Translate this page into:
Micromeria biflora mediated gold and silver nanoparticles for colourimetric detection of antibiotics and dyes degradation
⁎Corresponding authors. naeem@kust.edu.pk (Naeem Khan), nargisjamila@sbbwu.edu.pk (Nargis Jamila), rullah@ksu.edu.sa (Riaz Ullah)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The study reports on synthesis of aqueous extract mediated gold and silver nanoparticles of M. biflora (MBAuNPs and MBAgNPs) via hydrated chloroauric acid and silver nitrate salts. The nanoparticles (NPs) were produced in 1:15 (MBAuNPs) and 1:6 (MBAgNPs) ratios under sunlight displaying localized surface plasmon resonance (LSPR) peaks at 541 and 431 nm, respectively. The sizes characterized by transmission electron, scanning electron, and atomic force microscopic (SEM, TEM, AFM) techniques were respectively 26.73 nm and 53.81 nm. The subject NPs demonstrated application in the degradation of methylene blue, Congo red, Rhodamine B, methyl orange, ortho-nitrophenol, and para-nitrophenol ranging from 65 to 86 %. For detection of levofloxacin, amoxicillin, and azithromycin antibiotics, the MBAuNPs and MBAgNPs exhibited efficiency in real water and biological (blood plasma and urine) samples. Conclusively, the MBAuNPs and MBAgNPs applications for dyes degradation and antibiotics detection was found as simple and cost-effective analytical method.
Keywords
Micromeria biflora
MBAuNPs
MBAgNPs
Methylene blue
Amoxicillin
1 Introduction
Designing simplest structures of nanoparticles (NPs) of size from 1 to 100 nm generate a mechanism with novel research and fundamental functionalities, strategies, and methodologies. These are the extreme economically altering material features, and generally of proficient degree for advanced in addition to molecular levels (Ansari et al., 2022). Green synthesis protocols, in particular, by using various common and low-cost sources such as plant and fruit extracts, as stabilizing agents for preparation of nanomaterials is the modern research trend of the scholars from around the world. The plant extract mediated synthesis of NPs have several key advantages over chemical nano-synthesis methods as being economically efficient and environmental friendly (Pirtarighat et al., 2019). Using aqueous extract of various plants in the synthesis of several metal nanoparticles have been reported as an acceptable alternative to physicochemical processes of NPs synthesis (Salem & Fouda, 2021).
Metals such as gold and silver are mostly used to synthesize stable dispersed nanoparticles, which are useful in photography, catalysis, optoelectronics, bio-marking, nanoscale biotechnology, and biomedical sciences (Báez et al., 2021). Gold nanoparticles (AuNPs) have been used in the detection of a polynucleotide or protein, immunoassay, cancer nanotechnology (especially cancer cell detection), and electrophoresis (Pallares et al., 2019). The antimicrobial properties of silver nanoparticles (AgNPs) have resulted in their applications in various fields of medicine, industries, cosmetics, health, animal husbandry, packaging, accessories, and military (Gandhi et al., 2021; Jamila et al., 2021; Kaplan et al., 2022). This increased demand of nanotechnology, motivated the researchers throughout the globe to synthesize novel plants mediated metal nanoparticles instead of using hazardous chemical processes.
Many industrial untreated effluents containing hazardous organic dyes which must also be degraded. It has been reported that dyes are continuously polluting freshwater resources and, thus, posing serious threat to life (Danner et al., 2019). Degradation of dyes via nanoparticles has been reported in literature as easy safe, nontoxic and cost effective method (Kapoor et al., 2021; Ravichandran et al., 2022). Recently researchers have reported serious threats about antibiotics contamination which needs to be monitored and controlled (Liu et al. 2021). Colourimetric sensing technique in which nanoparticles are used as a probe is used to detect antibiotics, is believed low-cost, high sensitive, and easy to use (Abedalwafa et al., 2019).
Antibiotics treat antibacterial infections such as urinary tract, ear, lungs, throat and chest infections, tonsils, bronchitis, and many other types of bacterial infections. However, improper use of antibiotics leads to contaminate water and environment. The intake of these antibiotics by humans through different routes causes serious health effects. Therefore, for their removal, cost-effective and affordable methods needs to be developed.
Micromeria biflora is a flowering plant belonging to Labiatae family which is also known as lemon-scented thyme, Jangli Ajwan, Dhawa Jari, and Shamakay (Duru et al., 2004; Sajad et al., 2020). In Pakistan, this plant is found in Swat, Shogran, Kaghan, Dir, and Hazar Nao Hills of Malakand (Ummara et al., 2013; Zeb et al., 2015; Zeb et al., 2016). It has a specific scent due to the presence of terpenoids and a great amount of essential oil (Ding et al., 1994; Mallavarapu et al., 1997; Slavkovska et al., 2005) and also is a source of tannins, flavonoids, and coumarins (Uddin et al., 2016). The plant is traditionally used to treat nosebleed, decay of teeth, wounds healing, headache, different skin infections, treating common cold and sinusitis conditions (Sajad et al., 2020). Considering the unique medicinal applications and novelty of untapped nanostructural study on the subject M. biflora plant, this study was therefore aimed to synthesize gold (MBAuNPs) and silver nanoparticles (MBAgNPs) using aqueous extracts of the M. biflora plant under conditions of sunlight, stirring, incubation, and heating. The synthesized NPs were characterized and evaluated for their potential in degradation of dyes, and antibiotics, which includes levofloxacin, amoxicillin, and azithromycin, and detection in biological (blood and urine) and water samples.
2 Methods and materials
2.1 Samples collection
Enough aerial parts of M. biflora plant was collected from Swat, during the flowering season (June to August 2021) from Swat valley, KPK province, Pakistan. The plant was taxonomically identified at the Botany Department, Peshawar University, Pakistan. The plant was washed to clean from any dust or other contaminants. The samples were then air dried in shade, powdered, kept in dry and cleaned, plastic bags, properly labelled, and stored in refrigerator until analysis. Approval from ethical committee, Kohat University of Science & Technology was obtained for working with biological samples including blood plasma and urine.
