Translate this page into:
Mimosa pudica alleviates streptozotocin-induced diabetes, glycemic stress and glutathione depletion in Wistar Albino Rats
⁎Corresponding author. subashis@srmist.edu.in (Subashini Thirukkalukundram Singarapriyavardhanan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Diabetes mellitus is a chronic metabolic disease that progresses to many microvascular complications. The phytoconstituents derived from herbs are thought to be important sources of anti-diabetic drug candidates. Mimosa pudica is one of such traditional herbal medicines having wide usage in treating diabetes mellitus. This study aims to validate the protective role of aqueous and alcoholic extracts of whole plant of Mimosa pudica in streptozotocin induced diabetic wistar albino rats.
Methods
The overnight fasted wistar rats were initially treated with nicotinamide followed by streptozotocin in order to induce type 2 diabetes. After 72 h, the fasting blood glucose analysis was done and animals were further allocated to 5 different groups - Control group (no STZ administration); STZ control (with STZ administration); Group III (M. pudica ethanol extract 400 mg/kg; ME group); Group IV (M. pudica aqueous extract 400 mg/kg; MA); Group V (metformin 200 mg/kg). The treatment drugs were administered once a day for three weeks. The fasting blood glucose on the initial (day 0) and final (21st) day of the experiment was determined. Parameters like plasma insulin and hepato-renal function parameters, lipid peroxidation and antioxidants were estimated using standard kits. The animals were then sacrificed and liver tissues were collected for tissue analysis.
Results
Single-dose streptozotocin (STZ) significantly increased blood glucose level, HbA1c, liver enzymes and lipid peroxidation, whereas repeated administration of extracts of Mimosa pudica significantly indicated a strong protective effect in the treated animals. The cellular pool of reduced glutathione and subsequent reduction in the lipid peroxidation status and improved insulin production are also well-corroborated. The plant extracts have shown statistically significant reduction of oxidative stress thereby showing antioxidant property by restoring the enzymatic and non-enzymatic antioxidant molecules to nearly normal levels.
Conclusion
Overall, the study concludes that the phytoconstituents of M. pudica significantly controls the hyperglycaemic stress and glutathione depletion thereby provide therapeutically efficacious drug molecules for treating diabetes mellitus and its complications.
Keywords
Anti-diabetic activity
Mimosa pudica
Streptozotocin
Oxidative stress
Hyperglycemia
Glutathione
- ALP
-
Alkaline phosphatase
- ALT
-
Alanine transaminase
- AST
-
Aspartate transaminase
- CAT
-
Catalase
- CPCSEA
-
The committee for the Purpose of Control and Supervision of Experiments on Animals
- GPx
-
Glutathione peroxidase
- GSH
-
Reduced glutathione
- GST
-
Glutathione-S-transferase
- γ-GTP
-
γ-Glutamyl transpeptidase
- IAEC
-
Institutional Animal Ethics Committee
- MA
-
Aqueous extract of Mimosa pudica
- ME
-
Ethanolic extract of Mimosa pudica
- SOD
-
Superoxide dismutase
- STZ
-
Streptozotocin
- TBA
-
Thiobarbituric acid
Abbreviations
1 Introduction
The current century is dominated by chronic degenerative diseases compared to the last century of infectious diseases. Among the chronic diseases, diabetes mellitus is the prominent one with a higher morbidity rate and accounting for large mortality across the globe. The diseases in well-characterized with altered insulin sensitivity and increased glycemic load (Chen et al., 2017). The impaired glucose metabolism is often associated with higher glycemic load and subsequent activation of polyol pathway enzymes and thereby inducing glycemic stress (Luo et al., 2016). These oxidative damages induced to the different body parts leads to the development of secondary microvascular complications including nephropathy and retinopathy (Ginter and Simko, 2012). In the past few decades, there is an alarming increase in the diabetic population around the world and especially in developing countries including India (Saeedi et al., 2019; Lin et al., 2020). Recent epidemiological surveys indicated that over 150 million diabetic patients exist all over the world and which is projected to increase above 300 million by 2025 (Standl et al., 2019). Similarly, it has been proposed that the number of diabetic patients may be projected to be above 57 million by 2025 (Geldsetzer et al., 2018). Lack of early diagnosis and timely interventions may cause diabetic complications. Persistent hyperglycemia and hypertension are responsible for the development of microvascular complications like diabetic retinopathy, neuropathy and nephropathy (Ahmed et al., 2017). Thus the loss of life from premature death among persons with diabetes is greatest in developing countries.
