Translate this page into:
Migratory behavior of free-living marine nematodes surrounded by sediments experimentally contaminated by mixtures of polycyclic aromatic hydrocarbons
⁎Corresponding author. fehmiboufahja@fsb.u-carthage.tn (Fehmi Boufahja)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
An experiment was conducted using microcosms with connected sedimentary compartments to assess the effects of three polycyclic aromatic hydrocarbons (PAHs) on free-living marine nematodes from Bizerte Bay (northeastern Tunisia). Over 30 days, the nematofauna were exposed to four treatment sediments, including one with chrysene (150 ppb), chrysene (150 ppb) plus fluoranthene (75 ppb), chrysene (150 ppb) and phenanthrene (15 ppb), and an uncontaminated reference. Numerical and qualitative community simplifications occurred in contaminated sediments relative to the reference. The diversity of nematodes differed based on hydrocarbon combinations. Multivariate analyses revealed communities in contaminated compartments differed from those in the initial natural sediment and reference. Differences in sensitivities of nematodes to hydrocarbons and in migratory abilities occurred. Rhabditis sp., Calamicrolaimus honestus, and Oncholaimus campylocercoides were in all compartments and classified as tolerant to PAHs. Nematodes observed only in control compartments, including Parasphaerolaimus paradoxus, Encheliidae (sp.), Trichotheristus mirabilis, and Theristus pertenuis were considered sensitive.
Keywords
Microcosm
Free-living marine nematodes
Polycyclic aromatic hydrocarbons
Migration
Abundance
Species diversity
1 Introduction
Free-living marine nematodes represent an excellent group for experimental and ecotoxicological studies because of their small size (1–5 mm), high abundance (up to 23 million per m2), and short life cycles (Mahmoudi et al., 2005; Moreno et al. 2011; Semprucci and Balsamo, 2012). To date, their migratory behavior has been studied in artificially defaunated sediments with different grain size to clarify the granulometric preference of these organisms (Schratzberger et al., 2004) and to determine if differences in the availability of organic matter drive their colonization patterns (Zhou, 2001). Focusing on this latter aspect, Gallucci et al. (2008) showed that deep-sea nematodes tended to preferentially colonize sediments with higher amounts of organic matter than those not similarly enriched.
Bazzicalupo et al. (1994) showed that nematodes detected and produced chemical signals for feeding, reproduction, and communication. In this context, contaminants, such as polycyclic aromatic hydrocarbons (PAHs), heavy metals, or pesticides, may act as chemical signals that induce reactions, such as the escape from harmful environmental conditions.
Various studies (Carman et al., 2000; Hägerbäumer et al., 2015; Mahmoudi et al., 2005) in closed microcosms have shown the toxic effect of PAHs on marine meiofauna, inducing a loss of abundance and nematode diversity. Thus, the disappearance of sensitive species has been observed in favor of more tolerant species, implying a major change in community structure (Muyssen et al., 2006). However, an approach based on closed enclosures does not simulate in situ conditions because it does not allow the progressive escape of nematodes from hotspots of harmful chemicals, thereby hindering the determination of the actual tolerance of a target species to a specific contaminant. In contrast to closed systems, open experimental enclosures allow the organisms to escape, if desired, making this approach closer to approximating in situ conditions.
In the present study, the migratory behavior of nematodes was investigated in defaunated sediment with different mixtures of PAHs. The use of open experimental enclosures allowed for the observation of spontaneous migration patterns that reflected the ability of nematodes to withstand contaminants and, in turn, their upper limit of tolerance.
2 Material and methods
2.1 Collecting site
On March 12, 2015 (7 A.M.), the sediments were collected from the subtidal upper edge of a site in Bizerte Bay (37°16′6.24″N, 9°52′59.24″E, depth = 60 cm). The 10 cm upper layer of sediment was collected using 10 cm2 hand-cores and placed in a bucket. The collecting was restricted to the first 10 cm because in most coasts over than 90% of nematodes are found in the surface 1–2 cm (Coull and Chandler, 1992).
2.2 Experimental set-up
To investigate any possible horizontal migration of nematodes, three microcosms were specially designed. Each microcosm consisted of several polyvinyl chloride (PVC) tubes (10 cm in diameter) assembled in a Ψ shape and closed at their upper ends with lids as shown in Fig. 1.
For each Ψ shaped microcosm:
- The central compartment was filled by sediment from the field with its natural meiofauna and no further treatment for use at the beginning of the experiment (I) (Fig. 1). At the end of the experiment, a second overview was obtained for the natural substrate and its final nematofauna, E.
