Translate this page into:
Molecular characteristics and function of elliptical Kiwifruit
⁎Corresponding authors. csfuzzf@163.com (Zhongfeng Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Elliptical Kiwifruit (Actinidia chinensis Planch) is a popular fruit among consumers. It has abundant nutrition. And it also has medicinal value and economic value. In our research, we detected and analyzed the chemical components by Fourier transform infrared spectroscopy (FT-IR) and gas chromatography mass spectrometry (GC–MS) technologies. In FT-IR analysis, we known that there it main had O-H stretching vibration, C-H stretching vibration, C⚌C stretching vibration, benzene ring stretching vibration, C-H C-O stretching vibration and anomeric carbon vibrational frequency absorption peak attribution. The types of compound that may be in this band is cellulose, carboxylic acid, alcohol, phenol, amine and ester. In GC–MS analysis, we known that the main chemical compounds were 5-hydroxymethylfurfural, 1,2,3,5-cyclohexanetetrol, quinic acid, d-alanine, N-propargyloxycarbonyl-, isohexyl ester, 4H-Pyran-4-one, cyclotetrasiloxane, octamethyl- and furfural. And these organic components can be used at food processing, medical treatment and light industry fields.
Keywords
Elliptical Kiwifruit
FT-IR
GC–MS
Chemical composition
Application
1 Introduction
Elliptical Kiwifruit (Actinidia chinensis Planch) belongs actinidiaceae, actinidia. Originally, it was a wild fruit produced in Yichang, Hubei Province (Takeoka et al., 1986). At the beginning of the last century, Kiwifruit was taken away from Yichang by New Zealanders and was artificially planted. Until now, many varieties have been developed. Kiwifruit is not only rich in water, soft, fragrant and tasty, but also rich in nutritional value. It has a high vitamin C content and also contains a lot of sugar, amino acids and minerals, for example: calcium, selenium, zinc, potassium, tellurium, fructose, malic acid, citric acid, antioxidants, flavonoids, carotenoids, anthocyanins, folate, and melatonin (Selman, 1983; Liu, 2001).
Kiwi has a very prominent medicinal value. It has the functions of heat-clearing and detoxifying, improving appetite, promoting digestion and anticancerous, etc. (Nødtvedt et al., 2017) reported that it may have some sleep improvement effect. Chan et al. (2007) reported that increase dietary fiber intake can effectively relieve chronic constipation and Kiwifruit had abundant in dietary fiber. Lim (2016) reported that hardy elliptical Kiwifruit contained high amount of ascorbic acid. Kiwifruit has clinical effect in atopic dermatitis and its fruit extracts have anticancer effect (Al-zaqri et al., 2017; Boussaid et al., 2018; Li et al., 2018; Maddi et al., 2018; Qadir et al., 2018; Yang et al., 2018). Hunter (2012) reported that gold Kiwifruit contains vitamins C and vitamins E, folate, carotenoids and polyphenols. These nutrients play an important role in enhancing immune function and alleviating symptoms of infection (Ge et al., 2017a). Kiwifruit can also reduce the duration and severity of upper respiratory infections in elderly patients. Kiwifruit was used as a medicine and it has been a long time in the history of China. Motohashi et al. (2002) reported that in the records of some ancient book, such as ‘neijing’, ‘Jin-shu’, ‘zhu-bingyuan-hou-lun’ and‘san yin-fang’ and so on, the ancients used kiwifruit as a prescription to treat many diseases that may be cancer, such as skin cancer, breast cancer and the cancer of digestive system.
In our study, we used FT-IR and GC–MS technology to analyze the chemical components and types of compounds for providing reference to other scholars and follow-up studies of our research group.
2 Material and methods
2.1 Experimental material
The material used for this study was the entire elliptical Kiwifruit. This elliptical Kiwifruit was produced in Xixia County, Nanyang City, Henan Province. Xixia County is located between longitude 111°48′ E, latitude 33°28′ N. It is rich in fruit resources, it is known as the hometown of kiwi. Kiwi varieties used in this experiment are shown in the following Fig. 1.
Experimental Materials.
2.2 Experimental methods
The elliptical Kiwifruit of this study used sample should clean by deionized water. Then crushed entire fruit flesh together with pericarp. Divided pulp into two parts, respectively added methanol and ethanol as solvents to perform extraction experiments (Ge et al., 2017b). In order to acquire more accurate experimental data, we put the leaching liquor into rotator evaporator to evaporate the water and redundant solvents. Finally, send the sample to do FT-IR and GC–MS detections.
