Translate this page into:
Mining of halo-tolerant plant growth promoting rhizobacteria and their impact on wheat (Triticum aestivum L.) under saline conditions
⁎Corresponding author. tahirnaqqash@gmail.com (Tahir Naqqash)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Plant growth promoting rhizobacteria have the potential to mitigate abiotic stressors to improve plant productivity. The current study was conducted to isolate and characterize halo-tolerant PGPR strains from the native plants of Khewra salt mine in Pakistan. Two halo-tolerant bacterial strains (SBN01 and SBN02) were purified, examined in vitro for their plant growth-promoting traits, and inoculated on wheat plants to evaluate their beneficial effects. Both isolates were able to produce indole-acetic acid in tryptophan-supplemented media, both were N2 fixers and capable of solubilizing phosphate. These isolates also showed positive results for catalase, gelatinase, and ammonia, except for protease activity. Based on 16S rRNA gene sequence analysis, SBN01 and SBN02 isolates showed 100% homology with Alcaligenes faecalis. Inoculation studies on wheat plant showed that these isolates maintained ionic imbalance by regulating Na+ and K+ ions and significantly increased growth parameters and plant biomass by decreasing reactive oxygen species induced lipid peroxidation while increased accumulation of osmolyte, photosynthetic pigments and improved photosystem II efficiency as compared to uninoculated plants. These results showed that isolated halo-tolerant rhizobacterial strains could improve plant growth and enhance tolerance during a stressful environment. Thus, they can be used as potential cost-effective candidates for growing wheat in salt-affected areas.
Keywords
Halo-tolerant rhziobacteria
Triticum aestivum L.
Alcaligenes spp.
NaCl stress
P-solubilization
PSII
- PGPR
-
plant growth-promoting rhizobacteria
- ACC
-
1-aminocyclopropane-1-carboxylic acid
- CFU
-
colony forming units
- OM
-
organic matter
- N
-
nitrogen
- EC
-
electrical conductivity
- K
-
potassium
- P
-
phosphorous
- NFM
-
nitrogen free malate media
- IAA
-
indole acetic acid
- PCR
-
polymerase chain reaction
- Chl
-
chlorophyll
- PSII
-
photosystem II
- MDA
-
lipid peroxidation
- CAT
-
catalase
- ROS
-
reactive oxygen species
- ANOVA
-
analysis of variance
Abbreviations
1 Introduction
Salt stress is one of the most damaging abiotic stresses that reduce the yield and growth of crops around the globe. Several plant cultivars are extremely salt-sensitive and cannot tolerate even a very low salt concentration (Bukhat et al., 2019). Excessive salts present in saline soils induce ionic stress, oxidative stress, and osmotic stress in crops that cause ionic imbalance, dehydration, and cell turgor loss, causing physiological dehydration. These conditions cause a reduction in plant growth and inhibit several physio-biochemical processes including respiration, seed germination, photosynthesis, protein synthesis, nitrogen contents of shoots, lipid, chlorophyll biosynthesis, and energy metabolism (Desoky et al., 2019). Salinity stress also reduces cellular membrane integrity and significantly increases the generation of reactive oxygen species (ROS), causing impairment of DNA molecules, proteins, lipids, and several anatomical properties. ROS production induces lipid peroxidation of the cellular membrane and causes nutrient imbalance, decreased membrane selectivity and fluidity (Elrys et al., 2020).
Soils in semi-arid and arid climatic zones are more susceptible to salinity. The salinized area has been increasing at a rate of 10% each year that will cause 50% of the arable area to be salinized till 2050 (Bukhat et al., 2019). Rapidly increasing urbanization, population, constant reduction of agricultural land, and decrease in fertility of soil urgently requires the development of practical approaches to transforming these saline lands into efficient viable entities. The production of salt-tolerant plant varieties is one of the practical and user-friendly options for saline farming, but the growth of these varieties needs a lot of investment, time, and input. In contrast, induction of salt tolerance through bio-fertilization of plants with salt-tolerant plant growth-promoting rhizobacteria (PGPR) can be a cost-effective and integrated solution for salt stress. The microbiome of rhizosphere has a direct effect on plant yield, nutrition, and growth, thus this rhizospheric microbiome can be engineered to induce salt tolerance in different plants (Abbas et al., 2019).
The microflora present in salt-affected soils has the potential for combating and overcoming the damaging effects of salinity stress. In these halo-tolerant microorganisms, 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase enzyme hydrolyzes ethylene precursor to ammonia and α-ketobutyrate, which causes a reduction in endogenous ethylene resulting in stimulated plant growth and root development. PGPR isolated from the salt-stressed environment possesses several plant beneficial characteristics such as phosphate solubilization, nitrogen fixation, and phytohormone production (Nakbanpote et al., 2014).
Inoculation of PGPR species as Pseudomonas, Agrobacterium, Azospirillum, and Bacillus, is an energy-efficient, economically feasible, and environmentally friendly approach for restoring salt-affected soil and improving biomass production (Majeed et al., 2015; Naqqash et al., 2016). PGPR application can help in developing strategies to promote the cultivation of wheat in salt-affected areas, as plant roots are colonized by PGPR. PGPR can locate and interact with plant roots exudates (amino acids and carbohydrates) through chemotaxis. PGPR increase growth by producing growth stimulating hormones and phosphate solubilization (Majeed et al., 2015). Salinity is the major constraint hampering wheat production worldwide as salt stress causes 65% yield loss in moderately saline areas. Different studies have reported the use of PGPR for alleviating salt stress in cereal plants (Jaemsaeng et al., 2018; Kruasuwan and Thamchaipenet, 2018). However, little information is available on the isolation of halo-tolerant PGPR from salt mines and their effect on wheat plants under saline stress.
Wheat (Triticum aestivum L.) is the staple crop worldwide, but ineffective fertilizer, lack of proper irrigation, and poor soils stop growers from achieving the maximum yield of wheat crop. The soils in such a zone have lower organic matter due to which these soils have poor structure and reduced soil fertility (Ullah et al., 2007). To establish a sustainable strategy for the cultivation of wheat, we have isolated halo-tolerant PGPR from native plants of Khewra salt mines of Pakistan and investigated inoculation results of these indigenous microflorae on productivity and growth of wheat in greenhouse conditions. Based on increasing salinity problems, the present research aimed to isolate indigenous halo-tolerant PGPR from soil of salt mines and further evaluate their effects on wheat under salt stress to be used as potential candidates in salt-affected soils.
2 Materials and methods
2.1 Soil sampling and bacterial isolation
Soil samples were taken from rhizospheric soil of halo-tolerant native plants (Sesbania aculeata and Atriplex lentiformis) of Khewra salt mine (N: 32° 38.883′, E: 73° 0.5′, 288 m above sea level) Pakistan. The collected samples were transferred to the Institute of Molecular Biology and Biotechnology (IMBB), Bahauddin Zakariya University (BZU) Multan, Pakistan, for further experimentation and stored at 4 °C in a sterilized plastic bag. Bacterial isolation was done by mixing 1 g rhizospheric soil with a 0.89% (9 ml) saline solution. Halo-tolerant bacteria were isolated by spreading bacterial culture on NaCl (0, 250, 500, and 1000 mM) supplemented LB agar plates and were incubated for 2 days at 28 ± 2 °C, and colony-forming units (CFU) were counted. The single bacterial colony was purified on the fresh LB agar and placed at −80 °C in a 10% glycerol solution (Vincent, 1970). Physiochemical properties of soil samples were investigated using standard protocols described in (Tahir et al., 2020).
2.2 Morpho-physiological characterization of bacterial isolates
The morphologicl charatcetristics of halo-tolerant bacterial isolates were observed under a light microscope following the protocol of Vincent (1970). To confirm protease production, isolated halo-tolerant strains were inoculated on a skim milk agar plate and incubated at 28 ± 2 °C for 24 h. The halo zone formation around the bacterial colony confirmed protease production (Abo-Aba et al., 2006). For checking the catalase activity, bacterial culture was placed on a clean glass slide, and few drops of H2O2 (3%) were applied to the bacterial culture. Production of bubbles after H2O2 addition showed catalase activity (Rørth and Jensen, 1967). Gelatinase activity was measured by inoculating isolated halo-tolerant strains into the gelatin tubes and incubated for 4–7 days at 28 ± 2 °C following refrigeration for 30 min (Rendic, 1975).
2.3 Bioassays for growth promoting traits
Diazotrophic bacterial strains were isolated by mixing root pieces and rhizospheric soil (0.5 g) with 1 ml nitrogen-free malate media (NFM) into eppendorf and incubated for 2 days at 28 ± 2 °C following streaking and purification on the NFM plate. The single purified bacterial colony was then transferred to LB agar plates (Okon et al., 1977). Qualitative analysis for phosphate solubilization ability of the isolates was checked on Piskovaskaya’s medium by spot inoculation while quantitative analysis was done a by phospho-molybdate blue-color method using a spectrophotometer at 882 nm (Pikovskaya, 1948). Indole acetic acid (IAA) production was investigated through Salkowski colorimetric test. Quantitatively auxin concentration was measured using a spectrophotometer at 540 nm (Sachdev et al., 2009). Bacterial isolates were added into peptone water for the determination of ammonia production. The supernatant (0.2 ml) was added in Nessler’s reagent (1 ml) that changed yellow color into brown (Marques et al., 2010).
