Translate this page into:
Mitigation of lead toxicity in Brassica juncea L. by sulphur application – Via various biochemical and transcriptomic strategies
⁎Corresponding author. hemanthyah72@gmail.com (Hemanthkumar Manne)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The present study was designed to elucidate whether exogenously applied sulphur (S) (S1; 100 ppm, S2; 200 ppm S) alleviate lead (Pb)-induced (Pb1; 100 ppm, Pb2; 200 ppm and Pb3; 300 ppm Pb) stress in the leaves of Brassica juncea. To study this effect, oxidative stress biomarkers (hydrogen peroxide and malondialdehyde), enzymatic antioxidants (ascorbate peroxidase (APX), glutathione reductase (GR)), non-enzymatic antioxidants (chlorophyll and carotenoids) and S-metabolic enzymes like adenosine triphosphate sulfurylase (ATPS), O-acetylserine(thiol)lyase (OASTL) were studied. Pb treatment dramatically inhibited plant development by increasing lipid peroxidation and hydrogen peroxide (H2O2) accumulation by 34.82 and 238.89 % respectively at 90 days plant. The overproduced oxidative biomarkers alter plant cell homeostasis. In contrast, APX (25.56 %) and GR (32.45 %) activities, chlorophyll (15.51 %) and carotenoids (30 %) contents increased under similar Pb treatments and regulated ROS-antioxidant balance. Exogenous S application, maintained the redox status of cell by further increasing the enzymatic activities. Pb treatment increased S assimilation in Brassica by elevating enzyme activities of ATPS and OASTL, which was further augmented by S supplementation to Pb-stressed plants. Besides gene expression of BjSULT, BjOASTL and BjGR1 exhibited slight increase under Pb stress, S application to the stressed plants doubled the expression and assisted the stress resistance. Overall, our findings demonstrate that S protect Pb-stressed Brassica seedlings, implying that S could be an ideal choice for reducing Pb toxicity in crops.
Keywords
Antioxidants
Brassica juncea
Gene expression
Lead stress
qRT-PCR
Sulphur
Transcriptomics
1 Introduction
Environmental pollution by heavy metals and deposition in soil is of great solicitude in agricultural production owing to the destruction on crop growth heavy metals were deposited in agricultural areas as a result of fast industrialization, improper trace metal consumption, disposal, and dumping into the environment, as well as excessive fertilizer and pesticide use (Amel et al., 2016) that posed a threat to human and environmental health. Plants succumb to stress and remodel their metabolic systems and gene expression in response to these metals absorption (e.g., lead, arsenic, cadmium) (Dixit et al., 2016). Under Pb stress, enhanced reactive oxygen species (ROS) production cause damage to cellular organelles (Hasan et al., 2019). The disturbed cellular homeostasis is regulated by APX and GR antioxidants. Besides, overproduced H2O2 and malondialdehyde (MDA) is counterattacked by chlorophyll and carotenoids (Dixit et al., 2016, Kohli et al., 2019).
Sulphur is an important macronutrient that assists in heavy metal detoxification, particularly Pb. Sulphur is transferred into plants by sulphate transporter genes (SULT) and assimilates into compounds like cysteine (Cys) and methionine (Met) by a enzymatic processes mediated by ATP sulfurylase (ATPS) and O-acetylserine (thiol) lyase (OASTL) enzymes (Ma et al., 2018). The GSH is a significant redox buffer and precursor of phytochelatins (PC), which bind to heavy metals and detoxifies in the form of PC-metal complexes, and transported to vacuoles through ABC transporters for metal sequestration (Song et al., 2010). Furthermore, multiple studies have discovered that providing additional S to plants, enhanced their antioxidant system, thiol metabolism, and metal toxicity (Liang et al., 2016). Further, to Cd toxic pakchoi, exogenous S promoted sulphur assimilation and boosted critical S genes involved in S metabolism and thiol pool (Lou et al., 2017).
Additionally, sulfate deficiency might make plants more stressed by heavy metals, whereas a sufficient sulfate supply can lessen the toxicity of heavy metals (Na and Salt, 2011; Astolfi et al., 2014). However, physiological processes that underpin the interactions between heavy metals and S is largely unknown. In plants, a number of genes transcriptional levels also regulate lead accumulation and sulfate absorption. In Arabidopsis thaliana L. ATP-binding cassette subfamily C transporter 1 (ABCC1) plays a key role in uptaking of heavy metals and translocating into vacuoles (Köhler and Neuhaus, 2000; Cao et al., 2009). Heavy metal exposure has been connected to alterations in the genetic level of herbaceous plants (Takahashi et al., 2011), but the transcriptional regulation of genes in mustard when exposed to Pb and/or varied S treatments remain mostly unknown.
Mustard (Brassica juncea (L.) Czern & Coss) is an important Indian oilseed. Brassica hyperaccumulates heavy metals, making it vulnerable to metals and damaging pigments, growth, and yield. Sulphur improved antioxidants and S-assimilating enzymes, reducing metal toxicity (Liang et al., 2016; Singh et al., 2020). Till date, S relationship to Pb remains unknown. Thus, current study examined how sulphur affects antioxidant enzyme activities, stress biomarkers, photosynthetic pigment concentration, and gene expression in lead-stressed Indian mustard (Brassica juncea (L.) Czern & Coss).
2 Materials and methods
2.1 Crop raising
Indian mustard [Brassica juncea (L.)] variety of RH-749 was grown in the screen house, Department of Biochemistry. Stress conditions were achieved by treating various concentrations (100, 200 and 300 ppm) of Pb [Pb(NO3)2] dissolved in water. To ameliorate Pb-stress, two different concentrations (100 and 200 ppm) of sulphur [Zn(SO4).7H2O] was sprayed. The experiment was established with 12 treatments (three replicates each treatment): control, Pb1 (100 ppm Pb), Pb2 (200 ppm Pb), Pb3 (300 ppm Pb), S1 (100 ppm S), S2 (200 ppm S) and following combinations were made for present study: Pb1 + S1, Pb1 + S2, Pb2 + S1, Pb2 + S2, Pb3 + S1 and Pb3 + S2. Here, Pb1, Pb2 and Pb3 treatments are with Pb alone, S1 and S2 treatments are with sulphur alone. Lead spray was done 5 days prior to sampling and sulphur spraying was done 3 days prior to sampling. Leaf samples were collected at vegetative stage −30 days after sowing (DAS), flowering stage (60 DAS) and pod filling stage (90 DAS) for further experimental analysis.