2.2.1 FT-IR analysis
The study uses infrared spectroscopy to analyze the structure and chemical bonds of molecules, and can identify the type of compound by the characteristic wave number of the chemical bond (Ge et al., 2017c). In this study, we used the polished KBr slice as the salt slice of infrared spectrometer. The recorded range of FT-IR spectra was all in 4000 cm−1-400 cm−1 (Sherazi, 2009; Li, 2015) (see Fig. 2).
The diagram of experimental process.
2.2.2 GC–MS analysis
Gas chromatograph has an effective separation ability for organic compounds. Under the control of the computer, it let compounds into the mass spectrometer ion source one by one, and satisfy the requirements of mass spectrometry for sample unity (Dauner and Sauer, 2000). In our study, the GC–MS analysis used 7890B-5977A (Agilent). Chromatographic column was HP-5MS (30 m × 250 μm × 0.25 μm). Capillary column of this experiment was elastic quartz. Carrier gas was high purity He, the flow rate was 1 mL/min and using shunting mode, the split ratio was 20:1. The temperature of GC program started at 50 ℃, Firstly rose to 250 ℃ at 8 ℃/min, secondly rose to 300 ℃ at 5 ℃/min (Peng et al., 2017). The temperature of ion source and quadrupole respectively were 230 ℃ and 150 ℃. Ionization voltage was 70 eV, ionization current (EI) was 150μA. MS program scanning quality range was 30–600 amu (Proestos et al., 2006; Ma et al., 2007; Wang et al., 2009a).
3 Results and discussion
3.1 Analysis of FT-IR
Based on the data obtained from FT-IR analysis, we can make a chart as Fig. 3, and referenced to existing books and other scholars’ research results, we made Table 1. According Fig. 4 we can find that as a whole, the absorption peaks of leaching liquor extracted by methanol (M) was stronger than the absorption peaks of leaching liquor extracted by ethanol (E). The phenomenon was because it extracted more chemical components by methanol solvent. According Table 1 and Fig. 3, we can know that in the range of 3650 cm−1 to 3200 cm−1 wave number, shown a wide and strong absorption peak of O-H stretching vibration. The types of compound that may be in this band was carboxylic acid, alcohol, phenol. In the range of 2975 cm−1 to 2840 cm−1 wave number, shown several absorption peak of C-H stretching vibration. At 1648 cm−1, the absorption peak was C⚌C Stretching vibration. At 1450 cm−1, the absorption peak was benzene ring stretching vibration. At 1420 cm−1, 1412 cm−1 and 1329 cm−1, the absorption peak was C-N stretching vibration. At 1383 cm−1, the absorption peak was C-H flexural vibration. In the range of 1110–1018 cm−1 wave number, shown several absorption peak of C-O stretching vibration. At 881 cm−1, the absorption peak was benzene ring stretching vibration. Some scholars reported that the characteristic absorption peak of cellulose was 2900 cm−1, 1425 cm−1, 1370 cm−1 and 895 cm−1. In our study, we can find absorption peak at 2930 cm−1, 1420 cm−1, 1383 cm−1 and 881 cm−1 in E. We can consider that these were characteristic absorption peak of cellulose and this kind of elliptical Kiwifruit has abundant cellulose (Schwanninger et al., 2004; Szymańska-Chargot et al., 2011) (see Table 2).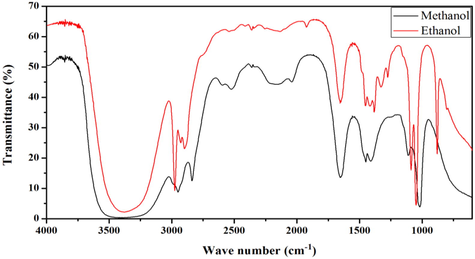
FT-IR curves of methanol extractives and ethanol extractives.
