Translate this page into:
Methanolic extract of Nepeta paulsenii as an ameliorative agent against CCl4 induced testicular damage in male albino rats
⁎Corresponding authors. shahidmahboob60@hotmail.com (Shahid Mahboob)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The issue of male sexual dysfunction arises due to numerous complications linked with sperm motility, imbalance of antioxidant enzymes and hormonal levels. Carbon tetrachloride (CCl4) is widely used as a standard toxin to induce chemical injury and toxicity. A lot of research has been performed on many species of the genus Nepeta in vitro and in vivo but the plant Nepeta paulsenii have been rarely used in this aspect. The current research was designed to explore the impacts of methanolic extract of the Nepeta paulsenii plant on CCl4 induced testicular impairment in male albino rats. Forty-eight rats were distributed equally into 8 groups; Control, Vehicle control, CCl4 treated (1 ml/kg), CCl4 + Silymarine treated, CCl4 + Nepeta paulsenii (200 mg/kg) treated, CCl4 + Nepeta paulsenii (400 mg/kg), Nepeta paulsenii (200 mg/kg) and Nepeta paulsenii (400 mg/kg) treated. Blood and testicles were obtained and analyzed. To check antioxidant enzymes, hormonal concentrations, daily sperm production (DSP), protein content and histopathology various analyses were performed. CCl4 exposure resulted in adverse morphological changes and significant (p < 0.05) reduction in testosterone, luteinizing hormone (LH), follicle stimulating hormone (FSH) concentrations, the activity of catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), glutathione-disulfide reductase (GSR) and glutathione S-transferase (GST) enzymes, daily sperm production (DSP), protein content and high-density lipoproteins (HDL). CCl4 increased the TBARS and H2O2 production along with the cholesterol, triglyceride and low-density lipoproteins (LDL) levels, but Nepeta paulsenii restored these changes back to their normal state significantly (p < 0.05). Therefore, it was concluded that Nepeta paulsenii extract has potential to ameliorate the CCl4 induced testicular damage.
Keywords
Nepeta paulsenii
Carbon tetrachloride
Testicular damage
Antioxidant enzymes
- NP
-
Nepeta paulsenii
- DSP
-
daily sperm production
- FSH
-
follicle stimulating hormone
- LH
-
luteinizing hormone
- CAT
-
catalase
- POD
-
peroxidase
- SOD
-
superoxide dismutase
- GSR
-
glutathione-disulfide reductase
- GST
-
glutathione S-transferase
Abbreviations
1 Introduction
Male sterility is a prominent clinical issue distressing 30% of people all over the world (Isidori et al., 2006). Several factors such as premature ejaculation, erectile dysfunction as well as quantity and quality of sperms are disturbing the male fecundity. These damages are prompted due to excessive free-radical production in the testicles. Many clinical disorders (such as hypertension) are mostly caused in response to reactive oxygen species (ROS) that give rise to premature ejaculation and erectile dysfunction. Male sexual physiognomies are controlled by androgens; hypogonadism incited with decline of natural antioxidant capability and intensification of ROS might instigate the reduced sexual desire in male adults that severely tamper the carnal erotic activities (Ojo et al., 2016).
Several investigational studies indicated that CCl4 as an absolute hepatotoxin which also induces testicular toxicity (Khan and Ahmed, 2009; Ojo et al., 2016). Metabolization of CCl4 in body tissues produces trichloromethyl free radicals (highly reactive) which leads to lipid peroxidation of phospholipids in plasma membrane and causes pathological anomalies due to the deposition of lipid originated oxidants. CCl4 exposure induces oxidative stress in testicular tissues, leading to reduction in antioxidant enzyme activity. Histopathological studies of testicles divulged the detrimental pathway of CCl4 in rats. The intoxication of CCl4 elicits the reduction of androgen in male rats. It is essential to explore antioxidants, which defy the disproportionate production of free radicals to deal with such clinical diseases and excessive ROS (Khan and Ahmed, 2009; Sahreen et al., 2013).
Plant based antioxidants have attained significant importance due to their potential medicinal properties. Several epidemiological reports have revealed that intake of plant-based foods having antioxidants are useful for health as it reduces many toxicant-induced deteriorating effects and can effectively fight with the cancer and cardiovascular diseases (Arabshahi-Delouee and Urooj, 2007). Nepeta L. (Lamiaceae family) includes around 300 species, that are present in Central and Southern Asia, Central and Southern Europe and in faraway East (Jamzad et al., 2003). Plants of the genus Nepeta, also known as Catmints, have been used as traditional medicines and some of their components showed medicinal properties. Extracts of various Nepeta species are used in traditional medicines (Grognet, 1990). Seeds of some species of Nepeta genus are used as medicine for the treatment of fever, rheumatic pains, contusions, as well as the cutaneous eruption due to their anti-asthmatic, antiseptic, and anti-inflammatory attributes (Baser et al., 2000; Miceli et al., 2005). Several researchers (in vitro and in vivo) have been performed on different species of the genus Nepeta but the plant Nepeta paulsenii is rarely used in this aspect. The present analysis was performed on male albino rats to explore the effects of methanolic extract of Nepeta paulsenii plant leaves on CCl4 induced testicular damage in male rats.
