Translate this page into:
MicroRNA-26b-3p inhibits human trophoblast cell proliferation, invasion and resistance to apoptosis via targeting SHBG
⁎Corresponding author at: Department of Gynecology and Oncology, Gansu Provincial Maternity and Child-Care Hospital, Qilihe North Street No 143, Qilihe District, Lanzhou City, Gansu Province 730050, China. Lixialongbio@yandex.com (Lixia Long)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aberrant increased apoptosis and dysfunction in migration and invasion of trophoblast cell play key role in preeclampsia. However, the mechanism remains unclear. Our study investigated the roles of microRNA-26b-3p (miR-26b-3p) in trophoblast cell growth by targeting sex hormone binding globulin (SHBG). Human trophoblast cell line HTR8-SVneo was used in this study. Gain- and loss-of-functions of miR-26b-3p and SHBG were performed, and then the cell viability was detected with CCK-8 and EdU assays. Cell apoptosis was detected using flow cytometry, and the cell invasion and migration were evaluated with transwell assays. The expression level of SHBG, caspase-3 and Bcl-2 after miR-26b-3p transfection was measured with RT-PCR and western blot analysis. Matching the sequence of miR-26b-3p and SHBG was analyzed through bioinformatic prediction and confirmed with dual luciferase reporter assay. It is found that overexpression of miR-26b-3p inhibited HTR8-SVneo cell proliferation, invasion and migration, while increased cell apoptosis and induced cell cycle arrest at the G0/G1 phase. Elvated level of miR-26b-3p decreased the expression of SHBG. Bioinformatic prediction and dual luciferase assay identified the relation of miR-26b-3p and HTR8-SVneo. Downregulation of SHBG exhibited similar effects on HTR8-SVneo cell growth with overexpression of miR-26b-3p. Conclusion: MiR-26b-3p targets SHBG and decreases SHBG expression. It inhibits human trophoblast cell proliferation, invasion and resistance to death of trophoblast cell. This study provided a mechanism for preeclampsia. Sex hormone binding globulin: SHBG; miR-26b-3p: microRNA-26b-3p; CCK-8: Cell-Counting Kit-8; RT-PCR: Real time polymerase chain reaction; NC: miR-26b-3p mimic negative control or SHBG negative control siRNA; inhibitor NC: negative control inhibitor; TBST: Tris-buffered saline with Tween.
Keywords
MicroRNA-26b-3p
Sex hormone binding globulin
Human trophoblast cell
Proliferation
1 Introduction
Differentiation and invasion of trophoblast cells are critical for placental development. Any disruption of this process leads to preeclampsia (Lu et al., 2017; Xue et al., 2019). Preeclampsia is one of the most incident disease in pregnancy and approximately 3–5% of the pregnancies are with preeclampsia. It is a major reason leading to maternal and fetal morbidity and mortality, particularly in developing countries (Lyall et al., 2013; Zou et al., 2013). Preeclampsia generally happens in the third trimester and is featured with edema, hypertension, and proteinuria (Sang et al., 2019; Luderer et al., 2019). The pathogenesis of preeclampsia remains unclear and there is no favorable clinical approach for preeclampsia treatment except delivery of the baby and placenta (Peter Stein et al., 2008; Xiao et al., 2017). Due to the poor obstetric outcome, it is critical to investigate the mechanism and there are researches have been made in the prediction and prevention of this disease (Lan et al., 2019; Ferreira and Motta, 2018). Aberrant increased trophoblast cell apoptosis and dysfunction in cell migration and invasion raised wide concerns in the field of preeclampsia studies (Xiao et al., 2017). However, the underlying mechanism remains largely unknown (Table 1). Note: RT-PCR, reverse transcription-quantitative polymerase chain reaction; miR, microRNA; SHBG, sex hormone binding globulin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; F, forward; R, reverse.
Gene
Primer sequence
miR-26b-3p
F: GGCCTGTTCTCCATTACTTGG
R: CGCTTCACGAATTTGCGTGTCAT
SHBG
F: CTGAGATCCAACTGCACAATCACT
R: ATCCACCTCCAGCAGCACA
Bcl-2
F: CCGTTGGCCCCCGTTGCTTT
R: CTGGCGGAGGGTCAGGTGGA
Caspase3
F: CTCGGTCTGGTACAGATGTCGATG
R: GGTTAACCCGGGTAAGAATGTGCA
U6
F: GCTTCGGCAGCACATATACTAAAAT
R: CGCTTCACGAATTTGCGTGTCAT
GADPH
F: ACCACAGTCCATGCCATCAC
R: TCCACCACCCTGTTGCTGTA
MicroRNAs (miRNAs) are short non-coding RNAs molecules composed of 20 ∼ 25 nucleotides. They work as negative regulators of post-transcriptional gene expression by binding to the mRNAs of target gene, leading to transcriptional degradation or translational inhibition (Shioya et al., 2010; Sun et al., 2017). MiRNAs emerged as critical factors in multiple fields of medicine and physiology, particularly as accessible biomarkers, or as regulators of cell differentiation (Doridot et al., 2013; Horn and Wendy, 2020). They also play crucial roles in key biological process including cell apoptosis, proliferation and invasion (Dong et al., 2018). Several miRNAs have been reported as critical roles in trophoblast cell proliferation and preeclampsia development (Ding et al., 2016; Jairajpuri and Almawi, 2016). Among these miRNAs, miR-26b has been revealed to promote granulosa cell apoptosis (Lin et al., 2012) and osteoblast differentiation (Lin et al., 2019). However, whether miR-26b-3p is important in preeclampsia remains unclear. In our study, we identified that miR-26b-3p negatively targets sex hormone binding globulin (SHBG). SHBG expression has been suggested to play key roles in trophoblasts growth (Yu et al., 2018; Konings et al., 2018). Besides, SHBG has been elucidated to decrease the incidence rate of preeclampsia. Taken these together, the current study was performed to investigate the roles of miR-26b-3p in the trophoblast cell growth and preeclampsia with the involvement of SHBG.
