Translate this page into:
Metal doped titania nanoparticles as efficient photocatalyst for dyes degradation
⁎Corresponding author. GSM: +923416062388. zahoor@uom.edu.pk (Muhammad Zahoor),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives: The present work aims at the synthesis of metal-doped and undoped titania photocatalysts for the degradation of methylene blue and neutral red dyes. Methods: Un-doped and metal-doped titania photocatalysts were synthesized by hydrothermal treatment, calcined at 673 K, and were evaluated by SEM, XRD, and XPS techniques for their phase composition. Results and conclusions: XPS studies of photo catalysts confirmed the presence of transition metal ions of Co, Ni, and Zn as dopants along with titania. XRD analysis indicated that all the synthesized photocatalysts were in the anatase phase. The morphology of prepared photocatalysts was confirmed through SEM analysis. The synthesized nanoparticle’s photocatalytic nature was examined through testing their degradation potentials of methylene blue and neutral red dyes under UV light irradiation. Under optimized conditions, like pH, contact time, initial dye concentration and catalyst amount metal-doped titania photocatalysts showed higher photodegradation efficiency than the pure parental material. Among the metal doped titania best results were obtained with Ni doped titania as 90% and 95% degradation were observed for neutral red and methylene blue dyes respectively. This photocatalyst can therefore be used successfully to degrade the selected dyes present in industrial effluents.
Keywords
Titania
Metal doping
Dyes degradation
Photocatalytic activity
1 Introduction
Water contamination is a serious global and environmental issue of this era as modern industries releases a number of contaminants like dyes (some of which are carcinogenic) pharmaceutical waste, and heavy metals that ultimately falls into water reservoirs. Dyes are considered one of the major contaminants that can adversely affect our environment when present beyond specified concentrations limit. Dyes are released from three main sources to water bodies: dyeing industries, household discharge, and dye manufacturing units. They affect the aquatic system by preventing the penetration of sunlight into water bodies. They also decreases the concentration of dissolved oxygen and reduces photosynthetic activities of aquatic plants. They react with other pollutants present in wastewater and produces toxic carcinogenic products that may cause severe health issues. Natural degradation of dyes by sunlight is a slow process because of faster accumulation than degradation. Biological degradation of dyes is difficult because most of the dyes have a large molecular size and stable aromatic structure. Neutral red belongs to the quinone-imine class of dyes and several studies have been carried out on its toxicity reduction through photocatalytic degradation using various catalyst (Devi and Dikdik, 2015). Neutral red is largely used in biological research for counterstaining and as a pH indicator in analytical laboratories (Mahmood et al., 2010). Methylene blue (MB) is a basic cationic dye used for dyeing silk, cotton, and wool. Direct contact of MB may cause severe health disorders including hyperhidrosis, vomiting, and nausea, local burning, breathe hazards, and mental disorders (Dariania et al., 2016).
Traditional techniques employed for the degradation of dyes in wastewater including chemical, physical, and biological methods but these methods are not efficient to convert these effluents into environment friendly end products (Abdellah et al., 2018). Photocatalytic degradation is a promising pollution treatment technology for water purification that causes the destruction of the chemical structure of contaminants present in water. Photocatalytic processes involves the excitation of a semiconductor material with an energy source higher than the band gap of semiconductor photocatalysts. Upon excitation electron holes pairs are generated that either recombine or may react with the target substance that may be either an electron acceptor (molecular oxygen) or an electron donor (hydroxide ions). Highly reactive species such as superoxide and hydroxyl radicals are generated during the photo degradation process (Bruno and Antoninho, 2019). These radicals are active that attacks the organic contaminants and microorganisms thus help in purification of water. Among various photocatalyst, the TiO2 semiconductor has received much attention because it is photo-stable, inexpensive, non-toxic and reusable. It can degrade chemical contaminants including dyes and microorganisms completely into carbon dioxide, mineral, and water (Chuanxi et al., 2017). Upon irradiation, by a light source, electron-hole pairs are formed on TiO2 surface, which either recombine or generate thermal energy or producing hydroxy radicals that mineralizes the organic pollutant to nontoxic substances. The high bandgap of TiO2 which is about 3.2 eV that allows it to be excited under UV light with λ < 387 nm and restricts its application in the presence of visible light because only a small fraction (3–5%) of sunlight could be utilized (Khairy and Zakaria, 2014).