2 Materials and methods
2.1 Plant collection
Nepeta paulsenii plant was collected at flowering stage in late spring in 2018 from the area of Ganga Choti, Pir-Panjal (Bagh), Pakistan administrated Kashmir.
2.1.1 Drug and plant dose preparation
The leaves of the Nepeta paulsenii plant were dried in the air, at room temperature and under shadow to remove moisture completely. These leaves were ground to a very fine powder and finely-ground powder (5 kg) of the plant was immersed for 72 hrs. in 10 L crude methanol for extraction. The extraction process was replicated twice with the above-mentioned process. Whatman number 1 filter was used for the filtration process and rotary evaporator was used to evaporate the methanol at 40 °C temperature under decreased pressure to acquire the sticky material. This material was fractionated on the rising polarity basis and dried under vacuum with the help of a rotary evaporator. An extract of the plant was stored at −20 °C for the quality maintenance.
CCl4 was purchased from Sigma-Aldrich, U.S.A. and diluted in olive oil for dose preparation (CCl4: Olive oil; 2:8 v/v). 1 ml/kg body weight dose of CCl4 was assigned keeping in view previous literature (Shah and Khan, 2017a,b).
2.2 Experimental layout
Male Albino rats were issued from the breeding and rearing center and kept in the Animal House, University of Agriculture, Faisalabad. Sexually mature rats (160–250 g) were kept at ambient temperature, served by tap water and normal diet (pellet) in 12 hrs. dark/light rotation in 26 ± 3 °C temperature for 25 days. Precise implementation of instructions by the National Institute of Animal Health (NIH) was ensured in the whole experimental trial. Ethical Committee of University of Agriculture, Faisalabad provided approval of this research study.
Rats were distributed into 8 groups; each group comprised of 6 rats. Rats of each group were placed in separate cages. Rats in group 1 (referred as a control group) were treated with normal pellet diet and tap water. Rats in group 2 were treated by giving dimethyl sulfoxide (DMSO) and olive oil (1:1; v/v) dose@1 ml/kg orally and group 3 was provided with CCl4 (CCl4: Olive oil; 2:8 v/v; 1 ml/kg). After twenty-four hours. of CCl4 treatment, silymarin dose@50 mg/kg in DMSO was given to group 4 rats. Group 5 was nursed with Nepeta paulsenii dose@ 200 mg/kg and 6 was served with 400 mg/kg in DMSO after twenty-four hours. of CCl4 treatment. The dose of Nepeta paulsenii extract 200 mg/kg and 400 mg/kg in DMSO was given to group 7 and 8 respectively (Sajid et al., 2016).
The rats were anesthetized and beheaded. Blood was collected in sterile falcon tubes, centrifuged and stored in the Refrigerator. Both of the testes were detached and washed with normal saline (NaCl, B.P. 0.9%). Tissues were minced, homogenized with distilled water and centrifuged (3000 rpm for about 15 min).
2.3 Hormone analysis
Samples of serum were collected from the experimental groups to determine the concentration of the testosterone hormone via enzyme-linked immuno sorbent assay (ELISA) kits. LH and FSH concentrations of each group were assessed from the serum samples with the help of Immunoassay Test Kits (GenWay Biotech Inc.).
2.4 Daily sperm production (DSP)
For the homogenization purpose, frozen testes were thawed at normal temperature and parenchyma was separated from tunica vaginalis. Then, its weighed amount was homogenized in five milliliter solution of 0.5% Triton X-100 and 0.9% NaCl for the 30 s via rotor, stator Homogenizer made by IKA-Werke, Staufen, Germany. Dilution of homogenate further carried out five times after preparation and 20 µL volume of the diluted homogeneous solution was shifted to Neubauer chamber and the count of spermatids of 19th stage was taken with the help of a light – microscope at 40X. An average of 3 readings was calculated for the estimation of spermatids count present in each sample. DSP was determined by obtaining the total number of spermatids/testis from these values and dividing them by 6.3 (total days these spermatids stay in the epithelial part of seminiferous tubule).
Y is the spermatid numbers existing in the homogenate
2.5 Enzymatic antioxidant status
CAT and POD activity was assessed in accordance with the methodology of Chance and Maehly (1955). SOD activity was estimated by noticing the color intensity as proposed by Kakkar et al. (1984). GSR activity was evaluated according to the method of Carlberg and Mannervik (1975) while activity of GST was measured by following the procedure of Habig et al. (1974).
2.6 Estimation of protein content
The total protein content of the testicular tissues was evaluated with the help of total protein kit (AMP Diagnostics, Austria). The values of total protein content were shown as mg/g of tissue.
2.7 TBARS (lipid peroxidation assay) and hydrogen peroxide (H2O2) estimation
Assessment of TBARS was carried out by the procedure of Kanter et al. (2003) with specific amendments. Assessment of H2O2 level was conducted by the methodology of Khan et al. (2015). Horseradish peroxidase enzyme facilitated by H2O2 was used.
2.7.1 Lipid profile
Estimation of HDL, LDL, triglycerides and total cholesterol with the help of AMP diagnostic kits (AMEDA labordiagnostik GmbH, Austria) by following all instructions mentioned by manufacturers on a chemistry analyzer.
