Translate this page into:
Microwave-assisted green synthesis of 1,5 benzodiazepines using Cu(II)-clay nanocatalyst
⁎Corresponding authors. shaikhiqbaln@gmail.com (Iqbal N. Shaikh), sfadil@ksu.edu.sa (Syed Farooq Adil)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Herein, we present a practical, more efficient, environmentally friendly method for the synthesis of 1,5-benzodiazepine via condensation of benzene-1,2-diamine with commercially available ketones. The reaction was performed using various heterogeneous clay based catalysts, including Montmorillonite-K10, Montmorillonite-KSF, Kaoline, and bentonite, and also other clay supported transition metals based catalysts such as, Cu(II)-clay, Pd/Montmorillonite-KSF, Ni/Montmorillonite-KSF, Co/Montmorillonite-KSF, Sn/KSF, and Zn/Montmorillonite-KSF. Among various metal-clay based catalysts tested, Copper (II) adsorbed on clay nanocatalyst was found to be very efficient and showed promising activity for the formation of nitrogen containing heterocycle compound i.e. 1,5-benzodiazepine with excellent yields. An assortment of structurally diverse 1,5-benzodiazepine was prepared in good to excellent yields from easily available starting materials by using this protocol. The catalyst was found to be applicable to aromatic, heteroaryl and aliphatic ketone such as cyclic ketones as well as acyclic ketones.
Keywords
Cu-clay
Nanocatalyst
Benzodiazepine
Microwave
Recyclable
1 Introduction
Benzodiazepines (BDZ’s) is a privileged heteroaromatic molecule and form an important class of pharmaceutically active compounds and their synthesis is of great importance for medicinal chemists and pharmacists in the field of medicinal and pharmaceutical chemistry owing to their application as analgesic, anticonvulsant, anti-inflammatory, sedative and hypnotic activity (Christian and Demeunynck, 2007; Pathak et al., 2007; Moody et al., 2007; Barrett et al., 2007; Puodziunaite et al., 2000). The analogues of 1,5-benzodiazepines are also used in pigments industries, dyes and paints industries as well as for acrylic fibers in photography related technology. Further, benzodiazepines scaffolds have been used as starting material for the synthesis of other compounds such as oxazinobenzodiazepines benzodiazepines, oxadiazolo, triazolo benzodiazepines and furano-benzodiazepines etc (Khanna et al., 2014; El-Snyed et al., 1999; Chimirri et al., 1990). Benzodiazepines are generally synthesized from of 1,2-diaminobenzene (OPD) by the condensation with simple ketones, α,β- unsaturated aromatic or aliphatic carbonyl compounds. So far different types of acidic reagents have been applied for this purpose, such as, PVP-FeCl3, Ag3PW12O40, Al2O3/P2O5, zeolite, MgO/POCl3, BF3-etherate, Yb(OTf)3, polyphosphoric acid, sodium borohydride (NaBH4), gallium triflate (Ga(OTf)3), (Lloyd et al., 1965; Younes, 2011; Climent et al., 2009; Baseer and Khan, 2012) lead nitrate (Pb(NO3)2, l-proline (amino acid), sulfated zirconia etc. apart from acetic acid as catalyst under microwave conditions and molecular iodine based catalysts have also been widely used (Ganai et al., 2006; Sivamurugan et al., 2004; Bennamane et al., 2008; Jadav and Kim, 2013; Chadha et al., 2011; Zangade et al., 2011; Sharma et al., 2011). However, it is found that many of the reagents suffer from one or more drawbacks due to drastic conditions such as long reaction times, harsh reaction conditions, formation of undesired side product or occurrence of many side reactions, tedious work-up procedure and low yields of the product. In addition, the previously used solid phase catalysts showed low surface area and low pore volume which do not prove to be more efficient for performance because of not effective adsorption on the surface and hence poor yield benzodiazepines.
Many of the reported methods suffer with drawbacks in terms of atom economy, substrate scope, reaction time, reaction conditions, unsatisfactory yields, elevated temperatures, and/or protected syntheses of substrates. Consequently, there is a need to develop environment friendly, operationally simple and mild methods to obtain this privileged pharmacophore from a broad class of readily available synthetic reagents.
