Translate this page into:
Mitigating the negative effects of lead toxicity on Vigna mungo: The promising role of rhizobacteria
⁎Corresponding author. shaima.6337@wum.edu.pk (Syeda Shaima Meryem),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Growing Vigna mungo in soils contaminated with lead (Pb) is a major concern due to its negative impact on plant growth and development. When lead enters through food in the human body, it can be accumulated in bones, liver, and kidneys. In severe cases, lead poisoning can cause cancer, seizures, coma, and even death. To address this issue in plants, phosphate-solubilizing bacteria (PSB) have been identified as a potential solution to enhance plant growth and mitigate the harmful effects of heavy metal toxicity. However, the effectiveness of PSB can be improved by isolating and characterizing new strains over time, which will aid in developing sustainable management strategies for Pb-contaminated soils. Thus, the current study aimed to investigate the impact of Pb contamination on V. mungo growth and the potential use of newly isolated microbial strains to enhance V. mungo growth and immobilize Pb. Three levels of Pb contamination were used: control (no Pb toxicity), 125Pb (125 mg Pb/kg soil), and 250Pb (250 mg Pb/kg soil). Six different microbial strains, RP01, RP02, RP03, RP04, RP05, and RP06, were used to inoculate V. mungo seeds. The results of the experiment demonstrated that RP02 was the most effective strain in improving various growth parameters such as shoot length, root length, seedling length, fresh weight, dry weight, chlorophyll a, chlorophyll b, and total chlorophyll. While RP03 also showed significant improvement in these growth parameters, its effectiveness was not as high as RP02 in some respects. The efficacy of RP02 in alleviating Pb toxicity was further confirmed by a significant decrease in Pb concentration in the seedling, which was attributed to the production of siderophore. Therefore, it can be concluded that RP02 has the potential to improve V. mungo growth under Pb contamination. However, further field investigations are needed to confirm RP02 as the best strain for enhancing V. mungo production under Pb toxicity.
Keywords
Rhizobacteria
Heavy metals
Chlorophyll contents
Growth attributes
Catalase
Citrate
1 Introduction
Macronutrients are essential for plant growth and agricultural production. A lack of any one of the 17 varieties of micronutrients may have detrimental effects on plant growth and yields. The three main macronutrient compositions are potassium, nitrogen, and phosphorous. Sulfur, calcium, and magnesium are essential minerals, while boron, iron, copper, manganese, zinc, molybdenum, and nickel are examples of elements. These elements are found in the reservoirs of carbon, hydrogen, and oxygen, including air and water (Malhotra et al., 2018).
Phosphorus (P) is particularly important for plant growth and development, as all plant cells contain phosphorus and can convert sunlight into development and upkeep (Malhotra et al., 2018). Inadequate P levels can hinder plant growth, delay maturation, and decrease production (Toleikiene et al., 2021). Both the respiration of plants and the fixing of nitrogen involve using phosphorus. However, despite the significant amount of total P in soil, only a small portion of it (1% in alkaline soil) dissolves, with around 17% of phosphate minerals being available in the soil (Billah et al., 2019).
On the other hand, among toxic heavy metals (Sheikh et al., 2023), lead (Pb) toxicity is a serious issue that can have detrimental effects on both human health and crop growth (Anwar et al., 2022). Pb is a naturally occurring element, but human activities such as mining, smelting, and industrial processes have led to an increase in its presence in the environment (Gillani et al., 2021). Pb can enter the soil through various pathways including the use of Pb-based pesticides, industrial emissions, and the disposal of lead-containing waste (Xiong et al., 2021). Once present in soil, Pb can be taken up by plants and enter the food chain, potentially impacting human health. Pb toxicity in soil can also have negative effects on crop growth and development, reducing yield and impacting the nutritional quality of the crop (Kaya and Eryiğit, 2021). Long-term exposure to Pb can cause damage to the nervous system, brain, and kidneys in humans, as well as developmental delays in children. Therefore, finding ways to mitigate the negative effects of Pb toxicity is crucial in order to protect human health and ensure sustainable crop cultivation (Duruibe et al., 2007).
V. mungo, also known as black gram, is a widely cultivated crop in tropical and subtropical regions. It is a staple food crop in many parts of Asia and is considered to be a valuable source of protein, carbohydrates, vitamins and minerals (Shabnum Shaheen, 2012). V. mungo is an excellent source of plant-based protein, with approximately 25 g of protein per 100 g of cooked dal. In addition, it is also rich in dietary fiber, which is essential for maintaining a healthy digestive system and regulating blood sugar levels (Shenoy et al., 2022). Furthermore, this legume is a good source of several important vitamins and minerals, including iron, magnesium, potassium, and folate. These nutrients are essential for maintaining overall health, as iron is necessary for hemoglobin production, magnesium and potassium are important for maintaining healthy muscles and nerves, and folate is essential for fetal development and can reduce the risk of certain birth defects (Shenoy et al., 2022). Additionally, V. mungo is low in fat and calories, making it an excellent food choice for individuals who are trying to lose weight or maintain a healthy weight. Overall, V. mungo is a nutritious and versatile food that can be an important part of a healthy diet (Shenoy et al., 2022). However, the cultivation of V. mungo is often affected by lead (Pb) toxicity due to the widespread use of Pb-based pesticides and industrial activities that lead to soil contamination (Siddiqui, 2012).
One potential solution to this issue is the use of phosphate solubilizing bacteria (PSB) for the management of phosphorus deficiency in plants. PSB are microorganisms that release soluble P from insoluble forms in the soil, increasing the availability of P for plants (Adnan et al., 2020). They have been shown to enhance plant growth and increase crop yields in low P soils (Toleikiene et al., 2021). The use of PSB as a management strategy for P deficiency in low P soils, as well as the isolation of new strains that can solubilize P in alkaline soils, is an area of ongoing research (Bibi et al., 2020). Therefore, there is a fundamental necessity to develop more effective techniques for employing phosphorus fertilizers in agricultural environments with low phosphorus levels, in addition to making their use financially viable. This research aims to investigate new isolation of PSB strains for the management of P deficiency in low-phosphorus level agricultural soils and alleviation of heavy metal Pb toxicity in crops. It is hypothesized that isolation and inoculation of new PSB strains in low-phosphorus level soils might improve P availability and crop growth while also reducing heavy metal stress on plants (see Fig. 1).
Bacterial isolates used in current study.
