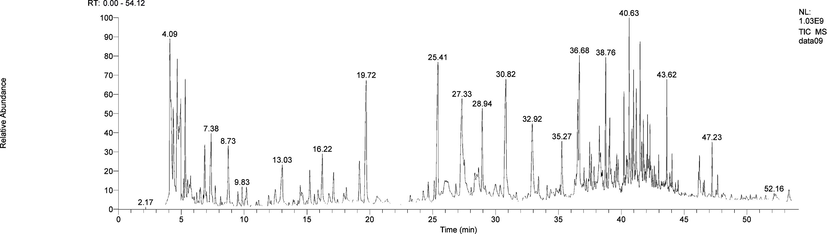
Total ion chromatograms of Wllow Bark by Py-GC–MS.
Through the PY-GC/MS method, the extract contains organic acids, alcohols, ketones, phenols and esters, etc., through the thermal gradient of the temperature gradient, the application of the extract will be expanded, it should be Its high quality and high efficiency to provide scientific basis.
4 Conclusion and discussion
By GC/MS most of the volatile organic compounds, such as 1,2-Benzenedicarboxylic acid, bis (2-methylpropyl) ester extract rich, the main function can be used as plasticizer. In addition, various beneficial volatile organic compounds, Ethanol, 2- (2-butoxyethoxy) -, acetate; Propanoic acid; Benzene, 1,2,3-trimethoxy-5- (2-propenyl) -; Dimethyl phthalate; Benzene, 1,2,3-trimethoxy-5- (2-propenyl) - 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate and Cedrol have a good use and development prospects.
FTIR results further confirmed that the total sample group structure and extracted residues were consistent with the chemical structure detected by TD-GC/MS, indicating that the willow bark contains etherics, organic acids, esters, alcohols and so on, which can be used for research. The total heat loss is two distinct stages: the first stage 50°Cto 100 °C, the second stage 150 °C to 250 °C, the mass loss is the second stage, the first stage. Throughout the experiment, the DTG curve reached a maximum of 245°Cto form a maximum value at a critical turning point of 245 °C, at this time the total mass of the temperature has undergone significant changes, this may be due to significant chemical changes that cleave large molecules and volatilize.
Further study the total mass loss treatment and high temperature pyrolysis composition changes, the results showed that the extracts contained different organic acids, alcohols, esters, ketones, amines, aldehydes and so on, many of which can be used for food spices, and some can be used as medicine or pesticide intermediates, organic synthesis, important chemical intermediates and analytical reagents, so we have to study the use of the future there is a lot of room for development.
Acknowledgement
This project is supported by the science and technology project of Hunan Province, China (2016NK2154), and the National Natural Science Foundation of China (31172257).
Declaration of Competing Interest
All the authors hereby agreed and confirm that there is no conflict of interest for this research work and publication of this paper.
References
- Synthesis of Di(2-(2-butoxyethoxy) ethanol) terephthalate catalyzed by br? nsted-lewis acidic ionic liquid. Fine Chem. 2015
- [Google Scholar]
- Rediscovery of physiological function of salicylic acid by foreign scholars. Plant Mag.. 1995;3:45.
- [Google Scholar]
- An analytical model for elastic modulus calculation of sic whisker-reinforced hybrid metal matrix nanocomposite containing sic nanoparticles. J. Alloys Compd.. 2018;767:632-641.
- [Google Scholar]
- Harutyunyan, A., Grigorian, L., Tokune, T., 2005. Mixing mixtures of iron, palladium, nickel and molybdenum acetates, with solvents such as 2-(2-butoxyethoxy) ethanol, then heating to form nanostructure particles, used for catalysis or drug delivery. EP.
- Treating n-butane by activated carbon and metal oxides. Toxicol. Environ. Chem.. 2017;99(5–6):753-759.
- [CrossRef] [Google Scholar]
- Catalytic hydrodeoxygenation of 2-methoxy phenol and dibenzofuran over Pt/mesoporous zeolites. Energy. 2014;81:33-40.
- [CrossRef] [Google Scholar]
- Molecules and functions of aesculus chinensis bunge bark volatiles. Emir. J. Food. Agr.. 2018;30(10):809-819.
- [CrossRef] [Google Scholar]
- Chemical structure characteristics of wood/lignin composites during mold pressing. Polym. Compos.. 2017;38(5):955-965.
- [CrossRef] [Google Scholar]
- Hardwood and softwood kraft lignins fractionation by simple sequential acid precipitation. Sep. Purif. Technol.. 2015;154:82-88.
- [CrossRef] [Google Scholar]
- In vitro and in silico characterization of angiogenic inhibitors from sophora interrupta. J. Mol. Model.. 2016;22(10):247.
- [CrossRef] [Google Scholar]
- Research on regional clustering and two-stage svm method for container truck recognition. Discrete Continuous Dyn. Syst. Series S. 2019;12(4–5):1117-1133.
- [Google Scholar]
- Application of LSSVM algorithm for estimating higher heating value of biomass based on ultimate analysis. Energy Source Part A. 2018;40(6):709-715.
- [CrossRef] [Google Scholar]
- Comparative studies on physico-chemical properties and GC-MS analysls of essential oil oh myristica fragrans. J. Stat. Softw.. 2013;62(2):345-346.
- [Google Scholar]
- Syngas production by catalytic co-gasification of coal-biomass blends in a circulating fluidized bed gasifier. J. Clean. Prod.. 2017;168:1513-1517.
- [CrossRef] [Google Scholar]
- Production of 2,5-hexanedione and 3-methyl-2-cyclopenten-1-one from 5-hydroxymethylfurfural. Green Chem.. 2016;18(10):3075-3081.
- [CrossRef] [Google Scholar]
- Quantitation of nonextractable anthropogenic quantitation of nonextractable anthropogenic sediments after chemical degradation. Clean - Soil, Air, Water. 2010;31(6):469-481.
- [CrossRef] [Google Scholar]
- Determination of in vivo estrogenic potential of Di-isobutyl phthalate (DIBP) and Di-isononyl phthalate (DINP) in rats. Environ. Sci. Pollut. Res. Int.. 2015;22(22):18197-18202.
- [CrossRef] [Google Scholar]
- A deep q-learning network for ship stowage planning problem. Polish Maritime Res.. 2017;24(SI):102-109.
- [Google Scholar]
- Characterization of Phthalate Plasticizer from BottledBeverages by GC-MS. Appl. Mech. Mater.. 2013;401–403:590-593.
- [CrossRef] [Google Scholar]
- Plasticizers DBP and DIBP product samples long-term storage of acidity changes and improvement of control points. Guangdong Chemi. In.. 2008;35(5):46-48.
- [CrossRef] [Google Scholar]
- Comparative analysis of VOCs in exhaled breath of amyotrophic lateral sclerosis and cervical spondylotic myelopathy patients. Sci. Rep.. 2016;6(1):26120.
- [CrossRef] [Google Scholar]
- Chemical composition of essential oils of two submerged macrophytes, Ceratophyllum demersum L. and Vallisneria spiralis L. Flavour Frag. J.. 2006;21(3):524-526.
- [Google Scholar]