2.8 Histopathological studies
CCl4 induced testicular injuries were assessed through the histopathological examination. Samples of testicular tissues were fixed in fixing solution containing absolute alcohol (70%), formaldehyde (20%) and acetic acid (10%) followed by inserting in paraffin wax. 4–5 μM thin slices were made precisely, stained with eosin/hematoxylin and analyzed under the DIALUX-20EB light microscope (40x).
2.9 Statistical analysis
Entire data were represented as mean ± SEM. Analysis of data was performed by one-way ANOVA and means were compared by applying Tukey’s test at the level of p < 0.05.
3 Results
3.1 Hormonal analysis
Concentrations of LH, FSH and testosterone were decreased significantly (p < 0.05) in CCl4 administered rats in comparison with rats of vehicle control and control groups. Silymerine + CCl4 served rats showed a significant increase in hormonal concentrations in comparison with CCl4 treated group. Likewise, concentrations of hormones in CCl4 + N. paulsenii treated rats were significantly improved compared to CCl4 treated rats. Significant (p < 0.05) escalation in the concentration of hormones was evinced in a dose dependent manner in N. paulsenii treated groups in comparison with the CCl4 treated rats (Table 1). Values having different superscripts from CCl4 treated group show significant difference with CCl4 group while values having no superscript show no significant difference between CCl4 and this group.
Groups n = 8
Plasma testosterone concentration (ng/ml)
LH (mlU/ml)
FSH (mlU/ml)
DSP × 106/g
Control
4.11 ± 0.05 a
2.78 ± 0.10 a
3.54 ± 0.06 a
18.90 ± 0.77 a
DMSO
4.11 ± 0.06 a
2.77 ± 0.10 a
3.56 ± 0.07 a
18.56 ± 0.80 a
CCl4
1.14 ± 0.03b
1.23 ± 0.06b
2.18 ± 0.06b
6.946 ± 0.29b
CCl4 + Silymarin
3.99 ± 0.06 a
2.77 ± 0.05 a
3.47 ± 0.08 a
17.24 ± 0.11 a
CCl4 + NP (200 mg/kg)
3.88 ± 0.05 a
2.05 ± 0.03c
2.93 ± 0.02 ac
16.96 ± 0.05c
CCl4 + NP (400 mg/kg)
4.02 ± 0.09 a
2.15 ± 0.04 a
3.02 ± 0.34 ac
17.47 ± 0.2 ac
NP (200 mg/kg)
3.87 ± 0.09 a
2.36 ± 0.04 ac
2.81 ± 0.33c
18.57 ± 0.12 a
NP (400 mg/kg)
4.05 ± 0.09 a
2.48 ± 0.04 ac
3.32 ± 0.12 a
18.88 ± 0.10 a
3.2 Biochemical analysis
Activity of antioxidant enzymes in comparison to control was significantly (p < 0.05) reduced in CCl4 induced rats in comparison to control. Antioxidant enzyme activity in the Nepeta paulsenii treated groups displayed no significant difference in comparison to vehicle control and control groups. CCl4 + Silymarin induced rats showed significant (p < 0.05) elevation in enzymatic activities when in comparison with CCl4 induced group. Similarly, CCl4 + Nepeta paulsenii induced groups (dose dependent) exhibited considerable (p < 0.05) upsurge in antioxidant enzyme activity in comparison to CCl4 treated group (Table 2). Values having different superscripts from CCl4 treated group show significant difference with CCl4 group while values having no superscript show no significant difference between CCl4 and this group.
Groups n = 8
CAT (U/mg protein)
POD (nanomole)
SOD (U/mg protein)
GSR (nm NADPH oxidized/min/mg tissue)
GST (U/ml)
Control
7.29 ± 0.23 a
8.33 ± 0.23 a
6.05 ± 0.09 a
4.54 ± 0.17 a
150 ± 3.42 a
DMSO
7.10 ± 0.15 a
8.25 ± 0.12 a
6.02 ± 0.07 a
4.47 ± 0.14 a
146 ± 2.66 a
CCl4
3.30 ± 0.20b
3.52 ± 0.25b
2.03 ± 0.05b
1.70 ± 0.11b
72.8 ± 2.39b
CCl4 + Silymarin
7.05 ± 0.05 a
7.62 ± 0.20 a
6.03 ± 0.08 a
4.17 ± 0.14 a
137 ± 3.60c
CCl4 + NP (200 mg/kg)
6.67 ± 0.11 a
8.03 ± 0.08 a
5.54 ± 0.15 a
4.03 ± 0.09c
132 ± 1.54 d
CCl4 + NP (400 mg/kg)
7.05 ± 0.04 a
8.07 ± 0.06 a
6.00 ± 0.13 a
4.09 ± 0.11c
135 ± 1.20 cd
NP (200 mg/kg)
7.13 ± 0.12 a
8.15 ± 0.09 a
6.11 ± 0.07 a
4.27 ± 0.07 ac
140 ± 1.39c
NP (400 mg/kg)
7.32 ± 0.17 a
8.32 ± 0.14 a
6.15 ± 0.10 a
4.31 ± 0.09 ac
147 ± 0.89 a
3.3 Analysis of protein content, TBARS and H2O2
A significant (p < 0.05) decline was noticed in the protein content in CCl4 administered rats when compared to vehicle control and the control group. While, protein content was regained in the CCl4 + Silymarine treated group, as well as significant (p < 0.05) amendments were perceived in CCl4 + N. paulsenii treated groups. However, no considerable changes in protein content were noticed in N. paulsenii treated rats in contrast to rats of the control group.