Clays are considered to be most cost-effective and environmentally friendly catalysts for organic transformations. The metal doped clays can be the hybrid materials with combined catalytic rewards of metal salts and clay catalysts. Thus, in continuation of our interests (Shaikh et al., 2017; Dar et al., 2015a; Farooqui and Baldev 2015; Dar et al., 2015b; Dar et al., 2014), we herein report the synthesis of benzodiazepine from readily available OPD and commercially available ketones using a heterogeneous Cu(II)/Montmorillonite clay catalyst. The advantages of the system include, short reaction times, catalyst recyclability, neat conditions, remarkable activity without any additives and amenable for synthesis of diverse benzodiazepines.
2 Materials and methods
2.1 Preparation of the Cu(II)-clay nanocatalyst
Cu(II)-clay nanocatalyst was prepared by introducing calculated amount of aqueous copper oligomer (prepared from copper(II) chloride precursor) to obtain a 10 wt% copper loading on to commercially available clay (Montmorillonite KSF) (purchased from Sigma-Aldrich). The as-prepared mixture was stirred for overnight (about 12–15 h). After the optimized time, it is filtered and thoroughly washed with distilled water several times to remove chloride ions. The solidified material in the form of cake is formed which is separated and dried in an oven at about 100–125 °C temperature over a period of 10–12 h, the obtained solid is then powdered and calcined in air at 450 °C for 2–3 h. The calcined product is referred to as Cu(II)-clay nanocatalyst and was used as such in all the reported experiments. Detailed preparation procedure is given in our previous publication (Bashir et al., 2018). The prepared catalyst was characterized by XRD analysis and the spectrum obtained was found to correspond to previously reported data (Fig. 1).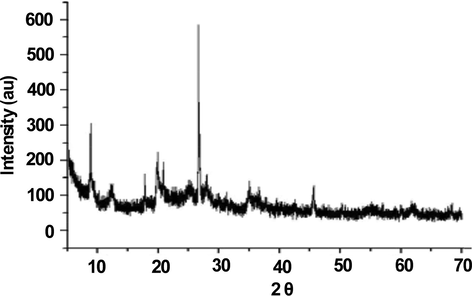
XRD pattern of fresh Cu(II)-clay nanocatalyst.
3 Results and discussion
In this method, we have employed Cu(II)-clay nanocatalyst with 10 wt% copper loading, which was pre-optimized. The best yield was found by using 10 wt% copper loading, this might be due to the optimum nano-size of the catalyst, which may contain increased number of active catalytic sites. Whereas, the higher copper loading may leads to the enhancement of particle size of CuO which adversely affects the surface area of the resultant catalysts and also lowers the formation of active sites. On the other hand, lowering the copper content to below 10%also decreases the activity of the catalysts, due to the absence of sufficient quantity of active material (Cu in this case).
In order to optimize the reaction condition different parameters were varied including the amount of reactants and catalyst, and following conditions were found to be best suited; 5 mg of Cu(II)-clay nanocatalyst, 2 mol of acetophenone, O-phenylendiamine (1.2 mmol). The reaction was completed within 10 min, which was confirmed by TLC (Scheme1).
Schematic representation of synthesis of 1,5-benzodiazepine.
To begin with, the reaction of acetophenone and o-phenylenediamine (OPD) in toluene as solvent was performed at room temperature which did not yield any product (Table 1 entry 1). This led us to increase the reaction temperature to 100 °C, which yielded the formation of corresponding benzodiazepine in about 12% yields. (Table 1 entry 2 based Scheme 1). These results warranted optimization of the reaction conditions.
No.
