Translate this page into:
Metabolic responses of plants to Meloidogyne species parasitism: A review on molecular events and functions
⁎Corresponding author. faheem.bt@amu.ac.in (Faheem Ahmad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The sedentary endoparasitic nematodes, Meloidogyne species, induce the formation of permanent feeding structures in the roots and alter the morpho-physiological and molecular events during parasitism. Several parasitism genes have been identified, indicating that effector proteins are important players in plant nematode interactions. During the interaction, many molecular events occur in host plants, such as pathogen-associated molecular patterns (PAMP)-triggered immunity (PTI) and effector triggered immunity (ETI) by which they suppress parasitism genes of nematodes. Plant-nematode interactions are complex and dynamic events that reflect the activation and suppression of expressed genes encoding various defence-related proteins, hormones, and enzymes. The omic studies such as metabolomics, proteomics, genomics, and transcriptomics helped us learn more about how nematodes and their hosts work together. This review highlights the metabolic response of host plants during root-knot nematode interaction. In addition, we review the role of effector protein in host parasitism and defence-related pathways and highlight the transcriptional analysis of differentially expressed genes that remain and play key roles against nematode parasitism. We also gathered information on how these knowledge gaps could be addressed and correlated their potential impact and application in the parasitism of the Meloidogyne species.
Keywords
Plant-nematode interaction
Parasitism gene
Effector protein
Metabolites profiling
1 Introduction
Meloidogyne species known as root-knot nematodes (RKNs) are plant-parasitic nematodes (PPNs) that infect plant roots, causing the formation of characteristic galls or knots on the roots. The pathogenicity of RKNs is known to inflict severe crop damage (Ahmad et al., 2022). They obtain nutrients from the host, by which they cause diseases in plants. Plant-parasitic nematodes cause an annual crop loss of 12.6 %, which for 20 essential crops amounts to USD 215.77 billion. An additional 20 crops worth at USD 142.47 billion, which are meant for food and export, have a yield loss of 14.45 %. The combined annual loss of these 40 crops is estimated to be 13.5 %, or USD 358.24 billion (Abd-Elgawad and Askary, 2015). These pathogens cause dramatic morphological and physiological changes and result in various ground symptoms such as yellowing of the leaves, stunting, and wilting in the host plant (Fig. 1). When nematodes successfully colonized the vascular tissue, they become immobile and spend the rest of their lives in the roots (Elhady et al., 2018). Feeding by RKN is responsible for the specific changes in the host plant, such as increased levels of metabolites, amino acids, and defence-related compounds, which are associated with the defence mechanism and nematode infestation reduction (Sato et al., 2021). Analysis is widely used to characterize genes, metabolites, and other defence-related components. These advances have significantly provided current knowledge of plant-nematode relationships and encouraged the identification of new components involved in plant responses against RKNs. Hence, recent advances in plant-nematode interaction have increased interest in effective approaches to knowing the roles of secreted metabolites of the host plant in response to RKN.
Slides showing the above and below-ground symptoms caused by RKNs on different plants. A. Cabbage; B. Eggplant aerial parts; C. Eggplant root; D. Okra root; and E. Mung bean.
2 Plant-nematode interactions
Plant-nematode interactions often begin in the soil, where the PPNs perceive various host cues using chemosensing, mechanosensing, thermosensing, redox potential sensing, humidity sensing, osmotic sensing, and electosensing (Chin et al., 2018). Obligate parasitic relationships mostly occur in nature in which parasitic nematodes develop a complex association with their host and cause severe crop effects. Therefore, these parasitic nematodes have socio-economic implications due to their wide host range. Some examples of parasitic nematodes with socio-economic implications are root-knot nematode (Meloidogyne spp. – tomato, potato, cotton), cyst nematode (Heterodera and Globodera spp. – soybean, wheat, potato), lesion nematode (Pratylenchus spp. – cereals, vegetables, legumes), burrowing nematode (Rhodopholus spp. – banana and citrus), reniform nematodes (Rotylenchulus reniformis - cotton), sugar beet cyst nematode (Heterodera schachtii – sugar beet) pine nematode (Bursaphelenchus xylophilus - pine forest.