2.2 Oxidative stress biomarkers
2.2.1 Hydrogen peroxide (H2O2)
The content of hydrogen peroxide was determined following the Velikova et al. (2000). Extract was centrifuged with trichloroacetic acid (TCA, 0.1 %), phosphate buffer (10 mM) and 1 M potassium Iodide was added and absorbance was recorded at 390 nm. Standard curve of 10–100 µmoles of H2O2 was used to find H2O2 content.
2.2.2 Lipid peroxidation
Lipid peroxidation was determined by TBARS content following Heath and Packer (1968) method. Extract was centrifuged with TCA (20 %), to 1 ml supernatant TCA containing thiobarbituric acid (0.6 %) was added. Mixture was heated at 95 °C for 30 min, absorbance was taken at 532 nm and at 600 nm. The peroxidation of lipids was estimated by the extinction coefficient 155 mM-1cm−1.
2.3 Enzymatic antioxidants
2.3.1 Preparation of enzyme extract
The leaves of B. juncea at three different stages viz., 30, 60 and 90 DAS were used for enzymatic studies. The leaves of 1 g was homogenized 5 ml phosphate buffer containing EDTA, β-mercaptoethanol and PVP. Whereas for ascorbate peroxidase the extraction buffer also contained ascorbate. Homogenate was centrifuged and supernatant was carefully transferred into the tubes and used for further analysis.
2.3.2 Glutathione reductase (GR) (EC: 1.6.4.2)
Glutathione reductase activity was estimated according to Schaedle and Bassham (1977). To the 200 µl extract, phosphate buffer, oxidized glutathione (GSSG), NADPH was added. The reaction was initiated by adding NADPH, absorbance measured at 340 nm and GR activity was estimated by using the extinction coefficient of NADPH 6.22 nM−1 cm−1.
2.3.3 Ascorbate peroxidase (EC 1.11.1.11)
Ascorbate peroxidase was estimated according to Nakano and Asada (1981). To 0.1 ml extract, phosphate buffer (pH 7.0), AsA, EDTA, H2O2 was added. Absorbance recorded at 290 nm. The APX activity was estimated by the extinction coefficient of ascorbate 2.8 mM−1 cm−1.
2.4 Sulphur assimilating enzymes
2.4.1 Atp-sulfurylase (ATPS/APS) (EC: 2.7.7.2)
ATPS (EC2.7.7.4) was estimated following Lappartient and Touraine (1996) procedure. Fresh tissue (0.1 g) was ground in Tris-HCl (pH 8.0) constituting EDTA, DTT and PVP and centrifuged at 20,000g for 10 min at 4 °C. The reaction contains MgCl2, Na2MoO4, Na2ATP and sulfate-free inorganic pyrophosphate in Tris-HCl (pH 8.0). A second portion was prepared without Na2MoO4. Phosphate present in mixture was estimated spectrophotometrically by incubating mixture for 15 min at 37 °C.
2.4.2 O-acetyl serine thiol lyase (OASTL) (EC: 4.2.99.8)
OASTL (EC 4.2.99.8) was estimated following Riemenschneider et al. (2005) method. Tissues were homogenized in ice-cold Tris-HCl before being centrifuged. To the 50 µl extract, OAS, Na2S, DTT, Tris-HCl (pH 7.5) was added and incubated (30 min, 37 °C). Afterwards, acidic ninhydrin addition terminates reaction and Cysteine was determined. Finally, colored solution was neutralized by 900 µl, 70 % ethanol addition to sample and absorbance recorded at 560 nm.
2.5 Non-enzymatic antioxidants
2.5.1 Carotenoid
The carotenoid content in the leaves was estimated according to Wellburn (1994). The carotenoids extracted in the DMSO were measured by reading the absorbance at 480 nm.
2.5.2 Chlorophyll
By using SPAD 502 chlorophyll meter, the chlorophyll content of the plants was measured instantly to reduce the risk of yield limiting deficiencies.
2.6 Gene expression analysis
The fresh seedlings of 30, 60 and 90 days B. juncea plants was used for RNA extraction. Trizol method was followed to extract the RNA. Treatment of RNA with RNAse free DNase was done before gene expression analysis and RNA was quantified by Nanodrop spectrophotometer. Out of total RNA first 1 µg was used for the synthesis of cDNA by using the qscript cDNA synthesis kit. For gene expression, actin gene in B. juncea used to normalise gene expression of the selected genes and the primers used are presented in Table 1. At each time point, the expression of a subset of genes was compared to that of the reference gene (β-actin gene), and the resulting expression ratio was calculated in terms of change in the fold expression. The PCR conditions used were initial denaturation at for 5 min, 40 cycles of denaturation at 95 °C for 15 sec, annealing at 59 °C for 30 sec and extension at 65 °C for 1 min. The qRT-PCR was done on Biorad system (CFX96 Touch system) by One-step real-time detector and using SYBR Green PCR Master Mix.
S. No.
Primer
Forward primer sequence (5′-3′)
Reverse primer sequence (5′-3′)
1
BjSULT
CGAAGGAAGGTTTGCTGAAG
TTGTACTCTCTGGCCCATCC
2
BjPb
GCAAGCATGGAGGAAACTG
AGGCCACCGTGTAACCAAGT
3
BjABCC
AGAATCGTGGAGCTGGAGAA
AAAAGAACCGGTGACTGTGG
4
BjATPS
GGATGCTGTCTTTGCTTTCC
ACCTTCTCGTGCTGCTTCAT
5
BjOASTL
CTTGAATCACCATGGCCTCT
CTTAGCAGCAACACGACCAA
6
BjGR1
GGACGTCCCTTCATTCCTGA
CCCATTAAAGATGCCCGCAA
7
Actin
CAGTACTGCTGACTGAGGCG
TGCGTCCACTAGCATATAGGG
2.7 Statistical analysis
The research data was analyzed with SPSS 24.0 statistical software package, as mean of three replicates using two factor analysis of variance (ANOVA) by completely randomized design (CRD) to evaluate the significant difference of means, using 5 % level of significance.