2 Materials and methods
2.1 Sampling and isolation of rhizobacteria
Specimens were gathered from factory area i.e., (feed mill; 30.2669425 N, 71.5828924E having Pb 0.28 mg/g soil) which was agricultural lead contaminated site. Initially, the temperature difference was measured using a handheld temperature gauge; next, samples were collected from a depth of 15 cm in a neat polyethylene sterilized bag; as well as finally, fully grown crop plants were uprooted and chosen to take towards the lab, where the roots were shaken, and indeed the soil was gathered using a clean spatula (Enya et al., 2019). For isolation of rhizobacteria, 1 g soil sample was removed from roots. The soil sample was taken in test tube and final volume was made 5 mL using sterilized water. Serial dilutions were made by taking 1 mL in each successive test tube and final volume was also maintained 5 mL. For culturing of rhizobacteria strains, streaking was done on Tryptone Soya Agar in zig-zag manner. Petri-dishes were incubated at 37 °C ± 2 °C for 24 h.
2.2 Soil characterization
The pH of the soil paste (1:1 soil and deionized water) was measured using the Inolab PH71110 vendor procedure (Page et al., 1983). The electrical conductivity of the soil extract (1:10 soil and deionized water) was measured using an automatic EC meter (Rhoades, 1996). The size distribution of the soil was determined through moisture screening and the hydrometer technique (Gee and Bauder, 1986). Potassium dichromate was used as oxidizing agent for the analysis of soil organic matter. Final titration was done with 0.5 M ferrous ammonium sulphate (Nelson and Sommers, 1982). For analysis of soil phosphorus Olsen reagent was used. Final value was computed on spectrophotometer (Kuo, 1996). To test potassium, ammonium acetate extracting reagent was used. Finally, flamephotometer was utilized for the computation of potassium in soil samples. The characteristics of soil are provided in Table 1.
No.
Attributes
Units
Value
1
pHs
–
8.01
2
EC
dS/m
2.82
3
Organic Matter
%
0.65
4
Available Phosphorus
µg/g
11.9
5
Extractable Potassium
µg/g
209
6
Sand
%
40
7
Silt
40
8
Clay
20
9
Texture
–
Loam
2.3 Rhizobacteria biochemical and morphological characterization
The bacteria were separated on an agar medium (Tryptone Soya Agar) and single colony were grown. The morphological, natural, biological, and chemical characteristics of the separated colonies were noted. The size, shape, color, transparency, edge, height, roughness, gram staining, and cell morphology were examined under a microscope (Gang et al., 2019) (Table 2).
Morphological Attributes
No.
Strains
Colony
Gram Staining
Color
Shape
Margins
Virtual
Texture
1
RP01
Cocci
-ve
Light yellow
Irregular
Undulate
Umbonate
Sticky
2
RP02
Bacilli
+ve
White
Irregular
Rhizoidal
Flat
Sticky
3
RP03
Bacilli
+ve
White
Irregular
Undulate
Umbonate
Sticky
4
RP04
Cocci
+ve
Red-offwhite
Circular
Undulate
Convex
Creamy
5
RP05
Cocci
+ve
Off white
Irregular
Undulate
Domed
Creamy
6
RP06
Cocci
-ve
Off white
Irregular
Undulate
Umbonate
Sticky
Biochemical Attributes
No.
Strains
Catalase
Citrate
MacConkey
IAA (µg/mL)
P- Solubilize
Biofilm Formation
Sidero-phore
1
RP01
+
+
-ve
0.91
+
–
+
2
RP02
+
+
Not able to grow
17.21
+
+
+
3
RP03
+
+
Not able to grow
13.43
+
+
+
4
RP04
+
+
Not able to grow
8.22
+
–
+
5
RP05
+
+
Not able to grow
9.41
+
–
+
6
RP06
+
+
-ve
11.96
+
+
+
2.4 Gram staining
To examine the cell structure, pure cultures were collected for 24 h. Gram's stain was used to examine the Gram response, cellular shape, and distribution of the cells. The stain was used to color the Gram segments on probiotic strains. A smear was made on the slide by mixing a small amount of the bacterial culture and spreading it on a small drop of water, then air-drying and heat-fixing it. Then, a droplet of crystal violet was added for 60 s, rinsed with distilled water, and then a droplet of iodine was added for one minute, followed by treatment with 70% ethanol for 4–5 min. Finally, a droplet of safranin was added, washed with distilled water, dried by air, and a small amount of absorbent was added to the smear before placing the coverslip. The colored stain was observed under a microscope with a 100 × lens (Holt et al., 1994) (Table 2).
2.5 Catalase test
A small sample of bacteria was taken from a cleaned colony and placed on a small, sterile glass slide for inoculation. The sample does not need to be grown on agar. Using a dropper or pipette, a droplet of H2O2 (%) was added to the microorganism on the glass slide. If bubbles form, it indicates a positive result for the catalase test. A weak result would show a small bubble and a negative result would show no or very few bubbles even after several tries (Das et al., 2018) (Table 2).
2.6 Test citrate
In the present study, the citrate test was employed to determine the ability of a specific bacterial strain to utilize citrate as a source of carbon and energy. The procedure involved inoculating a Petri plate with a culture of the strain and incubating it at room temperature for 16–18 h. A single colony was then streaked onto a citrate agar medium and incubated for 48 h at 35 °C. The presence of alkaline byproducts, such as carbonates and bicarbonates, produced during the hydrolysis of citrate caused the pH of the medium to rise, resulting in a change of color from green to blue. A positive result was indicated if the bacteria was able to utilize citrate, while a negative result was indicated if the bacteria was unable to utilize citrate. It should be noted that some microorganisms may take up to seven days to show visible growth. To avoid using liquid cultures as the inoculant supply and to limit the number of cells delivered to the agar slant, a syringe was used. The screw covers on the tube were loosely placed to allow oxygenation of the media (Table 2).
2.7 Test MacConkey
49.53 g of MacConker agar were suspended in 1000 mL of distilled water and mixed thoroughly before being heated to boiling point. The medium was then sterilized in an autoclave for 15 min at 121 °C and 15 lb of force. Once the temperature dropped to 45–50 °C, the medium was mixed again and poured into sterilized Petri dishes. This medium was used to identify Gram-negative microorganisms by differentiating between those that ferment lactose and those that do not, based on colony characteristics. Countercultures were carried out using biochemical tests to ensure accurate results. It should be noted that some strains may not have grown or thrived well on this substrate, which could have lowered the CO2 level during incubation of the Macconkey agar medium (Hinenoya et al., 2020) (Table 2).