- Directly linked to the central microcosm, four separated peripheral compartments were replenished with defaunated and treated sediment (Fig. 1). Treatments consisted of a reference (R) and three compartments with various combinations of PAH contamination:
-
Chrysene alone (C) at a concentration of 150.7 ng g−1 dry weight (DW),
-
A mixture of 150.7 ng g−1 DW of chrysene with 70.8 ng g−1 DW fluoranthene (CF),
-
A mixture of 150.7 ng g−1 DW of chrysene with 168 ng g−1 DW of phenanthrene (CP).
The concentrations of PAHs used in the contaminated compartments were those reported by Zrafi et al. (2010) as being in the sediments surrounding our collecting site.
-
-
The last portion of azoic and uncontaminated sediment was used to fill the furthest compartments from the center at the extremities of the Ψ-shaped PVC microcosm (RR, RC, RCF, and RCP) (Fig. 1). These referential compartments were the last solution for nematodes if they “decided” to escape the compartments R, C, CF, or CP, respectively, and go forward because the central part of the Ψ-shaped microcosm became distant after migration.
Five 0.5 L plastic bottles were inserted through the neck at holes placed at regular intervals in every Ψ-shaped PVC microcosm and then filled with filtered seawater (0.1 µm). These bottles were wrapped in aluminum foil to avoid photo-oxidation of the PAHs. They were also drilled and connected by pipes to an air pump to ensure water oxygenation. To efficiently separate the different compartments with ease, the upper half of the PVC tube at the limit between adjacent compartments was cut, clogged with silicone gel, and covered with cellophane tape.
2.3 Sediment contamination
The defaunation of the sediment was performed by repeated (3times) freezing to −20 °C for 12 h and thawing at room temperature for 48 h according to Schratzberger et al. (2004). The sediment was sieved through a 300 μm mesh to remove the coarser portion. This sieve size corresponded to the medium-grain size (Boufahja et al., 2015). Then, only the fine fraction (<300 µm) was contaminated by PAHs because of its higher retention potential (Boufahja et al., 2015).
Before contaminating the sediment, PAHs were solubilized in acetone according to Foss and Forbes (1997) for fluoranthene, Louati et al. (2014) for phenanthrene, and De Lange et al. (2006) for chrysene. Finally, uncontaminated coarse (≥300 μm) and contaminated finer sedimentary particles (<300 μm) were mixed to reach the desired concentrations of PAHs.
2.4 Sample processing
After 30 days from the commencement of the experiment (Millward et al., 2004; Beyrem et al., 2011; Boufahja et al., 2015), the microcosms were fragmented into the different compartments. First, the silicone gel and scotch tape that enclosed them were removed. Then, all parts were manually separated, one at a time, using a metallic circular cutter, starting from the periphery and moving to the center.
Meiofaunal taxa, defined here as metazoans that pass through a 1 mm mesh sieve and are retained on a 40 µm sieve (Vitiello and Dinet, 1979), were extracted from the sediment using the resuspension-decantation method followed by rapid sieving using the aforementioned sizes (Wieser, 1960). Thereafter, organisms were stained with Rose-Bengal (0.2 g L-1) (Elarbaoui et al., 2015) and fixed in 4% formalin (Schratzberger et al., 2004).
After extraction, nematodes from each compartment (up to a maximum of 100 individuals) were removed under a stereo-dissecting microscope and placed in 21% glycerol, evaporated to anhydrous glycerol, and then mounted on temporary slides for microscopic identification (Seinhorst, 1959).
The organisms were identified to the lowest taxonomic level possible using the generic keys of Platt and Warwick (1983, 1988) and Warwick et al. (1998), as well as species descriptions downloaded from the Nemys database (Vanaverbeke et al., 2015).
2.5 Statistical analysis
All data were tested for normality (Kolmogorov-Smirnov test) and equality of variance (Bartlett test). Data were log10 (x + 1) transformed to fulfil the requirements of parametric analyses (Clarke, 1993; Clarke and Gorley, 2001). The Plymouth Routines in Multivariate Ecological Research (PRIMER v.5) software package was used for data analysis. For each microcosm, the following univariate indices were computed: total abundance (N), species numbers (S), species richness (d), and the Shannon diversity (H') and Pielou’s evenness (J') indices, both loge. Subsequently, means, of all transformed (log10 (x + 1)) univariate indices, were analyzed using a one-way analysis of variance (ANOVA) across all compartments. A posteriori multiple comparisons were performed using the Tukey HSD test (software Statistica version 5.1). Differences were significant when p < 0.05. A non-metric Multidimensional Scaling ordination (nMDS) was performed on a Bray-Curtis similarity matrix based on square-root transformed species abundance to visualize any differences among compartments. Afterwards, an analysis of similarity (ANOSIM) was performed to determine significant differences among compartments (Clarke, 1993), whereas a one-way similarity percentage procedure (SIMPER, cut-off percentage: cut-off 70%) was applied to calculate the relative contribution of each taxon to the average dissimilarity among treatments.