3 Results
3.1 Oxidative biomarkers
3.1.1 Hydrogen peroxide
Lead stress of 100, 200 and 300 ppm caused significant H2O2 increase by 69.43, 201.59 and 303.34 % (30 DAS), 97.90, 193.13 and 317.39 % (60 DAS), 73.48, 144.89 and 238.89 % (90 DAS), respectively, in comparison to control (Fig. 1A). The S1 sulphur spray has decreased H2O2 by 30.73, 31.00 and 40.87 % at S2 spray H2O2 decreased by 40.13, 45.72 and 55.73 %, respectively at 30, 60 and 90 DAS, in respect to control. Maximum reduction of H2O2 was observed under S2 spray at Pb1 + S2 as 24.15, 28.35 and 27.96 % in comparison to Pb1 stress, whereas at Pb2 + S2, 11.19, 23.83 and 26.00 % in comparison to Pb2 stress, and at Pb3 + S2, 11.92, 19.29 and 18.62 % in comparison to Pb3 stress, at 30, 60 and 90 DAS, respectively.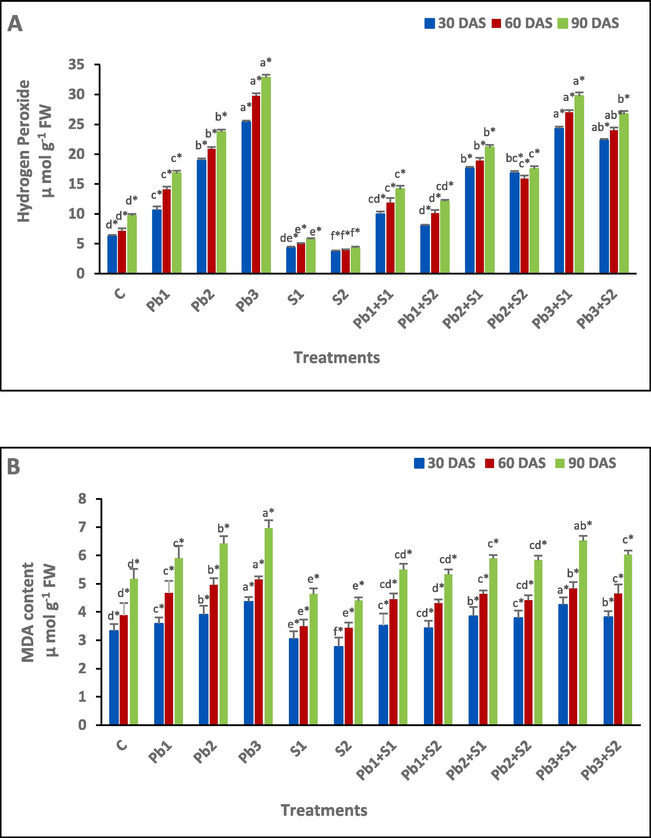
Determination of the impact of exogenous sulphur under lead stress on oxidative biomarkers (A) Hydrogen peroxide; (B) Malondialdehyde (MDA) in Indian mustard. Various letters indicate statistically significant difference (P < 0.05) between treatments within a developmental stage.
3.1.2 Malondialdehyde
The MDA content under 100, 200 and 300 ppm lead stress was increased by 7.51, 17.12 and 30.93 % at 30 DAS, 20.36, 27.84 and 32.73 % at 60 DAS and at 90 DAS, MDA content was enhanced by 14.12, 24.18 and 34.82 %, respectively, in respect to control. S1 spray reduced MDA content by 8.71, 9.79 and 10.25 % and 16.82, 11.34 and 14.89 % at S2 spray at 30, 60 and 90 DAS, respectively, in comparison to control. Sulphur treatment of 100 and 200 ppm to the Pb3 + S1, Pb3 + S2, MDA content showed a decrease of 2.52 and 12.61 % (30 DAS), 6.21 and 9.71 % (60 DAS), 6.46 and 13.49 % (90 DAS), respectively, in respect to Pb3 stress (Fig. 1B).
3.2 Enzymatic antioxidants
3.2.1 Ascorbate peroxidase (APX)
The Pb treatments of 100, 200 and 300 ppm has significantly increased APX activity by 22.75, 33.56 and 57.84 % at 30 DAS, and 14.35, 32.66 and 46.47 % increase at 60 DAS and at 90 DAS 8.34, 15.21 and 25.56 % increase, respectively, compared with control (Fig. 2A). APX activity increased by 14.84, 14.78 and 4.86 % at S1 spray, and 27.60, 23.55 and 7.03 % at S2 spray, respectively, at 30, 60 and 90 DAS, with respect to control. However, S2 enhanced APX activity by 10.40, 7.96 and 7.20 % at Pb1 + S2, and 13.19, 6.86 and 5.90 % at Pb2 + S2, and 8.26, 10.96 and 5.29 % in correspondence to Pb1, Pb2 and Pb3 stress, at 30, 60 and 90 DAS, respectively.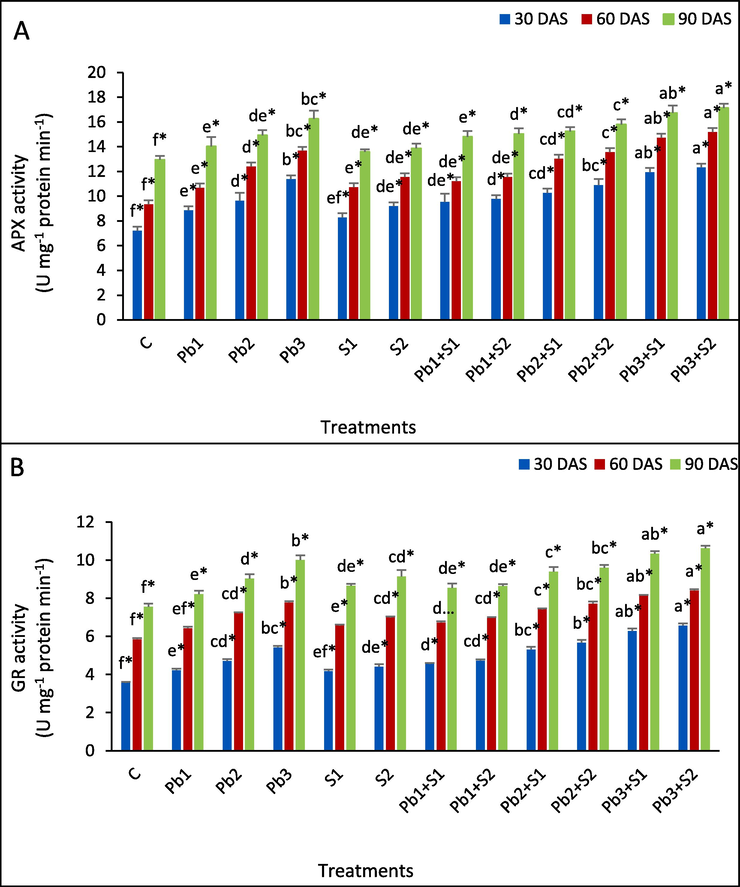
Determination of the impact of exogenous sulphur under lead stress on antioxidative enzymes (A) Ascorbate peroxidase (APX); (B) Glutathione reductase (GR) in Indian mustard leaves. Various letters indicate statistically significant difference (P < 0.05) between treatments within a developmental stage.