2.1 RKNs interaction provokes morphological reorganization of feeding cell
RKNs enter host cells by needle-like stylet and secrete the various cell wall degrading enzymes like endoglucanases, endoxylanases, and expansin-like proteins pectate lyases that dissolve the soft middle lamella of root cells (Wieczorek et al., 2014; Mitsumasu et al., 2015). Juveniles (J2s) of RKNs enter the vascular cylinder and migrate intracellularly to the plant cortex, where they target specific host cells and convert them into feeding sites composed of 5 to 7 enlarged cells, called giant cells that are multi-nuclei and hypertrophied. These cells are visible as galls or knots on the plant root (Bozbuga et al., 2018). These cells have a dense cytoplasm that is packed with a large number of organelles (Plastids, mitochondria, endoplasmic reticulum (ER), ribosomes, Golgi bodies) and serve as a nutrient source for developing RKN (Favery et al., 2016).They also have a high level of metabolic activity. RKNs secrete a specific molecule called an effector produced in the oesophageal glands that manipulate the host cells and induce the transformation of host cells into feeding cells (Table 1). These effectors help RKNs successfully parasitize by influencing plant metabolism, suppressing host defence mechanisms, and ensuring successful infestation (Vieira and Gleason, 2019). The transcription activator MYB3R4 was indicated to be necessary for gall formation in the host plant (Suzuki et al., 2021). Mejias et al. (2021) reported that M. incognita secretes an effector protein (MiEFF18) during the plant-nematode interaction that is localized by immunolocalization. The MiEFF18 causes the root cells of the host plant to change into feeding sites with many nuclei and modifies the gene expression of giant cell ontogenesis by modulation of SmD1 (plant core spliceosomal protein), which is essential for successful nematode development. SmD1 is necessary for the spliceosome in pre-mRNA splicing and alternative splicing. M. incognita is the most studied RKN species that comprises more than 100 effector proteins. In another study, Zhao et al. (2020) characterized an effector protein MiPDI1 (Meloidogyne incognita protein disulfide isomerase) in Solanaceae and Arabidopsis as a pathogenic factor promoting nematode parasitism, and upregulation of MiPDI1 protein interacts with a stress-associated protein (SAP), causing diseases development (Fig. 2).
Effector
Nematode
Host
Functions
References
Mi-CRT
Meloidogyne incognita
–
Feeding site formation via synthesis of calreticulin protein
(Jaubert et al., 2005)
Mg01965
M. graminicola
Oryza sativa
suppression of plant basal immunity and promotion of M. graminicola parasitism
(Zhuo et al., 2019)
MiPFN3
M. incognita
Arabidopsis thaliana
disrupts the plant’s actin filaments and promotes the parasitism
(Leelarasamee et al., 2018)
MgMO237
M. graminicola
Oryza sativa
interact with multiple host defence‐related proteins and suppress plant basal immunity
(Chen et al., 2018)
Mg16820
M. graminicola
Oryza sativa
immune suppressor and targets a protein involved in the stress response
(Naalden et al., 2018)
Mi-MSP18
M. incognita
Oryza sativa
enhance plant susceptibility and modulate host immunity
(Grossi-de-Sa et al., 2019)
MiIDL1 gene
M. incognita
A. thaliana
Promotes successful gall development
(Kim et al., 2018)
MiPDI1
M. incognita
A. thaliana
Interfere with stress-associated protein (SAP) and promotes nematode parasitism
(Zhao et al., 2020)
MYB3R4
M. incognita
A. thaliana
induced gall formation
(Suzuki et al., 2021)
MiEFF18
M. incognita
A. thaliana
Induces the redifferentiation of host root cells into multinucleate feeding sites by modulation of SmD1(plant core spliceosomal protein)
(Mejias et al., 2021)
MiEFF1/Minc17998
M. incognita
Glycine max
interferes with the soybean protein (GmHub6) and suppress the host response
(Mendes et al., 2021)
MiEFF1
M. incognita
A. thaliana
targets the giant cell nuclei through GAPCs are responsible for the nematode infection
(Truong et al., 2021)
Mj2G02
M. javanica
A. thaliana
Immune suppression via Gpa2/RBP-1-induced cell death by interfering with host jasmonic acid JA-responsive genes
(Song et al., 2021)
Minc03328
M. incognita
A. thaliana
Promotes successful parasitism through immune suppression.