TBARS level was significantly (p < 0.05) escalated in toxicant treated group in comparison to vehicle control and control groups. TBARS level was significantly (p < 0.05) diminished in the Nepeta paulsenii treated rats when compared with toxicant group. CCl4 + Silymarin induced rats also exhibited significant (p < 0.05) decline in TBARS level in comparison with CCl4 induced group. In a similar manner, CCl4 + Nepeta paulsenii treated groups (dose dependent) exhibited considerable (p < 0.05) decrease in TBARS level in comparison to CCl4 administered group.
A significant increase was observed in H2O2 concentration in CCl4 administered rats in comparison to vehicle control and control group. Concentration of H2O2 was normalized in the CCl4 + Silymarine treated group. In the same spirit, H2O2 concentration was significantly (p < 0.05) declined in CCl4 + N. paulsenii induced groups. There were not any remarkable variations in N. paulsenii treated rats in comparison to control group (Table 3). Values having different superscripts from CCl4 treated group show significant difference with CCl4 group while values having no superscript show no significant difference between CCl4 and this group.
Groups n = 8
Protein Content (mg/g)
TBARS (nm TBARS/min/mg tissue)
Hydrogen peroxide (H2O2)
Control
4.92 ± 0.03 a
14.65 ± 0.46 a
4.18 ± 0.13 a
DMSO
4.89 ± 0.01 a
14.58 ± 0.45 a
4.15 ± 0.14 a
CCl4
1.42 ± 0.06b
24.09 ± 0.59b
9.00 ± 0.06b
CCl4 + Silymarin
4.58 ± 0.02 a
15.65 ± 0.20 a
4.30 ± 0.12 a
CCl4 + NP (200 mg/kg)
4.20 ± 0.01 a
16.13 ± 0.16c
5.05 ± 0.09c
CCl4 + NP (400 mg/kg)
4.32 ± 0.02 a
16.02 ± 0.12c
4.92 ± 0.07 ac
NP (200 mg/kg)
4.52 ± 0.02 a
15.94 ± 0.07 ac
4.86 ± 0.07 ac
NP (400 mg/kg)
4.62 ± 0.03 a
15.85 ± 0.04 ac
4.85 ± 0.06 ac
3.4 Analysis of lipid profile
Numerous changes were observed in Lipid profile; HDL, LDL and triglycerides (mg/dl) in CCl4 induced rats when compared to vehicle control and control groups. LDL and triglyceride levels were significantly raised in CCl4 administered group in comparison to control and vehicle control group, whereas in co-treated groups, it overturns to normal state (i.e., similar to control group). N. paulsenii significantly decreased the levels of LDL and triglyceride in comparison to control group. Contrariwise, HDL level declined in CCl4 treated groups when compared to control, but recovered to normal in co-treated groups. Doses of N. paulsenii improved high density lipid profile in damaged groups and N. paulsenii (only) administered group. (Table 4). Values having different superscripts from CCl4 treated group show significant difference with CCl4 group while values having no superscript show no significant difference between CCl4 and this group.
Groups n = 8
HDL (mg/dl)
LDL (mg/dl)
Triglyceride (mg/dl)
Cholesterol Level
Control
33.99 ± 2.62 a
5.34 ± 0.17 a
57.4 ± 1.55 a
39.6 ± 0.88 a
DMSO
33.78 ± 2.65 a
5.34 ± 0.17 a
58.0 ± 1.15 a
44.0 ± 0.57c
CCl4
16.47 ± 0.26b
15.3 ± 0.11b
104 ± 3.79b
67.8 ± 0.43b
CCl4 + Silymarin
30.40 ± 0.35 ac