Reaction Condition
Solvent used
Time (in mins)
Catalyst (mole %)
Yielda (%)
1
Stirring at room T
Toluene
60
15
–
2
Stirring @120 °C
Toluene
60
15
35
3
Stirring @120 °C
CH3CN
60
15
60
4
Stirring @120 °C
MeOH
60
15
67
5
Stirring @120 °C
EtOH
60
15
52
6
Stirring @120 °C
DMF
60
15
63
7
Stirring @120 °C
DMSO
60
15
82
8
Stirring @120 °C
THF
60
15
46
9
Stirring @120 °C
Solvent free
60
15
14
10
Microwave
Solvent free
10
15
98
11
Microwave
Solvent free
8
15
98
12
Microwave
Solvent free
6
15
74
13
Microwave
Solvent free
8
10
98
14
Microwave
Solvent free
8
5
98
15
Microwave
Solvent free
8
3
79
16
Microwave
Solvent free
10
No catalyst
nil
The reactions is examines by using different solvents as medium for the reaction. We have observed that organic solvents various solvents includes methanol, dimethyl sulfoxide, THF, dichloromethane, chloroform and ethanol have been used and tested for the quality of reaction completion. We found that polar solvents give good to excellent yields. We moved for the solvent free reaction conditions and got exciting results. We investigated the reaction under sonication and microwave conditions. Interestingly, the reaction in microwave resulted in quantitative conversion to the products under solvent free conditions in 10 min to study the effect of the amount of catalysts needed for the transformation, various reactions were performed by varying the quantity of catalyst by keeping other parameters constant. It was revealed that 5 mg of catalyst was found to be efficient which yielded ∼98% of conversion (Table 1). Apart from this, the impact of other clay catalysts on the aforementioned reactions was also studied (Table 2). The catalysts such as Montmorillonite- K10, Montmorillonite-KSF, Kaoline, and bentonite failed to give any product. The Pd/Montmorillonite-KSF though gave corresponding product in low yields. The other montmorillonite based catalysts viz., Ni/Montmorillonite-KSF, Co/Montmorillonite-KSF, Sn/Montmorillonite- KSF, and Zn/Montmorillonite-KSF have also been tested and found to give the products in very less amounts. Furthermore, the use of PdCl2 and CuO as well did not offer any increased yield of product formation. However, the Cu(II)-clay nanocatalyst was found to show promising results compared to CuO for the model reaction under optimized conditions. This may be due to the high dispersion and the nano-sized character of Cu(II) ions on clay support. After investigating the various reaction procedures, it was confirmed that the use of 5 mole % Cu(II)-clay nanocatalyst is the optimum amount of catalyst, and the optimum reaction time is 10 min for best results.
No.
Catalyst
Catalyst loading (mole%)
Yieldsa (%)
1
Bentonite
5
–
2
Montmorillonite-K10
5
–
3
Montmorillonite-KSF
5
---
4
Kaoline
5
---
5
Cu/Montmorillonite-KSF[(Cu(II)-clay nanocatalyst]
5
98
6
Pd/ Montmorillonite-KSF
10
55
7
Sn/Montmorillonite-KSF
10
traces
8
Ni/Montmorillonite-KSF
5
12
9
Sn/Montmorillonite-KSF
5
traces
10
Co/Montmorillonite-KSF
5
13
11
CuO
5
54
12
PdCl2
5
traces
Efficiency of the catalyst can be judged from its recyclability property. The catalyst was recycled by using model reaction between commercially available acetophenone (2.0 mmol), o-phenylenediamine (1.2 mmol) and Cu(II)-clay nanocatalyst (5 mg) under microwave and solvent free conditions. Excellent result have been shown by the recycled catalyst for the above reaction. which depicts that there is persistent in activity of nanocatalyst without significant loss of activity and the catalyst could be easily recovered by simple filtration and reused, leading to corresponding 1,5-benzodiazepines in quantitative yield even up to five times reuse of the catalyst. (Fig. 2).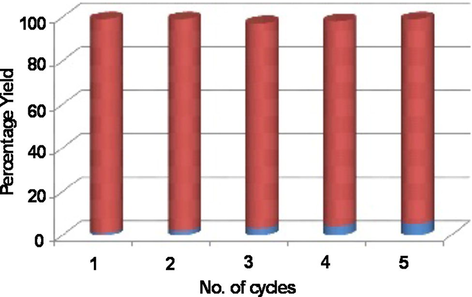
Recyclability of the Cu(II)-clay nanocatalyst for one pot synthesis of benzodiazepines. (red bars indicate product yield and blue bars indicate decrease in yield). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The promising and effective performance of the Cu(II)-clay nanocatalyst in the synthesis of 1,5-benzodiazepine stimulated us to use this protocol for the synthesis of substituted 1,5-benzodiazepines conjugates using different commercially available substituted benzene-1,2-diamine (o-PDA) and diversified ketones as precursor for heterocyclizatione (Scheme 2) and the results obtained are shown in Table 3. In all examples, the reactions proceeds with high rate and thus complete within very short reaction time viz.8-10mins. The nanocatalyst showed promising activity in all the types of precursor used in this transformation, affording 90–98% isolated yield of the corresponding derivatives of 1,5- benzodiazepine. Electron donating groups, e.g. halogens, methoxy, as well as electro withdrawing groups, e.g. nitro group, have no significant effect on the reaction yields. It was experimentally confirmed that the catalyst Cu(II)-clay nanocatalyst showed superior performance with high yields in a relatively shorter reaction time.