2.2 Aqueous extract preparation
For extraction, an established procedure from literature was followed (Jamila et al., 2020). Briefly, deionized water (500 mL) as extraction solvent was used to soak 25 g aerial parts of M. biflora powder. The mixture was stirred well at 500 rpm for 2 h and 40 °C temperature. The extract so obtained was filtered, and used to synthesize MBAuNPs and MBAgNPs.
2.3 Chemicals, reagents, and instrumentation
Gold(III) chloride salt as trihydrate (HAuCl4·3H2O), nitrate salt of silver (AgNO3), sodium borohydride (NaBH4), dyes [methylene blue (MB), Congo red (CR), Rhodamine (RdB), methyl orange (MO), and ortho- and para-nitrophenol (ONP, PNP)], antibiotic standards [levofloxacin (LFX), amoxicillin (AMX), and azithromycin were all purchased from Merck (Darmstadt, Germany) and Sigma-Aldrich (Steinheim, Germany). Aqueous plant extract and all reagent solutions were prepared with ultrapure deionized water obtained from Milli-Q apparatus (Millipore, MA, USA).
Instruments used in this research study included UV–1800 spectrophotometer (Shimadzu, Japan) for absorption spectra (200 to 800 nm) of the reaction mixture, FT-IR spectrometer (Bruker) for functional groups identification (400–4000 cm−1), field emission-scanning electron microscope (FE-SEM, S-4800, Hitachi, Japan), transmission electron microscope (Phillips CM12, Eindhoven, Netherlands) and atomic force microscopy (AFM) for size and morphology assessment.
2.4 Synthesis and characterization of MBAuNPs and MBAgNPs
The MBAuNPs and MBAgNPs were synthesised using M. biflora aqueous extract under different conditions involving ratios (1:5 to 1:15) of corresponding salt solutions (HAuCl4·4H2O and AgNO3) and aqueous extract, sunlight, incubation at 25 °C in dark, stirring, heating, pH (1–12) and time intervals (0 to 180 min). Synthesis of corresponding NPs were preliminary checked for any visible colour change from yellow (extract) to brown (AgNPs) and maroon/deep purple (AuNPs) (Fig. 1), which were then confirmed by UV–Vis analysis. The synthesized NPs from the solutions were collected via centrifugation and washing with water. The product so obtained was kept in sealed vials until required for analysis. The synthesized NPs were characterized by FE-SEM, FT-IR, AFM, and TEM techniques for functional group identification (involved in NPs synthesis), and size and morphology analysis.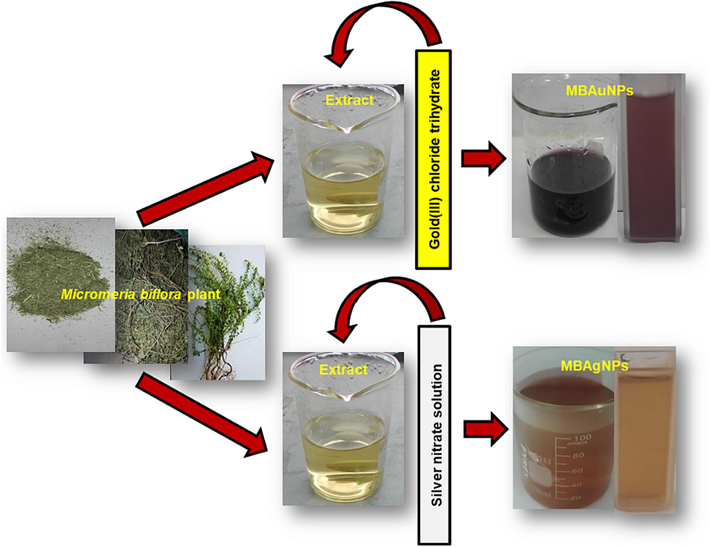
Colourimetric visible changes in the synthesis of MBAuNPs and MBAgNPs at sunlight.
2.5 Dyes degradation by MBAuNPs and MBAgNPs
Dyes degradation was evaluated using synthesized NPs (where they act as catalysts) following reported literature (Jamila et al., 2020). Briefly, 0.1 mM, 2.5 mL each of dyes and nitrophenols were mixed with 0.1 mM, 0.5 mL of NaBH4. In another experiment, 3 mg of NPs were mixed with 2.5 mL of solutions of dyes and nitrophenols and 0.5 mL NaBH4. The Ultraviolet–Visible spectra (200–800 nm) were recorded at a time intervals of 0 to180 min. The catalytic efficiency of NPs and degradation of dyes and nitrophenols was noted by discoloration and decrease in SPR absorbance. This decrease was calculated as reduction/decolorization via equation (1) given below.
Where; At means absorbance of dye at “t” time and A0 means absorbance of dye at 0 min.
2.6 Colourimetric antibiotics detection by MBAuNPs and MBAgNPs
In antibiotic detection in real samples, a slightly modified method (Makropoulou et al., 2020) was used. Depending on the solubility, the working solutions were set by taking the selected antibiotics and dissolving either in water or ethanol. The UV spectra of antibiotics working solutions (2.5 mL), a mixture of antibiotics (2.5 mL) and NPs (3 mg), and a mixture of antibiotics working solutions, NPs (3 mg) and real samples (blood plasma, urine, tape water, 1.5 mL) were recorded at 0 to180 min. To evaluate the percent removal of the applied antibiotics, the following equation was used.
Where C and C0 are the final and initial concentrations of the applied antibiotics, respectively.