6.72 ± 0.13c
60.6 ± 1.76 ac
45.2 ± 0.60c
CCl4 + NP (200 mg/kg)
28.22 ± 0.21c
7.05 ± 0.09c
64.6 ± 1.45c
50.2 ± 0.44 d
CCl4 + NP (400 mg/kg)
28.88 ± 0.17c
6.93 ± 0.05c
63.3 ± 0.88c
47.8 ± 0.20 cd
NP (200 mg/kg)
30.11 ± 0.06 d
6.12 ± 0.06 a
63.5 ± 0.28c
45.8 ± 0.45c
NP (400 mg/kg)
31.26 ± 0.21 d
5.83 ± 0.07 a
63.1 ± 0.51c
45.3 ± 0.33c
3.5 Analysis of histology and morphometric profile
Photomicrographs of male adult rat testes were studied under the lens of 40x magnification (Fig. 1). Tunica albuginea height displayed a significant (p < 0.05) reduction in CCl4 induced group when rivalled with the control group. Conversely, the vehicle control and the control group did not exhibit any significant decline in albuginea height. N. paulsenii treatment significantly (p < 0.05) elevated tunica albuginea height compared to CCl4 administered rats. In similar manner, dose dependent CCl4 + N. paulsenii nursed rats exhibited a significant increase in tunica albuginea height in comparison to CCl4 treated rats. A significant recovery in the tunica albuginea height of seminiferous tubules was perceived in co-treated rats when compared with CCl4 treated group.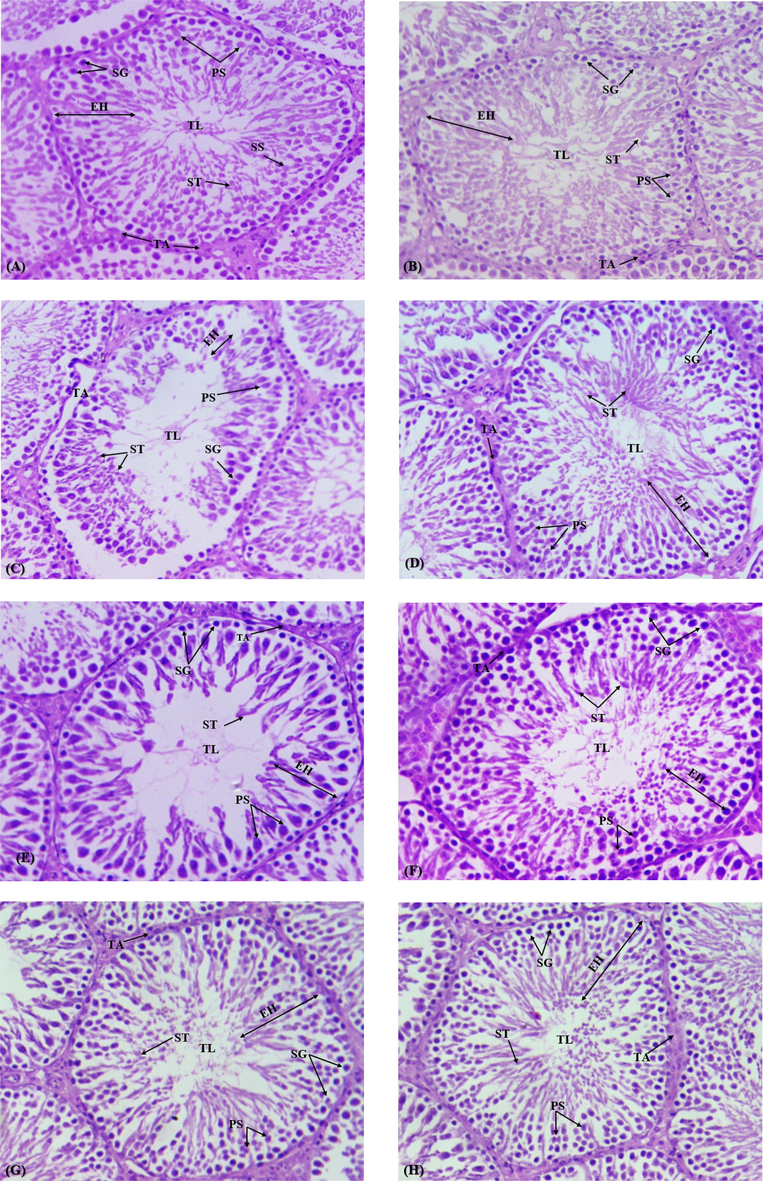
Photomicrographs of male adult rat testes (H&E, 40x): (A) Control group displaying standard morphometry of seminiferous tubules with active spermatogenesis; (B) Vehicle control group also showing normal morphology of tubule. (C) CCl4 treated group, showing increased interstitial spaces between seminiferous tubules and size of lumen (D) CCl4 + Silymarine treated group showing restoration of damaged tubule including lumen, Tunica albuginea, diameter and epithelial height; (E) Cotreated group CCl4 + N. paulsenii (200 mg/kg) presenting recovery of seminiferous tubules with germinal epithelium having proliferating germ cells at different stages such as (Spermatids, spermatocytes, spermatogonia: (F) Cotreated group CCl4 + N. paulsenii (400 mg/kg) presenting more recovery of tubular lumen and germinal epithelium, increased seminiferous tubule diameter, decreased interstitial spaces: (G) N. paulsenii (200 mg/kg) treated groups presenting increased diameter of germinal epithelium and seminiferous comparison with control: (H) N. paulsenii (400 mg/kg) treated group decreased in diameter of seminiferous tubule, proliferated number of germ cells and spermatogenesis improved comparison with control group. Tunica albuginea (TA), Primary spermatocytes (PS), Spermatogonia (SG), Secondary spermatocytes (SS), Tubular lumen (TL).
CCl4 treatment led to a significant (p < 0.05) diminution in the thickness of seminiferous tubules in comparison to vehicle control and control groups. The seminiferous tubule diameter in N. paulsenii induced groups was significantly (p < 0.05) escalated when contested with CCl4 administered rats. Likewise, significant expansion (p < 0.05) in diameter of seminiferous tubules in dose dependent CCl4 + Nepeta paulsenii treated groups was noticed in comparison to CCl4 induced rats.