2.4 Molecular characterization
2.4.1 16S rRNA gene amplification and phylogenetic analysis
For amplification of 16S rRNA, the inoculum was homogenized in sterilized polymerase chain reaction (PCR) water (200 µl) and incubated for five minutes at 95 °C for lysing bacterial cells. These lysed cells were used in the PCR master mixture as a template. These homogenized lysate cells of isolates were amplified with 785F and 907R primers in a thermocycler (Harris et al., 1998). The resulting PCR product was evaluated on ethidium bromide containing agarose gel (1%). Purified PCR products were sequenced from LGC Genomics, Berlin, Germany, and analyzed using NCBI BLAST and Sequence Scanner Software following submission to GenBank EMBL. The phylogenetic analysis was done using MEGA X software. The phylogenetic tree of the sequences was generated by employing the neighbor-joining tree method based on Nei’s 1983 similarity matrix. The evolutionary distances were calculated by the p-distance method (Kumar et al., 2018).
2.5 Plant inoculation studies
2.5.1 Green house experiment
To check the halo-tolerant activity of bacterial isolates (Alcaligenes faecalis SBN01 and Alcaligenes faecalis SBN02), greenhouse experiment was conducted in a completely randomized design involving nine different treatments with three replicates each. These treatments consist of water control, two different concentrations of sodium chloride (NaCl) salt (450 and 600 mM) along with inoculation of two bacterial strains (Alcaligenes faecalis SBN01 and Alcaligenes faecalis SBN02) i.e. T1 = Un-inoculated + 0 mM NaCl, T2 = Un-inoculated + 450 mM NaCl, T3 = Un-inoculated + 600 mM NaCl, T4 = SBN01 + 0 mM NaCl, T5 = SBN01 + 450 mM NaCl, T6 = SBN01 + 600 mM NaCl, T7 = SBN02 + 0 mM NaCl, T8 = SBN02 + 450 mM NaCl, and T9 = SBN02 + 600 mM NaCl. Seeds of wheat plant (variety PASBAN90) were surface sterilized with 100% ethanol and 10% NaOCl solution followed by washing with distilled water for 3 to 4 times. Sterilized seeds were dipped in the inoculum (108 CFU) for 20 min and sown in plastic pots (16 cm diameter and 18 cm depth) containing 800 g of loamy soil. The pots were placed in green house of BZU, Multan, Pakistan and watered with Hoagland nutrient solution. The second inoculation was done after twenty days following the third inoculation after forty days of germination, by mixing the inoculum in 1/2 strength of Hoagland nutrient solution. Salinity stress (450 and 600 mM) was given post 48 h of third bacterial inoculation, starting from 50 mM salt stress and gradually increased up to the required concentration. Plants were harvested after 48 h of last (600 mM) salt treatment (at day 60) and samples for further experimentation were collected. The harvested wheat plants were carefully uprooted from the plastic pots for separating shoots and roots. Measurements for physiological parameters was taken immediately, while for biochemical analysis, plants were stored at −80 °C in liquid nitrogen.
2.6 Biochemical assays
2.6.1 Chlorophyll and carotenoid content
Photosynthetic pigments were measured by grinding wheat leaves in 80% (v/v) acetone solution. The resultant mixture was centrifuged for 15 min at 4 °C and 14,000 rpm. Extract absorbance was measured at 480, 663 and 645 nm for estimation of chlorophyll (Chl) a, b, total Chl and carotenoid contents (Arnon, 1949).
2.6.2 Photosystem II photochemistry
Maximal efficacy of photosystem II (PSII) was determined by Chl fluorescence kinetics using Flour-Pen FP100 (PSI, CZ). Wheat plants were pre-darkened for half hour at room temperature following strong light (3000 µmolm−2 s−1) pulse exposure on 5 mm leaf area. Initially, the fluorescence was measured at 50 µs known as O-step following 300 µs, 2 ms, and 20 ms on intermediate K, J and I steps and at 300 ms (P-step), maximum fluorescence was measured. The following parameters were measured: PIABS, Fv/Fm, ABS/RC, TR/RC, DIo/RC and ETo/RC (Cessna et al., 2010).
2.6.3 Lipid peroxidation (MDA)
Salt stress induced lipid peroxidation was measured by grinding fresh leaves in 0.1% trichloroacetic acid following centrifugation for ten min at 5000 rpm. 0.5% thiobarbituric acid prepared in 20% trichloroacetic acid was mixed with supernatant. The resulting solution was placed in water bath at 100 °C for 30 min following immediate transfer on ice for stopping the reaction. After cooling, solution was again centrifuged for ten min at 5000 rpm. The absorbance of resulting supernatant was observed at 532 and 600 nm. The molar extinction coefficient (156 mmol−1 cm−1) was used for calculation of lipid peroxidation (Heath and Packer, 1968).
2.6.4 Proline content
Proline was measured by grinding wheat leaves in 3% (w/v) sulfosalicylic acid following filtration using Whatman filter paper. 2 ml of glacial acetic acid, filtrate and acid ninhydrin were mixed. Acid ninhydrin was prepared by mixing ninhydrin (1.25 g), glacial acetic acid (30 ml) and 6 M orthophosphoric acid (20 ml). The solution was incubated for one hour at 100 °C following immediate cooling on ice to cease the reaction. After cooling, toluene (4 ml) was added and the solution was kept on the shaker for forming two layers. The upper layer was taken and absorbance was measured using a spectrophotometer at 520 nm taking toluene as a blank (Bates et al., 1973).
2.6.5 Catalase activity
For measuring catalase activity, 0.2 g leaves were ground in potassium phosphate buffer (50 mM) of pH 7.0. The material was centrifuged for 10 min at 10,000 rpm. The resulting supernatant was mixed with H2O2 (5.9 mM) and potassium phosphate buffer (50 mM). The catalase (CAT) activity of solution was measured using a spectrophotometer at 240 nm after every 20 s up to 1 min (Aebi, 1984). One unit of activity of CAT is the variation in the absorbance of 0.01 units per minute which was measured with molar extinction coefficient (36 M−1 cm−1 µmol per min) of H2O2.
2.6.6 Protein content
Protein content was measured by grinding wheat leaves (0.2 g) in sodium phosphate buffer (4 ml) following centrifugation for five min at 10000 rpm. The upper layer of the mixture was homogenized with Bradford Reagent and was incubated at 37 °C for 20 min. The absorbance of the mixture was measured at 595 nm (Bradford, 1976).
2.6.7 Ions analysis
Sodium (Na+) and potassium (K+) ions were estimated by digesting dried wheat leaves (1 g) in a digestion mixture containing HNO3:HClO4:H2SO4 in the ratio of 10:3:1. This mixture was filtered and analyzed using a flame photometer (Richards, 1954).
2.7 Statistical analysis
In vitro quantification for plant inoculation studies was done on three independent replications. The data of these replicates were averaged and was statistically analyzed by ANOVA (analysis of variance) using STATISTIX 8.1 software. The significant difference between the treatments was measured by Fisher’s LSD test at 5% probability.
3 Results
3.1 Soil analysis
Soil samples of two native plants Sesbania aculeate and Atriplex lentiformis collected from Khewra salt mine showed sandy loamy soil texture having the same pH (8.0) and nitrogen content (300 mg kg−1). EC of soil ranged from 13.43 to 16.92 ds/m while organic matter (%), extractable potassium and phosphorous were (0.69%, 190 mg kg−1 and 9.5 mg kg−1, respectively) slightly greater in Sesbania aculeate as compared to (0.67%, 180 mg kg−1 and 8.8 mg kg−1, respectively) Atriplex lentiformis. CFU in Atriplex lentiformis soil sample (7.83) was more than Sesbania aculeate (6.69). All these physiochemical properties of both soil samples are given in Table 1.
Sample
Sesbania aculeate
Atriplex lentiformis
EC ds/m
13.43
16.92
pH
8
8
Organic Matter (%)
0.69
0.67
P mgkg−1
9.5
8.8
K mgkg−1
190
180
N mgkg−1
300
300
CFU
6.69
7.83
3.2 Isolation, characterization and identification of halo-tolerant isolates
The halo-tolerant bacterial strains (SBN01 and SBN02) were isolated on LB agar plates with varying salt concentrations. SBN01 bacterial isolate obtained from Sesbania aculeata rhizospheric soil showed round small off white colonies while cells were motile rod-shaped, whereas SBN02 obtained from Atriplex lentiformis showed cubical medium sticky white colonies with motile thin rods cells. Both isolates showed positive results for catalase and gelatinase activity, while for protease activity, only SBN01 isolate showed positive results (Table 2 and Fig. 1C, 2A & 2B).
Characteristics
Halo-tolerant bacterial isolates
SBN01
SBN02
Shape, size, and color
Round, small and off-white
Cubical, sticky white
Cell morphology
Medium rod
Medium thin rod
P-solubilization
84.32 µg/mL
73.68 µg/mL
IAA production
7.53 µg/mL
12.60 µg/mL
N2 fixer
+
+
Catalase production
+
+
Protease activity
+
–
Gelatinase activity
+
+
Ammonia production
+
+
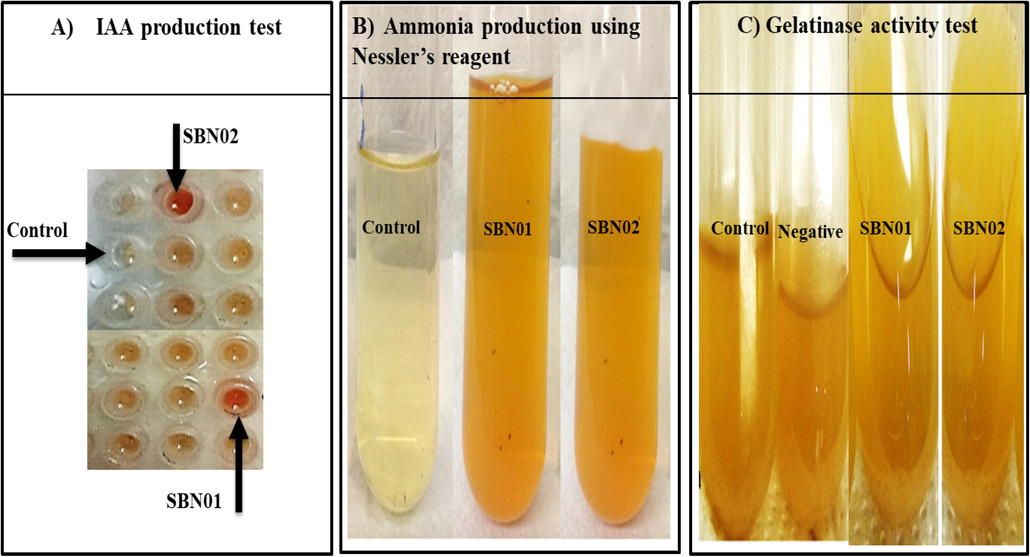
Potential of halo-tolerant PGPR for (A) IAA; (B) ammonia production; and (C) gelatinase activity test.