Alternative systems of medicines provide valuable information’s regarding medicinal herbs useful in the management of diabetics and their complications (Shweta and Arunaksharan, 2017). Many such documented herbs have shown significant effects on lowering the elevated blood sugar level and in manage diabetic complications. In the present scenario, the clinically employed drugs are expensive and are not devoid of any secondary clinical complications during prolonged application (Gor et al., 2020). Hence research is being conducted to discover a new hypoglycemic drug with a high efficacy with minimal side effects. Though insulin is a highly useful tool in the management of diabetes/ hyperglycemia, it is also known to be associated with various risk factors such as local lipodystrophy at the injection site and weight gain after chronic treatment (Home and Itzhak, 2020).
This has led to the quest for novel anti-diabetic molecules that are having lesser secondary issues with respect to patient health. Anti-diabetic properties of plants have been well documented and many of them are used in Ayurveda (Butala et al., 2017). Several scientific pieces of research in herbals revealed that the medicinal herbs have potential hypoglycemic activities and can be a better alternative to their synthetic counterparts. It is therefore important to utilize the traditionally engaged herbal medicines in the pharmaceutical arena for the successful treatment of diabetes and associated secondary complications.
Mimosa pudica L. (Fabaceae) is a common plant with higher levels of phytochemicals including tannins, steroids, triterpenes and flavonoids glycosides, and other phenolic constituents (Parvathy et al., 2021). The root extracts of M. pudica was used against cobra bite by traditional users in the Villupuram district of Tamil Nadu (Vijay Kumar, 2021). The extracts of M. pudica are widely used in the treatment of headache, migraine, stomach-aches, as a sedative, to stop menstruation insomnia, diarrhoea, dysentery, fever, diabetic mellitus, piles and fistula (Majeed et al., 2021). It has also been reported that the extracts of M. pudica beneficially modulate stress, learning and memory in mice (Patro et al., 2016). In arginine induced acute pancreatitis the extracts of M. pudica has been found to be having ameliorative potentials (Kaur et al., 2016). The protective effect of the plant is also evident in other models such as heavy metal toxicity or organ toxicities (Onyije et al., 2018).
Besides these benefits, the plant is also reported to have hypoglycemic efficacy in multiple models. Tunna et al. (2015) has indicated the use of M. pudica in the management of glycemic stress associated with diabetes by the traditional communities of South Africa. Further, experimental evidence has indicated that the leaves of the plant are good hypoglycemic agents (Parasuraman et al., 2019). However, the tested extracts were predominantly alcoholic and therefore the efficacy can’t be corroborated to its traditional uses. Hence, we attempted to compare the efficacy of ethanol and aqueous extracts of the M. pudica against the glycemic insults induced by STZ in murine models.
2 Materials and methods
2.1 Experimental animals
Wistar rats (140 ± 20 g) of either sex were supplied by the Tamil Nadu Veterinary and Animal Science University, Chennai, Tamil Nadu and housed with six in each group. The animals were cared for as per the CSPCSEA guidelines and had access to food and water ad libitum. The protocols used in the study were approved vide IAEC approval No. 07/034/04 and monitored by the institutional ethics committee.
2.2 Plant material
Mimosa pudica whole plants used in the study were collected from Chinthareddypalem in the outskirts of Nellore, Andhra Pradesh. The materials were authenticated by the chief Botanist and a voucher specimen was deposited (voucher No. 00591) in the institutional herbarium of Captain Srinivasa Murti Central Ayurveda Research Institute, Chennai, India.
2.3 Preparation of extracts
The whole plant of M. pudica was cut into smaller pieces, dried under shade and powdered. It was then mixed with ethanol or water separately (10%). Ethanol extraction was done by means of Soxhlet apparatus (ME) and aqueous extraction was conducted by cold percolation method (MA) and obtained extracts were concentrated using a vacuum concentrator. The yield of the ME (ethanolic extract) and MA (aqueous extract) was 10.2% and 6.9% respectively.
2.4 Experimental design of STZ induced diabetes in rats
The overnight fasted Wistar rats were randomly divided into two groups and of which one was kept untreated (Control). The other rats were initially treated with a dose of 120 mg/kg nicotinamide (i.p.). The animals were then administered with a dose of 60 mg/kg streptozotocin (STZ) after 15 min and supplied with a glucose solution for drinking. After 72 h, all the animals were subjected to fasting blood sugar analysis and further allocated to different groups (n = 6).
The grouping was as follows; control group (no STZ administration); STZ control (with STZ administration); Group III (M. pudica ethanol extract 400 mg/kg; ME group); Group IV (M. pudica aqueous extract 400 mg/kg; MA); Group V (metformin 200 mg/kg). The treatment drugs were administered once a day for three weeks. The fasting blood glucose on the initial (day 0) and final (21st) day of the experiment was determined using Accu-Chek Active blood glucometer. After blood glucose estimation, all the animals were anaesthetized, blood was collected using retro-orbital plexus. The serum and plasma were separated; parameters like plasma insulin and hepato-renal function parameters were estimated using standard kits. The animals were then sacrificed and liver tissues were collected for tissue analysis. A cleaned section of the liver tissue was used for histological studies and other parts were used for liver homogenate preparation for biochemical analysis (Narayanankutty et al., 2017).