2 Materials and methods
2.1 Cell culture and transfection
Human trophoblast cell line HTR8-SVneo were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in Dulbecco's Modified Eagle’s Medium (DMEM)/F12 complete medium supplemented with 10% fetal bovine serum (FBS), 100 μg/mL streptomycin and 100 U/mL penicillin in a 37 °C incubator with 5% CO2.
2.2 Dual luciferase reporter gene assay
A computer-based detection program (http://www.targetscan.org) was performed to predict the binding site of miR-26-3p and SHBG, and the binding relation was further identified using dual luciferase reporter gene assay. The wild-type SHBG (SHBG-WT) or SHBG mutant-type (SHBG-Mut) sequence was cloned to the downstream of firefly luciferase gene to produce pGL3-SHBG-WT or pGL3-SHBG-Mut luciferase reporter plasmid (synthesized by Guangzhou RiboBio Co., Ltd). HEK293T cells were obtained from Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and seeded into 24-well plates.
2.3 Real time quantitative polymerase chain reaction (RT-PCR)
Total RNA was extracted using Trizol (Invitrogen Inc., Carlsbad, CA, USA) 48 h after transfection, and the RNA concentration and purity were evaluated detected on a Nanodrop 2000 (Thermo Fisher Scientific Inc., Waltham, MA, USA). The RNA was reversely transcripted to cDNA using PrimeScript™ RT reagent kit with gDNA Eraser (Takara Holdings Inc., Kyoto, Japan). U6 was set as the internal control for miRNA while GAPDH for other genes in the RT-PCR detection. RT-PCR was performed using SYBR® Premix Ex Taq™ (TliRNase H Plus) assay kit (Takara).
2.4 Western blot analysis
Forty-eight hours after transfection, cells were washed twice with phosphate buffer saline (PBS), dried, lysed with sodium dodecyl sulfate (SDS) on ice for 10 min. The protein concentration was measured using a bicinchoninic acid assay kit (P0012, Beyotime Biotechnology Co., Ltd, Shanghai, China). 30 μg each sample were calculated and 5× loading buffer was added. The mixture was boiled for 5 min and separated with 10% SDS-polyacrylamide gel electrophoresis. The membranes were exposed using X-ray film, and the intensity of the bands was determined using Image J software (National Institutes of Health, Bethesda, Maryland, USA).
2.5 Cell-Counting Kit-8 (CCK-8) method
The HTR8-SVneo cells in each group were detached with trypsin, and then seeded into 96-well plates for 24 h incubation. A blank well was set as control. The culture medium was removed at 24 h, 48 h, 72 h and 96 h, respectively, after which the cells were added with 100 μL complete medium. Then each well was added with 10 μL CCK-8 solution (GlpBio, USA), and the plates were maintained in the incubator for 1–4 h, after which the optical density (OD) values at 450 nm were measured.
2.6 5-Ethynyl-2′-deoxyuridine (EdU) labeling assay
Cell proliferation was detected as per the instructions of the EdU staining reagent (Cat. No. C0081L, Beyotime). The EdU reagent was diluted in medium at a ratio of 1:500 to produce EdU working solution. Then the solution was added into the culture plates for 2 h of incubation. The cells were then washed twice with PBS, fixed with 4% paraformaldehyde for 15 min, washed with PBS for three more times, and treated with 0.3% Triton X-100 in PBS for 5 min.
2.7 Flow cytometry
Forty-eight hours after transfection, HTR8-SVneo cells in each group were successively detached with ethylene diamine tetraacetic acid-free trypsin, washed twice with phosphate buffer saline (PBS), centrifuged at 1000 rpm for 5 min and then collected for follow-up experiments. Cell apoptosis was measured in strict accordance with the apoptosis assay kit (No. 556547, BD Biosciences, San Jose, CA, USA).