Owing to the importance of titania as catalyst, the present study was aimed to prepare metal doped titania and used as catalyst for the degradation of neutral red and methylene blue.
2 Experimental
2.1 Materials
The chemicals used in this research work were: Urea (Sigma Aldrich), cobalt nitrate (BDH chemical England), nickel nitrate (Alfa Aesar), zinc nitrate (BDH chemical England), methylene blue (Sigma Aldrich), Neutral red (Alfa Aesar), and titanium isopropoxide (Sigma Aldrich). They were purchased from firms shown in brackets and were used as such without further processing.
2.2 Morphological and structural analysis
The morphology of synthesized doped and un-doped TiO2 were visualized with scanning electron microscopy (JSM 5910, Jeol, Japan). The elemental structure of the prepared catalyst was examined by energy dispersive X-rays (INC 200, Oxford, UK). The phase structure of catalysts was measured by X-rays diffraction (X-ray diffractometer Rigaku D/Max-II, Cu tube, Japan). The photodegradation potential of the prepared catalysts were evaluated against neutral red and methylene blue while the extent of degradation was estimated through UV/Visible spectrophotometer (UV-1800, Shimadzu, Japan).
2.3 Preparation of un-doped and doped titania
About 7.4 mL solution of titanium isopropoxide and 100 mL distilled water were mixed under vigorous stirring for 1 h at room temperature and a turbid solution of titanium hydroxide was obtained. After being washed several times with deionized water the precipitate were dried and re-dissolved in 1 M nitric acid solution to obtain a clear solution of [TiO(NO3)2]. Then the equimolar solutions of titanyl nitrate and urea was stirred for 1 h and was kept in a muffle furnace at 673 K to get titania. The synthesized titania was then kept in desiccators till further use. For the preparation of doped titania 1% solution by weight of Zn, Ni, Co were added separately to [TiO(NO3)2] solution. The reaction mixture was stirred and then titrated against urea. The mixture was then kept in a muffle furnace at 673 K. Finally the synthesized metal-doped titania was kept in a desiccators till further use (Zhang et al., 2017).
2.4 Photocatalytic test
To evaluate the photocatalytic activity of un-doped and doped titania the photodegradation of methylene blue and neutral red in aqueous solution was carried out at room temperature under UV light irradiation while to keep the mixture homogenous a magnetic stirrer was used. In a typical experiment, neutral red (20 ppm) and methylene blue (10 ppm) with an appropriate amount of catalyst (1 mg to 10 mg) was stirred magnetically at a constant temperature (using a hot plate). The suspension was then exposed to UV light (254 nm, 15 W) at a different time interval (10–100 min). After irradiation for a certain period of time sample were withdrawn for analysis with a pipette and immediately centrifuged to remove the photocatalyst particles from the heterogeneous solution before any absorbance measurement. Finally, the concentration of dye in the clear supernatant was determined by monitoring the absorption using a UV-visible spectrophotometer. The decomposition (Mohammad-Salehi et al., 2019) of both dyes in aqueous solution was determined using the following formula:
3 Results and discussion
3.1 SEM characterization
The morphology of metal-doped and un-doped titania was visualized through SEM analysis. Fig. 1 (b–d) represents the photographs of Zn, Co, and Ni doped titania respectively. It is clear from photographs that the titania is in dispersed form that is agglomerates at some point. The selected metals are well deposited on the surface of titania. Also the doped titania particles are bigger than pure titania. Hassan and Sayed, (2016) reported that the presence of dopant increases the catalyst particle size and causes particles to agglomerate.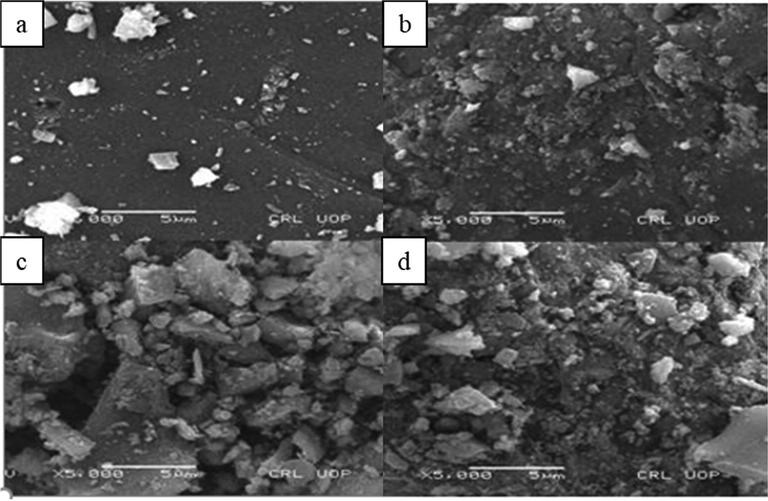
SEM images of titania photocatalyst a) Pure TiO2, b) Co/TiO2, c) Ni/TiO2, d) Zn/TiO2.