2.5 Analysis of antioxidant enzyme activities and non-enzymatic antioxidant level estimation
The activities of radical reaction termination enzymes such as superoxide dismutase (SOD- superoxide scavenging enzyme) (Narayanankutty et al., 2016), catalase (CAT- peroxide scavenging enzyme, glutathione peroxidase (GPx- peroxide scavenging enzyme utilizing reduced glutathione) and glutathione-s-transferase (GST- glutathione dependent xenobiotic detoxification enzyme) were determined by the standard protocols (Ravi et al., 2019). Non-enzymatic parameters such as lipid peroxidation or thiobarbituric acid reactive substances, reduced glutathione, vitamin E (α-Tocopherol), Vitamin C was determined using specific biochemical methods determined previously (Narayanankutty et al., 2020). The level of glycated haemoglobin was determined using the TBA method (Khalili et al., 2013).
2.6 Statistical analysis
The results obtained from the analysis were entered and sorted with the help of MS office Excel and further processing was carried out using one way-ANOVA/ Tukey’s multiple comparison test (SPSS windows student version 7.5).
3 Results
3.1 Effectiveness on the different Mimosa pudica extracts on glycemic status
Intra-peritoneal administration of STZ caused a significant elevation in the basal (on day 0) random blood glucose level in comparison with non-STZ treated murines. Oral administration of ME and MA for 21 days causes a marked reduction in serum glucose levels compared to the STZ alone group. Reference standard metformin administration was also induced a significant diminution in the blood sugar levels. However, it was noteworthy that there were no significant changes between ME and MA treatment group with that of metformin treatment group (Fig. 1).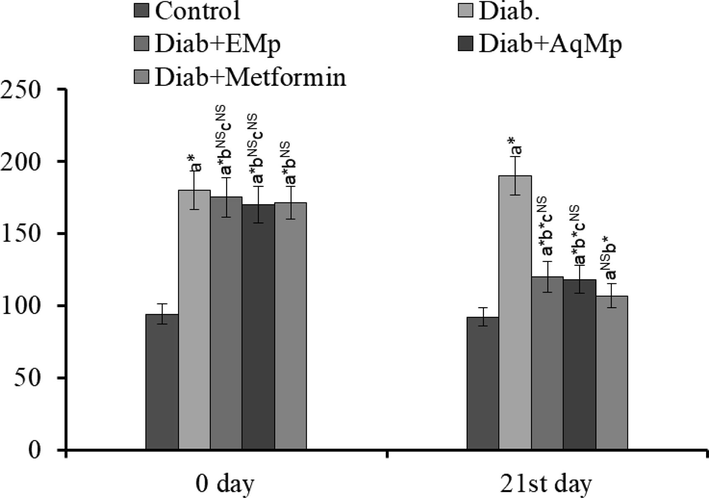
Changes in the initial fasting blood glucose levels and at the end of 21st day in the diabetic rats and the protective effect of Mimosa pudica ethanol (ME) and aqueous (MA) extracts.
3.2 M. pudica extract treatment and changes in the fasting plasma insulin levels
Results indicated a marked and statistically significant reduction in the fasting insulin levels of diabetic rats compared to the normal animals (P < 0.001). However, pre-treatment with the MA and ME successfully improved the fasting plasma insulin levels along with the metformin (p < 0.05) (Fig. 2).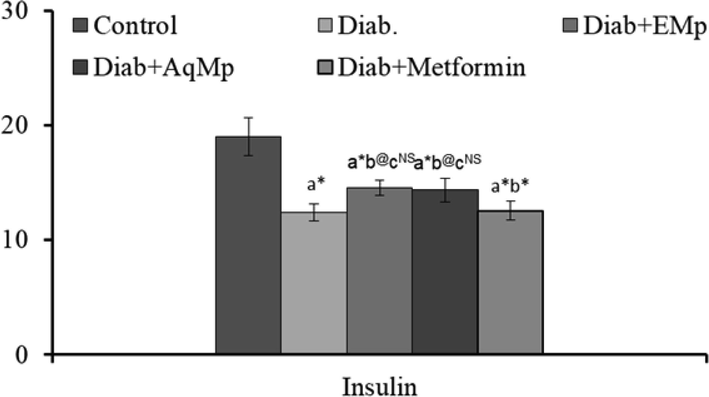
Changes in the levels of plasma insulin in streptozotocin induced diabetic rats and the protective effects of Mimosa pudica ethanol (ME) and aqueous (MA) extracts.