CCl4 treatment brings about a significant (p < 0.05) shrinkage in mean seminiferous germinal epithelial thickness as compared to control. The thickness of germinal epithelium in the vehicle control group didn’t show a significant change from the rats of the control group. However, in CCl4 + Silymarin induced group, the height of the tubular germinal epithelium exhibited significant (p < 0.05) elevation as compared with CCl4 administered group. Nevertheless, no remarkable alteration was displayed in the thickness of germinal epithelium in Nepeta paulsenii induced groups in comparison to control while in CCl4 + Nepeta paulsenii treated groups, which were dose contingent, significant (p < 0.05) recovery was observed in tubular germinal epithelium height when compared with CCl4 administered rats.
Tubular luminal diameter revealed a significant (p < 0.05) escalation in the CCl4 induced rats in comparison to control. There was not any significant difference in the vehicle control group as compared with control. CCl4 + Silymarin induction displayed a significant decrease in tubular luminal diameter in comparison to CCl4 treated group. Nepeta paulsenii induced rats exhibited a non-significant difference in comparison with control, but a significant (p < 0.05) difference was detected as compared with CCl4 considered group. However, CCl4 + Nepeta paulsenii treated groups exhibited significant (p < 0.05) decline in diameter of lumen as compared with CCl4 administered rats.
A significant (p < 0.05) escalation was observed in the interstitial spaces between the tubules in CCl4 administered rats in comparison to control and other groups. In vehicle control, interstitial spaces among tubules remained same as in the control. Therefore, there was not any significant difference between control and vehicle control groups. While, interstitial spaces among tubules were significantly (p < 0.05) decreased in the CCl4 + Silymarin and CCl4 + Nepeta paulsenii treated groups in comparison to toxicant induced rats. Interstitial spaces between the tubules displayed a significant (p < 0.05) reduction in N. paulsenii treated rats when compared with CCl4 treated rats (Table 5). Values having different superscripts from CCl4 treated group show significant difference with CCl4 group while values having no superscript show no significant difference between CCl4 and this group.
Groups n = 8
Tunica Albuginea (µm)
Diameter of Seminiferous Tubule (µm)
Epithelial height of Seminiferous Tubule (µm)
Tubular Lumen (µm)
Interstitial Spaces (µm)
Control
27.6 ± 0.88 ac
177 ± 1.76 a
75.6 ± 1.85 a
9.66 ± 1.20 a
8.66 ± 0.88 a
DMSO
26.0 ± 0.57 a
176 ± 1.45 a
76.6 ± 1.20 a
9.33 ± 1.20 a
8.33 ± 1.45 a
CCl4
14.3 ± 1.85b
163 ± 2.40b
29.6 ± 3.48b
66.0 ± 2.64b
12.3 ± 0.88b
CCl4 + Silymarin
24.6 ± 0.88 a
174 ± 1.85 ac
56.3 ± 2.90c
23.0 ± 2.08c
9.66 ± 0.88 ab
CCl4 + NP (200 mg/kg)
25.0 ± 1.00 a
175 ± 1.73 ac
60.0 ± 2.08 cd
22.0 ± 1.52c
7.33 ± 0.88 a
CCl4 + NP (400 mg/kg)
26.3 ± 0.66 a
178 ± 2.08 a
65.6 ± 0.88 ad
17.0 ± 1.15 ac
6.66 ± 0.66 ac
NP (200 mg/kg)
28.3 ± 1.76 ac
180 ± 1.15c
77.6 ± 1.33 d
8.66 ± 1.76 a
6.00 ± 0.57 ac
NP (400 mg/kg)
30.3 ± 1.45c
182 ± 0.88c
78.3 ± 1.45 d
10.6 ± 0.88 a
5.33 ± 0.33c
CCl4 induction significantly (p < 0.05) reduced number of spermatogonia, primary spermatocytes, secondary spermatocytes as well as spermatids in comparison to control and vehicle control groups. Whereas, doses of CCl4 + N. paulsenii and Silymerin + CCl4 significantly improved the number of these germ cells in these co-treated groups in comparison with CCl4 treated rats. Significant (p < 0.05) increase in germ cell count was observed in a dose dependent manner in N. paulsenii medicated groups as compared with CCl4 administered rats (Table 6). Values having different superscripts from CCl4 treated group show significant difference with CCl4 group while values having no superscript show no significant difference between CCl4 and this group.