3 Results and discussion
3.1 Synthesis and characterization of MBAuNPs and MBAgNPs
M. biflora mediated NPs were synthesized using various ratios of extract and corresponding salt solutions under sunlight, stirring, incubation, and heating techniques. Applying these conditions to mixtures of 1:5, 1:10, and 1:15 ratios, for MBAuNPs, no visible colour change was noticed, and from UV–Vis study there was no surface plasmon resonance band seemed, which indicates that no NPs in the subject mixtures/ratios are produced at stirring, incubation, and heating as depicted from Fig. 2 (A-C). The prominent SPR peak appeared at λmax 300–350 nm shown in Fig. 2 is attributed to aggregation of particles of sizes larger than 100 nm (Dzimitrowicz et al., 2019). Proceeding the synthesis in sunlight, MBAuNPs were produced at a slow rate in ratio of 1:5 with the more intense SPR at λmax 300–350 nm than at λmax 541 nm (Fig. 2D). However, in 1:10 and 1:15 ratios, significant MBAuNPs were produced as indicated by visible colourimetric change in color from light yellow to purple, and appearance of SPR band at 541 nm (Fig. 2E & F). An intense absorption peak at λmax 541 nm was observed for MBAuNPs produced at the ratio of 1:15, indicating the presence of reasonable AuNPs.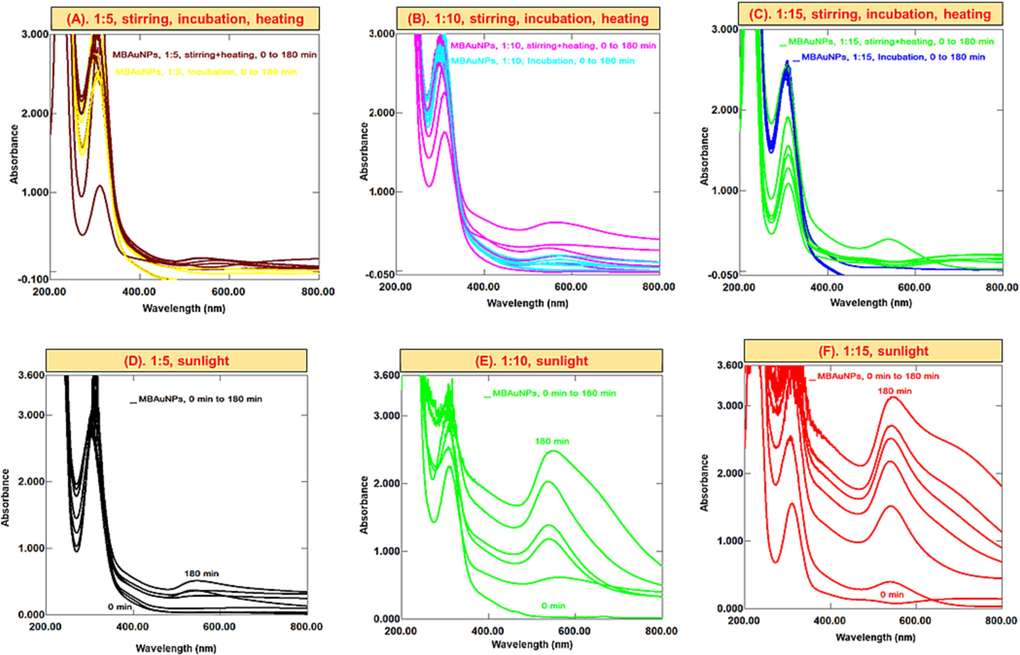
Successive UV–Vis spectra of MBAuNPs using aqueous extract in ratios of (A) 1:5, (B) 1:10 and (C) 1:15 at stirring, heating, incubation, (D) 1:5 sunlight, (E) 1:10 sunlight, and (F) 1:15 sunlight.
In MBAgNPs synthesis, the colorimetric change from yellow to brown at the ratios of 1:5, 1:6, and 1:7 keeping in sunlight play a crucial role in preparation of MBAgNPs, which exhibited SPR at λmax 431 nm (Fig. 3A & B). A 1:6 ratio demonstrated an intense and sharp SPR band, which indicate the production of significant and well dispersed AgNPs. In a ratio of 1:7, a bathochromic/red shift from 431 to 579 nm and broadened SPR band was observed. Furthermore, due to boosted interactions between MB aqueous extract with excessive amounts of phytocompounds and silver ions, this aggregation ultimately resulted in the decrease of stable MBAgNPs (Lee et al., 2019; Rani et al., 2020). Proceeding with increased salt concentration to 1:10, 1:12, and 1:15, at sunlight, the AgNPs showed weak SPR band (Fig. 3C) and produced at a slow rate, which indicate its formation in small amount. Following the AgNPs synthesis with all the subject ratios at stirring, incubation, and heating, no SPR excitations at 420–450 nm were seen, which corresponded to no AgNPs production (Fig. 3D).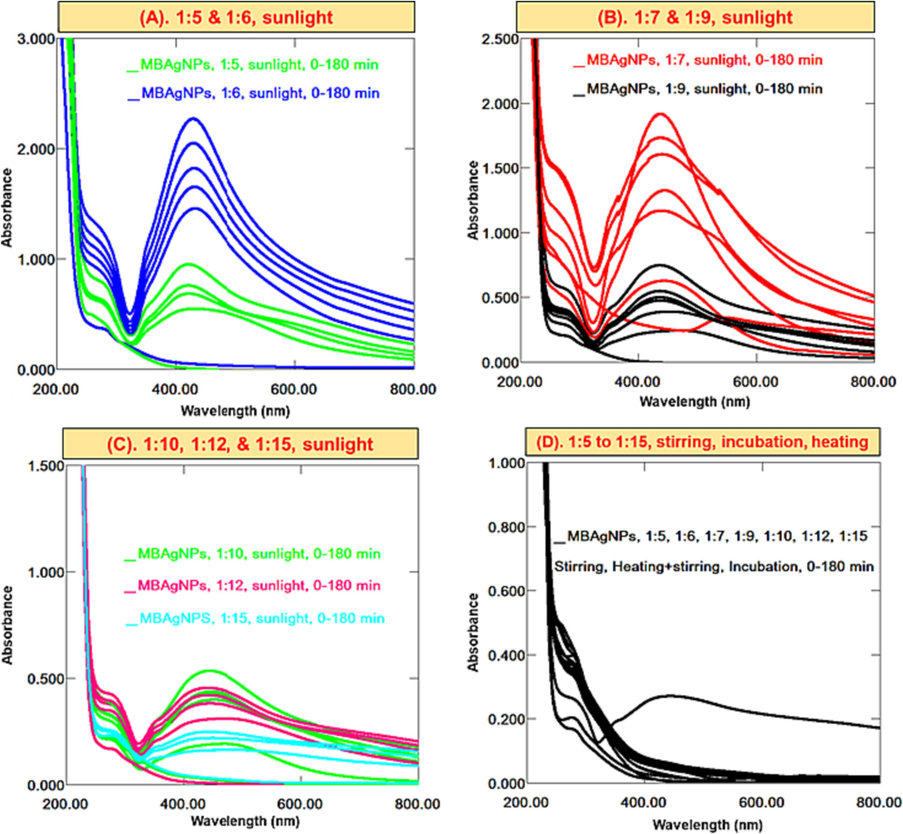
Successive UV–Vis spectra of MBAuNPs using aqueous extract in ratios of (A) 1:5 & 1:6 sunlight (B) 1:7 & 1:9 sunlight, (C) 1:10, 1:12 & 1:15 sunlight, (D) 1:5 to 1:15 at stirring, heating, and incubation.