3 Results
Overall, 28 species belonging to 28 genera were observed during the experiment (Table 1). Each compartment was characterized by a specific sub-community of nematodes. Although Rhabditis sp. presented a relatively high relative abundance in all treatments, this ubiquitous taxon was variably associated with other nematode species according to the compartment: Calamicrolaimus honestus in N (16.60 ± 4.38%), R (18.46 ± 2.78%), and CF (20.88 ± 3.08%), Cyartonema germanicum (14.14 ± 7.94%) in RR, and Oncholaimus campylocercoides (15.5 ± 3.94%) in RCP. Furthermore, Rhabditis sp. was the dominant taxon in almost all compartments; in particular, in RCF, it accounted for 98.41 ± 2.74% of the entire assemblage. The only two exceptions were C and CP, where O. campylocercoides and, to a lesser extent, Calamicrolaimus honestus had a higher relative abundance than that of Rhabditis sp.
Species
I
E
R
RR
C
RC
CF
RCF
CP
RCP
Odontophora villoti
0.49 ± 0.86
1.58 ± 2.74
3.21 ± 2.08
–
5.55 ± 9.62
–
–
–
1.96 ± 3.39
–
Cyartonema germanicum
4.36 ± 1.62
2.86 ± 2.52
9.75 ± 3.01
14.14 ± 7.94
–
11.11 ± 19.24
–
–
7.07 ± 8.61
–
Synonchiella edax
1.82 ± 1.98
5.12 ± 4.44
2.08 ± 2.4
–
6.66 ± 11.54
–
1.5 ± 1.3
–
1.51 ± 2.62
–
Prochromadorella longicaudata
1.88 ± 0.64
–
–
–
–
–
0.79 ± 1.37
–
–
–
Longicyatholaimus longicaudatus
0.51 ± 0.88
–
–
–
–
–
2.95 ± 1.13
–
–
–
Microlaimus cyatholaimoïdes
6.51 ± 6
5.43 ± 5.79
4.54 ± 5.24
–
5.55 ± 9.62
–
0.74 ± 1.28
1.58 ± 2.74
–
–
Calamicrolaimus honestus
21.85 ± 4.26
16.60 ± 4.38
18.46 ± 2.78
–
20.55 ± 4.19
27.77 ± 25 0.45
20.88 ± 3.08
–
19.12 ± 6.94
2.77 ± 4.81
Spirinia parasitifera
5.97 ± 1
9.58 ± 1.92
2.08 ± 2.4
1.23 ± 2.13
2.77 ± 4.81
–
2.95 ± 1.13
–
1.51 ± 2.62
–
Nudora gerlachi
0,93 ± 0.81
–
2.27 ± 2.27
–
–
–
–
–
3.47 ± 3.08
–
Encheliidae sp.
0.49 ± 0.86
–
1.13 ± 1.31
–
–
–
–
–
–
–
Oncholaimus campylocercoïdes
28.14 ± 5.31
1.28 ± 2.22
3.21 ± 2.36
–
23.33 ± 8.81
11.11 ± 19.24
2.75 ± 2.22
–
21.7 ± 13.1
15.5 ± 3.94
Metoncholaimus pristiurus
4.78 ± 0.41
–
–
–
–
–
–
–
1.85 ± 3.2
–
Oncholaimellus calvadosicus
0.43 ± 0.75
–
–
–
–
–
1.41 ± 2.45
–
5.66 ± 5.55
–
Enoplolaimus longicaudatus
0.95 ± 0.83
–
–
–
2.77 ± 4.81
11.11 ± 19.24
–
–
1.51 ± 2.62
–
Halalaimus gracilis
1.31 ± 2.27
–
–
–
–
–
–
–
–
–
Thalassironus britannicus
1.01 ± 0.87
–
1.13 ± 1.31
–
–
–
0.7 ± 1.22
–
–
–
Phanodermopsis sp.
0.93 ± 0.81
–
–
–
–
–
–
–
3.81 ± 3.3
1.85 ± 3.2
Bathylaimus sp.