Schematic representation of synthesis of 1,5-benzodiazepine using nanocatalyst.
S. No.
Diamine
Ketone
Product
Time (min)
% Yields
1
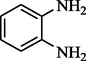
Acetophenone
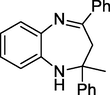
3a
8
98
2
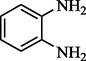
4-methyl Acetophenone
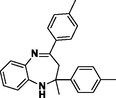
3b
10
94
3
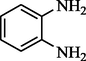
4-chloro Acetophenone
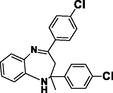
3c
10
96
4
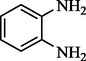
3-acetyl thiophene
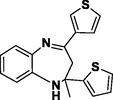
3d
10
98
5
2-acetyl thiophene
3e
8
98
6
Acetone
3f
8
94
7
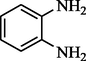
Butanone
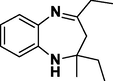
3g
8
94
8
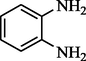
2-pentanone
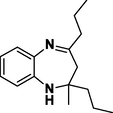
3h
10
95
9
Methyl Iso Butyl Ketone
3i
10
96
10
Cyclopentanone
3j
12
96
11
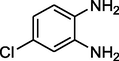
Acetophenone
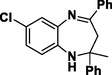
3k
10
98
12
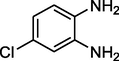
4-methyl Acetophenone
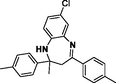
3l
10
97
13
4-chloro Acetophenone
3m
10
98
14
3-acetyl thiophene
3n
8
96
15
3-acetyl thiophene
3o
8
95
16
Acetone
3p
10
95
17
Butanone
3q
8
94
18
2-pentanone
3r
10
92
19
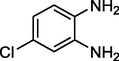
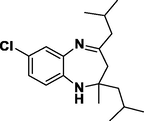
Methyl Iso Butyl Ketone
3s
8
93
20
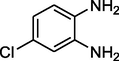
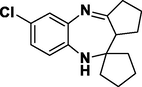
Cyclopentanone
3t
10
90
4 Experimental
4.1 General procedure for the synthesis of 1,5-benzodiazepines
A mixture of o-phenylenediamine (OPDA) (1) (1 mmol) with commercially available ketone (2) (2.5 mmol) and catalyst (5 mol %) were taken into a synthesizer used for microwave governed reaction i.e. CATA - R I operating at 300 W, and irradiated for 8 min. The progress of the reaction was checked by thin layer chromatography by using ethyl acetate polar solvent (10%) and pet ether used as non polar solvent (90%) for mobile phase After completion of the reaction, reactions was quenched by adding ethyl acetate and filtering gave product which on purification by column chromatography subjected for physical and spectral data analysis. The filtrate was concentrated under high vacuum. The crude products were purified by crystallization and were further characterized by nuclear magnetic resonance spectroscopy (NMR) and mass analysis. The characterization data of the as-synthesized compounds were compared with the reported examples in literature.
The crude product extracted by ethyl acetate was purified by column chromatography and crystallized by using alcohol water system. The spectral data of all the compounds are described in the following sections.