3.3 Impact of Mimosa pudica on antioxidant parameters in diabetic rats
Streptozotocin induced diabetes caused significant down regulation of antioxidant enzyme activities (p < 0.001). Repeated administration of ME and MA caused significant preservation of antioxidant enzymes in comparison with the STZ control group (p < 0.001, p < 0.05). Metformin administered group rats showed a comparable level of all the enzymatic activities with that of normal control and also attenuated the diabetes-associated decrease in the cellular enzymatic antioxidants (p < 0.01) (Table 1). Symbols represent statistical significance: * = p < 0.001, # = p < 0.01, @ = p < 0.05 and NS = Not significant.
Parameters
Control
STZ group
M. pudica ethanol extract
M. pudica aqueous extract
Standard Metformin
SOD (Units/mg protein)
9.00 ± 0.69
5.30 ± 0.37
a*7.90 ± 0.66
a@ b* cNS
7.50 ± 0.54
a# b* c@
8.73 ± 0.63
aNS b*
CAT (µmoles of H2O2 Consumed/mg protein/ min)
60.60 ± 4.94
42.00 ± 3.32
a*55.00 ± 4.01
aNS b* cNS
52.00 ± 3.51
a# b# c@
59.20 ± 4.50
aNS b*
GPx (µg of glutathione utilized/mg protein/min)
17.80 ± 1.38
11.50 ± 0.88
a*16.60 ± 0.94
aNS b* cNS
15.30 ± 1.34
a@ b* c@
17.40 ± 1.50
aNS b*
GST (Units/mg protein)
0.50 ± 0.03
0.33 ± 0.02
a*0.46 ± 0.02
aNS b* cNS
0.44 ± 0.02
a@ b* c@
0.50 ± 0.03
aNS b*
The non-enzyme antioxidants molecules like GSH, vitamin E, vitamin C were shown to have a marked decline in the rats administered with streptozotocin (60 mg/kg) (p < 0.001). Both ME and MA administration led to significant attenuation of streptozotocin induced decrease in GSH (p < 0.05), vitamin E (p < 0.05) and vitamin C (p < 0.001). Likewise, compared to the STZ control animals, metformin administered rats have significant elevation in the antioxidant content in their hepatic tissues (p < 0.001) (Table 2). Symbols represent statistical significance: * = p < 0.001, # = p < 0.01, @ = p < 0.05 and NS = Not significant.
Parameters
Control
STZ group
M. pudica ethanol extract
M. pudica aqueous extract
Standard Metformin
GSH (µmoles of GSH/mg protein)
7.50 ± 0.54
5.50 ± 0.37
a*6.80 ± 0.66
aNS b* cNS
6.50 ± 0.32
a@ b@ c@
7.35 ± 0.51
aNS b*
Vitamin E (µg/mg protein)
6.06 ± 0.53
4.48 ± 0.28
a*5.53 ± 0.29
aNS b* cNS
5.23 ± 0.40
a# b@ c@
5.90 ± 0.36
aNS b*
Vitamin C (µg/mg protein)
1.26 ± 0.08
0.51 ± 0.03
a*1.11 ± 0.06
a@ b* cNS
1.05 ± 0.07
a* b* c#
1.22 ± 0.09
aNS b*
3.4 M. pudica extract administration and changes in serum biochemical parameters
The serum biochemical parameters tested were urea, uric acid, creatinine and blood urea nitrogen were elevated during the induction STZ-mediated diabetes. Further, 21-day treatment with MA and ME was efficiently mitigated the elevation in the renal function marker and caused an attenuation in the elevated renal function markers (p < 0.001). In contrast, the serum protein was showing a marked reduction during STZ treatment; whereas, the drug treatment also mitigated such a decline in serum protein content (Table 3). Symbols represent statistical significance: * = p < 0.001, # = p < 0.01, @ = p < 0.05 and NS = Not significant.
Parameters
Control
STZ group
M. pudica ethanol extract
M. pudica aqueous extract
Standard Metformin
Urea (mg/dL)
32.66 ± 2.16
45.00 ± 3.74
a*38.00 ± 2.89
a@ b# cNS
39.66 ± 3.72
a# b@ c#
33.50 ± 2.34
aNS b*
Uric acid (mg/dL)
3.20 ± 0.23
4.20 ± 0.32
a*3.60 ± 0.20
a@ b# cNS
3.70 ± 0.20
a# b# c@
3.30 ± 0.17
aNS b*
Creatinine (mg/dL)
1.29 ± 0.07
1.68 ± 0.08
a*1.39 ± 0.07
aNS b* cNS
1.47 ± 0.07
a# b* c@
1.34 ± 0.07
aNS b*
Blood Urea Nitrogen (mg/dL)
21.91 ± 1.42
31.00 ± 2.36
a*25.08 ± 1.42
a@ b* cNS
26.58 ± 1.42
a* b* c#
22.91 ± 1.42
aNS b*
Protein (g/dL)
6.70 ± 0.57
4.80 ± 0.37
a*6.42 ± 0.48
aNS b* cNS
6.25 ± 0.48
aNS b* cNS
6.56 ± 0.52
aNS b*
3.5 Effect of M. pudica administration on liver function marker enzymes
Serum marker enzymes like AST, ALT, ALP, ACP and γ-GT were significantly increased after single intraperitoneal injection of streptozotocin. Repeated administration of both ME and MA led to significant attenuation of streptozotocin induced increase in AST, ALT, ALP, ACP and γ-GT to a significant level. Further, compared to the STZ-control rats, metformin treatment caused a significant lowering effect on the values of AST, ACP ALT, ALP and γ-GT (Table 4). Symbols represent statistical significance: * = p < 0.001, # = p < 0.01, @ = p < 0.05 and NS = Not significant.