1.01 ± 0.87
–
–
1.96 ± 3.39
–
–
–
–
–
–
Theristus modicus
0.49 ± 0.86
–
–
–
–
–
–
–
–
5.78 ± 5.57
Paramonhysteria proteus
10.58 ± 0.16
2.86 ± 2.52
6.72 ± 6.24
8.35 ± 8.86
11.11 ± 9.62
–
1.44 ± 1.25
–
12.5 ± 3.84
10.64 ± 2.12
Valvaelaimus maior
1.37 ± 1.31
–
1.13 ± 1.31
–
2.77 ± 4.81
–
1.53 ± 1.33
–
–
2.08 ± 3.6
Trichotheristus paramirabilis
1.01 ± 0.87
–
1.13 ± 1.31
–
–
–
–
–
–
–
Theristus pertenuis
0.51 ± 0.88
–
2.08 ± 2.4
–
–
–
–
–
–
–
Daptonema fallax
0.93 ± 0.81
–
5.2 ± 5.46
8.05 ± 7.03
–
–
–
–
1.96 ± 3,39
–
Parasphaerolaimus paradoxus
1.01 ± 0.87
–
3.21 ± 1.07
–
–
–
–
–
–
–
Sabatieria splendens
0.43 ± 0.75
–
–
–
6.66 ± 11.54
11.11 ± 19.24
–
–
–
–
Rhabditis sp.
0.43 ± 0.75
50.79 ± 4.08
–
49.42 ± 13.49
12.22 ± 10.71
27.77 ± 25.45
–
98.41 ± 2.74
16.32 ± 6.66
42.59 ± 12.83
Nematode abundance varied significantly among compartments and values ranged between 2.6 ± 0.5 individuals throughout the RC compartments and 69.3 ± 5.8 individuals throughout the initial field community (I) used to start the experiment (Fig. 2). At the end of the experiment, the compartments presented the following descending numerical gradient in abundance: I-R-CF-E-RCF-RR-CP-RCP-C-RC. Post-hoc significant differences were determined by the Tukey HSD test between central samples (i.e., I and E) and those contaminated with PAHs (Fig. 2).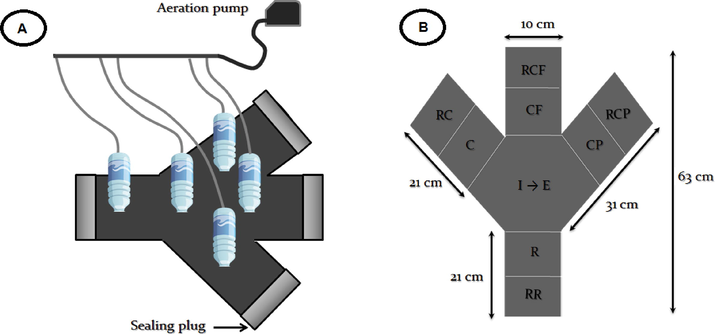
Microcosm design (A) and horizontal distribution of the different sedimentary compartments into the microcosm. Natural sediment collected to start the experiment (I); natural sediment at the end of the experiment (E); defaunated sediments contaminated with chrysene (C); defaunated sediments contaminated with a mixture of chrysene and fluoranthene (CF); defaunated sediments contaminated with a mixture of chrysene and phenanthrene (CP); (RC, RCF, RCP, R, and RR) defaunated and uncontaminated sediments.
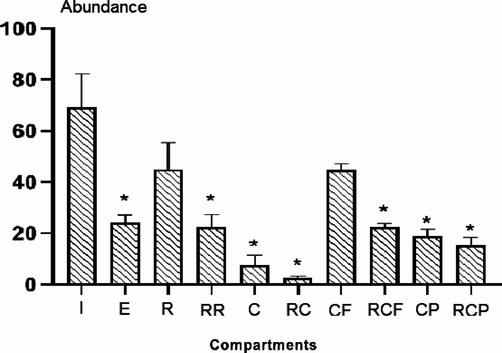
Abundance of free-living marine nematodes in the different sedimentary compartments (see Fig. 1 for the meaning of the abbreviations coding for the compartments).
The species numbers were significantly lower in both contaminated and uncontaminated compartments than at the beginning of the experiment (I), as indicated by one-way ANOVA results and post-hoc Tukey HSD comparisons (Fig. 3). In particular, overall nematode assemblages in the reference compartments (R, RR, RC, RCF, and RCP) were less diverse than in that in I (Fig. 3).
The values for the Shannon diversity index were lower at the end of the experiment than at the beginning (I). Paired Tukey HSD test outputs showed significant differences between the mean Shannon values in different compartments, especially for I vs. RR, RC, CF, and RCF and E vs. RC, RCF and C vs. RC and CF vs. RCF (Fig. 3). Comparable results were observed for Margalef’s species richness (d).
In the case of evenness (J’), no discernible differences from the initial community were recorded. However, multiple comparisons with the Tukey HSD test highlighted significant differences for I vs. RCF, E vs. RCF, and CF vs. RCF.