M. biflora is a rich source of chemical constituents/secondary metabolites such as phenolics, tannins, flavonoids, and coumarins (Chandra et al. 2013). The hypothesised mechanism for the NPs synthesis by the interaction of these secondary metabolites with metal ions include interaction of OH/COOH/NH groups, enol synthesis by liberating reactive hydrogen, and then oxidation of hydroxyl to carbonyl group through adsorption on the metal surface (Kumar et al., 2017). The metal ions M+/++ thus are reduced to M0, and eventually NPs creation. According to Lee et al. (2019), nanomaterials synthesized via these bioactive components, which have hydroxyl groups as reducing agents follow the mechanism as shown below.
Depending upon the extent and position of both hydroxyl and carbonyl functional groups in the plant constituents, the NPs synthesis and their biological activities are greatly affected. Regarding the synthesis of MBAuNPs, the interaction of Au+ with phenolics of M. biflora extract, the colorimetric colour change from yellow to purple indicate the reduction of Au+ to Au, and hence, MBAuNPs production. MBAuNPs synthesized under sunlight were significantly produced in 1:10 and 1:15 ratios, exhibiting surface plasmon resonance (SPR) band at 541 nm, as indicated by previous literature (Dzimitrowicz et al., 2019; Rani et al., 2020). In 1:15 ratio, MBAuNPs were produced in the highest extent. Hence, it is the optimal ratio displaying a sharp absorption peak at λmax 541 nm. This indicates that the salt solution (HAuCl4) is rapidly reduced by the chemical constituents of M. biflora to produce homogeneous nuclei, which results in tiny AuNPs rather than aggregates.
Concerning MBAgNPs in 1:6 ratio under sunlight, which display a sharp peak at λmax 431 nm, shows that this ratio is the optimal condition for the synthesis of MBAgNPs. However, proceeding to higher ratios, a bathochromic/red shift from 431 to 579 nm and a broadened SPR band was observed, which indicates salt induced MBAgNPs aggregation. Also due to greater interactions between M. biflora aqueous extract with excessive amounts of reducing phytoconstituents and accessible silver ions, this aggregation finally resulted in the reduction of stable MBAgNPs which is consistent to the published literature (Lee et al., 2019). Similarly, Rani et al. (2020) has reported about Ostwald slow ripening process to enhance the production of AgNPs and result in size enlargement. Owing to several properties, many of the NPs have been used for the pollutants detection; dyes, antibiotics (Zhang et al., 2020; Aguilar-Pérez et al., 2021).
The functional groups of the phytochemicals (tannins, flavonoids, coumarins) present in M. biflora, which involve in the synthesis of M. biflora nanoparticles (MBNPs) were evaluated using FT-IR technique. The FT-IR spectra of plant extract (MBAE) are labelled by comparing with literature (Fig. 4A & B). The broad and intense absorption bands at 3500–3200 cm−1 is the stretching vibration assigned to hydroxyl (–OH), and or amine (–NH) groups however the absorption frequencies at 1700–1540 cm−1 and 1030–13010 cm−1 are attributed to –C = O and –C–O stretching vibrations. It has been observed that the subject absorption bands in the spectra of MBAuNPs and MBAgNPs either reduced or disappeared, thus concluding the contribution of the different functionalities in MBNPs synthesis (Jamila et al., 2020).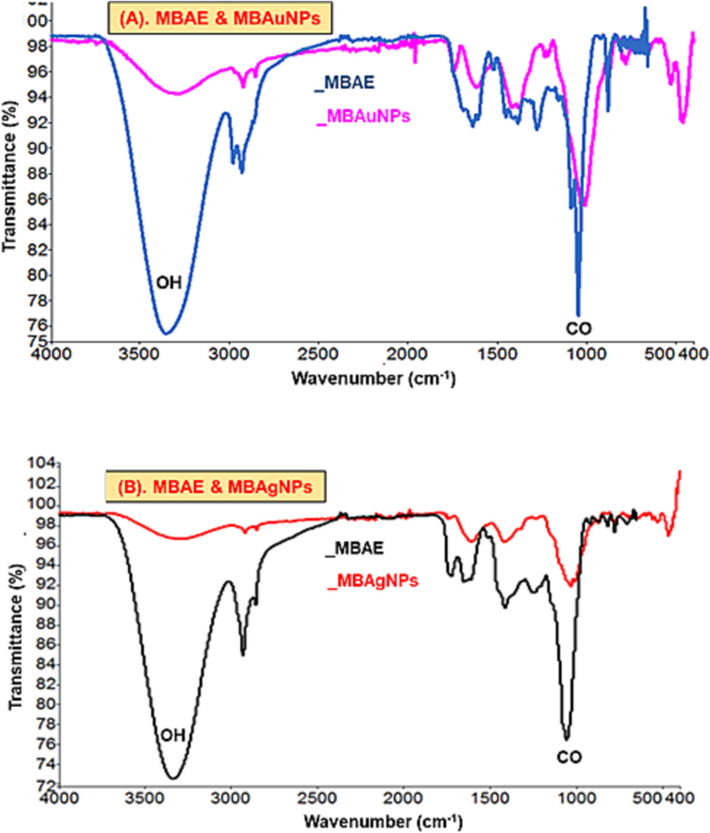
FT-IR spectra of (A) MBAE and MBAuNPs, and (B) MBAE and MBAgNPs.