Entry 1: (E)-2,3-dihydro-2-methyl-2,4-diphenyl-1H-benzo[b][1,5]diazepine(3a): Color : Yellowish crystalline in nature , melting point : 145–147 °C. IR (KBr)cm−1:νmax 3280, 2959,1466, 749
1HNMR (300 MHz, Chloroform-d (CDCl3): δ 1.29 (s, 3H, –CH3), 2.17 (d, 1H, J = 12.8 Hz), 2.5 (d, 1H, J = 12.4 Hz), 3.34 (broad singlet, 1H, -), 7.05–7.3 (m, 4H, ArH),6.6–6.7 (m, 3H,), 7.4–7.4 (m, 4H, ArH), 7.9–8.0 (m, 3H,) ppm. MALDI-MS: m/z [M+] = 312. M.F. C22H20N2.
Entry 2: (E)-2,3-dihydro-2-methyl-2,4-dip-tolyl-1H-benzo[b][1,5]diazepine(3b): pale Colour: Yellowish melting Point:. 199–201 °C. IR(in KBr)cm−1: νmax 3307, 2974, 1603, 1471, 759.1HNMR (300 MHz, Chloroform-d (CDCl3): δ 1.3 (s, 6H, –CH3), 2.4 (s, 3H, CH3), 2.7–2.7 (s, 2H, –CH2), 3.03 (br singlet, 1H,), 6.7–6.8 (m, 1H,), 6.9–7.0 (m, 3H,), 7.2–7.3 (m, 5H,), 7.8–7.9 (m, 3H,) ppm. MALDI-MS: m/z [M+] = 340. M.F. C24H24N2.
Entry 3: (E)-2,4-bis(4-chlorophenyl)-2,3-dihydro-2-methyl-1H-benzo[b][1,5]diazepine(3c): Color: Yellow Coloured, crystalline solid, Melting Point14–14 °C. IR (KBr)cm−1: νmax 3330, 2974, 1607, 1468, 762. 1HNMR (Chloroform-d (CDCl3)300 MHz,: δ 1.7 (s, 3H, CH3), 2.8 (d, 1H, J = 13.2 Hz), 3.04 (d, 1H, J = 13.2 Hz), 6.8–6.8 (m, 1H), 3.4 (broad singlet,), 7.0–7.1 (m, 2H), 7.15–7.21(m, 4H, 7.17.20 (m, 1H,), 7.39–7.49 (m, 4H,) ppm. MALDI-MS: m/z [M+] = 380.M.F. C22H18Cl2N2.
Entry 4: (E)-2,3-dihydro-2-methyl-2,4-di(thiophen-2-yl)-1H-benzo[b][1,5]diazepine(3d):Color: pale yellow, melting point.111–113 °C. IR (KBr) cm−1 νmax 3303, 2920, 1600, 1499, 765. 1HNMR (Chloroform-d (CDCl3, 300 MHz,): δ 1.70 (s, 1H), 1.74 (s, 1H), 2.54 (s, 3H, CH3), 6.78–7.30(m, 6H), 3.46 (br singlet, 1H,), 7.26–8.04 (m, 4H,) MALDI-MS: [M+] = 324. M.F. C18H16N2S2.
Entry 5: (E)-8-chloro-2,3-dihydro-2-methyl-2,4-di(thiophen-3-yl)-1H-benzo[b][1,4]diazepine: 1HNMR (300 MHz, Chloroform-d (CDCl3)): δ 1.82 (s, 3H, CH3), 3.00 (1H,d, J = 12.4 Hz), 3.07 (1H,d, J = 12.4 Hz), 3.59 (1H,broad singlet,), 6.79–6.87 (1H, m, Ar H), 6.89–6.92 (2H,m, ArH), 6.99–7.12 (4H,m,), 7.32–7.42 (1H,m,), 7.61–7.65 (1H,m, ArH) ppm. IR (KBr): νmax 3334, 2977, 1587, 1471, 715 cm-MALDIMS: [M+] = 358. M.F C18H15ClN2S2.
Entry 6: (Z)-8-chloro-2,3-dihydro-2,2,4-trimethyl-1H-benzo[b][1,4]diazepine(3f): IR (KBr) in cm−1: 3295, 2964, 1633, 1591, 1475, 770. 1HNMR (, Chloroform-d (CDCl3300 MHz: δ 1.25 (s,6H, 2CH3), 2.14 (2H,s)- 2.28 (3H, s), 3.39 (1H, broad singlet,), 6,64 (1H, d, J = 8.19 Hz), 6.88 (2H, d, J = 3.1 Hz) 7.04 (1H, d, J = 8.19 Hz)MALDI-MS: m/z [M + ] = 188. M.F. C12H16N2.