2.8 Test for indole acetic acid
A colony of a strain of glycerol stock was introduced into the pre-culture medium. The pre-culture medium was incubated at 30 °C and 120 rpm to generate a new culture for isolating the liquid used to test for indole acetic acid (IAA). 100micro l of the new culture was added to a test tube and vortexed to create a homogenous solution. The test tube was then incubated in the dark at 120 rpm and 30 °C (covered with aluminum foil). The length of incubation was determined by the strain's ability to produce IAA (24–96 h). The production of IAA by the strain typically lasts for 72 h. After 24 h, 1.5 mL of the broth culture sample was taken and placed in an Eppendorf test tube. It was centrifuged for 5 min at 16,278 g using a mini centrifuge. Carefully, 1 mL of the pellet was transferred to a new test tube. The same amount of Salkowski reagent was added, mixed vigorously and then incubated in the dark at 30 °C for 30 min. The contents of the tube were combined with 1 mL of the Salkowski reagent and 1 mL of the control uninoculated media. After 30 min, the appearance of pinkish or red tints indicates the presence of IAA (Gang et al., 2019) (Table 2).
2.9 Biofilm formation
10 mL of NB medium with different NaCl concentrations (0, 50, 100, and 150 mM) were inoculated with a loop full of bacteria strains into test tubes. The test tubes were incubated at 28 °C for two days. After that, the excess was removed and discarded, and the test tubes were rinsed with phosphate buffer saline (pH 7). The test tubes were then stained for 15 min using 0.1% crystal violet, and biofilm development was measured by observing the tubes. The colored glassware was rinsed with distilled water after being dyed (Table 2).
2.10 Test for phosphate solubilization
In the present study, a solitary bacterial colony was selected and streaked onto Pikovskaya's agar plates containing tricalcium phosphate. The plates were incubated at 37 °C, and after the colonies began to form halos or regions surrounding them, they were used to isolate strains of bacteria. Screening conditions were then employed to evaluate and compare the ability of the isolated strains to solubilize phosphorous (P) from the tricalcium phosphate in the agar medium (Chawngthu et al., 2020) (Table 2).
2.11 Seeds collection, sterilization and sowing
Seeds of V. mungo (NM-98) were collected from local certified Punjab Seed Corporation dealer. Initially debris or non-seed material were removed manually. After that seeds were surface sterilized by applying sodium hypochlorite (5%) solution for 10 min. Finally seeds were rinsed several times with sterile distilled water to remove any remaining bleach solution. On 23–06-2022, 15 sterilized seeds were placed in each pertri dish. Ten mL of inoculum was poured on seeds having optical density maintained 0.5 which was maintained at 535 nm wavelength (Nadeem et al., 2009).
2.12 Nutrition
Standard Hoagland solution was made. After that 2 mL of solution was added in each petri dish for provision of macro and micronutrients (Hoagland and Arnon, 1950).
2.13 Harvesting and data collection
The data for seeds germination was collected after 7 days. Seedlings from each petri dish were harvested after 28 days of sowing. Morphological attributes i.e., vigor index, seedlings length, shoot length, root length, seedlings fresh and dry weight were recorded soon after harvesting.
2.14 Chlorophyll contents
Ten mL of 80% acetone was added in a piston mortar and 0.5 g fresh leaf sample was grinded. Filtration was done to remove residues of tissues. After that final volume was made 10 mL using 80% acetone. The mixture was allowed to sit in the dark for 30 min to allow the chlorophyll to dissolve. After 30 min, the absorbance of the solution at 663 nm and 645 nm was measured using a spectrophotometer and recorded (Arnon, 1949).
2.15 Lead analysis
Dry leaf samples were digested by using di-acid mixture (nitric and perchloric acid) at 280 °C using hot plate (Miller, 1998). After dilutions of samples 1000 times samples were run on pre-calibrated atomic absorption spectrophotometer and data was collected for Pb concentration (Hanlon, 1998).
2.16 Statistical analysis
A statistical analysis was conducted to evaluate the experimental results (Steel et al., 1997). A Two-way factorial ANOVA was performed to evaluate the effects of multiple factors on the response variable. The Fisher LSD test was utilized to determine the significant differences between the treatments. The results of the statistical analysis were further visualized using Principal Component Analysis (PCA) and parallel plots generated using Origin 2021 software (OriginLab Corporation, 2021). Paired comparisons were also conducted using Origin 2021 to identify specific differences between the treatments.
3 Results
The influence of different phosphate solubilizing bacterial strains (RP) under variable toxic levels of lead (Pb) was significant on the germination, vigor index, shoot and root length. Treatment RP02 and RP03 were statistically similar and caused significant enhancement in germination compared to RP01, RP04, RP05, and RP06 under control Pb (0Pb = 0 mg/kg Pb). However, RP01 and RP04 also remained significantly better than RP05 and RP06 for an increase in germination under control Pb. In the case of 125Pb, RP02 and RP03 differed significantly better than RP05 and RP06 for enhancement in germination. Treatments RP01, RP04, RP05, and RP06 caused no significant change in germination when compared with each other under 125Pb. Furthermore, the performance of RP02 and RP03 remained significantly best at 250Pb over RP01, RP04, RP05, and RP06 for germination percentage. Maximum enhancement in germination was observed where RP02 was inoculated under control, 125Pb and 250Pb than RP06, respectively. Among the six microbial strains tested, RP02 showed the highest percentage increase in plant growth parameters, with an average of 93.89% for shoot length, followed by RP03 with 93.28%, and RP01 with 92.11%. In contrast, RP06 showed the lowest percentage increase in plant growth parameters with an average of 86.94% for shoot length. Furthermore, when the microbial strains were tested under different concentrations of lead (125Pb and 250Pb), the percentage increase in plant growth parameters decreased with increasing concentrations of lead. For instance, inoculation with RP02 resulted in an average percentage increase in shoot length of 86.66% and 81.11% under 125Pb and 250Pb, respectively. In contrast, inoculation with RP06 resulted in an average percentage increase in shoot length of 84.72% and 70.22% under 125Pb and 250Pb, respectively (Fig. 2A).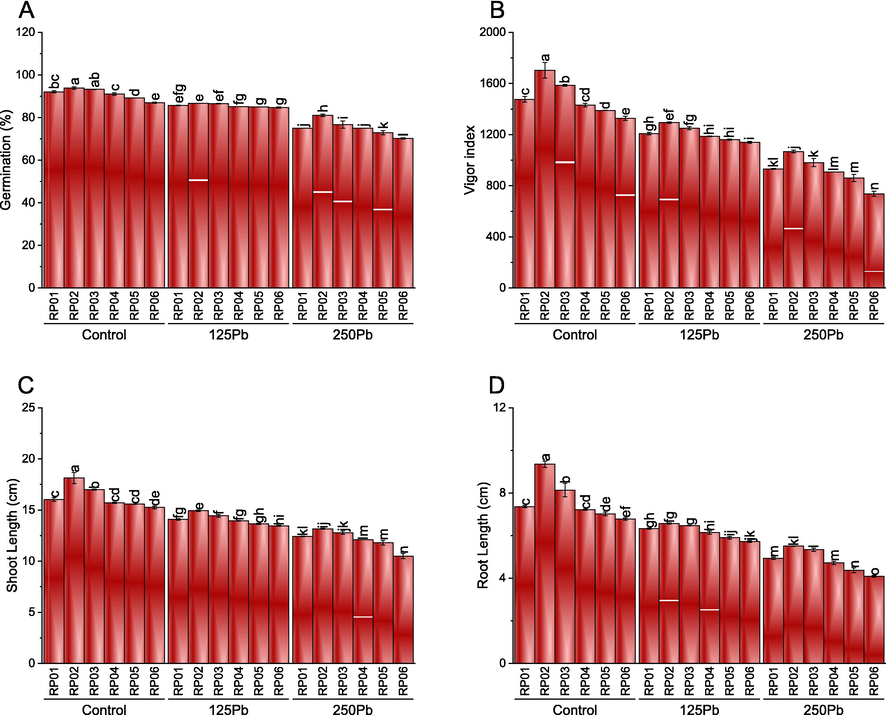
Effect of different PSB rhizobacterial isolates (RP01, RP02, RP03, RP04, RP05 and RP06) on germination (A), vigor index (B), shoot length (C) and root length (D) of V. mungo cultivated under control Pb, 125Pb and 250Pb lead toxicity. Bars are means of six replicates ± SE. Different letters are showing significant alteration at p ≤ 0.05; Fisher LSD. RP01:RP01, RP02:RP02, RP03:RP03, RP04:RP04, RP05:RP05 and RP06:RP06.