3.3 Biochemical characterization and 16S rRNA gene based identification of bacterial isolates
Both halo-tolerant bacterial isolates showed IAA production giving pink color by adding the FeCl3 solution, however, the production of IAA by SBN02 bacterial isolate (12.60 µg/mL) was greater than SBN01 isolate (7.53 µg/mL). Moreover, both halo-tolerant bacterial isolates also showed positive results for ammonia production (Table 2 and Fig. 1A & 1B). In the present study, both SBN01 and SBN02 halo-tolerant bacterial isolates showed phosphate solubilizing potential by forming a clear halo zone on phosphate solubilizing media. Out of both isolates, SBN01 showed maximum phosphate solubilization and solubilized 84.32 µg/mL of phosphorous while SBN02 solubilized 73.68 µg/mL of phosphorous. Both halo-tolerant isolated showed positive results for nitrogen fixing assay by producing a pale yellow color as shown in Fig. 2C.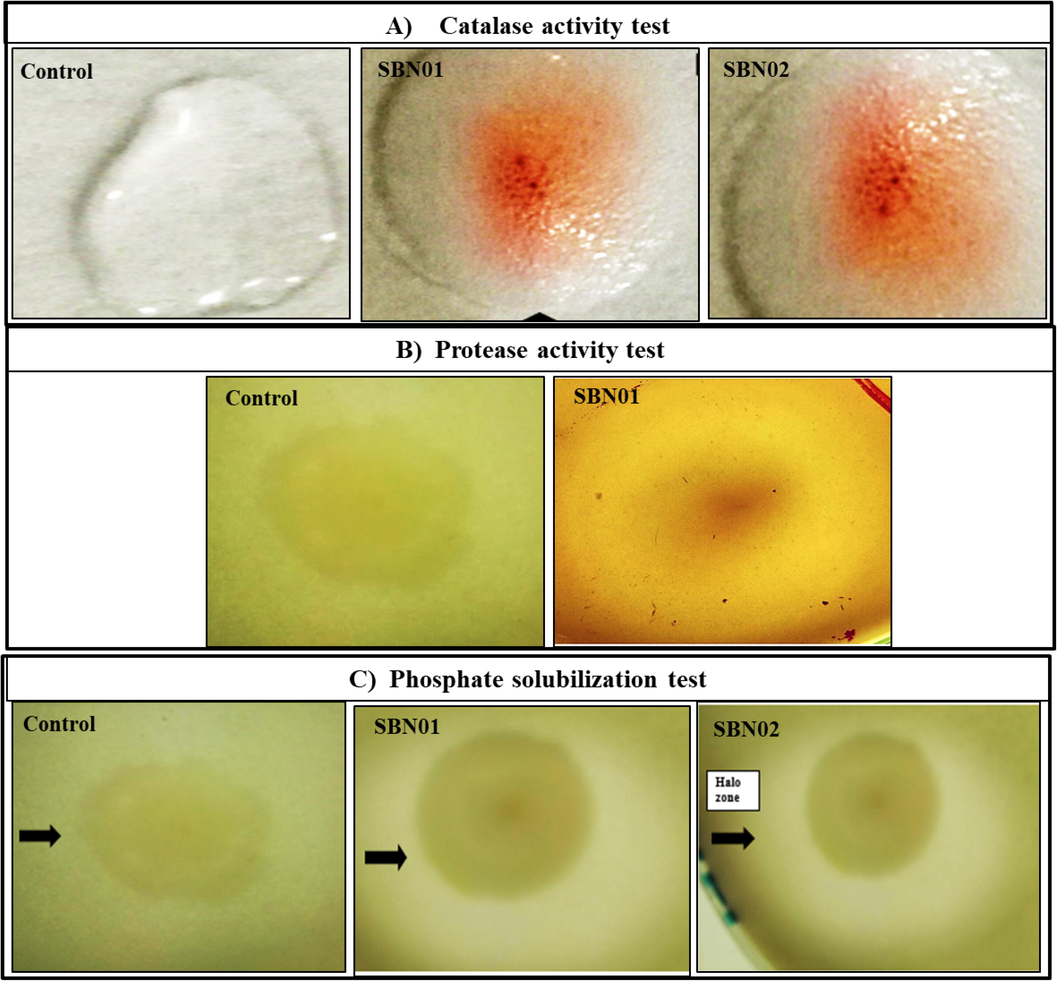
Potential of halo-tolerant PGPR for (A) catalase activity; (B) protease activity of SBN01; and (C) P-solubilization.
Based on sequence analysis of the 16S rRNA gene, SBN01 and SBN02 strains were identified as Alcaligenes spp., both showing 100% similarity with Alcaligenes faecalis strain C_9 (KT748643.1) with the relevant sequence in GenBank. The 16S rRNA gene sequences of SBN01 and SBN02 halo-tolerant isolates strains are submitted to GenBank under the accession numbers MT765076 and MT765077, respectively. The evolutionary history or phylogenetic analysis was inferred using the Neighbor-Joining method having the bootstrap value greater than 70 (Fig. 3). Both halo-tolerant isolates showed clustering with Alcaligenes faecalis (CP033861), Alcaligenes faecalis (MK312671), Alcaligenes faecalis (CP021079), Alcaligenes faecalis (KT748643), Alcaligenes faecalis (GQ438851), and Alcaligenes faecalis (CP023667). The phylogenetic tree with the sum of branch length (0.0195) is shown in Fig. 3.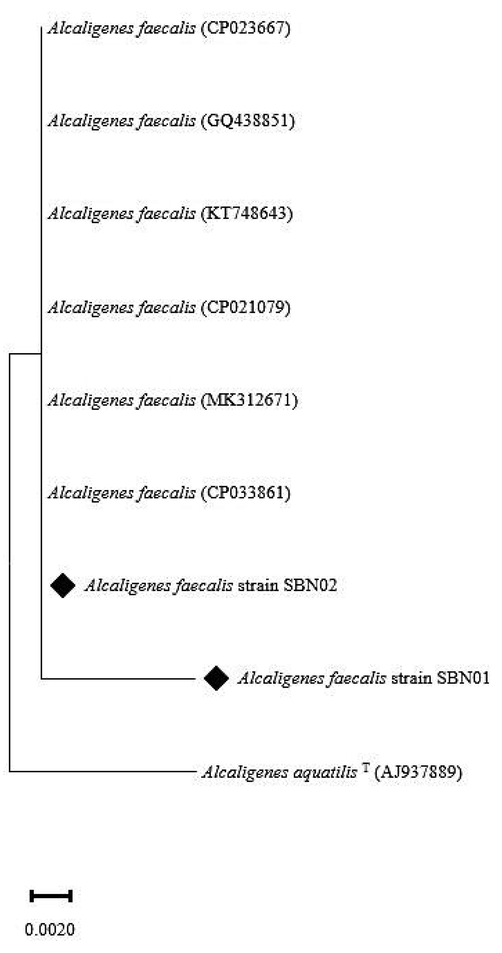
16s rRNA based neighbour joining phylogenetic tree of halo-tolerant PGPR isolated from native plants of Khewra salt mine (869 bp). Isolated strains (♦) and their closely related strains were obtained from GenBank. Alcaligenes aquatilis T(AJ937889) served as root. Bars represent 0.002 nucleotide sequence divergence.