The nMDS results (stress = 0.18) (Fig. 4) indicated a very weak impact of the PAHs on the specific distribution. The most important effect was noted for contaminated compartments C and RC. Additionally, the ANOSIM analysis did not indicate significant differences in the composition of nematodes among the compartments, most likely because of the low numbers of replicates (n = 3). Despite this, a dissimilarity exceeding 60% was noted between the initial community (I) and communities from all compartments after 30 days of exposure (Table 2). Furthermore, the average dissimilarities for C vs. E and C vs. RC exceeded 60% (Table 2).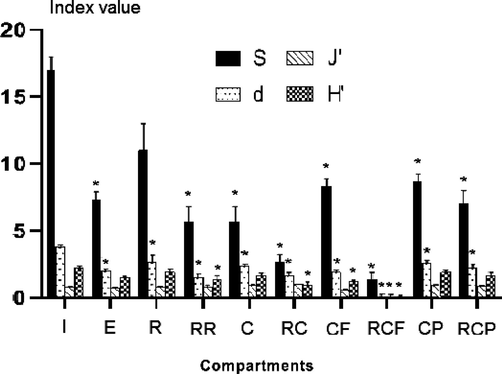
Values of univariate indices at each sedimentary compartment (see Fig. 1 for the meaning of the abbreviations coding for the compartments). Species number (S); Shannon diversity index (H'); Margalef’s species richness (d); Pielou’s evenness (J’). The stars above bars indicate significant differences when compared with the starting nematofauna ‘I’.
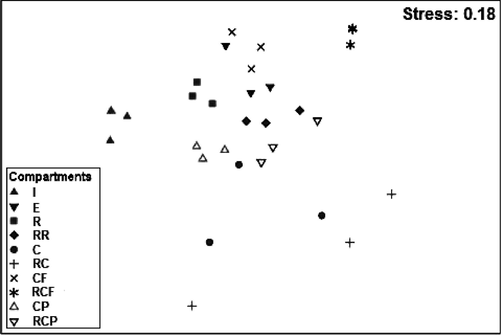
nMDS ordination based on the abundances of nematode species (see Fig. 1 for the meaning of the abbreviations coding for the compartments).
I vs. E (79.69%)
I vs. R (60.42%)
I vs. RR (79.97%)
Oncholaimus campylocercoides (26.08%) -
Oncholaimus campylocercoides (25.77%) -
Oncholaimus campylocercoides (23.46%) -
Rhabditis sp. (16.32%) +
Rhabditis sp. (22.92%) +
Calamicrolaimus honestus (18.72%) -
Calamicrolaimus honestus (14.70%) -
Calamicrolaimus honestus (8.67%) -
Rhabditis sp. (14.36%) +
Microlaimus cyatholaimoides (5.84%) -
Paramonohystera proteus (7.74%) -
Spirinia parasitifera (5.13%) -
I vs. C (84.95%)
I vs. RC (94.87%)
I vs. CF (74.70%)
Oncholaimus campylocercoides (27.96%) -
Oncholaimus campylocercoides (28.80%) -
Rhabditis sp. (32.42%) +
Calamicrolaimus honestus (20.53%) -
Calamicrolaimus honestus (21.09%) -
Oncholaimus campylocercoides (21.91%) -
Paramonohystera proteus (9.83%) -
Microlaimus cyatholaimoides (10.87%) -
Paramonohystera proteus (7.86%) -
Microlaimus cyatholaimoides (6.43%) -
Spirinia parasitifera (6.25%) -
Calamicrolaimus honestus (6.56%) -
I vs. RCF (98.90%)
I vs. CP (67.59%)
I vs. RCP (81.08%)
Rhabditis sp. (24.24%) +
Oncholaimus campylocercoides (25.97%) -
Oncholaimus campylocercoides (25.52%) -
Oncholaimus campylocercoides (21.84%) elim
Calamicrolaimus honestus (18.98%) -
Calamicrolaimus honestus (21.41%) -
Calamicrolaimus honestus (16.57%) elim
Paramonohystera proteus (8.40%) -
Rhabditis sp. (8.93%) +
Microlaimus cyatholaimoides (7.25%) elim
Paramonohystera proteus (8.33%) -
Spirinia parasitifera (5.93%) -
E vs. R (42.61%)
E vs. C (73.61%)
E vs. CF (44.37%)
Calamicrolaimus honestus (6.07%) +
Rhabditis sp. (48.76%) -
Rhabditis sp. (50.27%) =
Rhabditis sp. (12.21%) +
Calamicrolaimus honestus (10.42%) -
Calamicrolaimus honestus (17.40%) -
Paramonohystera proteus (10.96%) +
Spirinia parasitifera (8.72%) -
Cyartonema germanicum (9.88%) +
Microlaimus cyatholaimoïdes (6.20%) =
Daptonema fallax (6.07%) +
Spirinia parasitifera (6.07%) -
E vs. CP (59.36%)
R vs. RR (44.68%)
C vs. TC (65.72%)
Rhabditis sp. (36.16%) -
Calamicrolaimus honestus (25.84%) -
Oncholaimus campylocercoïdes (20.19%) -
Oncholaimus campylocercoïdes (15.22%) +
Rhabditis sp. (17.60%) -
Paramonohystera proteus (12.79%) elim
Spirinia parasitifera (7.85%) -
Paramonohystera proteus (9.79%) -
Calamicrolaimus honestus (12.04%) -
Paramonohystera proteus (6.49%) +
Daptonema fallax (7.23%) =
Rhabditis sp. (10.69%) -
Cyartonema germanicum (5.78%) -
Sabatiera splendens (7.53%) =
Synonchiella edax (6.64%) -
CF vs. RCF (34%)
CP vs. RCP (51.03%)
Calamicrolaimus honestus (40.95%) elim
Calamicrolaimus honestus (18.75%) -
Rhabditis sp. (25.01%) -
Rhabditis sp. (18.74%) +
Oncholaimus campylocercoïdes (12.02%) -