2.8 Transwell assay
The 24-well Transwell plates were purchased from Corning Glass Works (NY, USA) and the matrigel was obtained from BD (CA, USA). The matrigel was diluted in serum-free medium at a ratio of 1:3 and then preserved at −20 °C. In the cell invasion assay, the apical chamber was pre-coated with 100 μL diluted matrigel at room temperature overnight, and then added with 100 μL serum-free medium at room temperature for 2 h. Forty-eight hours after transfection, the cells were detached with trypsin, centrifuged at 100 rpm for 5 min, washed twice with PBS, and added with serum-free medium to adjusted the confluence to 5 × 105 cells/mL.
2.9 Statistical analysis
The Statistical Package for the Social Sciences (SPSS 21.0, IBM Corp. Armonk, NY, USA) was applied for data analysis. Measurement data were expressed as mean ± standard deviation. Differences among multiple groups were analyzed with one-way analysis of variance (ANOVA). p was obtained from two-sided test, and p < 0.05 was considered as statistically significant difference.
3 Results
3.1 Overexpression of miR-26b-3p inhibits HTR8-SVneo cell proliferation
HTR8-SVneo cells were transfected with miR-26b-3p mimic or miR-26b-3p inhibitor. MiR-26b-3p mimic transfection led to significantly elevated level of miR-26b-3p while miR-26b-3p inhibitor led to markedly reduced miR-26b-3p expression (p < 0.05) (Fig. 1A–C).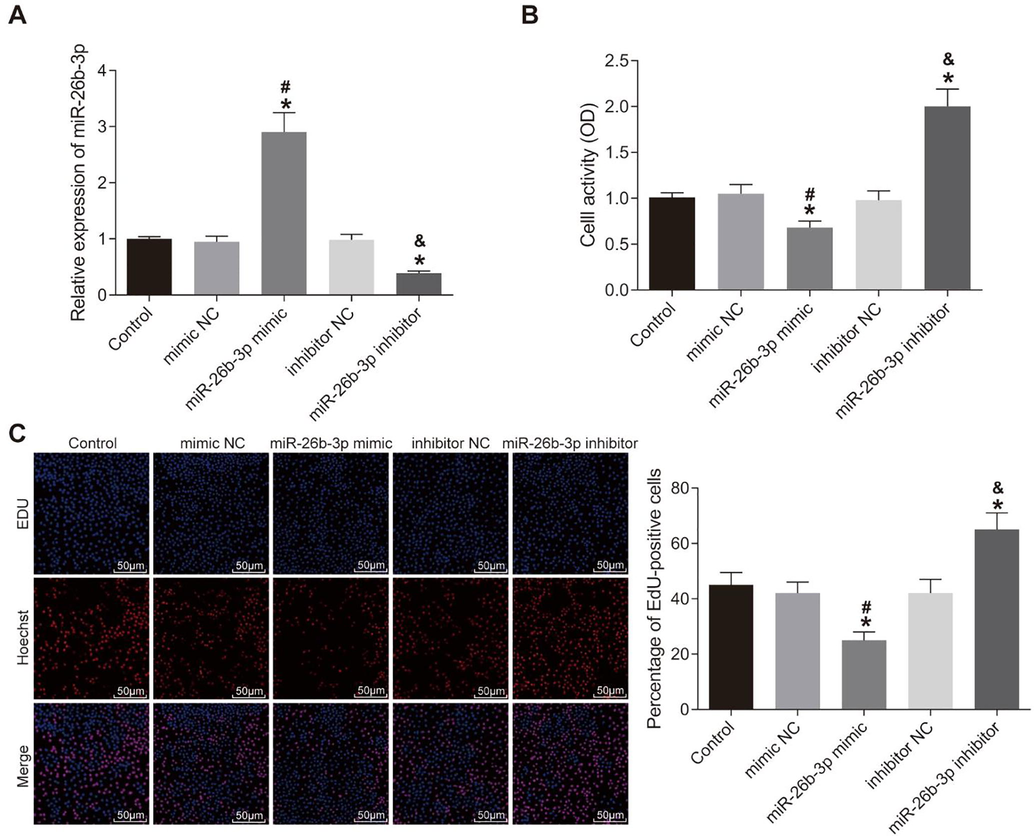
Over-expression of miR-26b-3p inhibits HTR8-SVneo cell proliferation. A, miR-26b-3p expression in cells measured using RT-PCR; B, cell viability measured using CCK-8 assay; C, cell proliferation detected using EdU assay; *, compared to the control group, p < 0.05; #, compared to the mimic NC group, p < 0.05; &, compared to the inhibitor NC group, p < 0.05; RT-PCR, reverse transcription-quantitative polymerase chain reaction; CCK-8, Cell-Counting Kit-8; EdU, 5-ethynyl-2′-deoxyuridine; NC, negative control.