3.4 Plant inoculation studies
3.4.1 Effect of bacterial inoculation and salt stress on growth parameters of wheat plant
As bacterial strains were isolated from the rhizospheric soil of native plants of the salt mine and both of them showed halophilic characters, thus their ability to promote wheat growth under salt stress was evaluated by conducting a greenhouse experiment. Salt stress in uninoculated control wheat plants significantly decreased all plant growth parameters while the inoculation of SBN01 and SBN02 strains significantly improved all these traits under saline and non-saline conditions (Fig. 4A to 4F). The 600 mM salt concentration causes much reduction in leaf area (34%), plant height (25%), number of spikelets per spike (50%), spike length (27%), number of reproductive tillers (42%), and vegetative tillers (65%) as compared to 450 mM NaCl salt stress (25%, 8.3%, 24%, 13%, 31.5% and 50%, respectively). At 450 mM NaCl salt stress, the inoculation of SBN01 halo-tolerant strain increased leaf area by 29.47%, plant height by 6.5%, number of spikelets per spike by 25%, spike length by 11.8%, and vegetative tillers by 90%, while the inoculation of SBN02 halo-tolerant strain increased all these parameters by 21.89%, 7.02%, 25%, 12.27%, and 70%, respectively, however, the increase in number of reproductive tillers by both isolates was similar to uninoculated control wheat plants. The inoculation of SBN01 and SBN02 halo-tolerant strains at 600 mM NaCl stress improved leaf area by 36.78% and 22.29%, plant height by 19.15% and 13.05%, number of spikelets per spike by 19.04% and 28.57%, spike length by 16.93% and 21.31%, and number of vegetative tillers by 114.28% and 42.85%, respectively (P ≤ 0.05).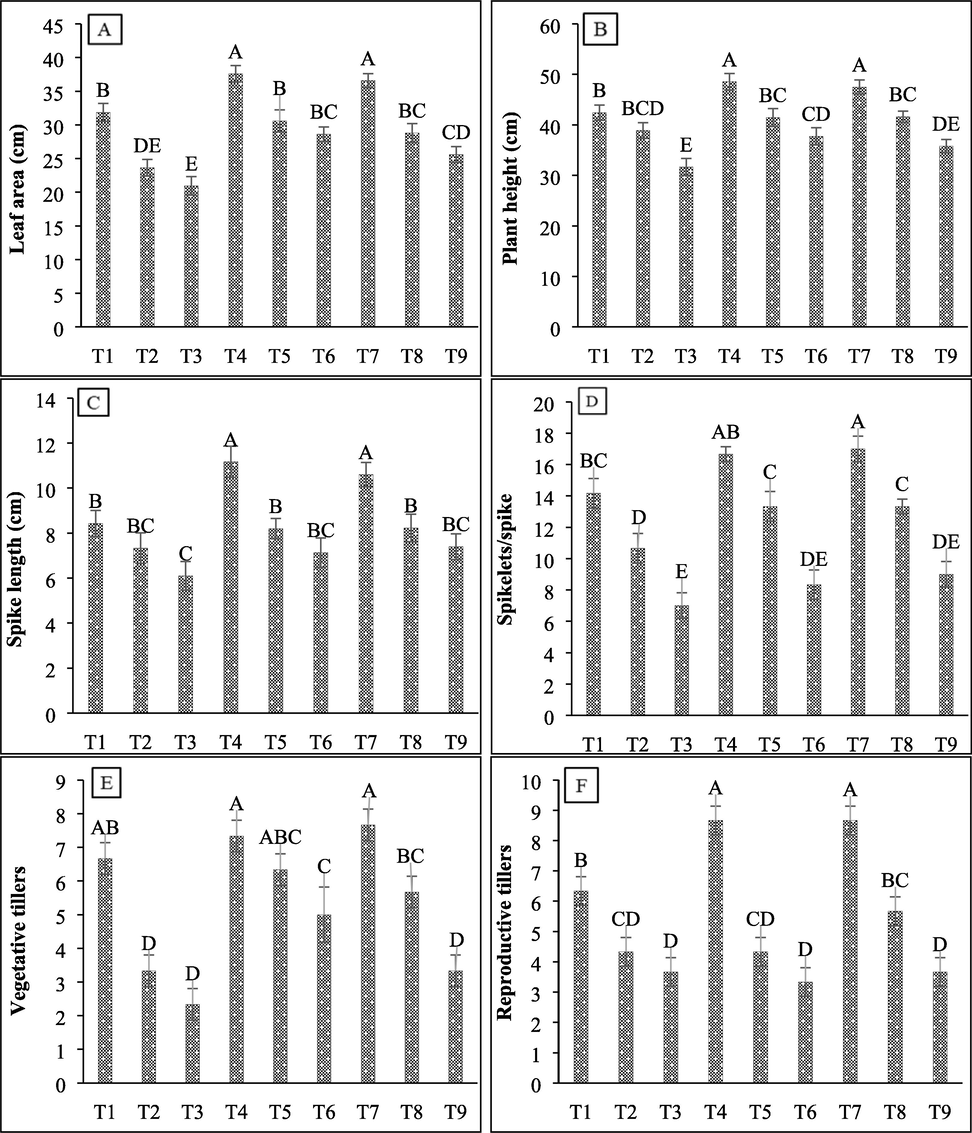
Effect of halo-tolerant PGPR on biomass and agronomic traits of wheat grown under varying salt concentrations (A) Leaf area; (B) Plant height; (C) Spike length; (D Spikelets per spike (E) Vegetative tillers; (F) Reproductive tillers. Values are mean of three biological replicates. Bars represent standard deviation of mean. TI = Un-inoculated + 0 mM NaCl, T2 = Un-inoculated + 450 mM NaCl, T3 = Un-inoculated + 600 mM NaCl, T4 = SBN01 + 0 mM NaCl, T5 = SBN01 + 450 mM NaCl, T6 = SBN01 + 600 mM NaCl, T7 = SBN02 + 0 mM NaCl, T8 = SBN02 + 450 mM NaCl, T9 = SBN02 + 600 mM NaCl, where SBN01 and SBN02 = Alcaligenes faecalis strains.
Moreover, the bacterial population of the rhizospheric region was also changed due to salt stress and bacterial inoculation as shown in Fig. 7E. The salt stress reduces the bacterial population at both concentrations of NaCl salt stress. The highest salt concentration (600 mM) causes a maximum decrease in colony forming units (3.166) of the bacterial population in the rhizosphere of wheat plants. The inoculation of both halo-tolerant bacterial isolates causes an increase in the bacterial population in both saline and non-saline conditions.
3.4.2 Effect of bacterial inoculation and salt stress on biochemical parameters
To evaluate the effects of halo-tolerant bacterial isolates on photosynthetic pigments in wheat plants under varying salt concentration, Chl a, Chl b, total Chl, and carotenoid contents were measured. Results of the present study showed that salt stress reduced Chl a, Chl b, total Chl, and carotenoid contents in wheat seedlings. However, the inoculation of halo-tolerant isolates SBN01 and SBN02 enhanced these photosynthetic pigments (Fig. 5). The highest salt concentration (600 mM) causes more damage to Chl a (59.62%), Chl b (5.36%), total Chl (28.9%), and carotenoid (44%) contents of wheat plants as compared to 450 mM NaCl concentration (27.35%, 3.48%, 13.87%, 22.89%, respectively) and. Both SBN01 and SBN02 halo-tolerant bacterial strains showed maximum increase in Chl a (83.82% and 83.34%), total Chl (22.41% and 21.68%), and carotenoid contents (39.94% and 33.35%, respectively) at 600 mM salt concentration, while in Chl b maximum increase by both isolates (3.57% and 2.87%) was observed at 450 mM salt stress.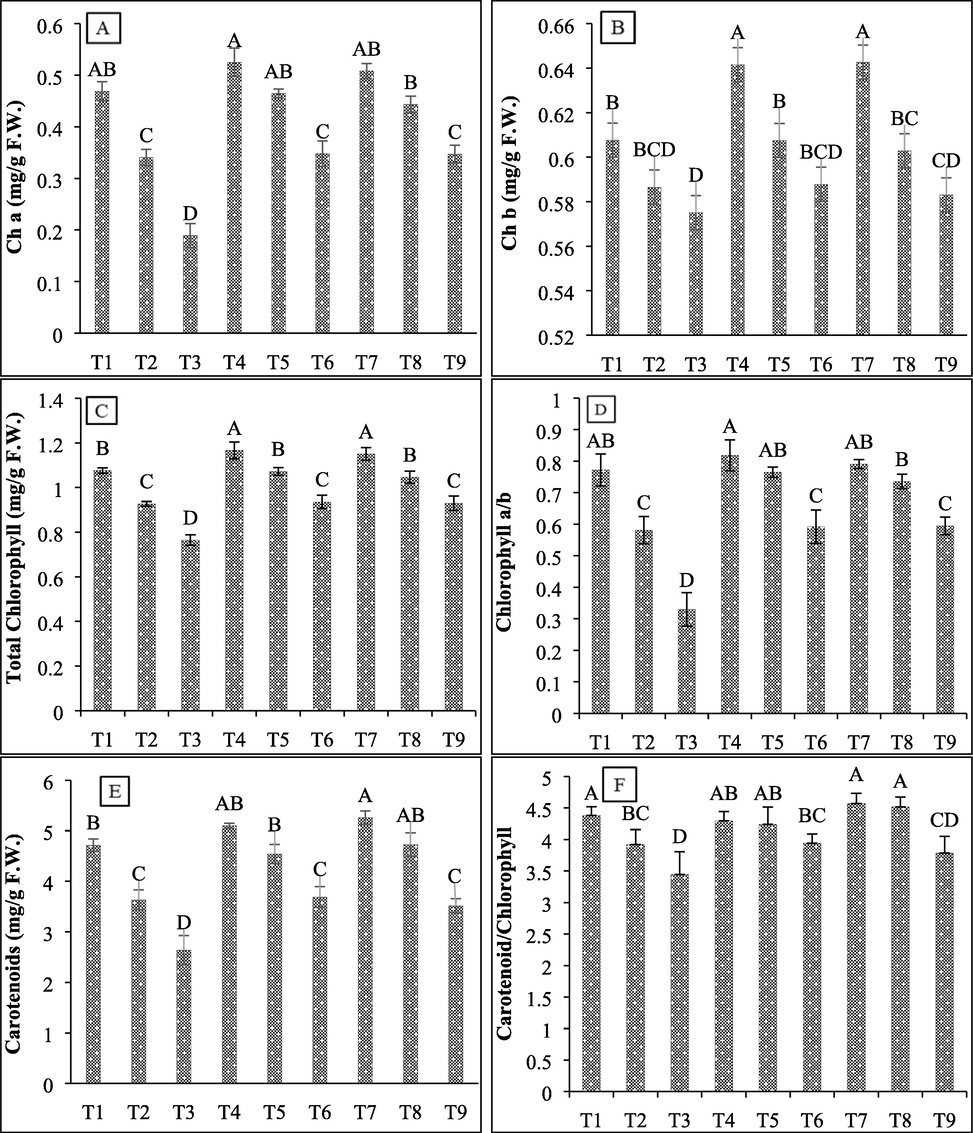
Effect of halo-tolerant PGPR on chlorophyll and carotenoid contents of wheat grown under varying salt concentrations (A) Chl a; (B) Chl b; (C) Total Chlorophyll; (D) Chl a/b ratio; (E) Carotenoid content; (F) Carotenoid/Chlorophyll ratio. Values are mean of three biological replicates. Bars represent standard deviation of mean. TI = Un-inoculated + 0 mM NaCl, T2 = Un-inoculated + 450 mM NaCl, T3 = Un-inoculated + 600 mM NaCl, T4 = SBN01 + 0 mM NaCl, T5 = SBN01 + 450 mM NaCl, T6 = SBN01 + 600 mM NaCl, T7 = SBN02 + 0 mM NaCl, T8 = SBN02 + 450 mM NaCl, T9 = SBN02 + 600 mM NaCl, where SBN01and SBN02 = Alcaligenes faecalis strains.