3.2 EDX analysis
The elemental composition of the doped and un-doped titania was determined through energy dispersive X-rays analysis (Fig. 2). Fig. 2 (a) shows the undoped photocatalyst that only consists of titania and oxygen, while the EDX spectra of cobalt, nickel, and zinc doped titania are given in Fig. 2 (b to d), which represents that the photocatalyst consists of titanium, oxygen and doped metals i.e. cobalt, nickel, and zinc as well in their chemical structure respectively.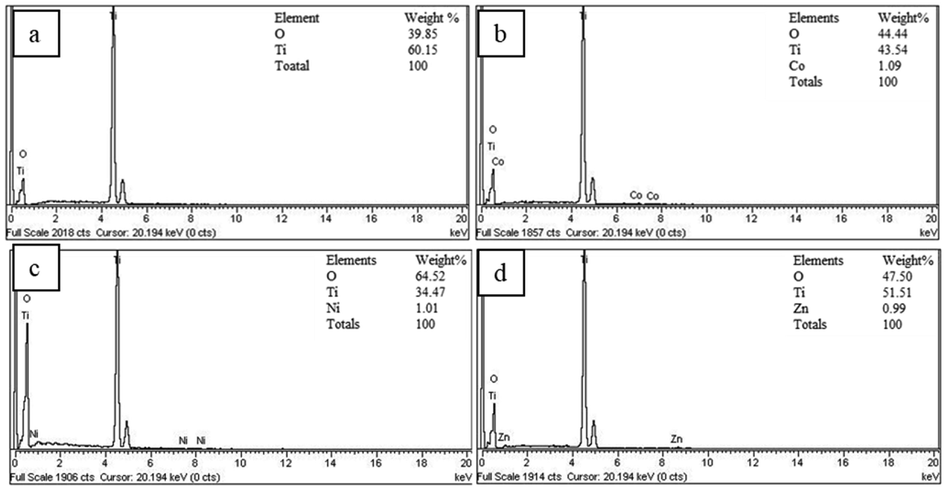
EDX spectra of the catalyst a) TiO2, b) Co/TiO2, c) Ni/TiO2, and d) Zn/TiO2.
3.3 XRD analysis
The XRD pattern of un-doped and doped titania is shown in Fig. 3. Strong diffraction peak at 2θ = 25.2°, 37.1°, 47.5°, 53.5°, and 62.3 are exhibited by anatase titania. All the peaks were comparable to the standard spectrum. It is cleared from Fig. 3 that all the samples show anatase phase regardless of metal content. XRD patterns of metal-doped titania samples do not exhibit any diffraction peaks of metals. This is may be due to low metal doped contents (1%) and also the metals are well dispersed within the titania crystal phase, as reported before (Shamalh et al., 2013; Balaram et al., 2016).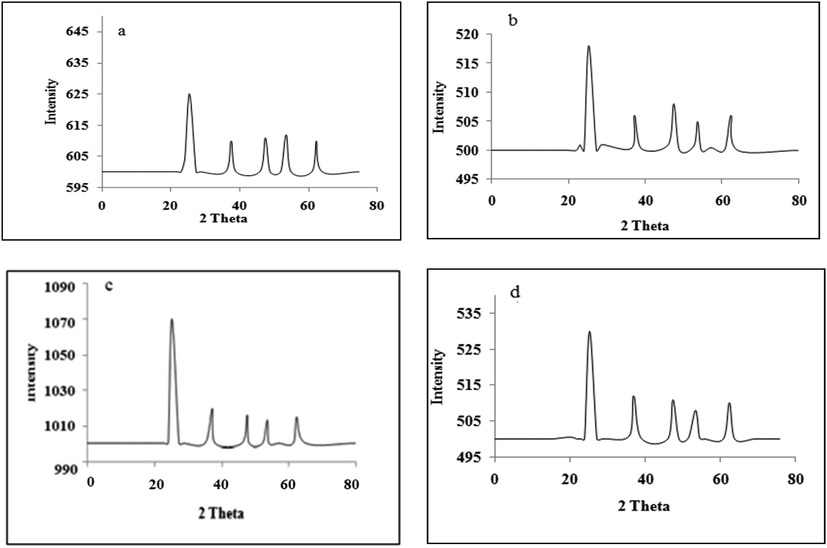
XRD analysis a) TiO2, b) Co/TiO2, c) Ni/TiO2, d) Zn/TiO2.