(Moreira et al., 2022)
microRNA
miR319/
TCP4
M. incognita
Solanum lycopersicum
feeding site formation via interaction with phytohormones
(Zhao et al., 2015)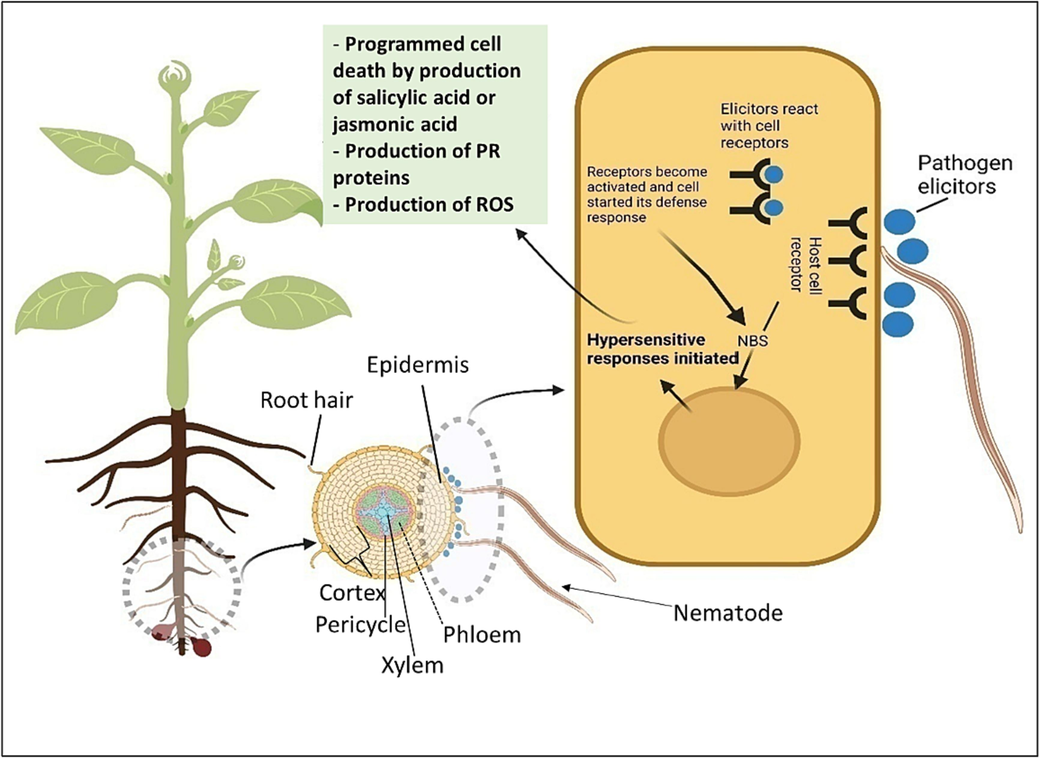
Schematic overview of plant and Meloidogyne species interaction and molecular response. Nematode elicitors are recognized by the plant's innate immune system, which triggers a defence response. At the site of nematode infection, the plant may develop a hypersensitive reaction, referred to as a localized cell death reaction. Elicitors can interfere with the plant's signalling pathways. PR- Pathogenesis-related protein, ROS- Reactive Oxygen Species, NBS- Nucleotide binding sequence.