Entry 7: (Z)-8-chloro-2,4-diethyl-2,3-dihydro-2-methyl-1H-benzo[b][1,4]diazepine(3): IR (KBr): νmax 3341, 2966, 1592, 1375, 750 cm−1 1HNMR (Chloroform-d (CDCl3300 MHz,): δ 0.93 (3H,t, J = 3.2 Hz, 0.98–1.02(6H, m,) 1.57–1.64 (2H, m), 2.11–2.19 (2H, m),2.54–2.62 (2H, m), 3.55 (1H, broad singlet), 6.69–6.72 (1H, m), 6.93–6.9992H, m), 7.8–7.14 (1H, m) MALDI-MS: [M+] = 216 M.F. C14H20N2.
Entry 8: (Z)-2,3-dihydro-2-methyl-2,4-dipropyl-1H-benzo[b][1,4]diazepine (3h): Colour: solid; yield): m.p. 138–140 °C. IR (KBr): 3341, 3060, 1589, 1371, 687 cm−1. 1HNMR (300 MHz, Chloroform-d): δ 0.93–0.99 (6H, m), 1.12 (3H, s), 1.16–1.34 (4H, m),1.51–1.60 (1H, m), 2.09–2.18 (1H, m), 2.52–2.58 (4H, m) 3.03(1H, broad singlet) 6.69–6.72 (1H m), 6.93–6.97 (2H, m), 7.10–7.13 (1H,m) MS: m/z [M+] = 244. Anal.Calcd.for C16H24N2.
Entry 9: (Z)-2,3-dihydro-2,4-diisobutyl-2-methyl-1H-benzo[b][1,4]diazepine(3i): IR (KBr): 3403, 2955, 2353, 1674, 1464, 750 cm−1. 1HNMR (Chloroform-d (CDCl3, 300 MHz,): δ 0.88–0.99 (12H, m), 1.31 (3H. s),1.43–1.55(2H, m), 1.69–1.71 (2H, m), 2.10–2.29 (2H, m), 2.42–2.45 (2H, m), 3.12 (1H, brs),6.66–6.69 (1H, m), 6.93–6.97(2H,m) 7.11–7.14 (1H, m) MALDI-MS: M+] = 272 M.F. C18H28N2.
Entry 10: 10-Spirocyclopentane −1, 2, 3, 9, 10, 10a -hexahydrobenzo [b] cyclopenta [e][1,5]-diazepine (3j): IR (KBr) cm−1: 3326, 2950, 1629, 1371, 676. 1HNMR (Chloroform-d (CDCl3, 300 MHz,): δ 1.21 (s, 1H), 1.62–2.63 (m, 14H), 2.78 (broad singlet, 1H, NH), 6.62–6.89 (1h, m), 7.21(2H, dd, J = 1.4 Hz), 7.81 (1H, d, J = 7,1 Hz), MALDI-MS: [M+] = 240, M.F. C16H20N2.
Entry 11: 7-chloro-2-methyl −2,4-diphenyl-2,3-dihydro-1H-1,5-benzodiazepine (3k): IR (KBr, cm−1): 3352, 3066, 1457, 835 1HNMR (Chloroform-d (CDCl3, 300 MHz,): δ 1.73 (3H, s), 2.92–2.96 (1H, d, j = 12.8 Hz), 3.0 (1H, d, J = 12.8 Hz), 3.59 (broad singlet, 1H,), 6.79–6.82 (1H, m), 6.95–7.03 (1H, m), 7.14–7.31 (8H, m), 7.52–7.56 (4H, m) MALDI-MS: [M+] = 346. M.F. C22H19ClN2.
Entry 12: 7-chloro-2methyl-2,4-dip-toluyl −2,3-dihydro-1H-1,5-benzodiazepine (3l): IR(KBr): νmax 3318, 2955, 1444, 817 cm−1. 1HNMR (300 MHz, Chloroform-d (CDCl3)): δ1.67 (3H, s), 2.17 (2H, s), 2.33 (6H, s) 2.99 (1H, brs), 6.79–6.81 (1H, m), 7.3–7.08 (5H, m), 7.42–7.51 95H,m) MALDIMS: [M+] = 374. M.F: C24H23ClN2.