3.4 Photodegradation of selected dyes
The basic steps involve in heterogeneous photo-catalysis includes the transfer of the organic reactants in the liquid phase to the catalyst surface, adsorption of reactants on the catalyst surface followed reaction in the adsorbed phase and then product desorption/removal of the final products into the liquid phase (Rauf et al., 2011). The absorption of a photon having an energy equal to or greater than the bandgap of titania initiate the photocatalytic mechanism by producing an electron-hole pair on the surface of titania as schematized in Fig. 4. The total number of free charge carriers on the surface of titania is determined by the rate of electron-hole pair generation, charge recombination, charge release, migration, and interfacial charge transfer. Transition metal ion dopant act as hole or electron traps (Azeez et al., 2018).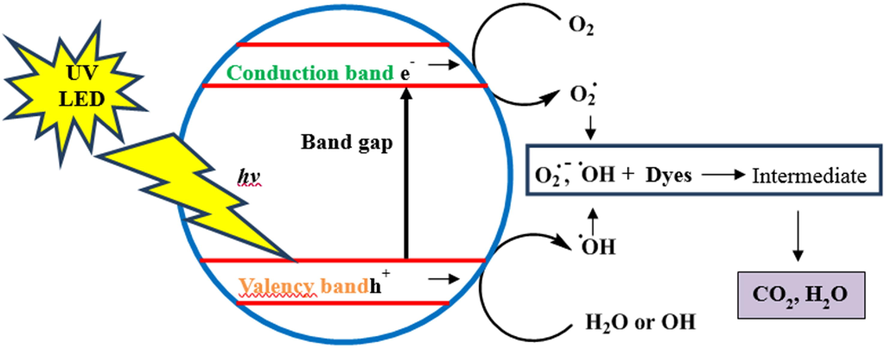
Possible mechanism of photodegradation process.
The recombination of pair of electron-hole decreases that results in an increase in the lifetime of charge carriers (Qian et al., 2019; Gesesse et al., 2019). The recombination rate of photo generated pair has to be precluded for a net chemical reaction to occur. In the indirect photocatalytic mechanism, the positive holes are trapped by a water molecule that results in the formation of hydroxyl radicals and hydrogen ion. The electrons in the conduction band can reduce molecular oxygen to superoxide radicals. The superoxide radical form hydrogen peroxide and subsequently form hydroxyl radical which take part in the degradation of organic dyes.
The following is the reaction sequence of titania semiconductor, activated by UV light in aqueous media
The radical species that are most active that participate in photodegradation are O2•−, HO2• and •OH, resulting in complete oxidation of the contaminant.