Absorption peak (cm−1)
Absorption peak attribution
Chemical composition
Methanol
Ethanol
3375
3375
O-H Stretching vibration
Carboxylic acid, alcohol, phenol
2950, 2840
2975, 2930
C-H Stretching vibration
Cellulose
1648
1648
C⚌C Stretching vibration
Alkene
1450
1450
Benzene ring stretching vibration
Aromatic hydrocarbon
1412
1420
C-N Stretching vibration
Cellulose, amine
1383
C-H Flexural vibration
Cellulose
1329
C-N Stretching vibration
Amine
1110, 1018
1088, 1049
C-O Stretching vibration
Acid, phenol, carboxylic acid, ester
881
Anomeric carbon vibrational frequency
Cellulose
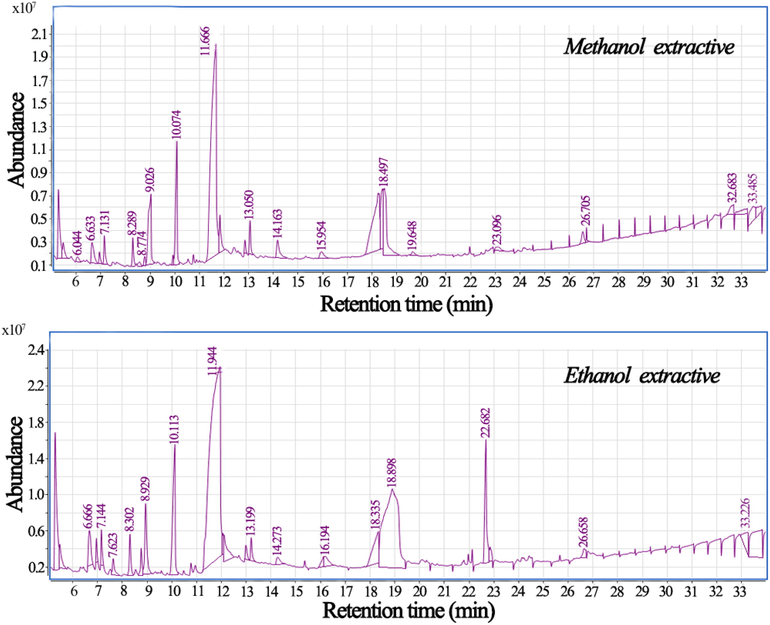
The total ion chromatogram of methanol and ethanol extractives by GC–MS.
No.
RT (min)
Peak area (%)
Compound
PubChem CID
Molecular formula
1
5.27
4.07
Furfural
7362
C5H4O2
2
5.46
1.06
Levoglucosenone
699486
C6H6O3
3
6.63
1.81
cis-Aconitic anhydride
65163
C6H4O5
4
7.13
1.24
2,4-Dihydroxy-2,5-dimethyl-3(2H)-furan-3-one
C6H8O4
5
8.29
1.21
1,3-Dioxane-5-methanol, 4,5-dimethyl-
C7H14O3
6
9.03
6.59
d-alanine, N-propargyloxycarbonyl-, isohexyl ester
C13H21NO4
7
10.07
6.52
4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl-
C6H8O4
8
11.67
34.93
5-Hydroxymethylfurfural
237332
C6H6O3
11.83
2.13
12.84
0.52
9
13.05
1.42
Ethyl propionylacetate
78656
C7H12O3
10
14.16
1.76
Cyclobutanecarboxylic acid, nonyl ester
568107
C14H26O2
11
15.95
0.91
Lactose
C12H22O11
12
18.26
12.29
Quinic acid
6508
C7H12O6
13
18.42
4.68
1,2,3,5-Cyclohexanetetrol
548226
C6H12O4
18.50
7.92
13
32.68
1.16
Cyclotetrasiloxane, octamethyl-
121111
C8H24O4Si4
33.24
1.26
33.49
2.68
33.80
1.30
3.2 Analysis of GC–MS
The result was shown that in GC–MS analysis, the leaching liquor of methanol solvent was detected 25 peaks and indentified 18 types chemical components. The main chemical compositions were 5-hydroxymethylfurfural (37.58%; PubChem CID: 237332), 1,2,3,5-cyclohexanetetrol (12.60%; PubChem CID: 548226), quinic acid (12.29%; PubChem CID: 6508), d-alanine, N-propargyloxycarbonyl-, isohexyl ester (6.59%), 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- (6.52%), cyclotetrasiloxane, octamethyl- (6.40%; PubChem CID:121111), furfural (4.07%; PubChem CID: 7362), cis-aconitic anhydride (1.81%; PubChem CID: 65163), cyclobutanecarboxylic acid, nonyl ester (1.76%; PubChem CID: 568107) and ethyl propionylacetate (1.42%; PubChem CID: 78656) and so on (see Table 3).