For vigor index, treatment RP02 remained significantly better than all other treatments when applied at control Pb. Application of RP03 also caused a significant change in vigor index compared to RP01 at control Pb. However, no significant change was observed where RP04 was applied than RP01 under control Pb. A significant decline in the vigor index was observed in RP05 and RP06 over RP01 under control Pb. At 125Pb and 250Pb, only RP02 caused significant enhancement in the vigor index compared to RP01. No significant variation was noted among RP01, RP03, and RP04 for vigor index under 125 Pb and 250 Pb. However, RP06 caused a significant decline in vigor index compared to RP01 under 125Pb and 250Pb. Among the six strains tested, RP02 showed the highest percentage increase in the vigor index with a 43.07% increase, followed by RP03 with a 33.86% increase, and RP01 with a 25.84% increase. In contrast, RP06 showed the lowest percentage increase in the vigor index with only an 0.09% increase. Under heavy metal stress conditions, the percentage increase in the vigor index decreased with increasing concentrations of lead. For example, inoculation with RP02 resulted in a 15.26% and 24.76% increase in the vigor index under 125Pb and 250Pb, respectively. On the other hand, inoculation with RP06 resulted in a 0.44% and 44.31% increase in the vigor index under 125Pb and 250Pb, respectively (Fig. 2B).
In the case of shoot length, RP02 and RP03 differed significantly better than RP01 under control Pb. The addition of RP06 caused a significantly decreased shoot length than RP01 under control Pb. However, RP01, RP04, and RP05 remained statistically similar to each other under control Pb for shoot length. The highest shoot length was observed in the plants inoculated with RP02, which showed an increase of 13.05% compared to the control group. Similarly, the plants inoculated with RP03 and RP01 showed an increase of 6.67% and 5.49%, respectively, compared to the control group. In contrast, the lowest shoot length was recorded in plants inoculated with RP06, showing a decrease of 34.38% compared to the control group. Plants treated with 125Pb and 250Pb showed a decrease in shoot length compared to the control group. The decrease in shoot length was more prominent in plants treated with 250Pb, showing a decrease of 34.61% compared to the control group (Fig. 2C).
Results showed that RP02 and RP03 caused significant enhancement in root length compared to RP01 under control Pb. No significant alteration was present among RP01 and RP04, yet RP05 and RP05 caused a significantly lower root length than RP01 under control Pb. Application of RP02, RP03, RP04 and RP01 showed no significant change in root length under 125Pb. However, RP05 and RP06 caused a significant decrease compared to RP01 in root length under 125Pb. Under 250Pb, RP02 and RP04 were significantly better than RP01 for enhancement in root length. Treatments RP04 and RP01 showed statistically similar results for root length under 250Pb. However, a significant decline was noted in RP05 and RP06 compared to RP01 at 250Pb. RP02 had the greatest effect with a 62.17% increase in root length, followed by RP03 with a 49.65% increase, and RP01 with a 14.11% decrease. For the 125Pb treatment, all strains showed a decrease in root length compared to the control, with RP01 having the greatest effect with a 13.92% decrease. For the 250Pb treatment, all strains showed a decrease in root length compared to the control, with RP01 having the greatest effect with a 32.98% decrease. Overall, the microbial strains had a varying effect on root length, with some strains increasing root length while others decreasing it. (Fig. 2D).