3.5 Effect of loading catalyst
The quantity of catalyst and its particle aggregation influences the rate of photodegradation of dyes. The enhancement of the photodegradation rate with increasing catalyst amount is the characteristics of heterogeneous catalysis. For neutral red dye photodegradation was increased up to 85.5% by increasing the catalyst amount from 1 to 2 mg. The rate of photodegradation became constant after 2 mg as shown in Fig. 5. The methylene blue degradation was increased up to 78.5% by increasing the catalyst amount from 1 to 10 mg and then leveled off as shown in Fig. 5. The number of active sites increases by increasing the catalyst dosage that results in an increase in hydroxyl radicals taking part in dyes degradation. Further increasing catalyst amount showed constant photodegradation rate because the incoming photons were scattered by the solid particles of the catalyst. Beyond a certain limit of photocatalyst amount, the solution becomes turbid and thus blocks UV that hindered the further catalytic process to occur; thereby decreasing the photodegradation rate (Lu et al., 2017).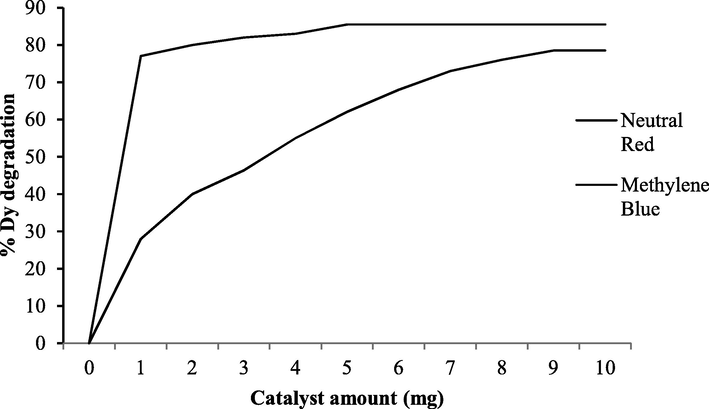
Percent degradation of neutral red and methylene blue at different catalyst amount.
3.6 pH effect
In photocatalytic degradation, solution pH is an important factor since it determines the surface charge of titania, adsorption of dyes molecules onto titania surface, the charge of dyes molecules, the number of hydroxyl radicals, and also the size of aggregates formed. The surface charge of titania changes by varying solution pH, and thus shifts the potential of photocatalytic reaction. Consequently, adsorption of dye on the surface is altered and the rate of reaction is affected. Under alkaline or acidic conditions the surface of titania can be deprotonated or protonated as shown by the following reactions (Guo et al., 2018).
Thus titania surface remain negatively charged in basic medium and positively charged in acidic media. Adsorption can be low or high in basic or acidic media depending upon the nature of the dye that’s to be adsorbed on the photocatalyst surface. The optimal photodegradation of neutral red was 82.84% at pH 8 and that of methylene blue was 81.15% at pH 11. No appreciable changes in the photodegradation were observed with further increase in pH of solution as shown in Fig. 6. The photodegradation of both dyes continuously decreased by decreasing the pH of the solution and was favorable in a slightly basic medium. In basic solution, titania acts as anion and neutral dye was adsorbed easily on the catalyst surface. Further increase in pH, cause repulsion between the dye molecules and negatively charged titania and hence reduced the rate of the photodegradation process. Under the acidic condition, the adsorbed cationic dye was repelled by the positive charge surface of titania that reduced the adsorption of dye and hence photodegradation efficiency (Xu et al., 2014).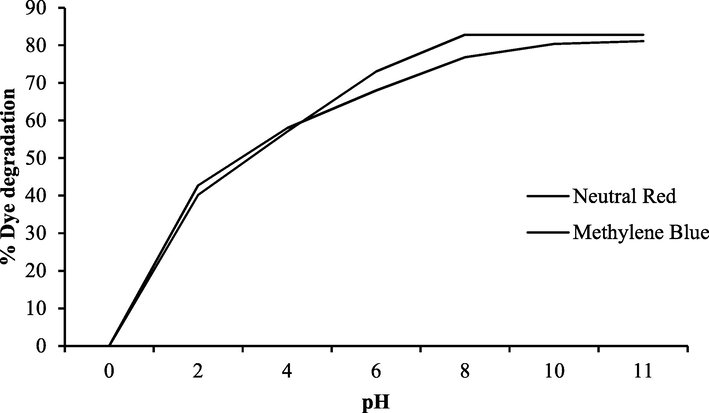
Percent degradation of neutral red and methylene blue at different pH.
3.7 The effect of initial dye concentration
In the present work, the percent dyes degradation increased up to 10 mg/L of dye concentration and then gradually decreased. It was noted that the process of photodegradation decreases with increasing dye concentration. High adsorption of dye molecule on the surface of titania causes a decrease in the number of hydroxyl radicals by occupying active sites (Raza et al., 2019).
3.8 Photocatalytic activity of metal doped titania at optimum condition
Metal ion dopants in low amounts produces structural defects on the surface of photocatalyst that acts as trap centers for photo generated charge pairs and greatly reduces the recombination process, hence increases the photocatalytic activity (Rumi et al., 2013).