Cyartonema germanicum (7.13%) +
Oncholaimellus calvadosicus (5.98%) elim
Microlaimus cyatholaimoïdes (5.76%) +
As shown in Table 2, SIMPER results confirmed that Rhabditis sp. was the main taxon that contributed to dissimilarity. Two other species, O. campylocercoides and Calamicrolaimus honestus, were also discernibly responsible for the dissimilarity between compartments. In particular, the average dissimilarity between I and all the communities at the end of the experiment was mainly caused by both a decrease in the abundance of O. campylocercoïdes, Calamicrolaimus honestus, and P. proteus and an increase in that of Rhabditis sp.
4 Discussion
This work was conducted to test the effects of PAHs that are primarily released from the oil industry in the Bizerte area, Tunisia (i.e., chrysene, phenanthrene, and fluoranthene) on the migratory behavior of a local free-living nematode community. An original microcosm design was then created using PVC tubes and compartments with different sediment qualities were directly connected.
Our results are in accordance with those of previous studies (Bazzicalupo et al., 1994; Troemel et al., 1995), which showed that the nematodes can detect relatively distant contamination hotspots and react by migrating from them at a distance dependent upon the taxon. After 30 days, Rhabditis sp. represented more than 50% of all nematodes in the natural sediments, although this taxon accounted for <1% at the beginning of the experiment. Because this taxon exhibited substantial abundance in the compartments treated with PAHs, Rhabditis sp. may be considered an opportunist and able to colonize contaminated sediments that would not be inhabited by more sensitive nematode species. Notwithstanding, the dominance of Rhabditis sp. in all compartments could also be ascribed to its small size and pointed and elongated tail. Indeed, according to Boufahja et al. (2016), the locomotion of a given species inevitably depends on its morphology, physiology, and lifestyle.
Two other species, Calamicrolaimus honestus and O. campylocercoides were widespread among all compartments. However, compared to Rhabditis sp., these two species were more represented in the initial natural sediment (I), as well as in the C and CP compartments. It is therefore likely that Calamicrolaimus honestus and O. campylocercoides are resistant to low levels of stress and compete with Rhabditis sp. in these compartments. However, these species failed to dominate Rhabditis sp. in the compartments with higher stress, namely a mixture of PAHs with four cycles, such as CF.
In the case of P. paradoxus, Encheliidae (sp.), Trichotheristus paramirabilis, and Theristus pertenuis, they seemed to avoid contaminated sediments because these organisms were observed only in uncontaminated sediments (I and E) and compartments (R). Conversely, M. pristiurus and L. longicaudatus were found only in one or more contaminated treatments, which resulted in them being almost absent from the controls. This finding may indicate that these organisms may preferentially migrate to contaminated sediments because in the uncontaminated ones the interspecific competition is excessive. Furthermore, this result suggests that they are, to some extent, tolerant to PAHs. On the other hand, some species exhibited avoidance behavior toward the contaminated compartments (C, CP, and CF). In this context, it is noteworthy that T. modicus, which avoided the stress in the CP compartment by moving forward until reaching the uncontaminated compartment, RCP. Additionally, the species C. germanicum also exhibited avoidance and migration; indeed, this species had a greater presence in the less stressful compartments (R, RR, RC, and RCP), but also was able to avoid the most stressful compartment with the heaviest PAHs, CF.