RKN interaction with the plants involves producing protein molecules that interfere with the plant-associated defence molecule for the nematode infection. Mendes et al. (2021) reported the interaction between M. incognita effectors and soybean proteins through in vitro and in vivo studies. Their findings revealed that Mi-EFF1/Minc17998 effector interferes with the soybean protein GmHub6 (Glyma.17G099100; TCP14), disrupting the transcription activity factor GmHub6 responsible for the cell cycle, and suppresses the host response to RKNs infection. Fitoussi et al. (2021) studied a comprehensive oxylipin profiling of tomato plants and identified differentially expressed genes that exhibited a fundamental role in the developmental process of plants. Oxylipin is a lipid molecule involved in the plant defence mechanism. These lipid molecules also regulate the nematode transcriptome and facilitate nematode parasitism, acting as communication signals between the tomato and the nematode Meloidogyne javanica. They are known as a mediator in host nematode relationships by interfering with signalling pathways. However, the work of effectors in parasitism is still not well known, but more evidence describes nematode effectors as being able to regulate the various signalling cascades that suppress cell death and promote nematode parasitism.
Some studies revealed the role of miRNAs, non-coding RNAs that induce host plant gene expression (Díaz-Manzanoet al., 2016; Jaubert-Possamai et al., 2019). During the plant nematode interaction, RKN suppresses the host RNA silencing through various activities necessary for successful nematode infestation (Walsh et al., 2017). Some evidence proved the role of miRNA in nematode parasitism. A study by Zhao et al. (2015) identified the jasmonic acid-associated micro RNAs and characterized the functions of the miR319/TCP4 in tomatoes with RKN interaction. The involvement of different phytohormones in the feeding site formation is well documented, and various excellent reports suggested the role of plant hormones in the plant nematode interaction (Table 1). Dowd et al. (2017) demonstrated that cytokinins are crucial in developing nematode feeding sites and induced giant cells in the host. Rutter et al. (2014) found a CLE motif in RKN called a MAP (Meloidogyne A virulence Protein), which is needed to form the nematode feeding site. Kim et al. (2018) reported a MiIDL1 gene of RKN, M. incognita, produces a functional IDA (Inflorescence Deficient in Abscission) mimic that exhibits a crucial role in the successful gall development of Arabidopsis.
2.2 Plant defence-related compounds regulate host-nematode interactions
In response to a nematode attack, the plant's first barrier cell wall is activated to accumulate various chemical compounds that are toxic to the nematode. Local defence response includes the phytoalexins biosynthesis and deposition of lignin (lignification) and induction of suberin as a physical barrier. In addition, the plant immune inducers beta-aminobutyric acid (BABA), thiamine, and sclareol, all contribute to the buildup of lignin in feeding sites.
Huang et al. (2016) reported that thiamine-induced defence activates the multifaceted defence response against the RKN (Meloidogyne graminicola) on rice (Oryza sativa cv. Nipponbare). The results showed that phenylalanine ammonia-lyase (PAL), the major enzyme in the phenylpropanoid pathway, is expressed at a higher rate after being treated with thiamine, leading to increased production of hydrogen peroxide and lignin. This inhibits root-knot invading and hampers nematode infection. Holbein et al. (2019) investigated the role of suberin and lignin-based Casparian strips in inhibiting nematode penetration and acting as a defence barrier against RKN. These are the apoplastic barriers or structural barriers to parasites that prevent invasion. Various reports suggested that the plant produces phytochemicals such as caffeic acid and chlorogenic acid, which also regulate the plant nematode interaction (D’Addabbo et al., 2013). The host plant develops complex molecular strategies to avoid RKNs invasion. In this interaction, the elicitor is the component of a specific signal recognized by the host and induces the gene expression and corresponding proteins involved in the defence response. The plant response can develop two pathway systems against the nematode invasion; the first recognition system is pathogen-associated molecular patterns (PAMPs) and NAMPs (nematode-associated molecular patterns) that trigger the PTI (pattern-triggered immunity), and the second system is effector-triggered immunity (ETI). In the host cell, pattern recognition receptors (PRRs) induce a network of cellular signalling processes by activating pathogen-associated molecular patterns (PAMPs).