Entry 13: 7-chloro-2,4-bis(4-chlorophenyl)-2-methyl-2,3-dihydro-1H-1,5-benzodiazepine (3m): IR (KBr): νmax 3265, 1588, 2967, 1475, 829 cm-1. 1HNMR (Chloroform-d (CDCl3300 MHz,)): δ 7.41–7.51(5H, m) 7.18–7.27 (4H, m), 6.98–7.04 (1H,m),6.6–6.7 (1H, m), 3.49 (1H, brs), 3.14 (‘1H, d), 2.86 (1H, d) 1.75 (3H, s), MALDI-MS: [M+] = 415. Anal. Calcd. For C22H17Cl3N2.
Entry 14: 7-chloro-2-methyl −2,4-di(thiophen-3-yl) −2,3-dihydro-1H-1,5-benzodiazepine (3n): IR (KBr): 3394, 2962, 1592, 1469, 781 cm-1. 1HNMR (Chloroform-d (CDCl3, 300 MHz,): δ 7.19–7.28(5H, M), 7.09–7.16 (1H,M), 6.89–7.01 (2H,m)6.69–6.76 (1H, m)3.42 (1H, brs), 2.92 (1H, d, J = 13.2 Hz)2.85 (1H, d, J = 13.1 Hz), 1.72 (3H, s), MALDI-MS: [M+] = 358. M.F: C18H15ClN2S2.
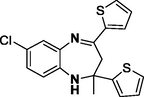
Entry 15: 7-chloro −2-methyl −2,4-di(thiophen-2-yl) −2,3-dihydro-1H-1,5-benzodiazepine (3o): IR (KBr, cm−1): 3303, 2967, 1577, 1471, 705. 1HNMR (Chloroform-d (CDCl3, 300 MHz,: δ7.62–7.67 1H, m) 7.29 = 7.37 (1H,m)7.01–7.10 (4H, m) 6.89–6.93 (2H,m), 6.80–7.82 (1 h, m), 3.57 (broad singlet, 1H, N–H), 3.07 (1H, d, J = 12.9 Hz), 3.0 (1H, d, J = 12.9 Hz) 1.83 (3H, s) MALDIMS: [M+] = 358. Anal. Calcd. for C18H15ClN2S2
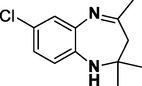
Entry 16: 2,2,4-Trimethyl −2,3-dihydro-7-chloro-1H-1,5-benzodiazepine (3p): IR (KBr, cm−1): νmax 3282, 2962, 1630, 1453, 750. 1HNMR (300 MHz, Chloroform-d (CDCl3)): δ 7.02–7.08 (1H, m), 6.89–6.92 (1H, m), 6.61–6.71 (1H,m), 3.01 (1H, brs) 2.33 (3H, s), 2.22 (2H,t) 1.33 (6H, m) MALDI-MS:[M+] = 222. M.F. C12H15ClN2.
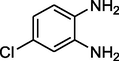
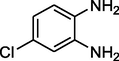
Entry 17: 7-chloro −2,4-diethyl- 2-methyl −2,3-dihydro-1H-1,5-diazepine (3q): IR (KBr, cm−1): 3424, 2971, 1596, 1499, 798.1HNMR (Chloroform-d (CDCl3, 300 MHz,): δ7.02–4.09 (1H,m), 0.91- (3H, t, J = 6.6 Hz) 1.23–1.25 (6H,m) 1.60–1.64 (2H, m) 2.23 (2H,m), 2.60 (2H, q, J = 3.1 Hz), 3.09 (1H, brs) 6.61–6.70 (1H,m), 6.89–6.93(1H, m) MALDI-MS: [M+] = 250. M.F: C14H19ClN2.