No.
RT (min)
Peak area (%)
Compound
PubChem CID
Molecular formula
1
5.47
1.10
3,5-Dimethylpyrazole
6210
C5H8N2
2
6.67
1.75
2,5-Furandione, 3-methyl-
12012
C5H4O3
3
8.30
1.36
1,3-Dioxepane, 2-pentadecyl-
C20H40O2
4
8.93
2.99
3-Methoxycarbonylpyrazole
565662
C5H6N2O2
5
10.11
6.21
4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl-
C6H8O4
6
11.94
38.82
5-Hydroxymethylfurfural
237332
C6H6O3
12.06
0.48
12.11
1.74
12.98
0.51
13.20
0.71
7
18.34
3.45
Quinic acid
6508
C7H12O6
18.90
22.72
8
22.68
4.73
Pyrazole-4-carboxaldehyde, 1-methyl-
C5H6N2O
9
33.23
1.63
Cyclotetrasiloxane, octamethyl-
121111
C8H24O4Si4
33.84
5.87
The result was shown that the leaching liquor of ethanol solvent was detected 29 peaks and indentified 21 types chemical components. The main chemical compositions were 5-hydroxymethylfurfural (42.26%; PubChem CID: 237332), quinic acid (26.17%; PubChem CID: 6508), cyclotetrasiloxane, octamethyl- (7.50%; PubChem CID:121111), 4H-pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- (6.21%), pyrazole-4-carboxaldehyde, 1-methyl- (4.73%), 3-methoxycarbonylpyrazole (2.99%; PubChem CID: 565662), 2,5-furandione, 3-methyl- (1.75%; PubChem CID: 12012), 1,3-dioxepane, 2-pentadecyl- (1.36%) and 3,5-dimethylpyrazole (1.10%; PubChem CID: 6210).
These organic components can be used at food processing, medical treatment and light industry fields. For example: 5-hydroxymethylfurfural has rich chemical property, and it can potential be acquired from carbohydrates, like inulin, cellulose, sucrose, fructose and glucose. It’s a kind of important chemical intermediate (Rosatella et al., 2011). For a long time, quinic acid has been used as antioxidant, and might have anticarcinogenic properties (Wang et al., 2009b). Cyclotetrasiloxane, octamethyl- (octamethylcyclotetrasiloxane) has been used in industrial applications, personal care consumer products and other industries for more than 40 years. It has thermal stability, chemical stability, UV radiation resistance and low surface tension (Zareba et al., 2002). Furfural is a raw material for preparing many medicine and industrial products. The chemical properties of furfural are active, numerous derivatives can be prepared by condensation reaction and oxidation reaction. And it is widely used in the synthesis of organic products such as resins, pharmaceuticals, varnishes, rubbers, coatings and pesticides. Its derivatives also are potential bio-fuel components worthy attention (Lange et al., 2012). Ethyl propionylacetate can be used in the essences and spices industry with a mild fruity fragrance. It is also used as a solvent for paints, varnishes, nitro spray paints and various resins.
According this study, we learned from books, literature, and experiments that Kiwifruit has rich nutritional, medicinal, and industrial value. In the report of FT-IR, we known that there were O-H stretching vibration, C-H stretching vibration, C⚌C stretching vibration, benzene ring stretching vibration, C-N stretching vibration, C-H flexural vibration, C-O stretching vibration and anomeric carbon vibrational frequency in our sample. The types of compound that may be in this band is cellulose, carboxylic acid, alcohol, phenol, amine and ester. In the report of GC–MS, we known that 25 peaks and indentified 18 types chemical components were detected in M and 29 peaks and indentified 21 types chemical components were detected in E. The main chemical compounds were 5-hydroxymethylfurfural (PubChem CID: 237332), 1,2,3,5-cyclohexanetetrol (PubChem CID: 548226), quinic acid (PubChem CID: 6508), d-alanine, N-propargyloxycarbonyl-, isohexyl ester, 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl-, cyclotetrasiloxane, octamethyl- (PubChem CID:121111) and furfural (PubChem CID: 7362). And these organic components can be used at food processing, medical treatment and light industry fields.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (31872697). The Hunan Science Fund for Distinguished Young Scholars (16JJ1028).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Comparative catalytic evaluation of nickel and cobalt substituted phosphomolybdic acid catalyst supported on silica for hydrodesulfurization of thiophene. J. Saudi Chem. Soc.. 2017;21:965-973.