The morphology and size of the subject MBNPs were analyzed using TEM, SEM and AFM techniques. These techniques showed that the size of MBAuNPs is 26.73 nm, and the surface shape represented nanorods as well as spherical shapes (Fig. 5A-C). Regarding MBAgNPs, the size by the subject techniques was found to be 53.81 nm with spherical shape (Fig. 5D-F).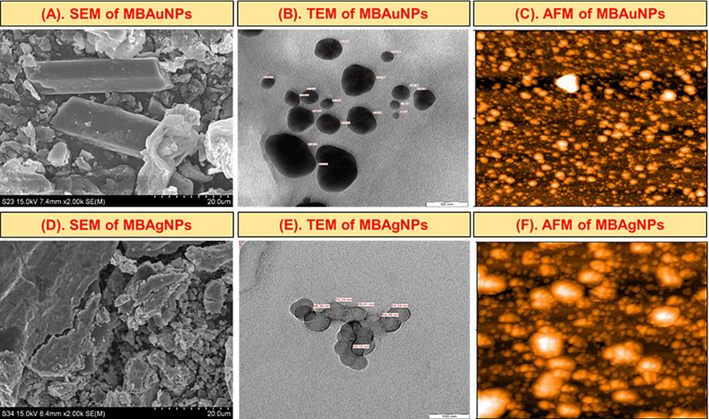
Size and morphology by (A, D) SEM (B, E), TEM (C, F), AFM images of the synthesized MBAuNPs and MBAgNPs.
3.2 Catalytic activity of MBAuNPs and MBAgNPs in dyes degradation
In the current study, CR, MB, MO, RdB, ONP, and PNP were reduced using NaBH4 and MBNPs. The combined UV–Vis spectra of dyes reduced with NaBH4, and MBAuNPs and AgNPs (Fig. 6) explained that dyes without MBNPs have a little variability in absorbance with no noticeable change in colour was observed, which indicates a slow rate of degradation of dyes reduction. However, MBAuNPs added dyes exhibited spectra where certain absorption peaks disappeared within 5 min after the addition of MBNPs. The results of the subject dyes (Fig. 6) showed that MB (cationic dye), which displays λmax at 653 nm, 591 nm, and 290 and inefficiently reduced by NaBH4, the absorbance at 653 and 290 nm weakened with a concurrent increase at 255 nm after the MBNPs addition (Fig. 6A). Similarly, the absorption maxima of CR (λmax at 487 and 349 nm), RdB (550 nm), and MO (λmax at 462 nm) decrease and shift to blue shift with the addition of MBNPs (Fig. 6B-D). The ortho-nitrophenol and para-nitrophenols (Fig. 6E & F) also have shown high degradation rate for ONP followed by PNP. MBAuNPs reduced CR to 80–85 %, MB 70–75 %, RdB 15–20 %, MO 65–80 %, ONP 80–85 %, and PNP 80–85 %. Regarding the % degradation of dyes by MBAgNPs (Fig. 7), the dyes were reduced as follows: CR (75–80 %), RdB (15–20 %), MB (60–70 %), MO (80–85 %), PNP (80–85 %), and ONP (83–85 %).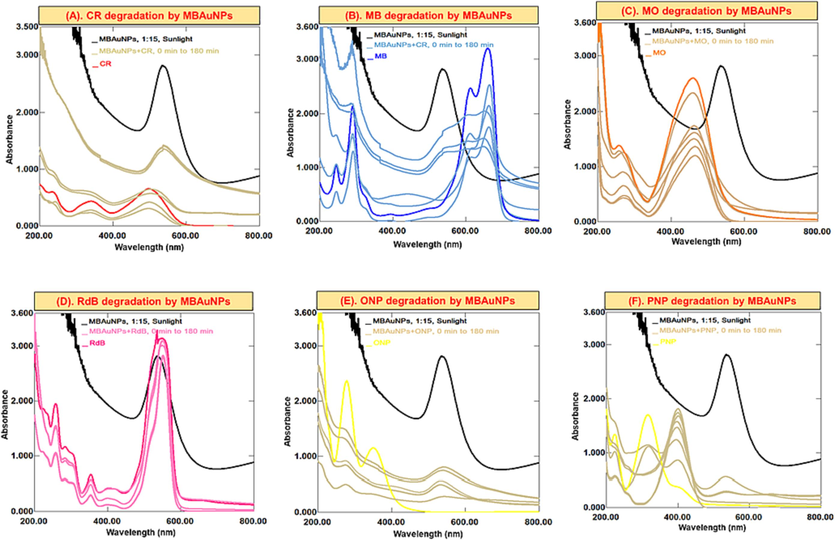
Successive UV–Vis absorption spectra for the catalytic potential of MBAuNPs in (A) CR, (B) MB, (C) MO, (D) RdB, (E) ONP, (F) PNP in dyes/nitrophenols degradation/reduction process.