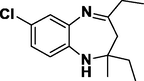
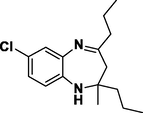
Entry 18: 7-chloro-2-methyl −2,4-dipropyl −2,3-dihydro-1H-1,5-benzodiazepine (3r): IR (KBr, cm−1): 3337, 2956, 1668, 1468, 806. 1HNMR (Chloroform-d (CDCl3, 300 MHz,: δ6.93–7.02 (1H, M)6.76–6.79 (1H, m)6.50–6.61 (1H, m) 3.11 (1H, BRS) 2.40–2.43 (2H, m), 2.02–2.10 (2H,m), 1.59–1.68 (2H, m), 1.23–1.49 (4H,m) 1.13 (3H, s) 0.80–0.91 (6H,m) MALDI-MS: [M+] = 278.
Entry 19: 7-chloro-10-Spirocyclopentane −1, 2, 3, 9, 10, 10apentahydrobenzo [b] cyclopenta [e] [1,5]-diazepine (3s): IR (KBr, cm−1): 3342, 2959, 1650, 1499, 837. 1HNMR (300 MHz, Chloroform-d (CDCl3)): δ7.87–7.90 (m, 1H, Ar-H),6.71–6.75 (1H,m), , 6.62–6.65 (1H, m), 3.05 (1H, broad peak0, 3.89–4.12 (2H, m), 2.89–2.94 (1H d, J = 13.2 hz), 1.88–1.99 (12H m), MALDI-MS: [M+] = 274.
Entry 20: 7-chloro-10-Spirocyclopentane −1, 2, 3, 9, 10, 10apentahydrobenzo [b] cyclopenta [e] [1,4]-diazepine (3t): IR (KBr): 3342, 2959, 1650, 1499, 837 cm–1 1HNMR (Chloroform-d (CDCl3, 300 MHz,): δ 7.88–7.91 (1H, m), 6.73–6.78 (1H,m), 6.66 (1H,m), 3.04 (1H, broad peak), 3.89–4.21 (2H, m),2.91–2.96 (1H, d, J = 13.1 Hz), 1.87–2.01 (12H, m). MALDI-MS: [M+] = 274. M.F: C16H19ClN2.
5 Conclusion
Clay supported transitional metal based materials have emerged as valuable catalysts for various organic transformations including the preparation of Benzodiazepines. Preparing such clay based catalysts supported by different transition metals introduces new possibilities for catalysis due to their excellent catalytic properties including selectivity and reusability. In this study, we have demonstrated the synthesis of Cu(II) supported clay based nanocatalysts, which was used for the synthesis of different conjugates of 1,5-benzodiazepines. Among various as-prepared nanocatalysts, Cu(II)-clay nanocatalyst has demonstrated excellent activity for the preparation of 1,5-benzodiazepine derivatives by condensation of o-phenylenediamine with commercially available ketones. In order to investigate the effect of parameters such as reaction time, and solvents on the quality and quantity of products various reactions were performed by varying these parameters.
Acknowledgment
The author INS is grateful to Savitribai Phule Pune University (SPPU) for providing financial assistance.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- J. Pharm. Biomed. Anal.. 2007;44(2):498-505.
- Eur. J. Chem.. 2012;9:407-414.
- Org. Commun.. 2008;3:62-68.
- J. Chem. Pharm. Res.. 2011;3:331-340.
- J. Heterocycl. Chem.. 1990;27(2):371-374.
- Chem. Eur. J.. 2009;15:8834-8841.
- Tetrahedron Lett.. 2014;55:1544-1548.
- Tetrahedron Lett.. 2015;56:136-141.
- Green Sustainable Chem.. 2015;5:15-24.
- Bulletin of Chemical Reaction Engineering & Catalysis. 2018;13(1):84.
- Synth. Commun.. 1999;29(20):3561-3572.
- Green Chem. Lett. Rev.. 2015;8:1-8.
- Synth. Commun.. 2006;26:803-807.
- RSC Adv.. 2013;3:5131-5140.
- Tetrahedron Lett.. 2014 Dec 3;55(49):6652-6654.
- J. Chem. Soc. 1965:3785-3793.
- Bioorg. Med. Chem. Lett.. 2007;17(8):2380-2384.
- Indian J. Chem.. 2007;46B:1191-1197.
- Arkivoc. 2000;1:512.
- IJSRST. 2017;9:264.
- J. Chem. Pharm. Res.. 2011;3:382-389.
- Synth. Commun.. 2004;34:3833-3846.
- Arkivoc 2011:322-330.
- Chem.. 2011;3:144-149.