3.2 Overexpression of miR-26b-3p promotes HTR8-SVneo cell apoptosis
In comparison to the negative control group, the induction of cell apoptosis dramatically enhanced in the miR-26b-3p mimic group. The cell apoptosis decreased in the miR-26b-3p inhibitor group (all p < 0.05) (Fig. 2A). Meanwhile, the cell cycle results exhibited similar trends as cell apoptosis. There was no difference in the cell cycle distribution among the control, mimic NC and inhibitor NC groups. The miR-26b-3p mimic group increased the cells at G0/G1 phase obviously while the miR-26b-3p inhibitor decreased this subpopulation (all p < 0.05) (Fig. 2B).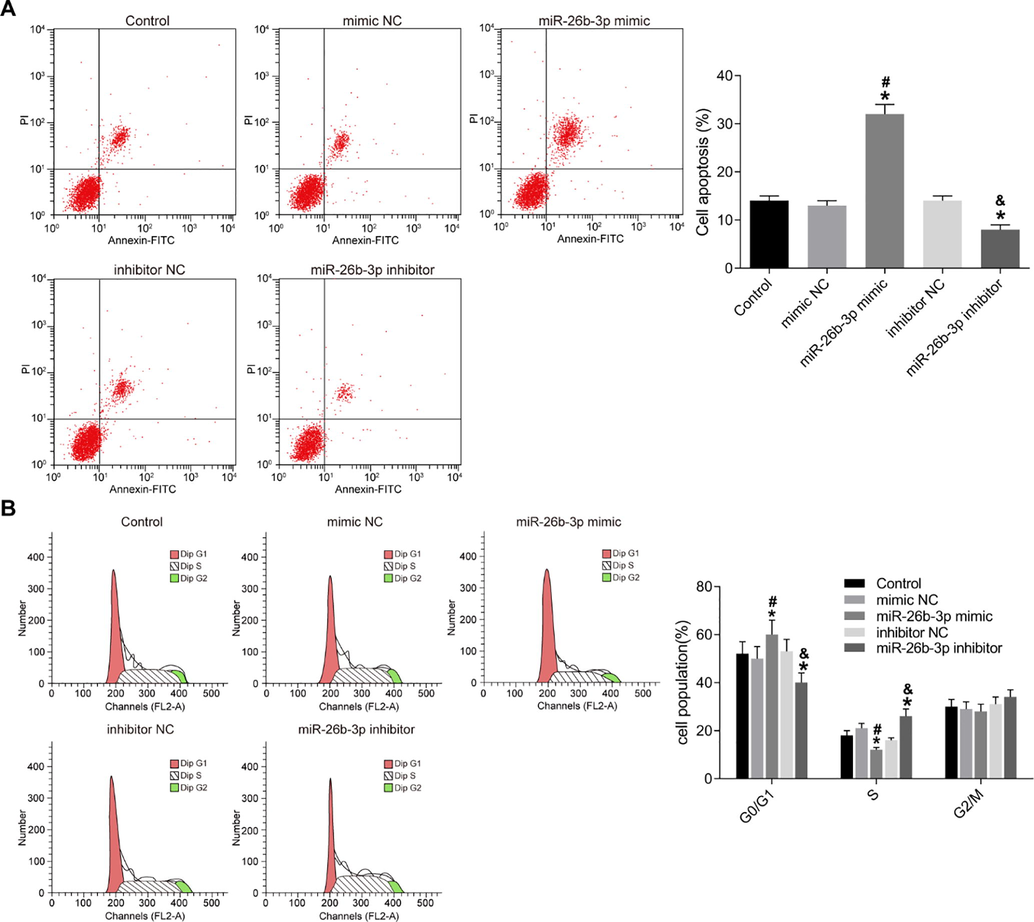
Over-expression of miR-26b-3p promotes HTR8-SVneo cell apoptosis. A–B, apoptosis (A) and cell cycle (B) of HTR8-SVneo cells detected using flow cytometry; C, bar charts of the cell apoptosis and cell cycle in each group of cells; *, compared to the control group, p < 0.05; #, compared to the mimic NC group, p < 0.05; &, compared to the inhibitor NC group, p < 0.05; NC, negative control.