The impact of treatment was significant for seedling length, fresh weight, and dry weight of seedlings. It was noted that RP02 performance remained significantly better than RP01 under control Pb, 125Pb, and 250Pb for seedlings length. The addition of RP03 caused a significant enhancement in seedling length compared to RP01 at control Pb and 250Pb, yet no significant change was noted at 125Pb. Compared to RP01, the addition of RP05 and RP06 remained significant for the decrease in seedling length at control Pb, 125 Pb, and 250 Pb. Control plants had an average seedling length of 23.39 cm, while those exposed to 125Pb and 250Pb showed a significant decrease in seedling length. The average seedling length for 125Pb treated plants was 20.25 cm, representing a 13.54% decrease compared to the control. The 250Pb treated plants showed a more severe reduction in seedling length, with an average of 16.64 cm, representing a 28.92% decrease compared to the control. Among the different concentrations of lead, RP02 and RP03 were found to have the highest and second-highest seedling lengths, respectively, in the control group. On the other hand, RP06 showed the lowest seedling length in the control group. When compared to the control, RP01 showed a 12.46% decrease in seedling length for 125Pb treatment and a 25.45% decrease for 250Pb treatment. Similarly, RP04 and RP05 showed a 12.05% and 13.44% decrease in seedling length, respectively, for 125Pb treatment and a 26.47% and 28.98% decrease, respectively, for 250Pb treatment. RP06 showed the highest reduction in seedling length, with a 23.11% and 33.98% decrease in length for 125Pb and 250Pb treatment, respectively (Fig. 3A).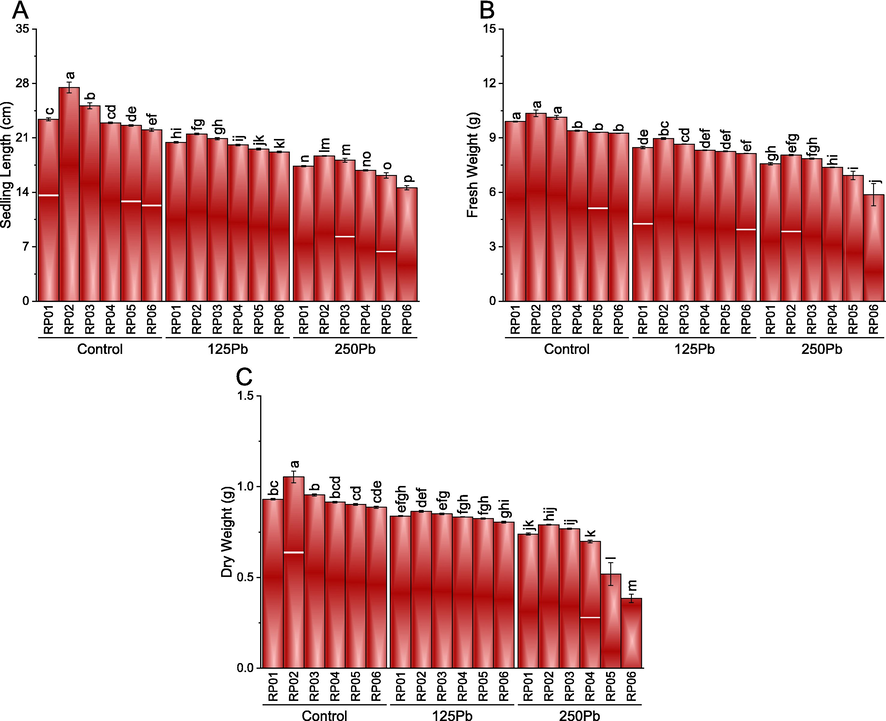
Effect of different PSB rhizobacterial isolates (RP01, RP02, RP03, RP04, RP05 and RP06) on seedlings length (A), fresh weight (B) and dry weight (C) of V. mungo cultivated under control Pb, 125Pb and 250Pb lead toxicity. Bars are means of six replicates ± SE. Different letters are showing significant alteration at p ≤ 0.05; Fisher LSD. RP01:RP01, RP02:RP02, RP03:RP03, RP04:RP04, RP05:RP05 and RP06:RP06.
For the fresh weight of seedlings, no significant change was noted between RP01, RP02, and RP03 under control Pb. Application of RP04, RP05, and RP06 significantly decreased the fresh weight of the seedlings compared to RP01 under control Pb. A significant enhancement in the fresh weight of seedlings was observed where RP02 was applied compared to RP01 under 125Pb. However, treatments RP04, RP05, and RP06 showed no significant variation compared to RP01 for the fresh weight of seedlings under 125 Pb. It was noted that RP01, RP02, RP03, and RP04 also remain statistically similar for fresh weight under 250 Pb. However, RP05 and RP06 caused a significant decrease in the fresh weight then RP01 under 250 Pb. The average fresh weight of seedlings in the control group was 9.81 g, whereas the fresh weight of seedlings exposed to 125Pb and 250Pb was considerably reduced. The average fresh weight of seedlings treated with 125Pb was 8.47 g, indicating a 13.92% decrease compared to the control group. The reduction in fresh weight was more pronounced for seedlings treated with 250Pb, with an average fresh weight of 7.15 g, representing a 27.12% decrease compared to the control group. In the control group, RP02 had the highest fresh weight, followed by RP03 and RP01, respectively. In contrast, RP06 had the lowest fresh weight in the control group. In comparison to the control group, RP01 displayed a 14.41% decrease in fresh weight for 125Pb treatment and a 22.97% decrease for 250Pb treatment. Similarly, RP02 and RP03 showed a 13.56% and 11.36% decrease in fresh weight, respectively, for 125Pb treatment and a 22.17% and 22.66% decrease, respectively, for 250Pb treatment. The highest reduction in fresh weight was observed in RP06, with a 17.44% and 36.52% decrease in weight for 125Pb and 250Pb treatment, respectively (Fig. 3B). In the case of dry weight, a significant increase was observed in RP02 was applied compared to RP01 under control Pb. Application of RP03, RP04, RP05, and RP06 showed no significant variation in dry weight than RP01 under control Pb. All the treatments remained statistically similar to each other under 125 Pb for dry weight. However, RP05 and RP06 caused a significant decrease in the dry weight then RP01 under 250 Pb. Compared to the control, the 125Pb treatment showed a percentage decrease in root dry weight ranging from 6.85% for RP02 to 13.81% for RP06. The 250Pb treatment had an even greater decrease, ranging from 21.32% for RP02 to 56.13% for RP06. On the other hand, the percentage increase in PSB was observed for all treatments when compared to the control. RP02 showed the highest percentage increase of 25.15%, followed by RP03 with 21.33%. RP01 showed the lowest percentage increase of 10.01%. The other treatments showed percentage increases ranging from 14.86% for RP06 to 19.51% for RP04. These results suggest that the use of PSB inoculation can be beneficial for plant growth and development, particularly in Pb-contaminated soil (Fig. 3C).