From the beginning of the experiment, the field sediments placed into the central part contained a nematode community (I), which was free to move within the microcosm in any direction. The exposure of the organisms to different mixtures of PAHs induced species-specific migratory behavior that was tightly associated with the tolerance of each taxon to contamination. Univariate analysis showed that all contaminated compartments appeared to have discernible effects on nematodes, probably in relation to the mortality of the most sensitive species (Beyrem and Aïssa, 2000; Carman et al., 2000) or their avoidance of the contaminated compartments. Overall, all peripheral control compartments (RC, RCF, and RCP) were characterized by assemblages with lower numbers of species than those observed in contaminated sediments (C, CF, and CP). Although sensitive species likely tended to remain in the natural sediments (E), the most tolerant ones seemed to migrate towards control associated areas by passing through contaminated compartments, i.e., a behavior observed for Cyartonema germanicum, T. modicus, and S. splendens. Focusing on contaminated compartments (C, CF, and CP), the lowest diversity characterized the assemblage exposed to chrysene, implying higher toxicity associated with this contaminant. Furthermore, possible antagonistic effects exerted between chrysene and the other two tested PAHs (fluoranthene and phenanthrene) may be inferred because it has been reported in previous studies (Morelis et al., 2007). Different effects on nematodes were exerted by the two tested mixtures; i.e., the assemblage exposed to phenanthrene and chrysene was more diverse than that observed in the sediments treated with fluoranthene and chrysene. This finding suggested that the antagonism between the first two contaminants was higher than that exerted by fluoranthene on chrysene.
5 Conclusion
The experimental design and protocols proposed in this work are totally original and the bioassay could be reproduced at low coast. Our experimental approach using connected compartments with different levels of stress allowed us to highlight several experimental findings. First, it was possible to highlight the “real” tolerance and sensitivity of nematode species vs. PAHs, and thus identified their natural distribution in the surrounding sediments based on their “own decisions.” Second, the observed distribution pattern of nematode species mirrored the species-specific affinity to PAHs contamination that was measurable in terms of biodiversity. The freed movement of the nematodes throughout all the compartments contaminated or not, allowed us to identify the most tolerant species, such as Rhabditis sp., and to less extent, Calamicrolaimus honestus and O. campylocercoides, that can be used in future ecotoxicological studies related to PAHs. The experiment allowed us to determine the most sensitive species, namely, S. paradoxus, Encheliidae (sp.), Trichotheristus mirabilis, and Theristus pertenuis, that existed only in uncontaminated compartments. These taxa could be classified as sentinels and their high abundance in a given water body could be indicative of pristine status.
Finally, our results suggested a possible antagonism between the PAHs used to start the experiment (chrysene, fluoranthene, and phenanthrene). Although chrysene appeared to exert a toxic effect on nematodes, its harmful effects appeared less pronounced when mixed with the other two PAHs, with more pronounced antagonism with phenanthrene than with fluoranthene.
Acknowledgments
Special thanks are due to Pr. Patricia Aïssa (Carthage University, Tunisia), for her invaluable assistance in identification of meiobenthic nematodes. We acknowledge the helpful comments by Pr. Marco Curini-Galletti (University of Sassri, Italy) on an earlier version of the manuscript. We are also grateful to two anonymous reviewers for their comments that improved the manuscript.
Funding.
This work was supported by Researchers Supporting Project number RSP-2019/17, King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Reference
- Les nematodes libres, organismes sentinelles de l'évolution des concentrations d'hydrocarbures dans la baie de Bizerte (Tunisie) Cah. Biol. Mar.. 2000;41:329-342.
- [Google Scholar]
- Laboratory study on individual and combined effects of cobalt- and zinc-spiked sediment on meiobenthic nematodes. Biol. Trace. Elem. Res.. 2011;144:790-803.
- [Google Scholar]
- Experimental evidence of the effects of an antimitotic agent, Colchicine, on a nematode community through a microcosm approach. Cah. Biol. Mar.. 2015;56:39-48.
- [Google Scholar]
- An experimental protocol to select nematode species from an entire community using progressive sedimentary enrichment. Ecol. Indic.. 2016;60:292-309.
- [Google Scholar]
- Does historical exposure to hydrocarbon contamination alter the response of benthic communities to diesel contamination? Marine Environ. Res.. 2000;49(3):255-278.
- [Google Scholar]
- Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol.. 1993;18:117-143.
- [Google Scholar]
- Clarke, K.R., Gorley, R.N., 2001. PRIMER v5: User manual/tutorial. PRIMER-E: Plymouth, UK. pp. 91.