3.2.2 Glutathione reductase (GR)
Lead treatments of 100, 200 and 300 ppm significantly increased GR content by 18.82, 32.30 and 51.97 % (30 DAS), whereas 9.91, 23.42 and 32.99 % (60 DAS) and 9.01, 19.74 and 32.45 % (90 DAS) respectively, in comparsion to control (Fig. 2B). S1 spray elevated GR content by 17.13, 12.14 and 14.70 % and S2 spray by 23.88, 19.32 and 21.19 % in comparison to control, at 30, 60 and 90 DAS respectively. S1 spray to Pb-stressed Brassica showed highest enhancement at Pb3 + S1 stress by 16.27, 4.63 and 3.40 % in comparison to Pb3 stress at 30, 60 and 90 DAS, respectively. S2 spray to Pb-stressed Brassica showed an enhancement of 21.44, 7.97 and 6.30 %at Pb3 + S2 in correspondence to Pb3 stress at 30, 60 and 90 DAS, respectively.
3.3 Sulphur-assimilating enzymes
3.3.1 ATP sulfurylase (ATPS)
The ATPS activity was increased by 3.53, 6.96 and 6.11 % (Pb1), 14.12, 13.04 and 10.69 % (Pb2), 21.18, 22.30 and 14.50 % (Pb3) at 30, 60 and 90 DAS, respectively, with respect to control (Fig. 3A). The ATPS activity was augmented upon exogenous S application of 100 and 200 ppm, by 11.76 and 14.12 % (30 DAS), 8.70 and 14.78 % (60 DAS) and by 3.82 and 8.40 % (90DAS), respectively, with respect to control. Further ATPS activity increased under S2 by 7.95, 5.69 and 2.88 % at Pb1 + S2, similarly, 5.49, 4.62 and 3.45 % increase at Pb2 + S2, and 7.77, 4.35 and 7.33 % increase at Pb3 + S2, the increase was in respect to Pb1, Pb2 and Pb3 stress, respectively, at 30, 60 and 90 DAS.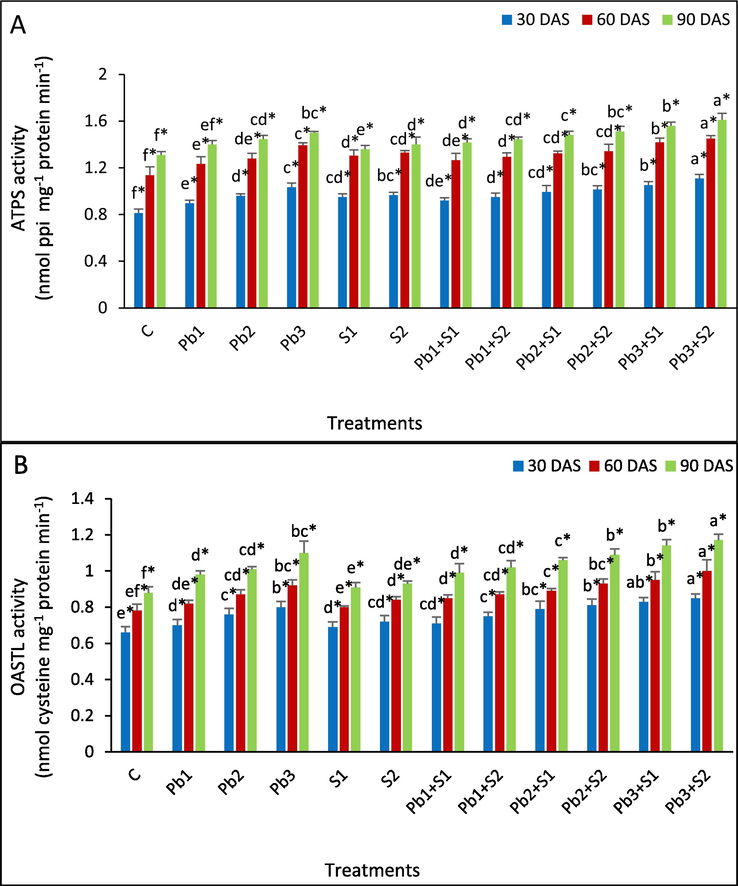
Determination of the impact of exogenous sulphur on S assimilating enzymes (A) ATP-Sulfurylase (ATPS); (B) O-acetylserine (thiol) lyase (OASTL) in Indian mustard leaves under lead stress. Various letters indicate statistically significant difference (P < 0.05) between treatments within a developmental stage.
3.3.2 O-acetylserine (thiol) lyase (OASTL)
Under Pb1, Pb2 and Pb3 stress, OASTL activity increased by 7.58, 15.15 and 21.21 % (30 DAS), 5.13, 11.54 and 17.95 % (60 DAS), 11.36, 14.77 and 25 % (90 DAS), respectively, in comparison to control. At S1 spray, OASTL activity increased by 4.55, 2.58 and 3.41 %, S2 spray showed 9.09, 7.69 and 5.68 % at 30, 60 and 90 DAS, respectively, in respect to control. Highest increase in OASTL activity under S1 spray to lead stressed plants was reported at Pb3 + S1 by 3.75, 3.26 and 3.64 % in respect to Pb3 stress, respectively at 30, 60 and 90 DAS. S2 application to Pb1 + S2 plants, activity increased by 7.14, 6.10 and 1.08 %, in respect to Pb1 stress at 30, 60 and 90 DAS. Similar increase was observed under Pb2 + S2 and Pb3 + S2.