- [Google Scholar]
- Physicochemical and bioactive properties of six honey samples from various floral origins from Tunisia. Arab. J. Chem.. 2018;11:265-274.
- [Google Scholar]
- GC-MS analysis of amino acids rapidly provides rich information for isotopomer balancing. Biotechnol. Prog.. 2000;16:642-649.
- [Google Scholar]
- Desulphurization characteristics of bamboo charcoal from sulfur solution. Saudi J. Biol. Sci.. 2017;24:127-131.
- [Google Scholar]
- Characteristics of activated carbon remove sulfur particles against smog. Saudi J. Biol. Sci.. 2017;24:1370-1374.
- [Google Scholar]
- Adsorption characteristics of sulfur solution by acticarbon against drinking-water toxicosis. Saudi J. Biol. Sci.. 2017;24:1355-1360.
- [Google Scholar]
- Furfural–a promising platform for lignocellulosic biofuels. Chemsuschem. 2012;5:150.
- [Google Scholar]
- Empirical research on the relationship between natural gas consumption and economic growth in the northeast Asia. Energy Environ.. 2018;29:216-231.
- [Google Scholar]
- Determination of trace elements in fruit of Actinidia chinensis planch. Trace Elem. Sci. 2001
- [Google Scholar]
- Ma, Q.Z., Peng, W.X., Zhang, D.Q., 2007. Study on Healthful function of superheated water extractives of Phyllostachys heterocycla by GC/MS, International Conference on Physical and Numerical Simulation of Materials Processing, pp. 1320–1324.
- Pyrolytic fractionation: a promising thermochemical technique for processing oleaginous (algal) biomass. ACS Sustainable Chem. Eng.. 2018;6:237-247.
- [Google Scholar]
- Cancer prevention and therapy with kiwifruit in Chinese folklore medicine: a study of kiwifruit extracts. J. Ethnopharmacol.. 2002;81:357.
- [Google Scholar]
- The effects of Kiwifruit consumption in students with chronic insomnia symptoms: a randomized controlled trial. Sleep Biol. Rhyth.. 2017;15:159-166.
- [Google Scholar]
- Characteristics of antibacterial molecular activities in poplar wood extractives. Saudi J. Biol. Sci.. 2017;24:399-404.
- [Google Scholar]
- Determination of phenolic compounds in aromatic plants by RP-HPLC and GC-MS. Food Chem.. 2006;95:44-52.
- [Google Scholar]
- anditin-silico anditstudy of potential carboxylic acid derivatives as d-glutamate ligase inhibitors in anditsalmonella typhiandit. Kuwait J. Sci.. 2018;45:100-107.
- [Google Scholar]
- ChemInform abstract: 5-hydroxymethylfurfural (HMF) as a building block platform: biological properties, synthesis and synthetic applications. Cheminform. 2011;42:754-793.
- [Google Scholar]
- Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc.. 2004;36:23-40.
- [Google Scholar]
- The vitamin C content of some kiwifruits (Actinidia chinensis, planch. variety hayward) Food Chem.. 1983;11:63-75.
- [Google Scholar]
- Sensing the structural differences in cellulose from apple and bacterial cell wall materials by Raman and FT-IR spectroscopy. Sensors. 2011;11:5543-5560.
- [Google Scholar]
- Volatile constituents of elliptical Kiwifruit (Actinidia chinensis Planch.) J. Agric. Food. Chem.. 1986;34:576-578.
- [Google Scholar]
- Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antiviral Res.. 2009;83:186-190.
- [Google Scholar]
- Volatile characteristics of 50 peaches and nectarines evaluated by HP-SPME with GC-MS. Food Chem.. 2009;116:356-364.
- [Google Scholar]
- Internet of things for smart ports: technologies and challenges. IEEE Instrum. Meas. Mag.. 2018;21:34-43.
- [Google Scholar]
- Percutaneous absorption studies of octamethylcyclotetrasiloxane using the human skin/nude mouse model. Skin Pharmacol. Appl. Skin Physiol.. 2002;15:184-194.
- [Google Scholar]