Groups n = 8
Spermatogonia
Primary spermatocytes
Secondary spermatocytes
Spermatids
Control
44 ± 2.08 a
41 ± 2.02 ab
32 ± 1.76 a
49 ± 1.20 ab
DMSO
44 ± 1.15 a
43 ± 0.88 ab
32 ± 1.00 a
48 ± 1.20 ab
CCl4
24 ± 1.73b
22 ± 2.08c
20 ± 1.52b
25 ± 1.85c
CCl4 + Silymarin
38 ± 1.76 a
38 ± 0.88b
29 ± 1.45 a
42 ± 1.52b
CCl4 + N. paulsenii (200 mg/kg)
44 ± 1.52 a
41 ± 1.20 ab
30 ± 0.88 a
44 ± 1.73 ab
CCl4 + N. paulsenii (400 mg/kg)
44 ± 2.33 a
44 ± 1.45 ab
32 ± 0.33 a
45 ± 2.72 ab
N. paulsenii (200 mg/kg)
45 ± 2.08 a
48 ± 1.45 a
34 ± 1.76 a
49 ± 1.45 ab
N. paulsenii (400 mg/kg)
48 ± 3.05 a
50 ± 1.45 a
35 ± 1.45 a
51 ± 1.15 a
4 Discussion
External or internal environmental disturbance due to various toxicants leads to fecundity issues in males. Control of free radicals and oxidative damages due to excess generation of ROS in the cells is a main topic of interest for scientists since last few years. The researches on natural or plant-based antioxidants have provided evidence on their health advantages in fighting against oxidative stress. This oxidative stress is among a major reason behind different disorders in living organisms (Masood et al., 2013). ROS generation stimulates hypogonadism in testicular tissues (Khan and Ahmed, 2009). CCl4 is widely utilized as a standard toxin to induce chemical injury in various body organs for experimental purposes (Cemek et al., 2010). Phase I cytochrome P450 converts the CCl4 to reactive metabolic trichloromethyl radical and peroxy trichloromethyl radical through bioactivation. These free radicals make a bond with polyunsaturated fatty acids for generating alkoxy and peroxy radicals, which may lead to production of lipid peroxides. These lipid peroxides affect the activities of enzymes and result in tissue damage due to their high reactive property (Bruckner et al., 2002; Weber et al., 2003). Importance of herbal compounds proved to treat multiple disorders in animals (Kiran et al., 2018). Number of plants of the Nepeta genus have been used as traditional medicines and some of their components showed curative abilities (Grognet, 1990).
CCl4, being a damaging compound, reduced the testosterone, LH and FSH concentrations and also decreased the DSP (Rajesh and Latha, 2004; Shah and Khan, 2017a,b). The low rate of sperm production in rats after CCl4 exposure may be due to reduced level of LH, FSH and testosterone, although these hormones are essential to ensure spermatogenesis. LH instigates Leydig cells to produce testosterone and FSH incites the spermatogenesis (Horn et al., 2006; Jaffat et al., 2014). Testosterone is secreted from the interstitial cells followed by LH stimulation. The reduction in the LH level by CCl4 exposure adversely affected the levels of plasma testosterone. CCl4 impaired steroidogenesis and impeded the levels of testosterone. On the basis of these investigations, it can be claimed that N. paulsenii extract improved the hormonal concentrations and increased the DSP in rats.
CCl4 produced damages due to free radicals by lipid peroxidation of cellular membrane, reduction of antioxidant enzymes along with their substrates, which ultimately leads to oxidative stress (Szymonik-Lesiuk et al., 2003). CCl4 intoxication significantly reduced CAT, POD, SOD, GSR, GST and protein content, while TBARS level was significantly increased in the reproductive system of male rats. Excess quantity of H2O2 in the testicles due to CCl4 attributes causes suppression of antioxidant enzymes and tissue damages (Khan and Ahmed, 2009; Sahreen et al., 2013; Ojo et al., 2016). Our other unpublished data confirm the presence of flavonoids, terpenoids, saponins and other antioxidants in the methanolic extract of N. paulsenii leaves. The presence of these bioactive compounds may be a key factor in reducing the free radicals. N. paulsenii impedes oxidation in the cells by reducing free radicals, which is revealed by low levels of H2O2, TBARS and escalation of total protein content along with antioxidant enzymes.
Lipid peroxidation in the testes is closely related to the dysfunction of the male reproductive system due to steroidogenesis impairment (Turner and Lysiak, 2008). Previous investigators have reported that CCl4 exposure induced hyperlipidemia (Hsu et al., 2009; Marimuthu et al., 2013). CCl4 intoxication significantly elevated the level of triglycerides, LDL and cholesterol, while the HDL level was reduced. Groups treated with N. paulsenii maintained the level of lipid profile somewhere close to control group.
CCl4 treatment caused significant reduction in germ cells belonging to various stages while improving cell count was observed in N. paulsenii treated groups. Deterioration of the seminiferous tubules and germ cells was noticed in CCl4 exposed testicles of rats. Flavonoids presence in N. paulsenii might be involved in recovering the CCl4 induced damages. Similar amendments were exhibited by other medicinal plant extracts to provide shielding effect against CCl4-instigated lesions on the testicles (Khan and Ahmed, 2009; Sahreen et al., 2013). Usage of N. paulsenii considerably increased germinal epithelial height of seminiferous tubules, enhanced diameter of seminiferous tubules as well as elevated tunica albuginea thickness was observed in rats treated with N. paulsenii extract.
5 Conclusion
The results of our investigations suggest that the N. paulsenii plant has curative effects against damages in testicular seminiferous tubules shape and apoptosis, histopathological lesions, hormonal disturbances as well as decreased antioxidant enzyme activity. The flavonoids terpenoids, saponins and other antioxidants present in N. paulsenii may have a potential to treat male sterility issues, caused by oxidative stress. Thus, it is suggested that N. paulsenii plant can be utilized to improve the performance of the male reproductive system and can play a major role for the betterment of humanity.