3.4 Non enzymatic antioxidants
3.4.1 Chlorophyll
The graphical representation (Fig. 4A) shows that chlorophyll decreased under lead stress by 11.14, 24.92 and 34.60 % (30 DAS), 4.68, 12.89 and 22.76 % (60 DAS) and 1.59, 7.30 and 15.51 % (90DAS) respectively, at 100, 200 and 300 ppm Pb, in respect to control. Further, S1 and S2 application increased chlorophyll by 9.54 and 16.83 %, 7.98 and 11.53 %, 7.58 and 14.81 %, respectively, at 30, 60 and 90 DAS in comparison to control. Highest increase of chlorophyll content was seen at Pb3 + S1 and Pb3 + S2 treated plants as 7.04 and 8.99 %, 5.49 and 7.48 %, 2.16 and 3.28 % at 30, 60 and 90 DAS, respectively, in comparison to Pb3 stress.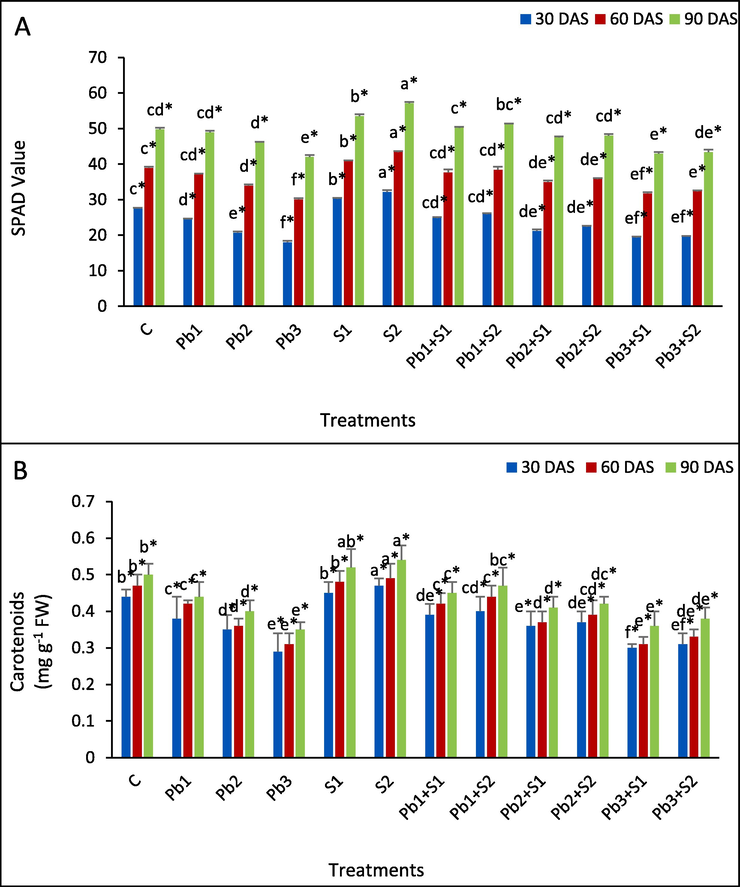
Determination of the impact of exogenous sulphur on non enzymatic antioxidants (A) Chlorophyll; (B) carotenoids in the leaves of Indian mustard under lead stress. Various letters indicate statistically significant differences (P < 0.05) between treatments within a developmental stage.
3.4.2 Carotenoids
Lead treatment of 100, 200 and 300 ppm resulted in decrease (Fig. 4B) of carotenoid content and highest decrease was 12.00, 20.00 and 30.00 % at 90 DAS respectively, in respect to control. Further, sulphur application increased carotenoid content by 2.27, 2.13 and 4.00 % at S1 spray, and at S2 spray 6.82, 4.26 and 8.00 %, increased respectively, at 30, 60 and 90 DAS, in respect to control. S spray to Pb-stressed plants showed an increased carotenoid and highest enhancement of carotenoids was noticed at Pb3 + S2, by 6.90, 6.45 and 8.57 %, respectively, at 30, 60 and 90 DAS, in comparison to Pb3 stressed plants.
3.5 Gene expression analysis
The gene expression of BjSULT showed the highest relative values of 0.95 and 0.91 fold under Pb2 and Pb3 stress respectively, compared to control of 30 DAS. S1 and S2 spray showed 1.82 and 1.95-fold increase at 90 DAS. Sulphur spray of 100 and 200 ppm on Pb3 + S1 and Pb3 + S2 resulted highest gene expression levels of 1.26 and 1.32 fold, respectively, at 90 DAS. The highest BjPb expression was seen at 90 DAS, with 2.42 and 2.83-fold increase under Pb2 and Pb3 stress, compared to control. S1 and S2 spray resulted a decreased BjPb expression. The BjPb expression had greatest reduction at 60 DAS when S2 was applied from a 2.58-fold increase under Pb3 stress to a 2.15-fold drop under Pb3 + S2 stress, relative to the control. The BjABCC expression was highest at 90 DAS, with fold changes of 1.39 and 1.46, respectively, under Pb2 and Pb3 stress, comparison to control. S1 and S2 spray resulted an increased expression 1.39 and 1.41 fold (90 DAS), respectively. Specifically, at 90 DAS, highest expression of BjABCC found to be 1.49 fold higher under Pb3 + S2 stress compared to control. The relative gene expression of BjATPS and BjOASTL increased by 1.78 and 1.84-fold, and 1.63 and 1.69-fold, respectively, under Pb2 and Pb3 stress at 90 DAS compared to control. Further spray of S1 and S2 at 90 DAS, showed a fold increase of 1.73 and 1.85 for BjATPS, and 1.56 and 1.61 for BjOASTL, respectively. Sulphur treatment to Pb-stressed Brassica resulted in greatest expression at 90 DAS, with fold increases of 2.03 and 2.42 at Pb3 + S1 and Pb3 + S2 stress, respectively, compared to the control. The gene BjGR1 exhibited greatest level of gene expression at Pb3 stress as 1.75-fold increase compared to 90 DAS control. The BjGR1 gene showed high upregulation of 1.84 folds change under S2 sulphur spray at 90 DAS. S2 application under Pb3 + S2 stress resulted in the greatest level of BjGR1 expression, with a fold increase of 1.81 at 90 DAS. Fig. 5A-F displays the findings of the investigation of gene expression.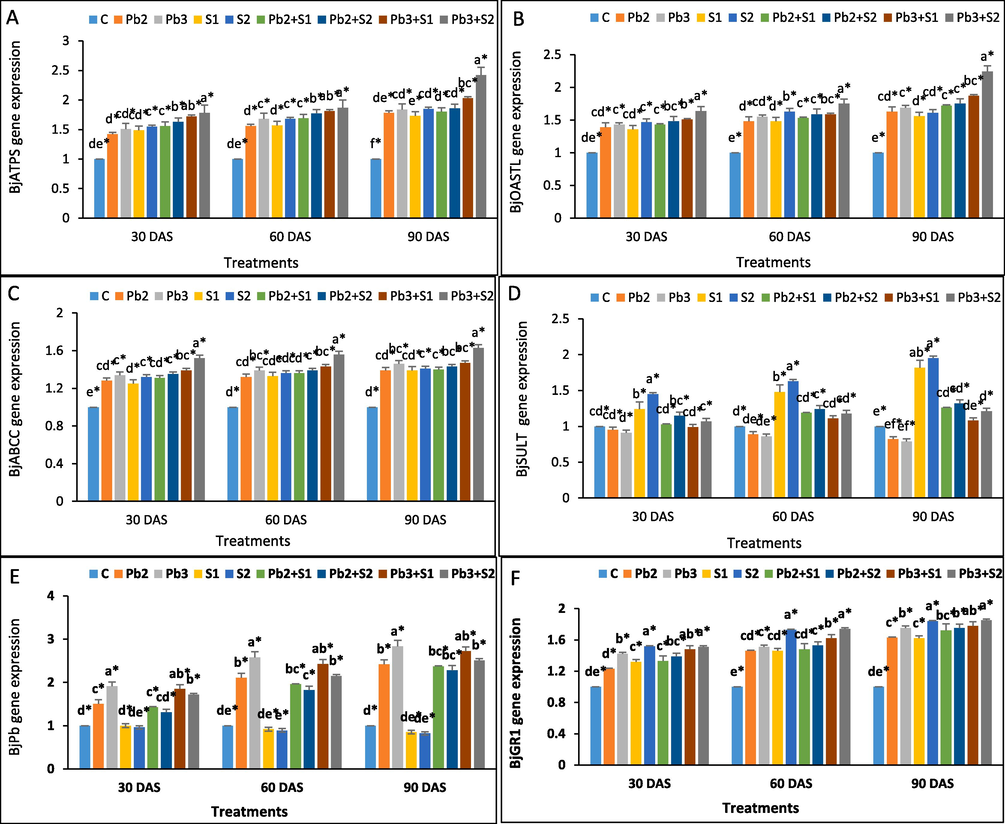
Effects of exogenous sulphur on transcript levels of the six genes encoding sulphur assimilation enzymes in the leaves of Indian mustard under lead stress. Transcript levels of BjSULT, BjABCC, BjATPS and BjOASTL (A,C,D,E) genes play a role in sulphur assimilation pathway, BjPb (B) gene in lead regulation and BjGR1 (F) gene in antioxidative system. Various letters indicate statistically significant differences (P < 0.05) between treatments within a developmental stage.