Parameters
Control
STZ group
M. pudica ethanol extract
M. pudica aqueous extract
Standard Metformin
AST (IU/L)
23.08 ± 2.01
32.00 ± 2.36
a*27.16 ± 1.96
a@ b# c@
28.16 ± 2.56
a# b@ c#
23.33 ± 1.47
aNS b*
ALT (IU/L)
40.16 ± 2.85
53.33 ± 2.73
a*46.14 ± 3.00
a# b# c@
47.33 ± 2.85
a# b# c#
41.33 ± 2.85
aNS b*
ALP (IU/L)
50.00 ± 3.74
66.33 ± 4.27
a*57.16 ± 3.60
a@ b# cNS
58.00 ± 3.74
a@ b# c@
51.00 ± 4.47
aNS b*
ACP (IU/L)
29.00 ± 2.36
40.00 ± 3.22
a*34.00 ± 2.36
a@ b# c@
35.00 ± 2.36
a# b@ c#
29.50 ± 1.41
aNS b*
γ-GTP (μmole of p-nitroanilide liberated/mg protein/min)
3.10 ± 0.24
6.80 ± 0.55
a*4.10 ± 0.29
a* b* c@
4.40 ± 0.32
a* b* c#
3.50 ± 0.24
aNS b*
3.6 M. pudica treatment and HbA1c levels in diabetic rats
The hemoglobin level was significantly decreased with an increase in the total glycosylated hemoglobin content in the STZ-induced diabetic rats. On contrary, treatment with ME and MA opposed the STZ-mediated Hb depletion and also in the elevation in glycosylated hemoglobin content over 21 days of ME/ MA administration (Fig. 3).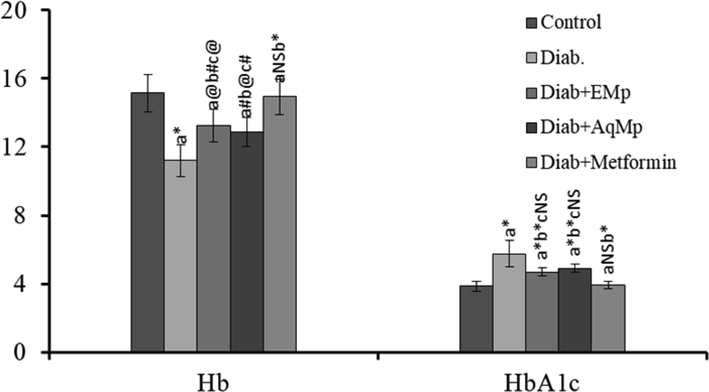
Changes in the haemoglobin and glycated haemoglobin levels of diabetic rats and the mitigating effect of Mimosa pudica ethanol (ME) and aqueous (MA).
3.7 Changes in the levels of lipid peroxidation products in STZ-induced diabetic rats and the protective efficacy of M. pudica extracts
Lipid peroxidation was significantly increased (p < 0.001) in streptozotocin induced diabetic control group, thereby indicating oxidative degradation of lipids. Repeated administration of ME and MA significantly attenuated the oxidative stress response in diabetic rats, as noted by reduced lipid peroxidation level (p < 0.001) (Fig. 4). Further, in basal, hydrogen peroxide, ferrous sulphate induced lipid peroxidation, both ME and MA treatment for 21 consecutive days led to significant attenuation of oxidative stress. Metformin administration has shown marginal reduction in the lipid peroxidation in comparison with the STZ induced diabetic rats.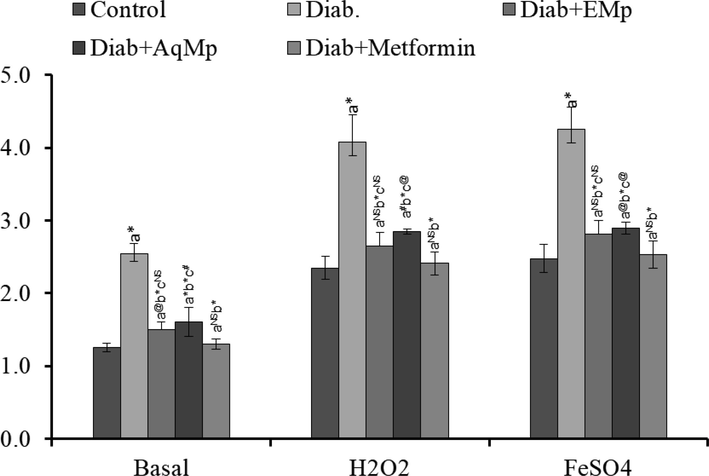
Changes in the lipid peroxidation status in the different treatment regimens including STZ control, protective effects of Mimosa pudica ethanol (ME) and aqueous (MA) extracts.