3.3 Over-expression of miR-26b-3p inhibits HTR8-SVneo cell invasion and migration
In the transwell assay, the invasion and migration of HTR8-SVneo cells showed very limited difference among the control, mimic NC and inhibitor NC groups, as expected. However, the invasion and migration of HTR8-SVneo cells markedly reduced in the miR-26b-3p mimic group but increased in the miR-26b-3p inhibitor group (p < 0.05) (Fig. 3).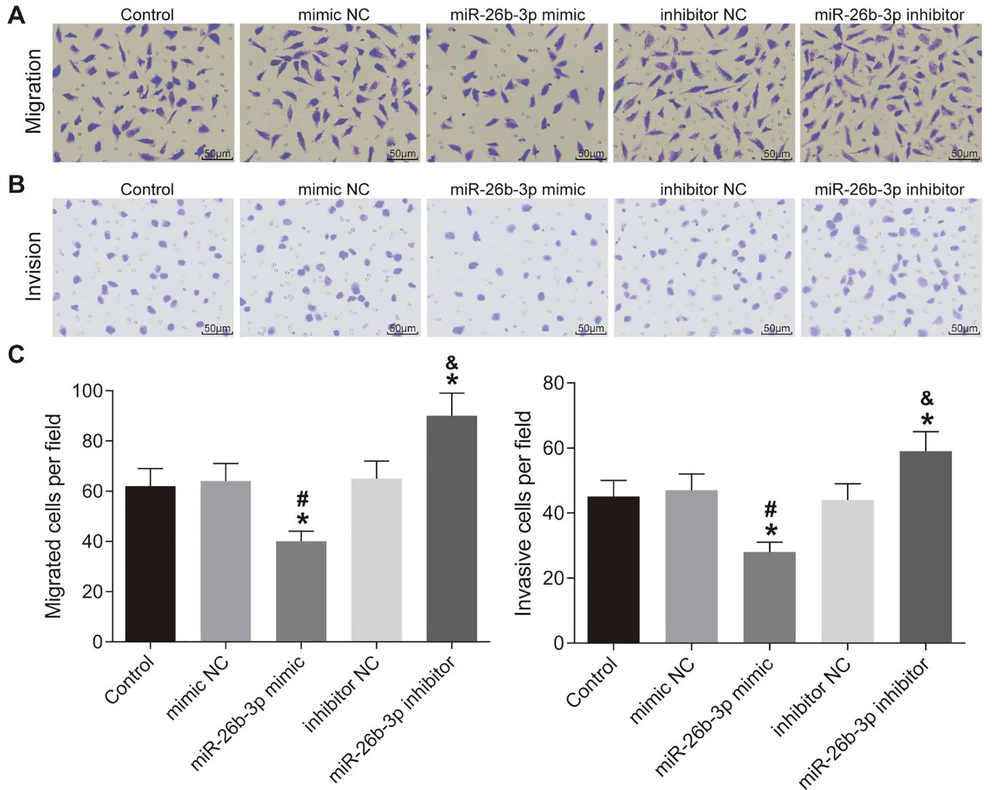
Over-expression of miR-26b-3p inhibits HTR8-SVneo cell invasion and migration. A–B, cell invasion (A) and migration (B) measured using Transwell assay and observed under a microscope; C, bar charts of the cell invasion and migration in each group; *, compared to the control group, p < 0.05; #, compared to the mimic NC group, p < 0.05; &, compared to the inhibitor NC group, p < 0.05; NC, negative control.
3.4 MiR-26b-3p inhibits SHBG gene expression and elevates caspase-3 expression while decreases Bcl-2 expression
To understand the mechanism how miR-26b-3p inhibits HTR8-SVneo growth, we detected the level of SHBG. Our study found that over-expression of miR-26b-3p led to significantly elevated mRNA and protein expression of SHGB, while down-regulation of miR-26b-3p led to an opposite trend (p < 0.05) (Fig. 4A). Then we further detected the levels of caspase-3 and Bcl-2 in cells using Western blot analysis and RT-PCR. Caspase-3 significantly elevated but the expression of Bcl-2 decreased in the miR-26b-3p mimic group. Meanwhile, in the miR-26b-3p inhibitor group, the caspase3 expression decreased while the Bcl-2 expression notably enhanced (all p < 0.05) (Fig. 4B and C).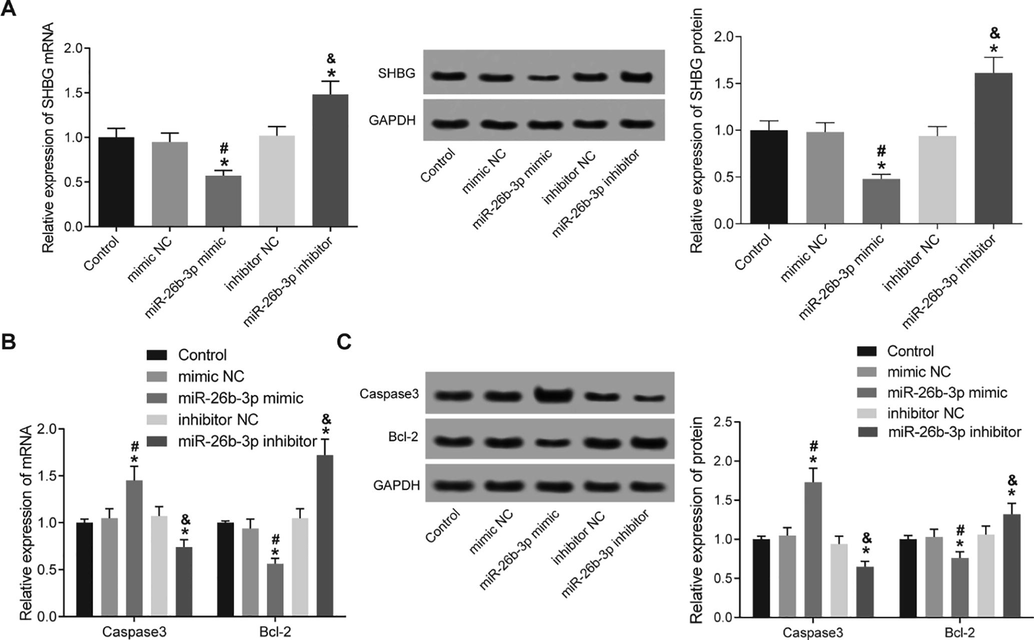
miR-26b-3p targets SHBG and elevates caspase-3 expression while decreases Bcl-2 expression. A, mRNA and protein expression of SHBG measured using RT-PCR and Western blot analysis; B-C, mRNA (B) and protein (C) expression of caspase-3 and Bcl-2 in cells detected using RT-PCR and Western blot analysis. respectively; *, compared to the control group, p < 0.05; #, compared to the mimic NC group, p < 0.05; &, compared to the inhibitor NC group, p < 0.05; SHBG, sex hormone binding globulin; NC, negative control.