4 Discussion
The presence of lead has detrimental effects on plant development, impacting several aspects such as physiological, biochemical, and molecular processes (Hasanuzzaman et al., 2018). Brassica is a very effective bioremediant, a nourishing cow supplement, and a valuable reservoir of beneficial phytochemicals such as vitamins and minerals (Xin et al., 2013). Hence, it was imperative to investigate the effects of Pb in Brassica plants and crucial elements involved in maintaining cell structure and the process of assimilating sulphur. The decrease in Brassica development is clear sign of lead poisoning, which can be attributed to elevated levels of Pb that generate ROS. The ROS might have disrupted the delicate balance between the production of ROS and the antioxidant defence mechanism that eliminates ROS (Ahmad et al., 2019). The elevated H2O2 and MDA levels of results corresponds with findings of Bharwana et al. (2013) and Singh et al. (2020) where an increased H2O2 and MDA levels were reported under Pb stress in Gossypium and Brassica juncea. Exogenous sulphur spray to Brassica plants under lead stress resulted in reduction in H2O2 and MDA levels, hence minimising oxidative damage to the cells. The use of sulphur spray effectively reduced the MDA by decreasing the accumulation of H2O2 therefore promoting growth of B. juncea under Pb stress. Sulphur spray has decreased the H2O2 and MDA in rice (Dixit et al., 2016) and maize (Cui and Wang, 2006) plants under cadmium and arsenic stress. The use of zinc sulphate as a foliar spray resulted in decrease of H2O2 and MDA levels in Brassica leaves that were treated with lead as shown in a study conducted by Ahmad et al. (2017). This corroborates usage of zinc sulphate as sulphur supplement to the Brassica plants.
Present study shows that Brassica exposed to lead stress showed an increase in APX and GR activity. The activity of APX increased following the administration of sulphur, indicating that the detoxification of H2O2 was effective. Ahmad et al. (2015) and Liang et al. (2016) reported that addition of S boosted APX activity in Brassica plants that were exposed to Pb and Cd. Sulphur enhanced activities of APX and GR in order to safeguard the leaf tissues of B. juncea from harmful effects of metal stress, as reported by Singh et al. (2020). S2 spray enhanced GR and APX activities in B. juncea. Vicia faba and tartary buckwheat exhibited comparable enhancement of metal stress defence when treated with sulphur (Wu et al., 2018, Lu et al., 2019). This study demonstrates that plants further treated with sulphur have enhanced ability to regulate oxidative stress. Finally this finding provides evidence that application of S enhances the activity of antioxidant enzymes, so offering a feasible approach to enhance plant tolerance against lead contamination.
The present study demonstrates a significant decrease in chlorophyll and carotenoid levels, in response to lead stress. Shahid et al. (2015) observed a reduction in chlorophyll levels in Vicia faba plants under Pb stress. Reduction in Chlorophyll is due to the change of chl central atom magnesium with Pb (Dixit et al., 2016). Exogenous sulphur spray mitigated harmful effects of Pb and enhanced the levels of photosynthetic pigments in Brassica plants, in comparison to plants that were not treated with sulphur spray. Similarly, the administration of S to plants under Pb stress led to an increase in the levels of chlorophyll and carotenoid content. Bharwana et al. (2014) found that treating Gossypium with Pb resulted in a reduction in its carotenoid concentration. The possible rationale for the augmentation of pigments in response to sulphur is the need to stabilize biomolecules using sulfolipids and integrate Fe-S clusters into apoproteins by repairing protein complexes that include Fe-S. Thus sulphur application has enhanced the activity of photosynthetic pigments n Brassica.