4 Discussion
Diabetes and associated secondary complications have emerged as an important concern in the arena of health management (Hafner et al., 2014). Considering the association of diabetes with other diseases like cancers, cardiovascular risk, fatty liver incidences, and neural degenerative diseases, it is important to control the glycemic load and associated stress (Benil et al., 2017). Underlying factors for the development of type-2 diabetes mellitus include glycemic load and insulin resistance. Hence, the anti-diabetic drugs are more focused on insulin sensitization. However, the diabetic complications are often associated with the polyol pathway and subsequent oxidative stress cascades. Hence, the ability of an anti-diabetic drug to control the oxidative insults are therefore important factor in controlling the secondary diabetic complications (Narayanankutty et al., 2018).
Streptozotocin is widely utilized as the model for experimental induction of type 2 diabetes mellitus in rats (Parasuraman et al., 2019). Single dose of 60 mg/kg STZ has been shown to induce hyperglycemia in all the experimental animals. Further, the treatment with aqueous and ethanol extract of M. pudica have shown to reduce the glycemic status in these rats, subsequently increasing the insulin sensitivity. The phenolic compounds present in the M. pudica are also reported to have anti-diabetic properties in individual experiments. Besides, the plasma insulin levels is also improved in the M. pudica extract treated animals over the 21 days of treatment. It is possible that the bioactive phytoconstituents present in the plant might have improved the insulin production and sensitivity in these rats.
Diabetes mellitus is often associated with increased glycemic load that eventually induce the polyol pathway signaling and subsequent induction of glycemic stress in the body (Luo et al., 2016). The glycemic stress is reported to be characterized by the increased oxidative damages in the tissues, subsequently resulting the generation of large volume of reactive radical moieties (Sheela et al., 2017). These radicals cause significant damages to the lipid macromolecules of the cell membrane and also modulate various pro-inflammatory signaling cascades (Tang et al., 2010). Corroborating with the reports, the present study also observed increased lipid peroxidation in the diabetic control group and which may be an indirect indicative of the glycemic load persisting in these animals. Besides, these animals also had significantly reduced intracellular glutathione pool; in diabetic conditions, the enhanced polyol activation may diminish cellular NADPH pool and thereby reduce the glutathione reductases activity (Tang et al., 2010). Due to the diminished GR activity, the salvage pathway of glutathione synthesis gets inhibited and causing the diminution in cellular reduced glutathione pool. It is therefore possible that the reduction in GSH pool in chronic diabetic rats may also explain the alteration in the plasma insulin levels.
Further, the results indicated a strong protective effect in the different extract treated animals during the diabetic conditions. The cellular pool of reduced glutathione and subsequent reduction in the lipid peroxidation status and improved insulin production are also well-corroborated. It is thus possible that the antioxidant molecules present in the M. pudica might have increased the de novo glutathione production. The phenolic compounds are well-known inducers of the glutathione biosynthesis mediated through the Nrf2/ ARE pathway (Narayanankutty et al., 2019). Further, phenolic compounds present in various plants are also reported to enhance the activity of glutathione biosynthetic enzymes and are also present in the M. pudica (Job et al., 2021). Apart from these the treatment with M. pudica extract also improved the cellular vitamin levels (Vit. E and Vit. C); the vitamins are important in preventing the insulin resistance and associated oxidative insults to the patients (Mason et al., 2021). Hence, the improvement in the non-enzymatic antioxidants may be partially contributed to the improved insulin sensitivity and reduction in glycemic stress in these animals.
Besides, the activities of enzymatic antioxidant molecules including CAT, SOD, GST and GPx are also altered in the diabetic rats. These results are in concordance with the previous literatures indicating the diminished antioxidant enzyme activities in preclinical and clinical models (Kala et al., 2015). These enzymes are important scavengers of reactive molecules that are involved in the cellular detoxification machinery and redox balancing (Li et al., 2016). Hence, the present observation in the improved antioxidant enzymes activities in rats treated with M. pudica extract may also contributed to the antidiabetic and diabetic complication reducing properties.