Interaction between PAMP and pattern recognition receptors leads to the activation of various complex signalling cascades such as mitogen-activated protein kinase (MAPK) signalling, ion fluxes pathway, Ca2+ cascade, production of defence hormones, and reactive oxygen species (Zhou and Zhang, 2020; Biere and Goverse, 2016). Mitogen-activated protein kinases (MAPK) phosphatases are involved in the phosphorylation of proteins and play a crucial key role in the signal transduction process during nematode parasitism by activation of complex signalling of defence-related compounds, hormones like salicylic acid (SA), jasmonic acid (JA), and ethylene (ET).
Brassinosteroids (BRs) are not able to induce the defence response by triggering jasmonic acid, salicylic acid, ethylene, or ABA signalling cascades, but they induce accumulation of ROS via respiratory burst oxidase homolog 1 (RBOH 1) and whitefly-induced 1 (WFI 1), mitogen-activated protein kinase-MPK1, MPK2, and MPK3 that BR-induced resistance to RKN is mediated via RBOH-dependent MPK activation (Song et al., 2018).
Sung et al. (2019) investigated the transcriptomic-based analysis of the sweet potato peroxidase gene in response to RKN, the susceptible cultivar Yulmi, and the resistant cultivar Juhwangmi. The study's findings identified the expression profiles of peroxidase genes and determined their crucial roles in protecting plants against the RKN, M. incognita infestation. In addition, plant defence response also triggered the nematode-associated molecular patterns (NAMPs) produced by nematodes known asascaroside, an evolutionarily conserved family of nematode pheromones. Manosalva et al. (2015) found that Ascr#18 are specific Ascarosides that pathogens stimulate mitogen-activated protein kinases, which in turn induce the expression of immunity-marker genes in plants' leaves and roots, and ascarosides play a key role in these processes including the SA- and JA-mediated signalling pathways. In the case of effector-triggered immunity (ETI), there are R genes or resistance proteins present in the host cell that recognize the effectors produced by nematode leading to the activation of transcription factors, including a range of pathogenesis-related genes. The resistant plant can recognize effector molecules by the intracellular nucleotide-binding site (NBS), and the leucine-rich (LRR) repeat domain and induces ETI that acceleration is faster than Pathogen triggered immunity (PTI). Pathogenesis-related (PR) proteins also induce a variety of nematodes, and their higher accumulation is associated with the long-distance immune response called systemic acquired resistance (SAR).
2.3 Changes in gene expression and metabolic pathways under nematode attack
After the attack, the host cell synthesizes various defence genes and enzymes that activate a new physiological status. Here, the important question is how the physiological pathway changes and metabolic activity are induced within the host cells. Kihika et al. (2020) investigated the influence of M. javanica in tomatoes through chemical analysis. The different compounds such as monoterpenes beta-pinene, alpha-phellandrene, and beta-phellandrene, (+)-(2)-carene and methyl salicylate and (Z)-methyl dihydro jasmonate are released after the nematode attack. This interfered with the chemoreception of the nematode and subsequently reduced the nematode response. In the past decades, the metabolic changes during the formation of giant cells have not been well understood, but lately, various genomic and transcriptomic analyses have been applied to realize host-nematode interactions better. Among molecular studies, metabolic profiling is a well-established method to measure host cell's metabolite level and physiological status after nematode infestation.