Plant life in severe settings relies heavily on the uptake and assimilation of sulphur (Fig. 6). The present investigation found that ATPS and OASTL activities increased slightly when B. juncea was exposed to Pb stress. This study corroborates the previous reports of Brassica juncea L. under Selenium stress (Gupta and Gupta, 2016) and Brassica chinensis L. cadmium stress (Liang et al., 2016). ATPS was the first enzyme to incorporate sulphur in plants (Lappartient and Touraine, 1996), whereas OASTL synthesizes cysteine, a precursor to glutathione (GSH) and other S-containing compounds (Takahashi et al., 2011). The enhancement of ATPS and OASTL enzyme activity under stress is evident with the elevation in transcript level of BjATPS and BjOASTL gene. However, elevated ATPS and OASTL activities in S2 spray, suggest that BjATPS and BjOASTL expression enhances S assimilation in high S-applied Brassica to minimize Pb toxicity. Various research found that sulphur-assimilating ATPS and OASTL enzymes enhanced heavy metal tolerance (Lou et al., 2017). ATPS gene overexpression in Indian mustard increased tolerance to Cd-stress trough phytoextraction (Wangeline et al., 2002). Even while OASTL did not affect S assimilation, overexpression of various OASTL isoforms in metal-stressed plants increases Cd tolerance (Nakamura et al., 2014). The results of the present study shows an increased BjSULT expression under lead stress and sulphur translocation in plants depends on the SULT gene (Takahashi et al., 2000) and found that sulfate-assimilating transcriptional genes such SULTR1;1 and SULTR2;2 help plants to translocate sulphur. Hence, we can conclude that increased BjATPS and BjOASTL expression under Pb stress was due to increased sulphur translocation through BjSULT transporters. Further, sulphur spray to stressed plants increased sulphur assimilation through BjSULT transporters and paved way for the enhancement of BjATPS and BjOASTL genes to ameliorate the lead toxicity.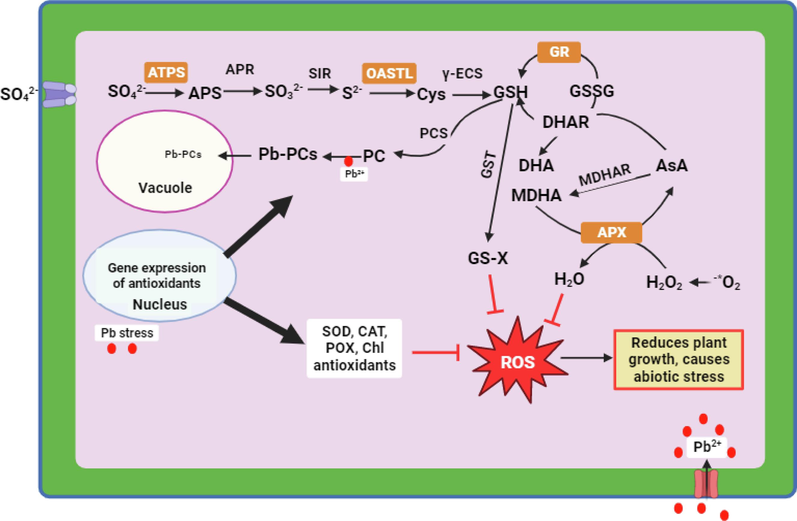
Schematic presentation of antioxidant defense mechanism and sulphur role in regulating lead accumulation and tolerance. SO42-available form of sulphur, ATPS-ATP sulfurylase, APS-adenosine 5′-phosphosulfate, APR-APS reductase, SO32--sulfite, SIR-sulfite reductase, S2--sulfide, OASTL-O-acetyl serine(thiol)lyase, Cys-cysteine, γ-ECS-γ-glutamylcsyteine synthetase, GSH-reduced glutathione, GSSG-oxidized glutathione, GR-glutathione reductase, PCS-phytochelatin synthetase, PC-phytochelatins, Pb-PCs-lead associated with phytochelatins, GST-glutathione-S-transferase, GS-X-glutathione containing compounds, DHAR-dehydroascorbate reductase, DHA-dehydroascorbate, AsA-ascorbate, MDHA-mono dehydroascorbate, MDHAR-mono dehydroascorbate reductase, APX-ascorbate peroxidase, H2O-water, H2O2-hydrogen peroxide, •2O2-superoxide anion, ROS-reactive oxygen species, SOD-superoxide dismutase, CAT-catalase, POX-peroxidase, Chl-chlorophyll, Pb-lead, Pb2+-ionic form of lead.
The current study found that BjPb expression increased under Pb stress and showed a reduced expression under sulphur spray, indicating that sulphur spray reduced lead transport into the plant's cellular system and increased Pb tolerance through the augmentation of BjATPS and BjOASTL genes. In present study, expression of BjABCC vacuole transporter enhanced under Pb stress. Exogenous sulphur spray to the lead stressed plants, increased BjABCC transporter expression resulting more sequestration of lead into the vacuoles. Similar results were found upon S supplementation to P. deltoides where, ABCC1.1 transporter sequestered PCs-Pb into the vacuole (Ma et al., 2018). The results of increased GR activity correlated with the increased BjGR transcript under lead stress. Similar, enhanced GR expression results was observed by Kaur et al. (2018) in B. juncea to various salt stress. In present study, sulphur spray to lead treated plants exhibited an increased expression of BjGR transcripts. Sulphur spray has mitigated the ROS content by enhancing gene transcript levels of BjGR in Cd stressed B. chinensis (Lou et al., 2017). Enhanced lead content entered plants through BjPb transporters and led to increased ROS. Sulphur application in present study might be the possible reason for the enhanced expression of BjGR and have lowered ROS and increased efficiency of photosynthetic pigments. Further sulphur treatment, S entered plants through BjSULT transporters and incorporated sequestration of Pb by enhancing BjATPS and BjOASTL level. This significant change in the alteration of S-assimilation pathway resulted in the excess sequestration of Pb through S-mediating enzymes and might be the reason for the reduced Pb content under sulphur spray.
5 Conclusions
In the current study, high sulphur application has resisted the Pb toxicity by decreasing the stress biomarkers and enhanced plant growth by strengthening antioxidant defense system. These data demonstrated that sulphur supplementation fine-tuned Brassica plants response to excess Pb including enhanced control of Pb distribution and ROS removal. Further S application has also alleviated the sulphur assimilating enzymes and sulfur related genes; therefore, S played a major role in maintaining the redox status of the cell. Furthermore, experiments are needed to explore the S combinational secondary metabolites at proteomic expression level in plants under different stress condition.
Declaration of Funding: No external funding.
Ethics approval: Not applicable.
CRediT authorship contribution statement
Hemanthkumar Manne: Conceptualization, Formal analysis, Methodology, Writing – original draft. Nisha Kumari: Data curation, Project administration, Supervision. Shikha Yashveer: Methodology, Supervision, Visualization. Sonia Nain: Investigation, Resources, Writing – review & editing. Jyoti Duhan: Visualization, Writing – review & editing. Ram Avtar: Investigation, Software. Sushil: . Minakshi Jattan: Investigation, Methodology. Babita Rani: Software, Validation. Abdulaziz Abdullah Alsahli: Conceptualization, Writing – original draft, Writing – review & editing. Sajid Ali: Methodology, Validation.
Acknowledgement
The research was financially supported by the Oilseeds section, Department of Genetics and Plant breeding and Department of Biochemistry, CCSHAU, India. The authors would also like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2024R236), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alleviation of cadmium toxicity in Brassica juncea L. (Czern & Coss) by calcium application involves various physiological and biochemical strategies. PLOS One.. 2015;10:0114571.