For evaluating the effects of salt stress and bacterial isolates in wheat plants PSII functioning was also assessed. Results showed that the increasing (450 mM and 600 mM) salt concentration decreased significantly performance index (PIABS) by 14% and 21.82%, and quantum yield (Fv/Fm) of PSII by 3% and 12.9%, respectively. The inoculation of both Alcaligenes faecalis (SBN01 and SBN02) strains at 450 mM salt stress increased PIABS by 31.29% and 63.64%, while for Fv/Fm both isolates showed a similar increase by 4%. At 600 mM salt stress, both SBN01 and SBN02 isolates increased PIABS by 34% and 45.22%, and Fv/Fm by 13% and 14%, respectively (Fig. 6A & 6B). Moreover, highest NaCl salt concentration (600 mM) increased TRo/RC by 36% and ABS/RC by 40%, while decreasing ETo/RC by 32% in wheat plants (Fig. 6C, 6D & 6E). The inoculation of SBN01 and SBN02 halo-tolerant isolates at 450 mM salt concentration showed a similar response in reducing ABS/RC by 17% and reduced TRo/RC by 1.3% and 4.17%, respectively, while increased ETo/RC by 12.3% and 11%, respectively. At 600 mM salt stress, both isolates reduced TRo/RC by 9% and 13%, ABS/RC by 25% and 20%, while increasing ETo/RC by 55 and 8%, respectively. Highest salt concentration also causes a greater increase in absorbed heat dissipation energy (DIo/RC) by 85% as compared to 450 mM concentration (79%), while the inoculation of bacterial isolates significantly reduced heat dissipation energy under saline and non-saline conditions (Fig. 6F). Both isolates showed a similar response in alleviating the damaging effects of salt stress on PSII photochemistry by 19% on 450 mM concentration and by 9% on 600 mM salt stress.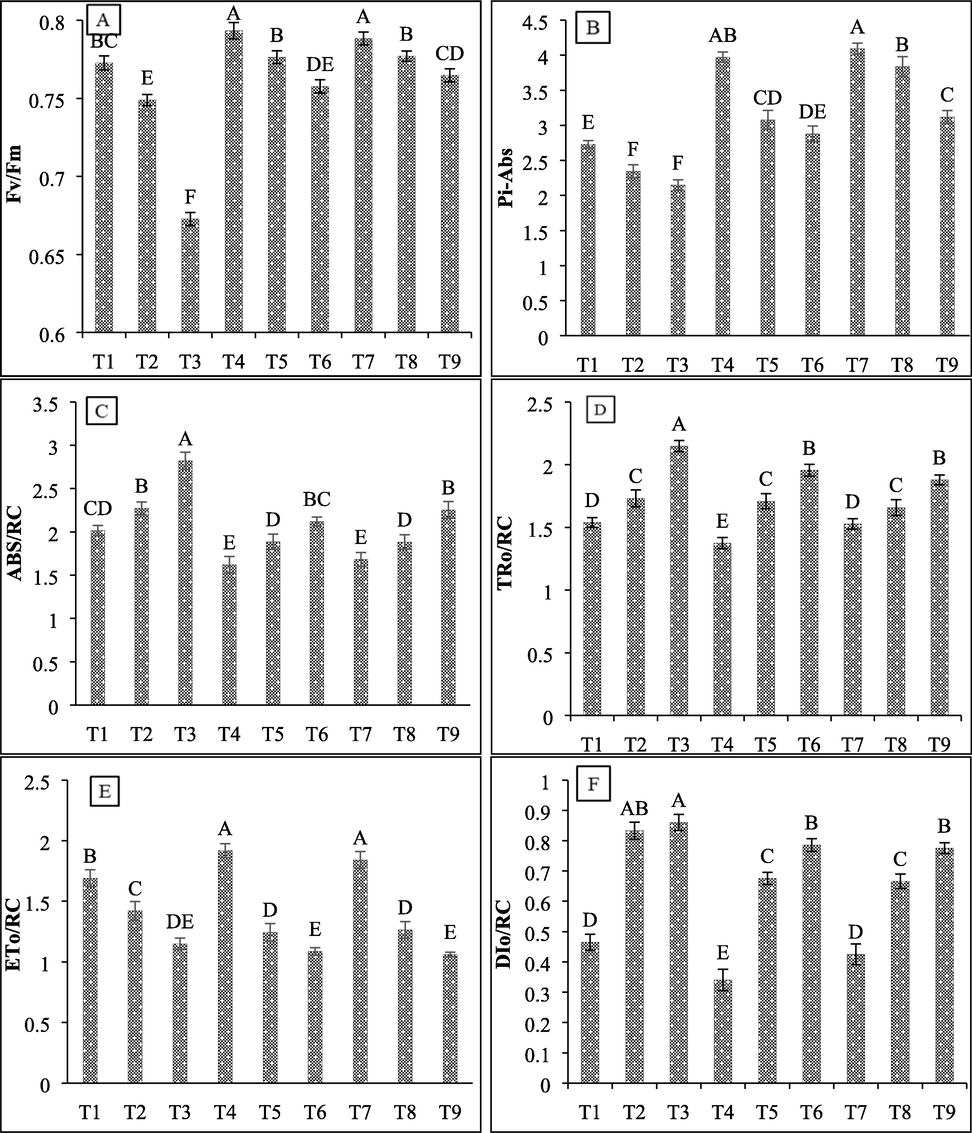
Effect of halo-tolerant PGPR on photochemistry of PSII of wheat grown under varying salt concentrations (A) Fv/Fm; (B) Pi_ABS; (C) ABS/RC; (D TRo/RC; (E) ETo/RC; (F) DIo/RC. Values are mean of three biological replicates. Bars represent standard deviation of mean. TI = Un-inoculated + 0 mM NaCl, T2 = Un-inoculated + 450 mM NaCl, T3 = Un-inoculated + 600 mM NaCl, T4 = SBN01 + 0 mM NaCl, T5 = SBN01 + 450 mM NaCl, T6 = SBN01 + 600 mM NaCl, T7 = SBN02 + 0 mM NaCl, T8 = SBN02 + 450 mM NaCl, T9 = SBN02 + 600 mM NaCl, where SBN01and SBN02 = Alcaligenes faecalis strains.
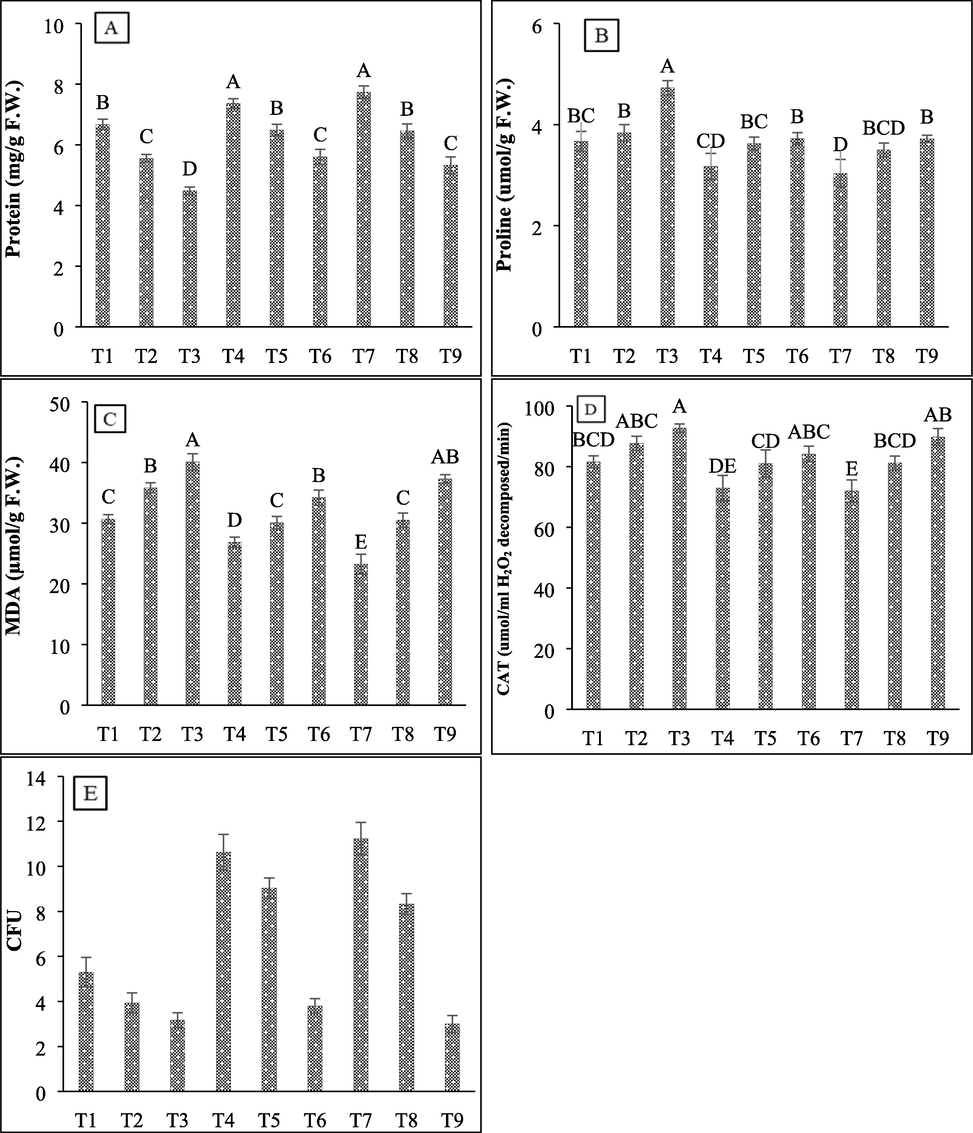
Effect of halo-tolerant PGPR on protein content, proline content, MDA, antioxidant activity of catalase, and CFU of wheat grown under varying salt concentrations (A) Protein content; (B) Proline content; (C) MDA; (D Catalase; (E) CFU after harvesting. Values are mean of three biological replicates. Bars represent standard deviation of mean. TI = Un-inoculated + 0 mM NaCl, T2 = Un-inoculated + 450 mM NaCl, T3 = Un-inoculated + 600 mM NaCl, T4 = SBN01 + 0 mM NaCl, T5 = SBN01 + 450 mM NaCl, T6 = SBN01 + 600 mM NaCl, T7 = SBN02 + 0 mM NaCl, T8 = SBN02 + 450 mM NaCl, T9 = SBN02 + 600 mM NaCl, where SBN01and SBN02 = Alcaligenes faecalis strains.