Martins et al. (2020) compared the resistant and susceptible peanut cultivars and identified a new candidate gene, AsMLP34, for Meloidogyne arenaria resistance in wild Arachis. The research found that some of the genes found to be differentially abundant (DAPs) encoded proteins associated with plant defence responses. These proteins include MLP-like protein 34 (MLP34), alcohol dehydrogenase (ADH), eukaryotic translation initiation factor 5A (eIF5A), enolase (ENO) and cinnamoyl-CoA reductase 1 (CCR1). Postnikova et al. (2015) reported the transcriptome analysis of resistant (cv. Moapa 69) and susceptible (cv. Lahontan) Alfalfa cultivars infected with RKN, M. incognita. Bioinformatic analysis of these cultivars revealed several differentially expressed genes in the susceptible cultivar and showed a more complex defence pathway to nematode infection. The development of gall in the vascular tissues is one of the RKN-specific features. Many shifts occur due to Populus and M. incognita interacting with one another. Genes encoding transcription factors involved in hormone signalling, production of salicylic acid, and secondary cell wall deposition were identified using comparative transcript profiling of poplar root (Baldacci-Cresp et al., 2020). Xing et al. (2017) employed the root transcriptome analysis of resistant (Yuyan 12) and susceptible (Changbohuang) tobacco (Nicotiana tabacum) varieties infected with RKN, M. incognita infection. The RNA-seq study identified a significant number of genes that had varied levels of expression (DEGs), in which six genes encoded pectinesterase/pectinesterase inhibitor (PMEU1), endoglucanase (GUN9), beta-glucosidase (BGL), polygalacturonase (PGLR4), glucan endo-1, 3-beta-glucosidase (E1312), and leucine-rich repeat extensin-like protein (LRX4) are up-regulated in the susceptible varieties of tobacco. These genes encode defence enzymes related to cell wall modification, defence-related hormone (Auxin), ROS scavenging route, and transcription factor in response to nematode infection (Fig. 3).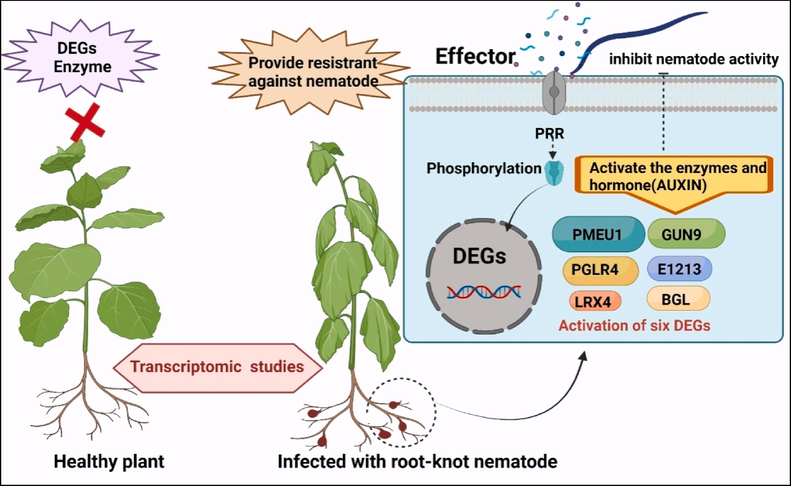
Diagram representation shows molecular changes in response to the root-knot nematode within plant.
Eloh et al. (2016) used the GC−MS metabolomics analysis for tomato plants infected with M. incognita to identify characteristic metabolites and biomarkers. The study indicated that metabolomics is sensitive and a specific approach can recognize an infected plant from controls. Their findings suggested that local changes in plant metabolome are responsible for the induction of metabolites and defence compounds in tomato leaves. β-alanine, phenylalanine, and melibiose in response to RKN attack and the case of the stem, fructose, glucose ribose and sucrose are up-regulated. This modification of metabolites is involved in the nematode response pathways. In the last decades, focusing on the fusion gene construct improved performance, enhanced the plant’s resistance against nematode invasion, and negatively reduced attraction. Another recent study by Hada et al. (2021) first reported the multiple effectors silencing in M. incognita using two fusion cassettes Mi-msp1, Mi-msp16, Mi-msp20, and two FMRF amide-like peptides, Mi-fp14, Mi-fp18, and Mi-msp20 in Nicotiana tabacum alter the nematode behaviour. They alter reproduction and root invasion that show better function than the one gene knockdown, leading to the development of the highly resistant plant. Nowadays, it is well known and recognized that host epigenome could change host genotypes and parasitic interaction and determine the outcome of host-nematode interaction. Beesa et al. (2022) demonstrated that phenylalanine ammonia-lyase (PAL), polyphenol oxidase (PPO) and peroxidase (POD) activity in resistant cultivars decreased the nematode activity that contributes to the formation of defence barriers in rice. However, research on the host root metabolic response on RKN is still progressing, and their result is very conflicting.