- [Google Scholar]
- Zinc application mitigates the adverse effects of NaCl stress on mustard [Brassica juncea (L.) Czern & Coss] through modulating compatible organic solutes, antioxidant enzymes, and flavonoid content. J. Plant Interact.. 2017;12:429-437.
- [Google Scholar]
- Revisiting the role of ROS and RNS in plants under changing environment. Environ. Exp. Bot.. 2019;161:1-3.
- [Google Scholar]
- Phytoremediation of soil contaminated with Zn using Canola (Brassica napus L.) Eco. Eng.. 2016;95:43-49.
- [Google Scholar]
- Cadmium exposure affects iron acquisition in barley (Hordeum vulgare) seedlings. Physiol. Plant.. 2014;152:646-659.
- [Google Scholar]
- Alleviation of lead toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes suppressed lead uptake and oxidative stress in cotton. J. Bioremed. Biodeg.. 2013;4:187.
- [Google Scholar]
- Glycine betaine-induced lead toxicity tolerance related to elevated photosynthesis, antioxidant enzymes suppressed lead uptake and oxidative stress in cotton. Turkish J. Bot.. 2014;38:281-292.
- [Google Scholar]
- The Arabidopsis ethylene-insensitive 2 gene is required for lead resistance. Plant Physiol. Biochem.. 2009;47:308-312.
- [Google Scholar]
- Physiological responses of maize to elemental sulphur and cadmium stress. Plant Soil Environ.. 2006;52:523-529.
- [Google Scholar]
- Reduced arsenic accumulation in rice (Oryza sativa L.) shoot involves sulfur mediated improved thiol metabolism, antioxidant system and altered arsenic transporters. Plant Physiol. Biochem.. 2016;99:86-96.
- [Google Scholar]
- Alleviation of selenium toxicity in Brassica juncea L.: salicylic acid mediated modulation in toxcity indicators, stress indicators and sulfur-related gene transcripts. Protoplasma. 2016;253(6):1515-1528.
- [Google Scholar]
- Melatonin inhibits cadmium translocation and enhances plant tolerance rgulating sulfur uptake and assimilation in Solanum lycopersicum L. J. Agril. Food Chem.. 2019;67(38):10563-10576.
- [Google Scholar]
- Exogenous glutathione attenuates lead-induced oxidative stress in wheat by improving antioxidant defense and physiological mechanisms. J. Plant Interact.. 2018;13:203-212.
- [Google Scholar]
- Photoperoxidation in isolated chloroplasts kinetics and stoichiometry of fatty acid peroxidation. Archi. Biochem. Biophys.. 1968;125:189-198.
- [Google Scholar]
- Homobrassinolide regulates antioxidant enzyme activities and gene expression to salt and temperature-induced oxidative stress in Brassica juncea. Sci. Rep.. 2018;8:8735.
- [Google Scholar]
- Characterisation of calmodulin binding to cyclic nucleotide-gated ion channels from Arabidopsis thaliana. FEBS Lett.. 2000;471:133-136.
- [Google Scholar]
- Assessment of subcellular ROS and NO metabolism in higher plants: multifunctional signaling molecules. Antioxidants.. 2019;8(12):641.
- [Google Scholar]
- Demand-driven control of root ATP sulfurylase activity and SO42−uptake in intact canola. Plant Physiol.. 1996;111:147-157.
- [Google Scholar]
- Sulfur decreases cadmium translocation and enhances cadmium tolerance by promoting sulphur assimilation and glutathione metabolism in Brassica chinensis L. Ecotoxicol. Environ. Safety.. 2016;124:129-137.
- [Google Scholar]
- Sulfur protects Pakchoi (Brassica chinensis L.) seedlings against cadmium stress by regulating ascorbate-glutathione metabolism. Int. J. Mol. Sci.. 2017;18:1628.
- [Google Scholar]
- Effects of exogenous sulfur in alleviating cadmium stress in tartary buckwheat. Sci. Rep.. 2019;9:7397.
- [Google Scholar]
- Sulfur nutrition stimulates lead accumulation and alleviates its toxicity in Populus deltoides. Tree Physiol.. 2018;38:1724-1741.
- [Google Scholar]
- The role of sulfur assimilation and sulfur containing compounds in trace element homeostasis in plants. Environ. Exp. Bot.. 2011;72:18-25.
- [Google Scholar]
- An improved tolerance to cadmium by overexpression for cysteine synthesis in tobacco. Plant Biotechnol.. 2014;31:141-147.
- [Google Scholar]
- Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol.. 1981;22:867-880.
- [Google Scholar]
- Impact of reduced acetylserine (thiol) lyase isoform contents on potato plant metabolism. Plant Physiol.. 2005;137:892-900.
- [Google Scholar]
- Role of metal speciation in lead-induced oxidative stress to Vicia faba roots. Russ. J. Plant Physiol.. 2015;62:448-454.
- [Google Scholar]
- Sulphur and calcium attenuate arsenic toxicity in Brassica by adjusting ascorbate-glutathione cycle and sulphur metabolism. Plant Growth Regul.. 2020;91:221-235.
- [Google Scholar]
- Song, W.Y., Park, J., Mendoza-Cozatl, D.G., Suter-Grotemeyer, M., Shim, D., Hortensteiner, S., Geisler, M., Wede, B., Rea, P.A., Rentsch, D., Schroeder, J.I., Lee, Y., Martinoia, E., 2010. Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. pp. 21187–21192.
- The roles of three functional sulphate transporters involved in uptake and translocation of sulphate in Arabidopsis thaliana. Plant J.. 2000;23:171-182.
- [Google Scholar]
- Sulfur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes. Ann. Rev. Plant Biol.. 2011;62:157-184.
- [Google Scholar]
- Oxidative stress and some antioxidant system in acid rain treated bean plants. Plant Sci.. 2000;151:59-66.
- [Google Scholar]
- Overexpression of ATP sulfurylase in Indian mustard. J. Environ. Qual.. 2002;33:54-60.
- [Google Scholar]
- spectral determination of chlorophyll a, b, as well as total carotenoids using various solvents with spectrophotometer different resolution. J. Plant Physiol.. 1994;144:303-313.
- [Google Scholar]
- Sulfate supply enhances cadmium tolerance in Vicia faba L. Plants.. 2018;25:33794-33805.
- [Google Scholar]
- Studies on Brassica carinata seed. Carbohydrate molecular structure in relation to carbohydrate chemical profile, energy values, and biodegradation characteristics. J. Agri. Food Chem.. 2013;61:10127-10134.
- [Google Scholar]