The results indicated no significant difference in chlorophyll a level between RP01 and RP02 under control Pb conditions. However, the use of RP03, RP04, RP05, and RP06 resulted in a significant decrease in chlorophyll compared to RP01 under control Pb conditions. At 125 Pb, RP01, RP02, RP03, RP04, and RP05 were comparable in terms of chlorophyll a level, but RP06 showed a significant decrease compared to RP01. Additionally, it was found that RP02 and RP03 were significantly more effective in improving chlorophyll a level than RP01 under 250 Pb conditions. In comparison, the use of RP05 and RP06 resulted in a significant decrease in chlorophyll compared to RP01 under 250 Pb conditions (Fig. 4A). Under control, 125 and 250 Pb, application of RP02 remained significantly best for enhancement in chlorophyll b compared to RP01. Treatment RP02 was significantly better compared to RP01 under control and 250 Pb but did not show any significant variation under 125 Pb for chlorophyll b. A significant decline in chlorophyll b was observed in RP05 and RP06 over RP01 under control 125 and 250 Pb (Fig. 4B). For total chlorophyll, the performance of RP02 and RP03 was significantly better compared to RP01 under control Pb. Treatments RP04, RP05, and RP06 caused a significant decrease in total chlorophyll than RP01 under control Pb. No significant change in total chlorophyll was observed where RP01, RP02, RP03, and RP04 were applied under 125 and 250 Pb. Application of RP05 and RP06 decreased the total chlorophyll significantly compared to RP01 under 125 and 250 Pb (Fig. 4C). In the case of Pb concentration, no significant variation was noted under control Pb among all the applied treatments. However, RP02 and RP03 caused a significant decrease in the Pb concentration compared to RP01 under 125 and 250Pb. It was observed that RP04, RP05, and RP06 caused significant enhancement in the Pb concentration compared to RP01 under 125 and 250Pb (Fig. 4D).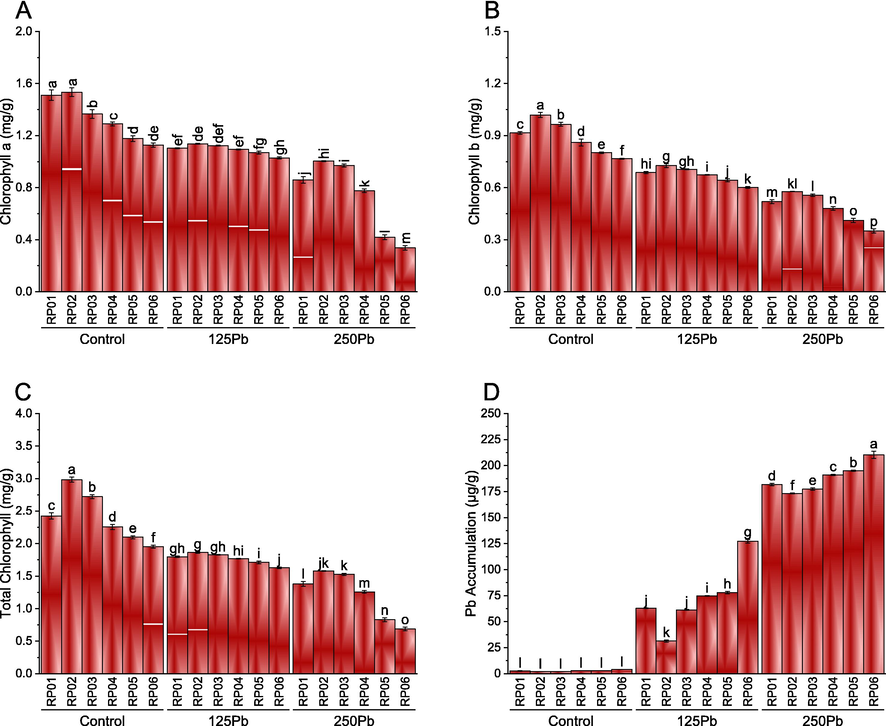
Effect of different PSB rhizobacterial isolates (RP01, RP02, RP03, RP04, RP05 and RP06) on chlorophyll a (A), chlorophyll b (B), total chlorophyll (C) and Pb accumulation (D) in V. mungo cultivated under control Pb, 125Pb and 250Pb lead toxicity. Bars are means of six replicates ± SE. Different letters are showing significant alteration at p ≤ 0.05; Fisher LSD. RP01:RP01, RP02:RP02, RP03:RP03, RP04:RP04, RP05:RP05 and RP06:RP06.
The results of the PCA also revealed that the first principal component (PC1) accounted for 94.9% of the variance and was primarily influenced by the variable “Germination (%)” with a loading value of 0.3017. The second principal component (PC2) explained only 2.2% of the variance and was mostly influenced by the variable “Vigor index” with a loading value of −0.16762. The cumulative percentage of variance for the first two principal components was 97.1%, indicating that these two components accounted for most of the variance in the data. Among the other variables, “Shoot Length (cm)” had the highest loading value (0.30638) on PC1, followed by “Seedling Length (cm)” (0.30626), “Chlorophyll a (mg/g)” (0.29663), and “Total Chlorophyll (mg/g)” (0.30548). On the other hand, “Pb Accumulation (µg/g)” had a loading value of −0.28755 on PC2. Overall, the results of the PCA indicated that the variables related to the growth and development of seedlings were strongly associated with the first principal component, while Pb accumulation was mostly independent of these variables and was associated with the second principal component. The findings of this study could be useful for understanding the factors that influence the growth and development of seedlings and their response to Pb contamination (Table 3; Fig. 5).
Principal Component Number
Eigenvalue
Percentage ofVariance
(%)Cumulative (%)
PC1
(94.9%)PC2
(2.2%)
Germination (%)
10.43707
94.88244
94.88244
0.3017
−0.11021
Vigor index
0.24044
2.1858
97.06824
0.30741
−0.16762
Shoot Length (cm)
0.16416
1.49241
98.56065
0.30638
−0.11947
Root Length (cm)
0.06114
0.55578
99.11643
0.30331
−0.14632
Seedling Length (cm)
0.04247
0.38612
99.50255
0.30626
−0.13092
Fresh Weight (g)
0.03065
0.27865
99.7812
0.30205
0.11214
Dry Weight (g)
0.01443
0.13116
99.91236
0.29157
0.59197
Chlorophyll a (mg/g)
0.0073
0.0664
99.97876
0.29663
0.47473
Chlorophyll b (mg/g)
0.00211
0.01922
99.99798
0.30753
−0.0963
Total Chlorophyll (mg/g)
2.22405E-4
0.00202
100
0.30548
0.14049
Pb Accumulation (µg/g)
1.2776E-30
1.16145E-29
100
−0.28755
0.5381
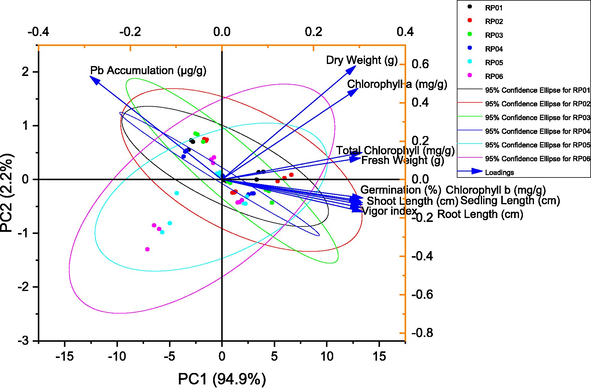
Principal component analysis for studied attributes.