3.5 miR-26b-3p directly targets SHBG
A bio-informatic program (http://www.targetscan.org) predicted the binding relationship between miR-26-3p and SHBG (Fig. 5A). The binding relationship was further identified using dual luciferase reporter gene assay. Co-transfection of miR-26b-3p and SHBG-WT plasmids significantly decreased fluorescence intensity (p < 0.05), while co-transfection of miR-26b-3p and SHBG-Mut showed no major difference compared to the NC group (Fig. 5B).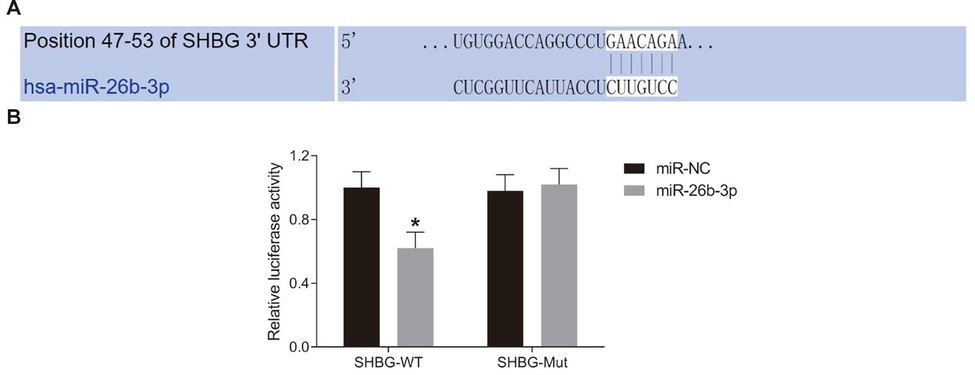
miR-26b-3p directly targets SHBG. A, binding sites between miR-26b-3p and SHBG predicted via the bio-information system (http://www.targetscan.org); B, target relation between miR-26b-3p and SHBG identified with dual luciferase reporter gene assay; *, compared to the miR-NC group, p < 0.05; SHBG, sex hormone binding globulin; NC, negative control.
3.6 Overexpression of SHBG (or knockdown) has the similar effect as miR-26b-3p inhibitor (or miR-26b-3p mimic) on HTR8-SVneo cells
To further investigate the roles of SHBG in HTR8-SVneo growth, we overexpressed or knocked down the SHBG expression in HTR8-SVneo cells. We further detected cell viability, apoptosis, invasion and migration. Compared to the NC group, the viability and invasion of cells significantly reduced while cell apoptosis enhanced in the si-SHBG group (all p < 0.05), which was consistent with the miR-26b-3p mimic treated cells. Cells in the oe-SHBG group showed an opposite trend, in which the cells significantly decreased cell viability and invasive ability and reduced cell apoptosis (all p < 0.05), and this was coincident with cells in the miR-26b-3p group (Fig. 6A–C).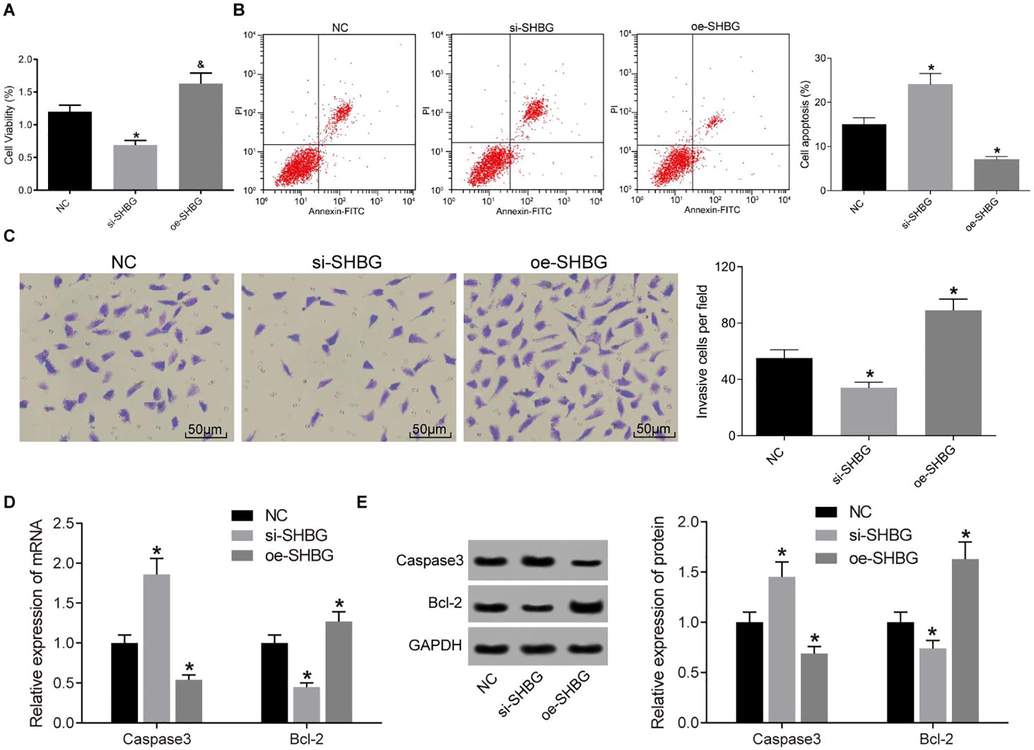
Over-expression of SHBG promotes cell viability, invasion and resistance to death of HTR8-SVneo cells. A, cell viability detected using CCK-8 method; B, cell apoptosis detected by flow cytometry; C, cell invasion measured by Transwell assay; D-E, mRNA (D) and protein (E) expression of caspase 3 and Bcl-2 in cells assessed by Western blot analysis; *, compared to the NC group, p < 0.05. SHBG, sex hormone binding globulin; CCK-8, Cell Counting-Kit 8; NC, negative control.