- Pollution and meiofauna: field, laboratory and mesocosm studies. Oceanogr. Mar. Biol.. 1992;30:191-271.
- [Google Scholar]
- Avoidance of polycyclic aromatic hydrocarbon-contaminated sediments by the freshwater invertebrates Gammarus pulex and Asellus aquaticus. Environ. Toxicol. Chem.. 2006;25:452-457.
- [Google Scholar]
- Effect of crude oil exposure and dispersant application on meiofauna: an intertidal mesocosm experiment. Environ. Sci. Process. Impact.. 2015;17(5):997-1004.
- [Google Scholar]
- Effects of the polycyclic aromatic hydrocarbon fluoranthene on growth rate and nucleic acid composition of Capitella sp. I. Mar. Biol.. 1997;129:489-497.
- [Google Scholar]
- Active colonisation of disturbed sediments by deep-sea nematodes: evidence for the patch mosaic model. Mar. Ecol. Prog. Ser.. 2008;367:173-183.
- [Google Scholar]
- Experimental studies with nematodes in ecotoxicology: an overview. J. Nematol.. 2015;47:11-27.
- [Google Scholar]
- Biostimulation as an attractive technique to reduce phenanthrene toxicity for meiofauna and bacteria in lagoon sediment. Environ. Sci. Poll. Res.. 2014;21:3670-3679.
- [Google Scholar]
- Effects of hydrocarbon contamination on a free living marine nematode community: Results from microcosm experiments. Mar. Poll. Bull.. 2005;50:1197-1204.
- [Google Scholar]
- Mixtures of metals hydrocarbons elicit complex responses by a benthic invertebrate community. J. Exp. Mar. Biol. Ecol.. 2004;310:115-130.
- [Google Scholar]
- Competition between phenanthrene, chrysene, and 2,5-dichlorobiphenyl for high-energy adsorption sites in a sediment. Chemosphere. 2007;68:2028-2032.
- [Google Scholar]
- The use of nematodes in assessing ecological quality status in the Mediterranean coastal ecosystems. Ecol. Indic.. 2011;11:328-336.
- [Google Scholar]
- Mechanisms of chronic waterborne Zn toxicity in Daphnia magna. Aquat. Toxicol.. 2006;7:393-401.
- [Google Scholar]
- Free-living marine nematodes.Part I. British Enoploids. London: Cambridge University; 1983.
- Platt, H.M., Warwick, R.M., 1988. Free-living marine nematodes. Part II. British Chromadorids. Synopsis of the British fauna (New Series) No. 38, E.J. Brill/W. Backhuys, Leiden.
- Colonisation of various types of sediment by estuarine nematodes via lateral infaunal migration: a laboratory study. Mar. Biol.. 2004;145:69-78.
- [Google Scholar]
- A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica. 1959;4:67-69.
- [Google Scholar]
- Key role of free-living nematodes in the marine ecosystem. In: Boeri F., Jordan A.C., eds. Nematodes: Morphology, functions and management strategies. Hauppauge, NY: NOVA Science Publishers, Inc.; 2012. p. :109-134.
- [Google Scholar]
- Divergent seven transmembrane receptors are candidate chemosensory receptors in Coenorhabditis elegans. Cell.. 1995;83:207-218.
- [Google Scholar]
- Vanaverbeke, J., Bezerra, T.N., Braeckman, U., De Groote, A., De Meester, N., Deprez,T., Derycke, S., Gilarte, P., Guilini, K., Hauquier, F., Lins, L., Maria, T., Moens, T.,Pape, E., Smol, N., Taheri, M., Van Campenhout, J., Vanreusel, A., Wu, X., Vincx, M., 2015. NeMys: World database of free-living marine nematodes.
- Définition et échantillonnage du méiobenthos. Rapp. Comm. Int. Mer. Medit.. 1979;25:279-283.
- [Google Scholar]
- Warwick, R.M., Platt, H. M., Somerfield, P.J., 1998. Free-living marine nematodes. Part III. British monohysterids. Synopsis of British fauna (new series) No. 53, Field Studies Council, Shrewsbury.
- Benthic studies in Buzzards Bay. II. The meiofauna. Limnol. Oceanogr.. 1960;5:121-137.
- [Google Scholar]
- Effects of leaf litter addition on meiofaunal colonization of azoic sediments in a subtropical mangrove in Hong Kong. J. Exp. Mar. Biol. Ecol.. 2001;256:99-121.
- [Google Scholar]
- Distribution and sources of polycyclic aromatic hydrocarbons around a petroleum refinery rejection area in Jarzouna-Bizerte (Coastal Tunisia) Soil. Sed. Contam.. 2010;19:292-306.
- [Google Scholar]