The results of the present study showed that 450 mM and 600 mM salt stress significantly decreased protein contents by 16.87% and 32.8%, respectively, while the inoculation of wheat plants with halo-tolerant SBN01 and SBN02 PGPR strains (Alcaligenes faecalis) significantly increased the soluble protein contents of wheat plants (Fig. 7A). At 450 mM NaCl concentration, SBN01 and SBN02 bacterial isolates showed a similar response in increasing the protein contents by 16%, while at 600 mM salt stress, protein contents increased by 29.94% and 18.67%, respectively. Both isolates showed a maximum increase in protein contents at the highest salt concentration.
Moreover, in our results, salt stress significantly increased proline, lipid peroxidation, and catalase activity with increasing salt concentration as compared to non-saline plants. Maximum increases in all these parameters were observed at highest NaCl concentration of 600 mM by 28.8%, 30.71%, and 13.62%, respectively as compared to 450 mM NaCl concentration (4.54%, 16.67%, and 7.52%, respectively). However, the inoculation of both halo-tolerant isolates significantly reduced proline, MDA contents, and catalase activity in PGPR inoculated salt stressed wheat plants (Fig. 7B, 7C & 7D). The inoculation of both SBN01 and SBN02 bacterial isolates at 450 mM salt stress reduced proline by 5.57% and 8.89%, MDA contents by 15.97% and 14.81%, while both showed a similar reduction in catalase activity by 7%, however, at 600 mM concentration similar decrease in proline (21%) was observed by both isolates. The decrease for MDA contents was 14.71% and 7%, and for catalase activity, 9.19% and 3%, respectively reduction was observed.
The effect of salt stress and bacterial inoculation on ionic imbalance was analyzed by measuring Na+ ions, K+ ions, and their ratio. Results showed that 450 mM and 600 mM salt stress significantly increased Na+ content by 62.08% and 104.13%, respectively, while the inoculation of SBN01 and SBN02 bacterial isolates significantly reduced Na+ accumulation under non-saline (22.33% and 9.51%, respectively) and saline conditions. At 450 mM NaCl salt stress, the inoculation of SBN01 and SBN02 halo-tolerant strains decreased leaf Na+ content by 19.75% and 17.82%, respectively, while at 600 mM salt stress, the decrease of 28.36% and 21.68% was observed by both isolates (Fig. 8A). The significant decrease in K+ ions was observed with increasing salt concentrations by 8.77% and 13.19%, respectively. The inoculation of bacterial isolates (SBN01 and SBN02) significantly increased K+ ions by 12.02% and 5.23%, respectively, under controlled conditions. During saline conditions, both halo-tolerant bacterial isolates showed a similar response in increasing K+ ions by 7% and 3%, respectively, compared to their respective controls (Fig. 8B). The highest salt stress concentration showed a maximum decrease in the K+/Na+ ratio by 57.95% compared to 450 mM NaCl salt stress (43.86%). The inoculation of SBN01 and SBN02 strains under controlled conditions significantly increased the K+/Na+ ratio by 44.69% and 15.78%, respectively. At 450 mM NaCl stress, both isolates increased the K+/Na+ ratio by 33.87% and 31.29%, respectively, while at 600 mM salt stress, both isolates showed 44.14% and 32.06% increase in ionic ratio (Fig. 8C).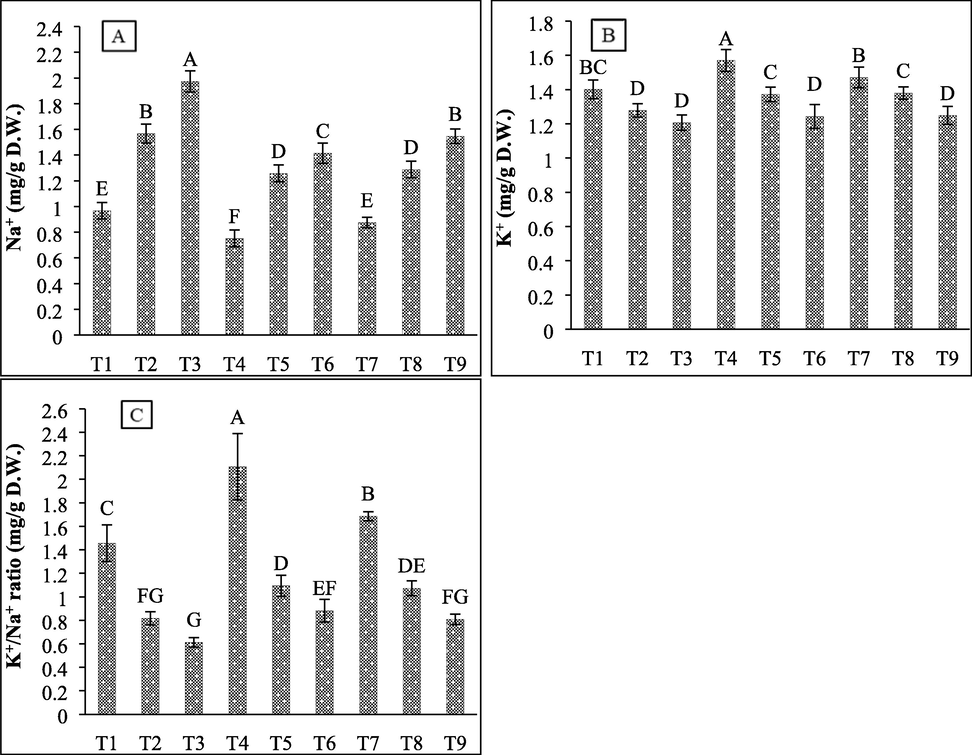
Effect of halo-tolerant PGPR on Na+ ions, K+ ions, and K+/Na+ ratio of wheat grown under varying salt concentrations (A) Na+ ions; (B) K+ ions; (C) K+/Na+ ratio. Values are mean of three biological replicates. Bars represent standard deviation of mean. TI = Un-inoculated + 0 mM NaCl, T2 = Un-inoculated + 450 mM NaCl, T3 = Un-inoculated + 600 mM NaCl, T4 = SBN01 + 0 mM NaCl, T5 = SBN01 + 450 mM NaCl, T6 = SBN01 + 600 mM NaCl, T7 = SBN02 + 0 mM NaCl, T8 = SBN02 + 450 mM NaCl, T9 = SBN02 + 600 mM NaCl, where SBN01and SBN02 = Alcaligenes faecalis strains.
4 Discussion
Salt stress significantly affects plant physiology and morphology through multiple and complex mechanisms that are directly or indirectly related to several metabolic pathways associated with different key organs. Being a decisive factor, the crop yield and plant growth decrease drastically due to constant increase in saline areas (Desoky et al., 2019). The process of developing salt-tolerant plant species or halophytes through genetic engineering or breeding remains largely ineffective and unsuccessful due to the physiological and genetic complexity of salt tolerance characteristics. The rhizospheric region of the halophytic plant acts as the reservoir for different types of halo-tolerant rhizobacteria that may increase crop development and growth in salt stress conditions. Similar to halophytic crops, salt-tolerant PGPR have developed different strategies for surviving in high salt areas. These bacteria display various stress-related characteristics that provide plant defensive potential and enhance growth during the inhibiting salt concentrations. PGPR mediated salinity tolerance in plants is one of the well-known established phenomena that provide an economically feasible strategy to combat salt stress globally (Jaemsaeng et al., 2018; Kruasuwan and Thamchaipenet, 2018). The current research was conducted to screen and isolate the halo-tolerant bacterial population from native plants of salt mine so that these isolates can be used for the cultivation of the economically important crops in salt-stressed areas.