3.8.1 Nickel doped titania
At the optimized conditions the dopant effected the degradation of the selected dyes. The photodegradation of neutral red in the presence of nickel doped titania was 90% and that of methylene blue was 95%. The photocatalytic activity of titania increased by Ni/TiO2. The nickel-metal as dopant greatly suppresses the recombination process on the surface of photocatalyst and provided more time for electrons and holes to produce maximum hydroxyl radicals, consequently, increases photodegradation (Devi et al., 2010).
3.8.2 Zinc doped titania
The difference between the conduction band and valence band of titania was reduced by doping titania with zinc metal which increased the photodegradation potential of the doped catalyst. Furthermore, the ionic radii of host Ti4+ (0.745 Å) are similar to that of Zn2+ (0.74 Å) ions. Therefore, Zn2+ can easily be substituted with Ti4+ in titania lattice, with no distortion in the crystal shape and thus stabilizing the doped phase over a range of metal ion concentration. The removal of neutral red achieved with zinc doped titania was 87.5% and that of methylene blue was 93.5% under optimized conditions. The presence of Zn2+ as a dopant act as follow:
The inclusion of Zn2+ metal ion as dopants causes an enhancement in number of O2−• and •OH on the solid electrical layer of titania that contributes to effective dye degradation (Devi and Dikdik, 2015).
3.8.3 Cobalt doped titania
About 88.75% neutral red dye gradation occurred when cobalt doped titania was used under light exposure while the percent removal of methylene blue dye obtained with Co/TiO2 was 93.5%. The wavelength of titania extended to the visible region of the spectrum and the phenomenon of red shift took place with cobalt used as a dopant. Cobalt also acts as an electron trapper and provide more time for radical formation, therefore increases the photodegradation efficiency of the catalyst.
3.9 Comparison between doped and un-doped titania
Photodegradation of neutral red in the presence of pure titania was 76.87%, while in the presence of nickel, cobalt, and Zn/TiO2 was 90%, 88.75%, and 87.5% respectively. The degradation of methylene blue achieved with pure TiO2 was 86%. The dye removal in the presence of Ni/TiO2 was 95%, while that for Co and Zn doped titania was 93.5%. From these results, it is clear that doped Titania shows greater photodegradation efficiencies as compared to pure titania as illustrated in Fig. 7. The metal ions accept the electrons from the valence band of titania and permit more time for charge separation inside the semiconductor material. Metal ion as dopants decreases the bandgap energy of titania as well as increasing the formation of radicals and hence photodegradation efficiency is increased. Among the three metal ion examined as dopants, excellent photocatalytic activity was observed for nickel ion suggesting the good dispersion of metal ion in the crystalline structure of titania (Mohseni-Salehi et al., 2018). A comparison of the doped catalyst has been shown in Table 1 while with other catalysts reported in literature is shown in Table 2.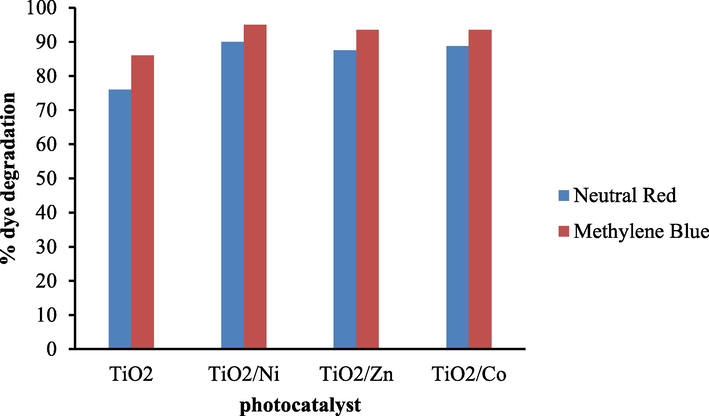
Comparison of photocatalytic efficiency of un-doped and metal doped titania for photo degradation of neutral red and methylene blue.