Acknowledgements
Authors acknowledge University of Agriculture, Faisalabad for providing research grant to accomplish this study. “The authors (KAAG, FAM and SM) express their sincere appreciation to the Researchers Supporting Project (RSP-2019-93), King Saud University, Riyadh, Saudi Arabia”.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antioxidant properties of various solvent extracts of mulberry (Morus indica L.) leaves. Food Chem.. 2007;102:1233-1240.
- [Google Scholar]
- Mechanisms of circadian rhythmicity of carbon tetrachloride hepatotoxicity. J. Pharmacol. Exp. Therap.. 2002;300:273-281.
- [Google Scholar]
- Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem.. 1975;250(14):5475-5480.
- [Google Scholar]
- Protective potential of Royal Jelly against carbon tetrachloride induced toxicity and changes in the serum sialic acid levels. Food Chem. Toxicol.. 2010;48:2827-2832.
- [Google Scholar]
- Assay of catalase and peroxidases. In: Methods in Enzymology. New York: Academic Press; 1955. p. :764-775.
- [Google Scholar]
- Glutathione S-Transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem.. 1974;249(22):7130-7139.
- [Google Scholar]
- Seminiferous epithelium of rats with food restriction and carbon tetrachloride-induced cirrhosis. Int. Braz. J. Urol.. 2006;32(1):94-99.
- [Google Scholar]
- Protective effects of seabuckthorn (Hippophae rhamnoides L.) seed oil against carbon tetrachloride-induced hepatotoxicity in mice. Food Chem. Toxicol.. 2009;47:2281-2288.
- [Google Scholar]
- Medical treatment to improve sperm quality. Reprod. Biomed. Online. 2006;12:704-714.
- [Google Scholar]
- Protective effect of Allium Ampeloprasum against toxicity induced by CCL4 in male white rats. Int. J. Sci. Eng. Res.. 2014;5(10):825-828.
- [Google Scholar]
- A modified spectrophotometric assay of superoxide dimutase. Ind. J. Biochem. Biophys.. 1984;21:130-132.
- [Google Scholar]
- Effects of Nigella sativa L. and Urtica dioica L. on Lipid Peroxidation, Antioxidant Enzyme Systems and Some Liver Enzymes in CCl4-Treated Rats. J. Vet. Med. A. Physiol. Pathol. Clin. Med.. 2003;50(5):264-268.
- [Google Scholar]
- Protective effects of Digera muricata (L.) Mart. On testis against oxidative stress of carbon tetrachloride in rat. Food Chem. Toxicol.. 2009;47:1393-1399.
- [Google Scholar]
- Modulation of carbon tetrachloride-induced nephrotoxicity in rats by n-hexane extract of Sonchus asper. Toxicol. Ind. Health. 2015;31(10):955-959.
- [Google Scholar]
- Anti-inflammatory and anticancer activity of pteris cretica whole plant extracts. Pak. Vet. J.. 2018;38(3):225-230.
- [Google Scholar]
- Protective role of ferulic acid on carbon tetrachloride-induced hyperlipidemia and histological alterations in experimental rats. J. Basic Clin. Physiol. Pharmacol.. 2013;24:59-66.
- [Google Scholar]
- Role of natural antioxidants for the control of coccidiosis in poultry. Pak. Vet. J.. 2013;33(4):401-407.
- [Google Scholar]
- Anti-Inflammatory Activity of Extract Fractions from Nepeta Sibthorpii Bentham. J. Ethno. Pharmacol.. 2005;97:261-266.
- [Google Scholar]
- Protective influence of Ficus asperifolia Miq leaf extract on carbon tetrachloride (CCl4)-induced testicular toxicity in rats. J. Appl. Pharm.. 2016;6(06):37-41.
- [Google Scholar]
- Protective activity of Glycyrrhiza glabra Linn. on carbon tetrachloride-induced peroxidative damage. Indian J. Pharmacol.. 2004;36:284-287.
- [Google Scholar]
- Ameliorating effect of various fractions of Rumex hastatus roots against hepato- and testicular toxicity caused by CCl4. Oxidative Med. Cell Longev.. 2013;2013:1-11.
- [Google Scholar]
- Proficiencies of Artemisia scoparia against CCl4 induced DNA damages and renal toxicity in rat. BMC Complement. Altern. Med.. 2016;16:117-122.
- [Google Scholar]
- Increase of glutathione, testosterone and antioxidant effects of Jurenia dolomiaea on CCl4 induced testicular toxicity in rat. BMC Complement. Altern. Med.. 2017;17:1-9.
- [Google Scholar]
- Increase of glutathione, testosterone and antioxidant effects of Jurenia dolomiaea on CCl4 induced testicular toxicity in rat. BMC Complement. Altern. Med.. 2017;17(1):206.
- [Google Scholar]
- Catalase, superoxide dismutase, and glutathione peroxidase activities in various rat tissues after carbon tetrachloride intoxication. J. Hepatobiliary Pancreat. Sci.. 2003;10:309-315.
- [Google Scholar]
- Oxidative stress: a common factor in testicular dysfunction. J. Androl.. 2008;29:488-498.
- [Google Scholar]
- Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit. Rev. Toxicol.. 2003;33:105-136.
- [Google Scholar]