Meanwhile, the expression of apoptosis-related factors caspase 3 and Bcl-2 was measured by RT-PCR and Western blot assays (Fig. 6D).
Studying the roles of miRNAs in preeclampsia have been widely performed. For instance, high expression of miR-136, miR-494 and miR-495 has been reported in preeclampsia patient serum (Motawi et al., 2018). Likewise, up-regulation of miR-202-3p has been observed in severe preeclampsia (Singh et al., 2017). MiR-128a may be involved in the pathogenesis of preeclampsia via promoting in HTR-8/SVneo trophoblast cell apoptosis (Ding et al., 2016). miR-144-3p has been disclosed to be lowly expressed in placentas from preeclampsia patients (Hu and Zhang, 2019). In our study, we found that overexpressed miR-26b-3p inhibited HTR-8/SVneo cell proliferation, invasion and migration, and promoted cell apoptosis. There is no report about miR-26b in trophoblast cells but it is reported in pregnancy. miR-26b could block PGDH expression in the chorion during human pregnancy (Sun et al., 2018). Reduced migration of trophoblast cells is well-known to result in the development and pathogenesis of preeclampsia. We also found that overexpression of miR-26b-3p led to enhanced level of cleaved caspase-3 while reduced level of Bcl-2. Cleaved caspase 3 is a well-recognized pro-apoptotic factor, while Bcl-2 is a critical apoptosis inhibitor (Renault et al., 2017). These results suggested that miR-26b-3p promoted trophoblast cell apoptosis. In brief, our study found that up-regulation of miR-26b-3p may lead to the initiation of preeclampsia.
The findings of the function of miR-26b-3p make it critical to understand its mechanism for potential targeting therapy. As aforementioned, miRNAs perform their functions through targeting the mRNA of their target genes. In this study, we identified that miR-26b-3p could directly bind to the 3′untranslated region of SHBG using a computer-based program and dual-luciferase reporter gene assay. Up-regulation of SHBG alleviated the roles of miR-26b-3p overexpression in trophoblast cells. SHBG is a circulating glycoprotein that synthetized in the liver and extrahepatic tissues, though most of the SHBG is originated from hepatic (Norfleet et al., 1976). Emerging studies have identified that SHBG may also play a specific role in the intracellular signaling regulated by SHBG-receptors located on the cell membranes, thus exerting various metabolic functions (Yasmine et al., 2016).
4 Conclusions
Our study provided evidence that overexpression of miR-26b-3p could suppress trophoblast cell migration, invasion and proliferation, and induce cell apoptosis via down-regulating the SHBG expression. This which may result in the initiation and development of preeclampsia. This study could provide novel insights into preeclampsia prevention and treatment. Further preclinical and clinical studies would be performed in the future by us and other researchers to validate our findings and develop efficient therapeutic options for preeclampsia treatment.
Acknowledgement
None.
Disclosure of funding
None.
Conflicts of interest
The authors declare no conflicts of interest.
References
- MicroRNA-128a-induced apoptosis in HTR-8/SVneo trophoblast cells contributes to pre-eclampsia. Biomed. Pharmacother.. 2016;81:63-70.
- [CrossRef] [Google Scholar]
- The neuroprotective role of MiR-124-3p in a 6-hydroxydopamine-induced cell model of Parkinson's disease via the Regulation of ANAX5. J. Cell. Biochem.. 2018;119(1):269-277.
- [CrossRef] [Google Scholar]
- Doridot, Ludivine, Francisco Miralles, Sandrine Barbaux, Daniel Vaiman. 2013. Trophoblasts, invasion, and microRNA. Front. Genet. 4 (248). doi: 10.3389/fgene.2013.00248.