Together with the altered antioxidant responses in diabetic rats, there observed a marked elevation in the liver function marker enzymes. Beyond that, the restoration of these hepatic marker enzyme activities to near-normal level by the M. pudica extracts may also indicate the protective function of the plant in diabetic model. Further, elevated activities of these transaminases are often reported to increase the diabetes-dependent ketogenesis in diabetic rats (Chen and Croxson, 2007). Hence, the reduction of hepatic transaminase activities may also indicate the possible reduction in secondary diabetic complications in these animals. The increased gluconeogenesis and ketogenesis observed in diabetes may be due to high level in activities of these transaminases. Hence, the transient reduction in the activity of these transaminases by M. pudica extracts may also responsible for the inhibition of diabetic complications in these rats.
Overall, the M. pudica extracts efficiently ameliorates the hyperglycemia and associated glycemic stress by re-establishing the cellular redox balance in these rats. Further, the possible role of M. pudica aqueous extract as antidiabetic compound may also supports its traditional uses as medicinal herbs.
5 Conclusion
The results are indicative of the potential of M. pudica extracts in preventing the glycemic load and associated oxidative damages in the rats. Further, the phytoconstituents in the extract also improved glutathione levels, possibly modulating GSH-biosynthesis. Hence, it is clear that the M. pudica can be a leading source of anti-diabetic drug candidate, which equally modulate the secondary diabetic complications.
Acknowledgments
The authors sincerely acknowledge Researchers Supporting Project No: RSP-2021/232, King Saud University, Riyadh, Saudi Arabia for funding this work. STS, PSTS and SKKN acknowledge Late Dr. A. Saraswathy, Former Director, Capt. Srinivasa Murti Central Ayurveda Research Institute, Arumbakkam, Chenna and Late Dr. M.P. Balasubramanian, Former Professor, Dept. of Pharmacology & Environmental toxicology, Dr. ALMPGIBMS, University of Madras, Taramani, Chennai for their constant support, motivation and guidance.
Conflict of interest
The authors declare no conflict of interest.
References
- The relationship between diabetic retinopathy and nephropathy in Sudanese adult with diabetes: population based study. Diabetes Metab. Syndr.. 2017;11(1):S333-S336.
- [Google Scholar]
- Benil, P.B., Lekshmi, R., Viswanathan, N., Jollykutty, E., Rajakrishnan, R., Thomas, J., Alfarhan, A.H. 2017. Combined efficacy of Vigna radiata (L.) R. Wilczek and Amorphophallus paeoniifolius (Dennst.) Nicolson on serum lipids in albino rats. Saudi J. Bio. Sciences 10.1016/j.sjbs.2017.03.003.
- Ayurvedic anti-diabetic formulation Lodhrasavam inhibits alpha-amylase, alpha-glucosidase and suppresses adipogenic activity in vitro. J. Ayurveda Integr. Med.. 2017;8(3):145-151.
- [Google Scholar]
- Triad: diabetic ketoacidosis, elevated liver enzymes and abdominal pain— think liver infarct! Pract. Diab. Int.. 2007;24(6):302-303.
- [Google Scholar]
- Clinical characteristics of type 1 diabetes mellitus in Taiwanese children aged younger than 6 years: a single-center experience. J. Formos. Med. Assoc.. 2017;116(5):340-344.
- [Google Scholar]
- Diabetes and hypertension in India: a nationally representative study of 1.3 Million adults. JAMA Int. Med.. 2018;178(3):363-372.
- [Google Scholar]
- Type 2 diabetes mellitus, pandemic in 21st century. Adv. Exp. Med. Biol.. 2012;771:42-50.
- [Google Scholar]
- Antidiabetic drug use trends in patients with type 2 diabetes mellitus and chronic kidney disease: a cross-sectional analysis of the National Health and Nutrition Examination Survey. J. Diabetes. 2020;12(5):385-395.
- [Google Scholar]
- Hyperglycemia, oxidative stress, and the diaphragm: a link between chronic co-morbidity and acute stress? Crit. Care. 2014;18(3):149.
- [Google Scholar]
- Toxic effects of fluoride in intestinal epithelial cells and the mitigating effect of methanol extract of coconut haustorium by enhancing de novo glutathione biosynthesis. Environ. Res.. 2021;200:111717.
- [Google Scholar]
- Epigenetic-based combinatorial resveratrol and pterostilbene alters DNA damage response by affecting SIRT1 and DNMT enzyme expression, including SIRT1-dependent gamma-H2AX and telomerase regulation in triple-negative breast cancer. BMC Cancer. 2015;15:672.
- [Google Scholar]
- Protective effect of Mimosa pudica L. in an L-arginine model of acute necrotising pancreatitis in rats. J. Nat. Med.. 2016;70(3):423-434.