The parallel plot effectively illustrates the interaction between treatments and attributes, providing valuable insights into the performance of each treatment in terms of growth attributes and Pb concentration reduction (Fig. 6). Upon examining the parallel plot, it is evident that the blue and green lines, which represent RP02 and RP03 treatments, respectively, performed significantly better than the red and orange lines of RP06 and RP05 under control, 125 and 250Pb. This finding is supported by the distinct pattern observed in the graph, indicating a clear difference in the performance of the treatments. These results provide compelling evidence that RP02 and RP03 treatments are highly effective in promoting plant growth and reducing the accumulation of Pb in seedlings.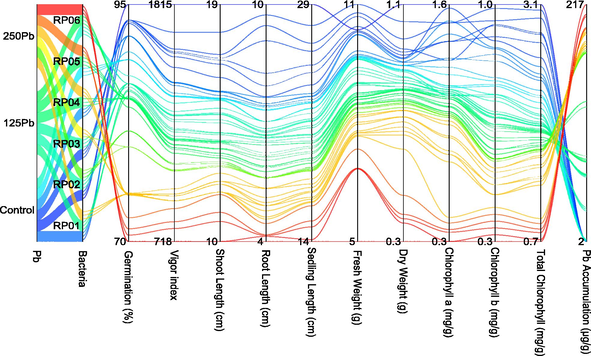
Parallel plot for studied attributes.
4 Discussion
The use of phosphate solubilizing bacteria (PSB) has been shown to be an effective strategy to improve plant growth and mitigate the negative effects of heavy metal toxicity. It was found that the application of PSB i.e., RP02 and RP03 led to an increase in shoot length, root length, and chlorophyll content of the V. mungo seedlings under control, 125 and 250Pb Pb. Additionally, the RP02 and RP03 PSB isolates were able to immobilize lead in the soil, reducing its bioavailability to the plants. One mechanism by which PSB can improve plant growth in lead-contaminated soils is through the solubilization of inorganic phosphates. Phosphorus is an essential element for plant growth and development, and its availability is often limited in soils contaminated with heavy metals (Lee et al., 2012). By expansion of cortex and aerenchyma space, PSB can increase root and shoot length and promote overall biomass accumulation, contributing to the overall health and productivity of plants. Rhizobacterial treatment has been shown to modify root plasticity in rice plants. One study found that plants inoculated with B. pyrrocinia had a thicker pericycle, which is associated with the development of lateral roots (Grover et al., 2021). The formation of lateral roots involves two main stages, including the reactivation of pericycle cell cycle and the establishment of a new meristem. By stimulating pericycle and protoxylem, plant growth-promoting rhizobacteria (PGPR) may lead to increased lateral root formation and ultimately promote the more vigorous establishment of rice plants (Rêgo et al., 2014). PSB are able to release inorganic phosphates from insoluble mineral sources, making them available for plant uptake. This increased availability of phosphorus can lead to improved growth and development of the seedlings (Yu et al., 2022). Our results also justified the above mechanisms because both RP02 and RP03 of current study were highly capable of P-solubilization. Another mechanism by which PSB can improve plant growth in lead-contaminated soils is through the production of various microbial secretions. These secretions can include enzymes, organic acids, and siderophores, which can help to chelate heavy metals and reduce their bioavailability to the plants (Bhadra et al., 2018). Additionally, these microbial secretions can also enhance the uptake of essential nutrients by the plants. Furthermore, the PSB can also improve the growth of seedlings by promoting the biosynthesis of phytohormones, such as indole acetic acid (IAA), ACC-deaminase and gibberellins. These hormones play a critical role in plant growth and development by regulating cell division, elongation, and differentiation. The increased production of these hormones can lead to an increase in shoot and root length, as well as an increase in chlorophyll content, which is an indicator of overall plant health (Waqas et al., 2012). The use of citrate and catalase as a strategy to improve growth and immobilize lead in lead-contaminated soils is promising, but further research is needed to fully understand the mechanisms involved. Studies have shown that the application of citrate can lead to an increase in shoot and root length and chlorophyll content, as well as reducing lead toxicity (Mallhi et al., 2019). Moreover, the application of catalase has been shown to be effective in reducing the negative effects of lead toxicity on the growth of seedlings by reducing the levels of ROS. The combination of citrate and catalase may have a synergistic effect, as citrate can chelate lead and catalase can detoxify ROS generated by lead toxicity (Min et al., 2010). Such arguments also justified our findings. The best results showing PSB of current study i.e., have potential for production of IAA, citrate and catalase in significant amount.
5 Conclusion
In conclusion, the use of PSB RP02 and RP03 have the potential to improve the growth of V. mungo under lead toxicity by reducing the bioavailability of heavy metals Pb. Additionally, the PSB RP02 have more potential than RP03 to enhance the growth of V. mungo, solubilization of inorganic phosphates and immobilization of lead. Growers are recommended to inoculate PSB for the achievement of better growth production under Pb contaminated soil when V. mungo is cultivated. More investigations and identification of genus and sp. are required for declaration of RP02 as best PSB isolate for production of V. mungo in Pb contaminated soils under different agroclimatic zones.
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No (RG-1441-362).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Coupling Phosphate-Solubilizing Bacteria with Phosphorus Supplements Improve Maize Phosphorus Acquisition and Growth under Lime Induced Salinity Stress. Plants. 2020;9:900.
- [CrossRef] [Google Scholar]
- Lead harms seed germination & growth of albizia lebbeck (L.) benth. and prosopis juliflora (sw.) dc. Pakistan J. Bot.. 2022;54:121-124.
- [CrossRef] [Google Scholar]
- Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol.. 1949;24:1.
- [Google Scholar]
- Immobilizing Siderophores on Solid Surfaces for Bacterial Detection. J. Electrochem. Soc.. 2018;165:B3017-B3022.
- [CrossRef] [Google Scholar]
- Effect of various application rates of phosphorus combined with different zinc rates and time of zinc application on phytic acid concentration and zinc bioavailability in wheat. Agric. Nat. Resour.. 2020;54:265-272.
- [CrossRef] [Google Scholar]
- Phosphorus and phosphate solubilizing bacteria: Keys for sustainable agriculture. Geomicrobiol. J.. 2019;36:904-916.
- [Google Scholar]
- Isolation and characterization of rhizospheric phosphate solubilizing bacteria from wetland paddy field of Mizoram, India. Geomicrobiol. J.. 2020;37:366-375.
- [Google Scholar]
- OriginPro. Northampton, MA, USA: OriginLab; 2021.
- Study of characterization of tamarind associated Rhizobium spp. and phosphate solubilizing bacteria and their potency for germination of tamarind seeds. Pharma Innov. J.. 2018;7:282-286.
- [Google Scholar]
- Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci.. 2007;2:112-118.
- [CrossRef] [Google Scholar]
- Heavy metal contamination status in soil-plant system in the Upper Mersey Estuarine Floodplain, Northwest England. Mar. Pollut. Bull.. 2019;146:292-304.