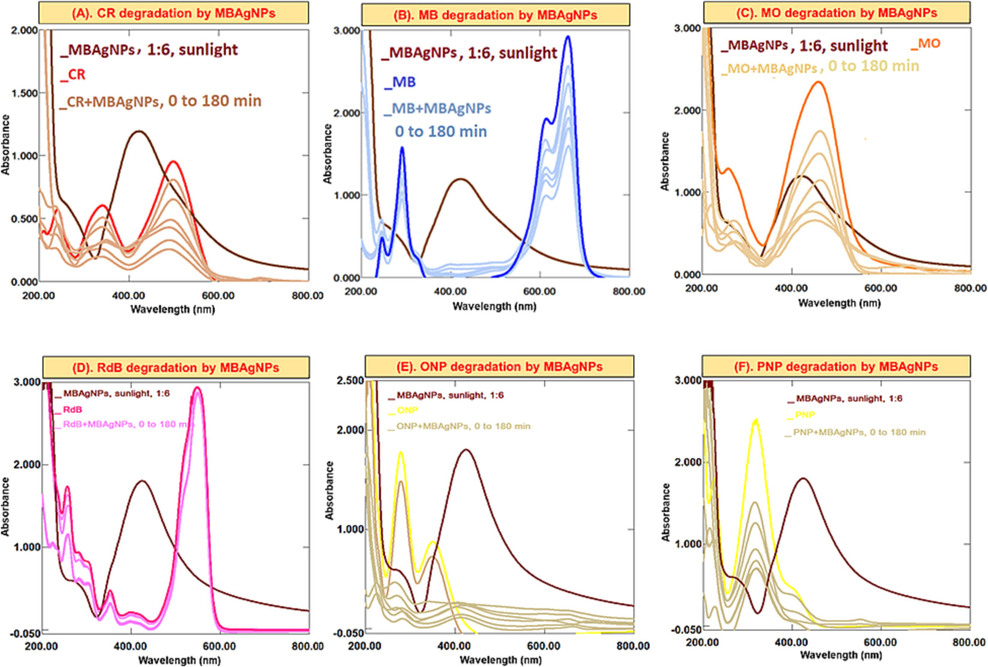
Successive UV–Vis absorption spectra for the catalytic potential of MBAgNPs in (A) CR, (B) MB, (C) MO, (D) RdB, (E) ONP, (F) PNP in dyes/nitrophenols degradation/reduction process.
Dyes are the organic synthetic pollutants, which release into the running water by various industries (Sadia et al., 2023). These dyes are polluting water and absorbed by soil thus affecting the environment, human health, and agriculture. Therefore, to control and cope this issue, recently, NPs of well enough catalytic efficiency have played key role as effective catalysts in the discolouration and elimination by decreasing the activation energy (Jamila, 2020; Khan et al., 2021; Yıldırım et al., 2022). The synthetic reducing agent (NaBH4), which is usually used to reduce dyes due to the large redox potential differences in the dye and BH4− ions, inefficiently reduce dyes. Therefore, due to the adsorption of dyes on NPs, the electron transfer from NaBH4 to dyes becomes easy and dye molecules decolorize. This is attributed to the high catalytic locations, lower the activation energy, and high volume to surface ratio of NPs. This diagram depicts MB-AuNPs' catalytic characteristics (Wang et al., 2021).
In this analysis, the λmax of dyes are decreased, which indicates the degradation of dyes. For example, for CR (Figs. 6 and 7A), showing λmax at 487 and 349 nm, the absorbance at the subject λmax decreased and the solution become faint. In MB dye (Figs. 6 and 7B) having λmax 653 and 290 nm, the absorbance decreased with an appearance of another absorption peak at 255 nm. This simultaneous decrease/increase in the λmax indicates dyes degradation.
3.3 Antibiotics detection by MBAuNPs and MBAgNPs
The most used antibiotics selected for this study including LFX, AMX, and AZM in water, blood plasma and urine samples were detected using both type of NPs as a probe. From the results (Figs. 8 and 9), it was found that upon the addition of MBNPs to the antibiotics solution, the concentration (absorbance) of the mixtures decreases. AMX solution displays absorption peaks at λmax 228 nm and 277 nm. Upon the addition of MBNPs, antibiotics are adsorbed on their surface and the λmax of antibiotics decreases. Regarding the other antibiotics, it can also be seen that the same changes/decrease in λmax occurs. In this study, it was found that compared to AZM, AMX and LFX capture by MBNPs was significant, and the removal % was in the range of 75–85 %. Proceeding the experiments with aqueous medium (tap water), blood plasma and urine samples, the recorded spectra obtained were irregular, and indicated that the λmax of antibiotics decreases, which in turn shows decrease in concentrations of antibiotics.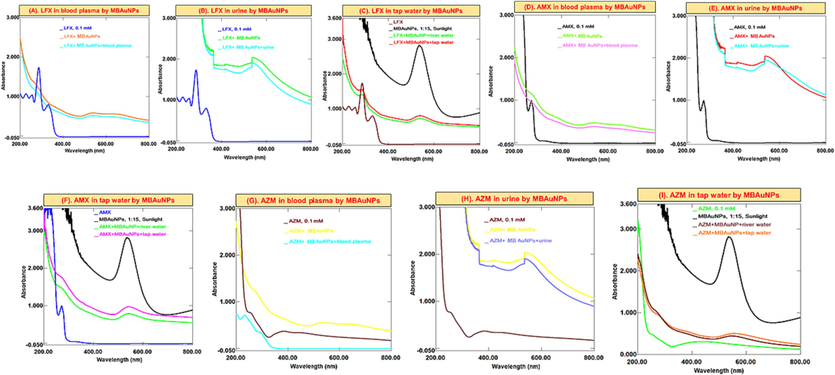
Successive UV–Vis absorption spectra of MBAuNPs interaction with (A-C) LFX (D-F) AMX, and (G-I) AZM in in blood, urine and water.