- [Google Scholar]
- Total Phenolic Content and In vitro Antioxidant Activity of Winged Bean (Psophocarpus tetragonolobus) Pakistan J. Nutr.. 2013;12(5):416-422.
- [Google Scholar]
- Manganese superoxide dismutase mediates anoikis resistance and tumor metastasis in nasopharyngeal carcinoma. Oncotarget. 2016;7(22):32408-32420.
- [Google Scholar]
- Lin, X., Xu, Y., Pan, X., Xu, J., Ding, Y., Sun, X., Song, X., Ren, Y., Shan, P.-F. 2020. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025, Sci. Rep. 10(1): 14790-14790.
- Hyperglycemic stress and carbon stress in diabetic glucotoxicity. Aging Dis.. 2016;7(1):90-110.
- [Google Scholar]
- A comprehensive review of the ethnotraditional uses and biological and pharmacological potential of the genus mimosa. Int. J. Mol. Sci.. 2021;22(14)
- [Google Scholar]
- Effects of Vitamin C supplementation on glycemic control and cardiovascular risk factors in people with Type 2 Diabetes: a GRADE-Assessed Systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2021;44(2):618-630.
- [Google Scholar]
- Vitamin E supplementation modulates the biological effects of omega-3 fatty acids in naturally aged rats. Toxicol. Mech. Methods. 2017;27(3):207-214.
- [Google Scholar]
- Glutathione, an antioxidant tripeptide: dual roles in carcinogenesis and chemoprevention. Curr. Protein Pept. Sci.. 2019;20(9):907-917.
- [Google Scholar]
- Virgin coconut oil reverses hepatic steatosis by restoring redox homeostasis and lipid metabolism in male Wistar rats: VCO ameliorates hepatic steatosis in rats. J. Sci. Food Agric.. 2018;98(5):1757-1764.
- [Google Scholar]
- Virgin coconut oil maintains redox status and improves glycemic conditions in high fructose fed rats. J. Food Sci. Technol.. 2016;53(1):895-901.
- [Google Scholar]
- Narayanankutty, S.P. Illam, V. Rao, S. Shehabudheen, A.C. Raghavamenon. 2020. Hot-processed virgin coconut oil abrogates cisplatin-induced nephrotoxicity by restoring redox balance in rats compared to fermentation-processed virgin coconut oil, Drug Chem. Toxicol.: 1-10.
- Mimosa pudica Protects the testes against cadmium-induced inflammation and oligospermia: potential benefits in treatment of heavy metal toxicity. Pathophysiology. 2018;25(4):293-297.
- [Google Scholar]
- Antidiabetic and antihyperlipidemic effects of methanolic extract of Mimosa pudica (Fabaceae) in diabetic rats. Egypt. J. Basic Appl. Sci.. 2019;6(1):137-148.
- [Google Scholar]
- ICP-MS assisted heavy metal analysis, phytochemical, proximate and antioxidant activities of Mimosa pudica L. Mater. Today: Proc.. 2021;45:2265-2269.
- [Google Scholar]
- Effects of Mimosa pudica L. leaves extract on anxiety, depression and memory. Avicenna J. Phytomed.. 2016;6(6):696-710.
- [Google Scholar]
- Protective effect of Garcinia pedunculata fruit rind in acetic acid induced ulcerative colitis. Farmacia. 2019;67(1):160-166.
- [Google Scholar]
- Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract.. 2019;157(107843):10.
- [Google Scholar]
- Coconut phytocompounds inhibits polyol pathway enzymes: implication in prevention of microvascular diabetic complications. Prostaglandins Leukotrienes Essential Fatty Acids (PLEFA). 2017;127:20-24.
- [Google Scholar]
- Traditional fruits of kerala: bioactive compounds and their curative potential in chronic diseases. Curr. Nutr. Food Sci.. 2017;13(4):279-289.
- [Google Scholar]
- The global epidemics of diabetes in the 21st century: current situation and perspectives. Eur. J. Prev. Cardiol.. 2019;26(2_suppl):7-14.
- [Google Scholar]
- Cardiac contractile dysfunction during acute hyperglycemia due to impairment of SERCA by polyol pathway-mediated oxidative stress. Am. J. Physiol. Cell Physiol.. 2010;299(3):C643-C653.
- [Google Scholar]
- Analyses and profiling of extract and fractions of neglected weed Mimosa pudica Linn. traditionally used in Southeast Asia to treat diabetes. S. Afr. J. Bot.. 2015;99:144-152.
- [Google Scholar]
- Phytochemical, pharmacological activities and ayurvedic significances of magical plant Mimosa pudica Linn. Mini-Rev. Org. Chem.. 2021;18(3):296-312.
- [Google Scholar]