- [Google Scholar]
- Analysis of indole-3-acetic acid (IAA) production in Klebsiella by LC-MS/MS and the Salkowski method. Bio-protocol. 2019;9:e3230-e.
- [Google Scholar]
- Particle-size analysis. In: Klute A., ed. Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods. Madison: Soil Science Society of America; 1986. p. :383-411.
- [Google Scholar]
- Mitigation of lead stress in Triticum aestivum L. seedlings by treating with salicylic acid. Pakistan J. Bot.. 2021;53:39-44.
- [CrossRef] [Google Scholar]
- PGPR Mediated Alterations in Root Traits: Way Toward Sustainable Crop Production. Front. Sustain. Food Syst.. 2021;4:618230
- [CrossRef] [Google Scholar]
- Elemental determination by atomic absorption spectrophotometery. In: Kalra Y., ed. Handbook of Reference Methods for Plant Analysis. Washington D.C.: CRC Press; 1998. p. :157-164.
- [Google Scholar]
- Development of XRM-MacConkey agar selective medium for the isolation of Escherichia albertii. Diagn. Microbiol. Infect. Dis.. 2020;97:115006
- [Google Scholar]
- The water culture method for growing plants without soil. In: University of California, College of Agriculture, Agricultural Experiment Station. Berkeley, CA: Circular; 1950. p. :374.
- [Google Scholar]
- Bergey’s manual of determinative bacteriology (9th ed.). Baltimore, MD, USA: Williams & Wilkins; 1994.
- Lead nitrate Pb (NO3)2 impacts on seed germination and seedling growth of different soybean (Glycine max L.) varieties. Pakistan J. Bot.. 2021;53:1617-1627.
- [CrossRef] [Google Scholar]
- Kuo, S., 1996. Phosphorus, in: Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E. (Eds.), Methods of Soil Analysis Part 3: Chemical Methods. John Wiley & Sons, Ltd, SSSA, Madison, Wisconsin, pp. 869–919. https://doi.org/10.2136/sssabookser5.3.c32.
- Mechanisms of Phosphate Solubilization by PSB (Phosphate-solubilizing Bacteria) in Soil. Korean J. Soil Sci. Fertil.. 2012;45:169-176.
- [CrossRef] [Google Scholar]
- Phosphorus nutrition: plant growth in response to deficiency and excess. In: Plant Nutrients and Abiotic Stress Tolerance. Springer; 2018. p. :171-190.
- [Google Scholar]
- Citric Acid Enhances Plant Growth, Photosynthesis, and Phytoextraction of Lead by Alleviating the Oxidative Stress in Castor Beans. Plants. 2019;8:525.
- [CrossRef] [Google Scholar]
- Nitric-Perchloric Acid Wet Digestion In an Open Vessel. In: Kalra Y., ed. Reference Methods for Plant Analysis. Washington, D.C.: CRC Press; 1998. p. :57-62.
- [Google Scholar]
- Downregulation of catalase by reactive oxygen species via hypermethylation of CpG island II on the catalase promoter. FEBS Lett.. 2010;584:2427-2432.
- [CrossRef] [Google Scholar]
- Rhizobacteria containing ACC-deaminase confer salt tolerance in maize grown on salt-affected fields. Can. J. Microbiol.. 2009;55:1302-1309.
- [CrossRef] [Google Scholar]
- Nelson, D.W., Sommers, L.E., 1982. Total Carbon, Organic Carbon, and Organic Matter, in: Page, A.L. (Ed.), Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties. American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, Madison, WI, USA, pp. 539–579.
- Soil pH and lime requirement. In: Page A.L., ed. Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 9.2.2/Agronomy Monographs. Madison: American Society of Agronomy, Inc. and Soil Science Society of America Inc; 1983. p. :199-208.
- [CrossRef] [Google Scholar]
- Morphoanatomical and Biochemical Changes in the Roots of Rice Plants Induced by Plant Growth-Promoting Microorganisms. J. Bot.. 2014;2014:1-10.
- [CrossRef] [Google Scholar]
- Rhoades, J.D., 1996. Salinity: Electrical Conductivity and Total Dissolved Solids, in: D.L. Sparks, A.L. Page, P.A. Helmke, R.H. Loeppert, P. N. Soltanpour, M. A. Tabatabai, C. T. Johnston, M. E. Sumner (Eds.), Methods of Soil Analysis, Part 3, Chemical Methods. Soil Science Society of America, Madison, WI, USA, pp. 417–435. https://doi.org/10.2136/sssabookser5.3.c14.
- Shabnum Shaheen, 2012. Comparative nutritional analysis between Vigna radiata and Vigna mungo of Pakistan. African J. Biotechnol. 11, 10.5897/AJB11.3496. https://doi.org/10.5897/AJB11.3496.
- Evaluating the effects of cadmium under saline conditions on leafy vegetables by using acidified biochar. Pakistan J. Bot.. 2023;55:1537.
- [CrossRef] [Google Scholar]
- Shenoy, A., Buttar, H.S., Dicholkar, P., Kaur, G., Chintamaneni, M., 2022. Role of nutraceuticals, functional foods, and spices in the management of metabolic syndrome and related disorders, in: Singh, R.B., Watanabe, S., Isaza, A.A. (Eds.), Functional Foods and Nutraceuticals in Metabolic and Non-Communicable Diseases. Elsevier, pp. 583–601. https://doi.org/10.1016/B978-0-12-819815-5.00017-3.
- Lead induced genotoxicity in Vigna mungo var. HD-94. J. Saudi Soc. Agric. Sci.. 2012;11:107-112.
- [CrossRef] [Google Scholar]
- Principles and Procedures of Statistics: A Biometrical Approach (3rd ed.). Singapore: McGraw Hill Book International Co.; 1997.
- Soybean Development and productivity in response to organic management above the northern boundary of soybean distribution in Europe. Agronomy. 2021;11:214.
- [Google Scholar]
- Endophytic Fungi Produce Gibberellins and Indoleacetic Acid and Promotes Host-Plant Growth during Stress. Molecules. 2012;17:10754-10773.
- [CrossRef] [Google Scholar]
- Effects of lead pollution on germination and seedling growth of turfgrass. Cynodon dactylon. Pakistan J. Bot.. 2021;53:2003-2009.
- [CrossRef] [Google Scholar]
- Identification of the Phosphorus-Solubilizing Bacteria Strain JP233 and Its Effects on Soil Phosphorus Leaching Loss and Crop Growth. Front. Microbiol.. 2022;13:892533
- [CrossRef] [Google Scholar]