The soil of two native plants from Khewra salt mine was sandy loamy, which showed similar pH and nitrogen content. Higher EC value of soil samples shows that the soils of Khewra salt mine contain excessive salts, which ultimately contain halo-tolerant rhizobacterial strains in its rhizosphere. These physiochemical properties influence plant growth, root development, microflora density in the rhizospheric region, and their interaction with plants. Both soil samples showed a different bacterial population in their rhizospheric region. The diversity of the bacterial population in a rhizosphere can be attributed to the soil characteristics that are important for their activities and in shaping rhizo-microbiome structure because it affects the quality and quantity of exudates released from the root (Schreiter et al., 2014). Therefore, isolated halo-tolerant PGPR from this high saline region can help plants to withstand salt stress while increasing growth and yield.
PGPR contains multiple plants beneficial traits that help them to thrive in unfavorable conditions. In this study, two different Alcaligenes faecalis strains (SBN01 and SBN02) were isolated that showed positive results for plant growth promoting (PGP) traits, expect for protease activity. IAA improves the growth of root by increasing length and number of the adventitious roots, which results in increased nutrient uptake, particularly in salt affected soil (Majeed et al., 2015; Naqqash et al., 2016). Another significant feature of PGPR is ammonia production that affects plant growth indirectly. Production of ammonia and IAA are found to be significant mediators for inducing stress tolerance and stimulating growth in a synchronized way (Singh and Jha, 2016).
Nitrogen fixing and phosphate solubilizing micro-organisms in the rhizosphere facilitate the bio-availability of nitrogen and phosphate for the growth of plants and are essential components for the manufacturing of biofertilizers. In the present study, both halo-tolerant bacterial isolates possess the potential of nitrogen fixation and phosphate solubilization. Studies showed that using halo-tolerant PGPR with the ability to fix nitrogen is regarded as an effective strategy for enhancing the growth and yield of salt-sensitive crop plants. In wheat plants, salt tolerance was increased when inoculated with a halo-tolerant strain of Bacillus licheniformis HSW16 having the potential of nitrogen fixation (Singh and Jha, 2016). Moreover, the study conducted by Upadhyay and Singh (2011) reported that phosphate solubilizing halo-tolerant strains promoted wheat growth in limited phosphate conditions. Two halo-tolerant strains of PGPR closely associated with Halobacillus spp. and Halomonas spp. having phosphate solubilizing potential showed increased pant growth during salt stress conditions (Desale et al., 2014). Based on sequence analysis of the 16S rRNA gene, SBN01 and SBN02 halo-tolerant isolates showed closeness to Alcaligenes faecalis, which is a well-known PGPR having growth promoting potential under various stress conditions. In Arabidopsis and wheat, inoculation of Alcaligenes faecalis improved salt tolerance by modulating ion transporters, ACC activity, and hormonal pathway (Bhattacharyya and Lee, 2017).
Being the phosphate solubilizing and nitrogen fixing diazotroph, Alcaligenes faecalis SBN01 and Alcaligenes faecalis SBN02 halo-tolerant isolates were evaluated for their growth-promoting ability in the wheat plant. The significant increase in growth parameters due to the Alcaligenes faecalis SBN01 and Alcaligenes faecalis SBN02 inoculation can be accredited to all PGP activities of these isolates. Previous studies also reported PGPR strains that stimulated the growth and yield of wheat crop under saline conditions. In salt stress, the extra release of bacterial auxin can also help plants for maintaining growth and increasing water uptake efficiency (Upadhyay et al., 2011; Singh and Jha, 2016). Thus, a correlation can be developed between PGP traits of halo-tolerant PGPR with improved biomass of wheat crop, suggesting that these traits not only mitigate salinity stress but also stimulate growth attributes.
The chlorophyll in the leaf is a significant physiological parameter that can be used as a plant stress indicator. Salt stress causes a reduction in crop yield and development by disrupting biosynthesis of a 5-amino-levulinic acid chlorophyll precursor, chloroplast structure, down regulating photosynthetic machinery, including synthesis of chlorophyll. Carotenoid pigments also regulate the photosynthetic process in plants by facilitating light harvesting and photoprotection (Bukhat et al., 2019). Several studies reported that salinity reduces chlorophyll and carotenoid contents in various plants such as cucumber, green bean, and basil (Tiwari et al., 2010; Golpayegani and Tilebeni, 2011). In the present study, salt stress reduced chlorophyll and carotenoid contents in wheat seedlings. The reduction in chlorophyll harmed the photosynthesis of plants that decreases biomass and growth parameters of wheat crop. However, the inoculation of halo-tolerant isolates (SBN01 and SBN02) enhanced chlorophyll contents. The inoculation of Azotobacter and Paenibacillus yonginensis strains in maize and ginseng plants increased chlorophyll and carotenoid contents during salt stress (Rojas-Tapias et al., 2012). Another study also showed similar results when paddy rice was inoculated with Bacillus pumilus and Pseudomonas pseudoalcaligenes in saline conditions (Jha and Subramanian, 2013). Thus, the improvement in chlorophyll and carotenoid can be attributed to the PGP traits that increase the biosynthesis of chlorophyll, which causes an increase in biomass and growth attributes.
Moreover, the photosynthetic capacity of a plant can also be assessed through PSII functional activity. Studies reported that the reduced activity of photosynthesis during salinity stress is caused by the functional and structural damage to electron carriers and photosystems. Mostly, long term exposure to high concentrations of salt stress causes structural damage to PSII (Bukhat et al., 2019). In this study, the increasing NaCl concentration decreased significantly PIABS and Fv/Fm of PSII while inoculation of Alcaligenes faecalis SBN01 and Alcaligenes faecalis SBN02 strains significantly increased these PSII parameters during salt stressed conditions. A positive interaction was recorded between primary photochemistry, the density of active RC, PIABS, and electron transport quantum yield. Thus, the inoculation of Alcaligenes faecalis SBN01 and SBN02 strains, improved the photosynthetic ability of salt stressed wheat plants by stabilizing electron transport and PSII activity. Inoculation of Pseudomonas fluorescens to Pinus halepensis showed increased efficiency of PSII (Rincón et al., 2008). It is well documented that alterations in PSII quantum yield are due to a decrease in oxidizing QA− pool and electron transport inhibition from the donor side or oxygen evolving complex (Bukhat et al., 2019). The increasing salt concentration in this study increased TRo/RC and ABS/RC while decreasing ETo/RC in wheat plants, which shows that the inactivation of RC causes an increase in antenna size. Salt stress also causes a greater decrease in QA− and also reduces electron transfer efficiency from QA− to QB. As a result, absorbed DIo/RC may have increased due to activated RC, which causes a reduction in overall PIABS. A study conducted in wheat plants showed that salt stress induced less damage on PSII acceptor end as compared to PSII donor end (Bukhat et al., 2019). Improvement in PIABS and Fv/Fm of PSII due to halo-tolerant PGPR inoculation can be due to increased active RC density per antenna chlorophyll, thus, decreasing TRo/RC and ABS/RC. Similar results were observed in Arabidopsis when inoculated with Pseudomonas knackmussii MLR6 during salt stress (Rabhi et al., 2018). Thus, from the results of the present study and previous findings, it can be concluded that salinity stress causes a reduction in the antenna of photosynthetic pigments and damages their conformation assemblies that results in reduced PSII efficiency.
Protein contents in this study were significantly decreased due to salt stress while the inoculation of wheat plants with halo-tolerant SBN01 and SBN02 PGPR strains significantly increased the soluble protein contents of plants that could be due to the increased N availability provided by PGPR. These results are similar to the study conducted in chickpea inoculated with Rhizobium that increases protein contents in salt stressed plants. The decrease in protein was also reported in different plants (Metwali et al., 2015). Furthermore, PGPR could assist plant growth by alleviating the deleterious impacts of salinity through the accumulation of glutamate and proline (Karlidag et al., 2013). In our results, salt stress significantly increased proline as compared to non-saline plants, while the inoculation of halo-tolerant isolates significantly reduced proline in PGPR inoculated salt stressed wheat plants. The increased accumulation of proline in the wheat plant suggests an outstanding mechanism for mitigating osmotic potential in the salt stressed plant. This validates the assumption that the accumulation of proline is a component of plant physiological response against severe stress (Metwali et al., 2015). Numerous PGPR strains as Bacillus, Burkholderia, and Arthrobacter increased proline synthesis and accumulation in salt stressed plants that help to maintain the water status of a cell, which facilitates the plant for coping with saline conditions (Karlidag et al., 2013).
Abiotic stresses trigger cellular level production of ROS such as hydroxyl radicals, hydrogen peroxide, and radical superoxide, resulting in membrane lipid peroxidation (Bukhat et al., 2019). Plants have various antioxidant enzymes having the ability to lower the amount of ROS and decrease oxidative stress. In the present study, salt stress significantly increased lipid peroxidation that was decreased significantly due to the inoculation of halo-tolerant bacterial isolates. Our results are similar to previous findings in different plants that showed decreased MDA contents when inoculated with Dietzia natronolimnaea, Enterobacter EN-21, Bacillus subtilis GB03 and Streptomyces spp. GMKU 336 (Jaemsaeng et al., 2018; Kruasuwan and Thamchaipenet, 2018). Increased proline accumulation also helps to reduce ROS induced damage by modulating antioxidant machinery.
Alleviation of oxidative damage caused by salinity with the help of antioxidant enzymes serves as a significant approach for crops to increase their tolerance against stresses. In the present study, catalase activity increased significantly during salt stress while the application of halo-tolerant PGPR strains reduced catalase activity in saline conditions as compared to uninoculated plants. The decreased activity of catalase by halo-tolerant PGPR is expected due to their ability to detoxify ROS through various plant protecting mechanisms such as proline accumulation. Moreover, it is expected that these bacterial isolates will be associated with the surface of the root and can modulate the activity of antioxidants as systemic resistance against salinity (Hashem et al., 2016). Thus, Alcaligenes faecalis induced salt stress tolerance can be linked with antioxidant capacity and osmoprotectant of plants.