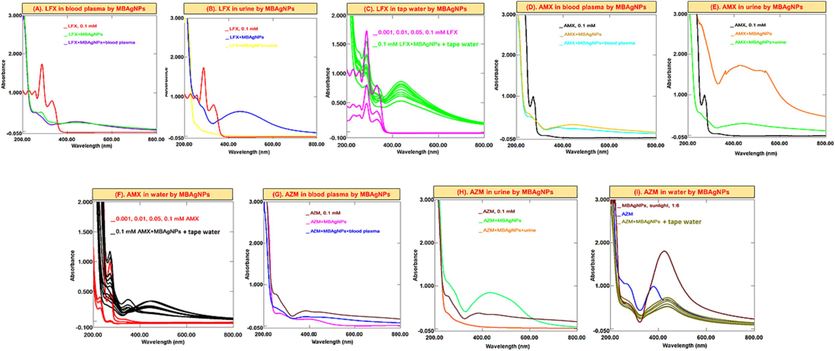
Successive UV–Vis absorption spectra of MBAgNPs interaction with (A-C) LFX (D-F) AMX, and (G-I) AZM in in blood, urine and water.
Antibiotics are also considered as the environmental pollutants from different pharmaceutical industries, various sewage treatment plants, hospitals, and others that can have an impact on microbial ecosystems. Therefore, it is crucial to assess and develop methods for their removal from the environment. Due to the outstanding affinity and optical absorbance of NPs with biomaterials, these have served as an effective technique to identify a variety of chemicals such as -agonists, dopamine, antibiotics, proteins, and DNA. Also, in current study, MBAuNPs and MBAgNPs were used for antibiotics detection in this study. The antibiotics (levofloxacin, amoxicillin, and azithromycin) have a high affinity for M. biflora phytoconstituents due to hydrogen-bonding interactions as previously reported by Lai et al. (2017). This results in cross-linking of AuNPs and AgNPs, leading to clear changes in absorbance features and colour. As given in Figs. 8 and 9, a colorimetric assay based on MBNPs as a probe is designed to detect antibiotics including LFX, AMX, and AZM in real samples including water, blood plasma and urine. From the results a decrease in the λmax of antibiotics was found, which is attributed to the interaction via hydrogen bonding of the carboxyl and hydroxyl (COOH and OH) functional groups with antibiotics.
From experiments, it was found that the decrease in the absorbance (concentration) of the LFX, AMX, and AZM solutions and MBNPs occurs. This shows that the concentration decrease because of the antibiotics adsorption on the MBNPs then ultimately lead to removal of the studied antibiotics from real samples as also suggested by published studies (Lai et al., 2017; Abedalwafa et al., 2019a; Makropoulou et al., 2020).
4 Conclusions
Conclusively, this study reported the green synthesis of AuNPs and AgNPs using M. biflora aqueous extract. The synthesized MBNPs were evaluated for their practical application in dyes/nitrophenols degradation, and antibiotics removal. The subject synthesized MBNPs degraded dyes to 60–87 %. Furthermore, the MBNPs also displayed efficient activity in removal of antibiotics from aqueous and biological media to an extent of 75–85 %. The subject study depicts that MBNPs could be efficient adsorbent dyes/nitrophenols degradation, and antibiotics removal both from aqueous as well as biological samples. Hence, it could provide a better way to control environmental/water pollution, and health problems for the developing countries specifically Pakistan.
Acknowledgement
Authors wish to thank researchers supporting project pumber (RSP2023R110) at King Saud University Riyadh, Saudi Arabia for financial support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Colorimetric sensor arrays for the detection and identification of antibiotics. Anal. Methods. 2019;11:2836-2854.
- [CrossRef] [Google Scholar]
- Nanoadsorbents in focus for the remediation of environmentally-related contaminants with rising toxicity concerns. Sci. Total Environ.. 2021;779:146465
- [CrossRef] [Google Scholar]
- Surface modified lanthanide upconversion nanoparticles for drug delivery, cellular uptake mechanism, and current challenges in NIR-driven therapies. Coord. Chem. Rev.. 2022;457:214423
- [CrossRef] [Google Scholar]
- Báez, D.F. Gallardo-Toledo, E., Oyarzún, M.P. Araya, E., Kogan, M.J. (2021). The influence of size and chemical composition of silver and gold nanoparticles on in vivo toxicity with potential applications to central nervous system diseases. Int. J. Nanomed. 16, 2187. 10.2147%2FIJN.S260375.
- Essential oil composition and pharmacological activities of Micromeria biflora (Buch.- Ham. Ex D. Don) Benth. collected from Uttarakhand region of India. J. Med. Plant Res.. 2013;7:2538-2544.
- [Google Scholar]
- Antibiotic pollution in surface fresh waters: occurrence and effects. Sci. Total Environ.. 2019;664:793-804.
- [CrossRef] [Google Scholar]
- Aromatic components of the essential oils of four Chinese medicinal plants (Asarum petelotii, Elsholtzia souliei, Eupatorium adenophorum, Micromeria biflora) in Yunnan. Z. Naturforsch. C.. 1994;49:703-706.
- [CrossRef] [Google Scholar]
- The constituents of essential oil and in vitro antimicrobial activity of Micromeria cilicica from Turkey. J. Ethnopharmacol.. 2004;94:43-48.