Dye
Photodegradation of dyes using doped and un-doped titania
TiO2
TiO2/Ni
TiO2/Zn
TiO2/Co
Neutral red
76%
90%
87.5%
88.75%
Methylene blue
86%
95%
93.5%
93.5%
Type of dye
Degradation (%)
Mode of degradation
Catalyst
References
Degradation (%) Current work
Neutral red
70
Adsorption
Co-oxide
Saeed and Khan, 2017
90
Methylene blue
65
Adsorption
Banana peel
Gautam and Khan, 2016
95
77
Adsorption
Zeolite
Nassar and Abdelrahman, 2017
95
81
Adsorption
Silver nanoparticles
Saha et al., 2017
95
3.10 Real sample applications
The performances of un-doped and doped TiO2 photocatalysts under the above mentioned optimized conditions were investigated on real wastewater samples coming from a textile industry to degrade the neutral red and methylene blue dyes present. The results obtained are presented in Fig. 8. The result shows that doping have increased the degradation ability of the catalyst. Similar results have been reported by other researchers as well (Nguyen et al., 2018).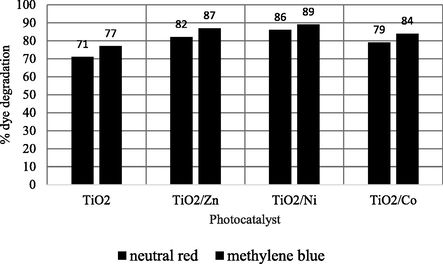
Real sample application of doped and un-doped photocatalysts.
4 Conclusion
Metal doped and un-doped titania photocatalyst were synthesized using titanium isopropoxide as titanium precursor. The catalytic efficiency of the selected dyes; neutral red and methylene blue were studied under UV irradiation. The photodegradation was higher for the metal-doped titania sample as compared to pure titania. Among the tested metal doped titania best results were obtained for Ni doped titania being 90% and 95% against the neutral red and methylene blue dyes respectively. It was observed that photodegradation is favorable under basic conditions. This improves catalytic activity was due to control of electron holes in the composite. The nickel doped catalyst could be effectively used in industries for the catalytic degradation of the mentioned dyes.
Acknowledgement
This project was supported by Researchers Supporting Project number (RSP-2020/283) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Photocatalytic decolorization of methylene blue using TiO2/UV system enhanced by air sparging. Alexandria Eng. J.. 2018;57(4):3727-3735.
- [CrossRef] [Google Scholar]
- The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles. Sci. Rep.. 2018;8(1):1-9.
- [CrossRef] [Google Scholar]
- Balaram, K.A., Siva, R.T., Sreedhar, B., 2016. Magnesium doped titania for photocatalytic degradation of dyes in visible light. J. Environ. Analyt. Taxicol. 6, doi:10.4172/2161-0525.1000358.
- Bruno, C.B.S., Antoninho V., 2019. Evaluation of the photocatalytic activity of SiO2@TiO2 hybrid spheres in the degradation of methylene blue and hydroxylation of benezene: Kinetic and Mechanistic Study. Brazilian J. Chem. Eng. 36, 1501–1518. doi: 10.1590/0104-6632.20190364s20190139.
- Chuanxi, Y., Wenping, D., Guanwei, C., Yingqiang, Z., Xifeng, S., Xinyuan, X., Bo, T., Weiliang, W. 2017. Highly-efficient photocatalytic degradation of methylene blue by PoPD-modified TiO2 nanocomposites due to photosensitization-synergetic effect of TiO2 with PoPD. Sci. Rep. 7, 3973. doi: 10.1038/s41598-017-04398-x.
- Photocatalytic reaction and degradation of methylene blue on TiO2 nano-sized particles. Optik. 2016;127(18):7143-7154.
- [CrossRef] [Google Scholar]
- Synthesis of Fe-TiO2 composite as a photocatalyst for degradation of methylene blue. Procedia Chem.. 2015;17:49-54.
- [CrossRef] [Google Scholar]
- Enhanced photocatalytic activity of transition metal Mn2+, Ni2+ and Zn2+ doped polycrystalline titania for the degradation of Aniline Blue under UV/solar light. J. Mol. Catal. A: Chem.. 2010;328:44-52.
- [CrossRef] [Google Scholar]
- Gautam, S., Khan, S.H., 2016. Removal of methylene blue from waste water using banana peel as adsorbent. Int. J. Sci. Environ. Technol. 5(5), 3230–3236. doi: 10.1016/j.ultsonch.2016.02.022.