The influx of potassium ions and efflux of sodium ions are the plant mechanisms for reducing the salt-induced ionic imbalance and oxidative stress (Bukhat et al., 2019). In our study, the inoculation of halo-tolerant bacterial strains decreased Na+ uptake while increasing K+ ions uptake; thus, favoring the K+/Na+ ratio. Our findings are congruent with the study conducted on wheat plants in which the inoculation of Stenotrophomonas maltophilia increased uptake of K+ ions by 20% to 28% and decreased Na+ ions accumulation by 25% to 32% (Singh and Jha, 2016). Another study reported that the co-inoculation of Arthrobacter sp. and Bacillus subtilis on wheat plants increases the potassium ions accumulation while decreasing the uptake of sodium ions and activity of antioxidant enzymes (Upadhyay et al., 2011). These changes in ion content can be correlated to PGP traits of bacterial strains in regulating plant growth and physiological mechanisms in diverse stressful conditions.
Both halo-tolerant bacterial isolates improved the growth and biochemical activities of the wheat plant under varying salt concentrations. The response of both isolates was more positive at 450 mM salt stress, which is statistically at par with uninoculated control plants. Thus, we can conclude that our isolates possess the mitigating potential against the damaging effects of salt stress.
5 Conclusion
The present work is fundamental research that gave insight into the halo-tolerant bacterial community in the rhizosphere of native plants of Khewra salt mine in Pakistan. The study has demonstrated several PGP traits in these halo-tolerant isolates such as IAA production, N2-fixation, and P-solubilization that not only help the plant to withstand salt stress but also facilitate the growth of wheat plants under varying salt concentrations. These traits are regarded as significant PGP characteristics that reduce detrimental effects of salt stress by increasing uptake of K+ ions, accumulation of osmolytes, enhancing photosynthetic ability, and PSII efficacy while decreasing lipid peroxidation and Na+ ions accumulation. Thus, these halo-tolerant bacterial strains offer the potential to be used as PGP agents for the cultivation of wheat in salt affected areas. For practical and useful applications of PGPR in a field, further research should be done on detailed functional and molecular characterization of halo-tolerant PGPR strains.
Author contribution
MB and TN designed the experimentation, SR and RA performed experiments and wrote the manuscript, KA, SR and HR analyzed the data, IM, MKH and TN critically revised the manuscript and approved it for publication. All the authors approved the contents of the paper and declared no conflicting interest.
Acknowledgments
This study was partially funded by Directorate of Research and External Linkages, Bahauddin Zakariya University, Multan, Pakistan under Non-PhD Research Grant Program via grant no. DR & EL/D-545.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Halotolerant PGPR: A hope for cultivation of saline soils. J. King Saud Univ. Sci. 2019:1195-1201.
- [Google Scholar]
- Enhanced production of extra cellular alkaline protease in Bacillus circulance through plasmid transfer. Res. J. Agric. Biol. Sci.. 2006;16:526-530.
- [Google Scholar]
- Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol.. 1949;24:1.
- [Google Scholar]
- Rapid determination of free proline for water-stress studies. Plant Soil.. 1973;39:205-207.
- [Google Scholar]
- A cocktail of volatile compounds emitted from Alcaligenes faecalis JBCS1294 induces salt tolerance in Arabidopsis thaliana by modulating hormonal pathways and ion transporters. J. Plant Physiol.. 2017;214:64-73.
- [Google Scholar]
- A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72:248-254.
- [Google Scholar]
- Salicylic acid induced photosynthetic adaptability of Raphanus sativus to salt stress is associated with antioxidant capacity. J. Plant Growth Regul. 2019:1-14.
- [Google Scholar]
- Exploring photosynthesis and plant stress using inexpensive chlorophyll fluorometers. J. Nat. Resour. Life Sci. Educ.. 2010;39:22-30.
- [Google Scholar]
- Plant growth promoting properties of Halobacillus sp. and Halomonas sp. in presence of salinity and heavy metals. J. Basic Microbiol.. 2014;54:781-791.
- [Google Scholar]
- Stimulating antioxidant defenses, antioxidant gene expression, and salt tolerance in Pisum sativum seedling by pretreatment using licorice root extract (LRE) as an organic biostimulant. Plant Physiol. Biochem.. 2019;142:292-302.
- [Google Scholar]
- Integrative application of licorice root extract or lipoic acid with fulvic acid improves wheat production and defenses under salt stress conditions. Ecotoxicol. Environ. Safety. 2020;190:110144
- [Google Scholar]
- Effect of biological fertilizers on biochemical and physiological parameters of basil (Ociumum basilicm L.) medicine plant. Am. Eurasian. J. Agric. Environ. Sci.. 2011;11:411-416.
- [Google Scholar]
- Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in US adults: the Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care.. 1998;21:518-524.
- [Google Scholar]
- The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress. Front. Microbiol.. 2016;7:1089.
- [Google Scholar]
- Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophy.. 1968;125:189-198.
- [Google Scholar]
- Molecular interaction of 1-aminocyclopropane-1-carboxylate deaminase (ACCD)-producing endophytic Streptomyces sp. GMKU 336 towards salt-stress resistance of Oryza sativa L. cv. KDML105. Sci. Rep.. 2018;8:1950.
- [Google Scholar]
- Paddy physiology and enzymes level is regulated by rhizobacteria under saline stress. J. Appl. Bot. Food Qual.. 2013;85:168.
- [Google Scholar]
- Plant growth-promoting rhizobacteria mitigate deleterious effects of salt stress on strawberry plants (Fragaria× ananassa) Hortscience. 2013;48:563-567.
- [Google Scholar]
- 1-aminocyclopropane-1-carboxylate (ACC) deaminase-producing endophytic diazotrophic Enterobacter sp. EN-21 modulates salt–stress response in sugarcane. J. Plant Growth Regul.. 2018;37:849-858.
- [Google Scholar]
- MEGA, X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018:1547-1549.
- [Google Scholar]
- Isolation and characterization of plant growth-promoting rhizobacteria from wheat rhizosphere and their effect on plant growth promotion. Front. Microbiol.. 2015;6:198.
- [Google Scholar]
- Assessment of the plant growth promotion abilities of six bacterial isolates using Zea mays as indicator plant. Soil Biol. Biochem.. 2010;42:1229-1235.
- [Google Scholar]
- Alleviation of salinity stress in faba bean ('Vicia faba'L.) plants by inoculation with plant growth promoting rhizobacteria (PGPR) Plant Omics.. 2015;8:449.
- [Google Scholar]
- Salt-tolerant and plant growth-promoting bacteria isolated from Zn/Cd contaminated soil: identification and effect on rice under saline conditions. J. Plant Interact.. 2014;9:379-387.
- [Google Scholar]
- Differential response of potato toward inoculation with taxonomically diverse plant growth promoting rhizobacteria. Front. Plant Sci. 2016:144.
- [Google Scholar]
- Methods for growing Spirillum lipoferum and for counting it in pure culture and in association with plants. Appl. Environ. Microbiol.. 1977;33:85-88.
- [Google Scholar]
- Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Mikrobiologiya. 1948;17:362-370.
- [Google Scholar]
- Pseudomonas knackmussii MLR6, a rhizospheric strain isolated from halophyte, enhances salt tolerance in Arabidopsis thaliana. J. Appl. Microbiol.. 2018;125:1836-1851.
- [Google Scholar]
- Resolution of (+/-)-alpha-(3-benzoylphenyl)-propionic acid (Ketoprofen) and diastereomer interactions of its enantiomers with some biological systems. Chimia. 1975;29:170-172.
- [Google Scholar]
- Diagnosis and Improvement of Saline and Alkali Soils. Vol 78. US Government Printing Office; 1954.
- Water stress responses of two Mediterranean tree species influenced by native soil microorganisms and inoculation with a plant growth promoting rhizobacterium. Tree Physiol.. 2008;28:1693-1701.
- [Google Scholar]
- Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays) Appl. Soil Ecol.. 2012;61:264-272.
- [Google Scholar]
- Determination of catalase activity by means of the Clark oxygen electrode. Biochim. Biophys. Acta. 1967;139:171-173.
- [Google Scholar]
- Isolation and characterization of indole acetic acid (IAA) producing Klebsiella pneumoniae strains from rhizosphere of wheat (Triticum aestivum) and their effect on plant growth. Indian J. Exp. Biol.. 2009;47:993-1000.
- [Google Scholar]
- Effect of the soil type on the microbiome in the rhizosphere of field-grown lettuce. Front. Microbiol.. 2014;5:144.
- [Google Scholar]
- A halotolerant bacterium Bacillus licheniformis HSW-16 augments induced systemic tolerance to salt stress in wheat plant (Triticum aestivum) Front. Plant Sci.. 2016;7:1890.
- [Google Scholar]
- First report of diazotrophic Brevundimonas spp. as growth enhancer and root colonizer of potato. Sci. Rep.. 2020;10
- [Google Scholar]
- Effect of salt stress on cucumber: Na+–K+ ratio, osmolyte concentration, phenols and chlorophyll content. Acta Physiol. Plant.. 2010;32:103-114.
- [Google Scholar]
- Effect of rice bean (Vigna umbellata) inter-cropping on the yield of perennial grass, Panicum maximum cv. Gaton under rain-fed conditions. J. Agric. Social Sci.. 2007;3:70-72.
- [Google Scholar]
- Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedosphere. 2011;21:214-222.
- [Google Scholar]
- A manual for the Practical Study of the Root-Nodule Bacteria. Blackwell Scientific Publ; 1970. 45 s. Z