- [CrossRef] [Google Scholar]
- Comparison of the characteristics of gold nanoparticles synthesized using aqueous plant extracts and natural plant essential oils of Eucalyptus globulus and Rosmarinus officinalis. Arab. J. Chem.. 2019;12:4795-4805.
- [CrossRef] [Google Scholar]
- Nanosilver reinforced Parmelia sulcata extract efficiently induces apoptosis and inhibits proliferative signalling in MCF-7 cells. Environ. Res.. 2021;199:111375
- [CrossRef] [Google Scholar]
- Piper longum catkin extract mediated synthesis of Ag, Cu, and Ni nanoparticles and their applications as biological and environmental remediation agents. Arab. J. Chem.. 2020;13:6425-6436.
- [CrossRef] [Google Scholar]
- Characterization of natural gums via elemental and chemometric analyses, synthesis of silver nanoparticles, and biological and catalytic applications. Int. J. Biol. Macromol.. 2020;147:853-866.
- [CrossRef] [Google Scholar]
- Hg (II) sensing, catalytic, antioxidant, antimicrobial, and anticancer potential of Garcinia mangostana and α-mangostin mediated silver nanoparticles. Chemosphere. 2021;272:129794
- [CrossRef] [Google Scholar]
- Biosynthesis and characterization of silver nanoparticles from Tricholoma ustale and Agaricus arvensis extracts and investigation of their antimicrobial, cytotoxic, and apoptotic potentials. J. Drug Deliv. Sci. Technol.. 2022;69:103178
- [CrossRef] [Google Scholar]
- Exploration of microbial factories for synthesis of nanoparticles–a sustainable approach for bioremediation of environmental contaminants. Front. Microbiol.. 2021;12:1404.
- [CrossRef] [Google Scholar]
- Synthesis of zero-valent Au nanoparticles on chitosan coated NiAl layered double hydroxide microspheres for the discoloration of dyes in aqueous medium. Spectrochim. Acta A. 2021;250:119370
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using Andean blackberry fruit extract. Saudi J. Biol. Sci.. 2017;24:45-50.
- [CrossRef] [Google Scholar]
- Chitosan-wrapped gold nanoparticles for hydrogen-bonding recognition and colorimetric determination of the antibiotic kanamycin. Microchim. Acta. 2017;184:2097-2105.
- [CrossRef] [Google Scholar]
- Bio-mediated synthesis and characterisation of silver nanocarrier, and its potent anticancer action. Nanomaterials. 2019;9:1423.
- [CrossRef] [Google Scholar]
- A review of the distribution of antibiotics in water in different regions of China and current antibiotic degradation pathways. Front. Environ. Sci.. 2021;9:692298
- [CrossRef] [Google Scholar]
- Photocatalytic facile ZnO nanostructures for the elimination of the antibiotic sulfamethoxazole in water. J. Process. Eng.. 2020;36:101299
- [CrossRef] [Google Scholar]
- Composition of the essential oil of Micromeria biflora. J. Essent. Oil Res.. 1997;9:23-26.
- [CrossRef] [Google Scholar]
- Manipulating immune activation of macrophages by tuning the oligonucleotide composition of gold nanoparticles. Bioconjug. Chem.. 2019;30:2032-2037.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostruct. Chem.. 2019;9:1-9.
- [CrossRef] [Google Scholar]
- Highly stable AgNPs prepared via a novel green approach for catalytic and photocatalytic removal of biological and non-biological pollutants. Environ. Int.. 2020;143:105924
- [CrossRef] [Google Scholar]
- Cost-effective and eco-friendly dye degradation by enzyme-powered ZnO nanomaterial: effect of process temperature. Bull. Mater. Sci.. 2022;45:1-5.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of MIPs for selective removal of textile dye acid black-234 from wastewater sample. Molecules. 2023;28(4):1555.
- [CrossRef] [Google Scholar]
- Evaluation of chromium phytoremediation potential of some plant species of Dir Lower, Khyber Pakhtunkhwa, Pakistan. Acta Ecol. Sin.. 2020;40:158-165.
- [CrossRef] [Google Scholar]
- Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview. Biol. Trace Elem. Res.. 2021;199:344-370.
- [CrossRef] [Google Scholar]
- Essential oil and its systematic significance in species of Micromeria Bentham from Serbia & Montenegro. Plant Systemat. Evol.. 2005;255:1-15.
- [CrossRef] [Google Scholar]
- Antinociceptive, antioxidant and phytochemical studies of Pakistani medicinal plants. Pak. J. Pharm. Sci.. 2016;29:929-933.
- [Google Scholar]
- Pharmacological study of Shogran valley flora, Pakistan. Int. J. Sci. Eng. Res.. 2013;4:1-9.
- [Google Scholar]
- Green synthesis of copper nanoparticles using green coffee bean and their applications for efficient reduction of organic dyes. J. Environ. Chem. Eng.. 2021;9:105331
- [CrossRef] [Google Scholar]
- Fabrication and characterization of copper nanoparticles anchored on sulfonated reduced graphene oxide as effective catalyst for the reduction of Thioflavine-T cationic dye in aqueous medium. Mater. Chem. Phys.. 2022;275:125212
- [CrossRef] [Google Scholar]
- Floristic composition and phytosociological studies of Hazar Nao hills, District Malakand, Khyber Pakhtunkhwa, Pakistan. Pak. J. Weed Sci. Res.. 2016;22:295-315.
- [Google Scholar]
- Antibacterial and antifungal activities of Micromeria biflora (Leaves) Int. J. Med. Biomed. Sci.. 2015;1:28-34.
- [CrossRef] [Google Scholar]
- Recent developments of two-dimensional graphene-based composites in visible-light photocatalysis for eliminating persistent organic pollutants from wastewater. Chem. Eng. J.. 2020;390:124642
- [CrossRef] [Google Scholar]