- Uterine function: from normal to polycystic ovarian syndrome alterations. Curr. Med. Chem.. 2018;25(15):1792-1804.
- [Google Scholar]
- Nutritional influences on reproduction: a functional approach. Integr. Func. Med. Nutr. Ther.: Principles Practice 2020:533-561.
- [Google Scholar]
- MicroRNA expression pattern in pre-eclampsia (Review) Mol. Med. Rep.. 2016;13(3):2351-2358.
- [CrossRef] [Google Scholar]
- Intracrine regulation of estrogen and other sex steroid levels in endometrium and non-gynecological tissues; pathology, physiology, and drug discovery. Front. Pharmacol.. 2018;9:940.
- [Google Scholar]
- Effect of early use of low-dose aspirin therapy on late-onset preeclampsia. J. Matern. Fetal Neonatal Med.. 2019;32(13):2137-2142.
- [CrossRef] [Google Scholar]
- miR-26b promotes granulosa cell apoptosis by targeting ATM during follicular atresia in porcine ovary. PLoS ONE. 2012;7(6):e38640
- [CrossRef] [Google Scholar]
- MiR-26b-3p regulates osteoblast differentiation via targeting estrogen receptor alpha. Genomics. 2019;111(5):1089-1096.
- [CrossRef] [Google Scholar]
- MicroRNA-137 affects proliferation and migration of placenta trophoblast cells in preeclampsia by targeting ERRα. Reprod. Sci.. 2017;24(1):85-96.
- [Google Scholar]
- Proposed key characteristics of female reproductive toxicants as an approach for organizing and evaluating mechanistic data in hazard assessment. Environ. Health Perspect.. 2019;127(7):075001
- [Google Scholar]
- Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension. 2013;62(6):1046-1054.
- [CrossRef] [Google Scholar]
- Role of mesenchymal stem cells exosomes derived microRNAs; miR-136, miR-494 and miR-495 in pre-eclampsia diagnosis and evaluation. Arch. Biochem. Biophys.. 2018;659:13-21.
- [CrossRef] [Google Scholar]
- Does bacteremia follow colonoscopy? II. Results with blood cultures obtained 5, 10, and 15 minutes after colonoscopy. Gastrointest. Endosc.. 1976;23(1):31-32.
- [CrossRef] [Google Scholar]
- Oxidative stress early in pregnancy and pregnancy outcome. Free Radical Res.. 2008;42(10):841-848.
- [CrossRef] [Google Scholar]
- A brewing understanding of the regulation of Bax function by Bcl-xL and Bcl-2. Mech. Ageing Dev.. 2017;161(Pt B):201-210.
- [CrossRef] [Google Scholar]
- Characteristics and outcome of severe preeclampsia/eclampsia concurrent with or complicated by acute pancreatitis: a report of five cases and literature review. J. Matern. Fetal Neonatal Med.. 2019;32(4):633-640.
- [CrossRef] [Google Scholar]
- Aberrant microRNA expression in the brains of neurodegenerative diseases: miR-29a decreased in Alzheimer disease brains targets neurone navigator 3. Neuropathol. Appl. Neurobiol.. 2010;36(4):320-330.
- [CrossRef] [Google Scholar]
- Up-regulation of microRNA-202-3p in first trimester placenta of pregnancies destined to develop severe preeclampsia, a pilot study. Pregnancy Hypertens.. 2017;10:7-9.
- [Google Scholar]
- Neuroprotective effects of miR-27a against traumatic brain injury via suppressing FoxO3a-mediated neuronal autophagy. Biochem. Biophys. Res. Commun.. 2017;482(4):1141-1147.
- [CrossRef] [Google Scholar]
- Reduced expression of hydrogen sulfide–generating enzymes down-regulates 15-hydroxyprostaglandin dehydrogenase in chorion during term and preterm labor. Am. J. Pathol.. 2018;188(1):63-71.
- [Google Scholar]
- miR-144 may regulate the proliferation, migration and invasion of trophoblastic cells through targeting PTEN in preeclampsia. Biomed. Pharmacother.. 2017;94:341-353.
- [CrossRef] [Google Scholar]
- Down-regulation of microRNA-34a-5p promotes trophoblast cell migration and invasion via targetting Smad4. Biosci. Rep.. 2019;39:(2).
- [Google Scholar]
- Undertaking the first online sexuality survey among private university students in Lebanon - Process, challenges, and lessons learned. J. Med. Liban.. 2016;64(4):205-210.
- [CrossRef] [Google Scholar]
- Establishing reference intervals for sex hormones and SHBG in apparently healthy Chinese adult men based on a multicenter study. Clin. Chem. Lab. Med.. 2018;56(7):1152-1160.
- [CrossRef] [Google Scholar]
- Upregulation of long noncoding RNA SPRY4-IT1 modulates proliferation, migration, apoptosis, and network formation in trophoblast cells HTR-8SV/neo. PLoS ONE. 2013;8(11):e79598
- [CrossRef] [Google Scholar]