- Enhanced photogenerated charge carriers and photocatalytic activity of biotemplated mesoporous TiO 2 films with a chiral nematic structure. Chem. Mater.. 2019;31(13):4851-4863.
- [CrossRef] [Google Scholar]
- Dredged-sediment-promoted synthesis of boron-nitride-based floating photocatalyst with photodegradation of neutral red under ultraviolet-light irradiation. ACS Appl. Mater. Interfaces. 2018;10(5):4640-4651.
- [CrossRef] [Google Scholar]
- Photocatalytic degradation of methyl orange and cyanide by using TiO 2/CuO composite. Desalin. Water Treat.. 2016;57(46):22029-22038.
- [CrossRef] [Google Scholar]
- Khairy, M., Zakaria, W., 2014. Effect of metal-doping of TiO2 nanoparticles on their photocatalytic activities toward removal of organic dyes. Egyptian J. Petrol. 23, 419–426. doi: 10.1016/j.ejpe.2014.09.010.
- Heterometallic lanthanide–titanium oxo clusters: a new family of water oxidation catalysts. Inorg. Chem.. 2017;56(3):1057-1060.
- [CrossRef] [Google Scholar]
- Adsorption and magnetic removal of neutral red dye from aqueous solution using Fe3O4 hollow nanospheres. J. Hazard. Mater.. 2010;181(1-3):1039-1050.
- [CrossRef] [Google Scholar]
- Visible-light induced photodegradation of methyl orange via palladium nanoparticles anchored to chrome and nitrogen doped TiO2 nanoparticles. J. Inorg. Organomet. Polym.. 2019;29(5):1457-1465.
- [CrossRef] [Google Scholar]
- Effect of dopant (Co, Ni) concentration and hydroxyapatite compositing on photocatalytic activity of titania towards dye degradation. J. Photochem. Photobiol., A: Chem.. 2018;356:57-70.
- [CrossRef] [Google Scholar]
- Hydrothermal tuning of the morphology and crystallite size of zeolite nanostructures for simultaneous adsorption and photocatalytic degradation of methylene blue dye. J. Mol. Liq.. 2017;242:364-374.
- [CrossRef] [Google Scholar]
- Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: efficiency and degradation pathways. J. Cleaner Prod.. 2018;202:413-427.
- [CrossRef] [Google Scholar]
- Charge carrier trapping, recombination and transfer during TiO2 photocatalysis: an overview. Catal. Today. 2019;335:78-90.
- [CrossRef] [Google Scholar]
- An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination. 2011;276(1-3):13-27.
- [CrossRef] [Google Scholar]
- Synthesis of visible light driven TiO2 coated carbon nanospheres for degradation of dyes. Arabian J. Chem.. 2019;12(8):3534-3545.
- [CrossRef] [Google Scholar]
- Rumi, C., Eiko, O., Katsumi, K., Hom, N.L., Katsuyuki, N. 2013. Effect of transition metal doping under reducing calcination atmosphere on photocatalytic property of TiO2 immobilized on SiO2 beads. J. Environ. Sci. 25. 1419–1423. doi: 10.1016/S1001-0742(12)60211-3.
- Efficient photodegradation of neutral red chloride dye in aqueous medium using graphene/cobalt-manganese oxides nanocomposite. Turk. J. Chem.. 2017;41(3):391-398.
- [CrossRef] [Google Scholar]
- A novel green synthesis of silver nanoparticles and their catalytic action in reduction of Methylene Blue dye. Sustainable Environ. Res.. 2017;27(5):245-250.
- [CrossRef] [Google Scholar]
- Shamalh, M., Rangabhatla, S.L.A., Rangabhatla, G.S.V., 2013. Photocatalytic effect of TiO2 and the effect of dopants on degradation of brilliant green. Sustainable Chem. Proc. 1, doi: 10.1186/2043-7129-1-4.
- Photocatalytic degradation of methylene blue by titanium dioxide: experimental and modeling study. Ind. Eng. Chem. Res.. 2014;53(38):14641-14649.
- [CrossRef] [Google Scholar]
- Hydrothermal preparation of Ag-TiO2 nanostructures with exposed {001}/{101} facets for enhancing visible light photocatalytic activity. Ceram. Int.. 2017;43(3):3118-3126.
- [CrossRef] [Google Scholar]