Recent years have seen tremendous advancements in the study of host-nematode interactions. Proteomics and metabolomics have shed light on the secretions and host responses, while transcriptome and genome studies have revealed the genes and regulatory components involved. Functional genomics methods such as CRISPR-Cas9 and RNA interference have made it possible to manipulate genes in nematodes and hosts precisely, which has aided in the research of certain genes. The discovery of immune cells and signalling pathways and our understanding of host immunological responses have advanced. It is now well-known which effector proteins nematodes use to manipulate their hosts, and studies on the influence of the microbiota have only started. These developments have an impact on a variety of fields, including immunology, genetics, and evolutionary biology, and present prospective paths for the efficient management of nematodes in agriculture and medicine.
3 Metabolites determining RKNs parasitism control
Nematode behaviour, development, reproduction, and even their very survival are all directly influenced by a variety of plant metabolites that are found in the roots and that are secreted from the roots into the rhizosphere (Wang et al., 2018). Various metabolites, the end products of typical metabolic plant processes, are introduced into the rhizosphere by the root system. Because of the osmotic and concentration discrepancies between the cell and soil solutions, metabolites can either actively secrete themselves or passively deposit themselves when transported to the rhizosphere (Sikder and Vestergård, 2020). In the past decade, the role of metabolites in disease management influenced nematode parasitism and development. Plants produce a wide range of active phytochemicals with antagonistic activity participating in plant defence against nematode attacks. In these metabolites, phenolic compounds, alkaloids, terpenes, and flavonoids are diverse secondary metabolites reported in response to invasion by a nematode (Fig. 4). Haroon et al. (2019) investigated the presence of active metabolites in plant extracts of Azadirachta indica and identified various compounds such as alkaloids, flavonoids, saponins, Hexane, 2, 4-dimethyl; Hexane, 2,2,5-trimethyl; Cyclohexane, benzamide, and ketones, which were effectively controlled egg hatching of RKN. Nasiou and Giannakou (2018) conducted research to investigate the effectiveness of terpene (geraniol), an alcohol monoterpene derived from plants, in combating M. javanica. They noticed that geraniol paralyzed the J2 stages (99.5 %) at a dose of 500 ppm, causing disease reduction in root tomato roots.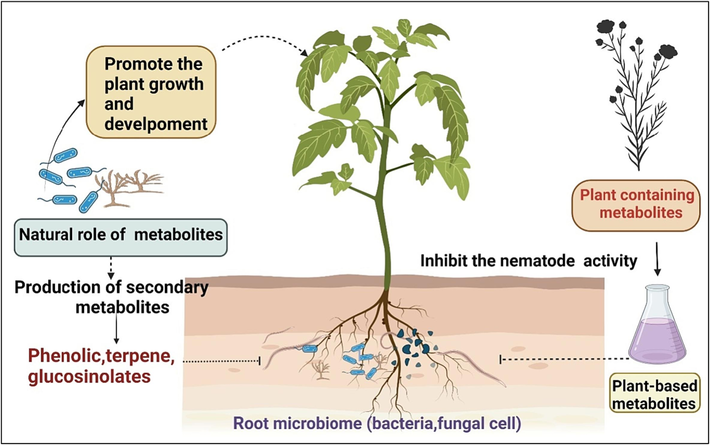
Recognition and response of metabolites of host plant during RKNs infection.
Researchers are trying to find novel components from different plant species against plant-parasitic nematodes. Plant-origin essential oils (EOs) and their derivative compounds, such as aldehyde, ketone, phenols, oxides, ethers, alcohols, and esters, have been reported as defence substances against RKN (D’Addabbo et al., 2021; Catani et al., 2023; Hammad and El-Sagheer, 2023). The biological effects of saponins and their specific interaction with the cell membranes of nematodes lead to molecular changes. Saponins are high molecular weight secondary metabolites extracted from the Medicago sativa that suppress the Cholesterol Biosynthesis of RKN and inhibit their development in tomato Seedlings. Cholesterol is a structural part of the nematode body membrane that controls the signalling functions in interacting with others. Interaction between saponins and cholesterol involves the formation of a saponin-cholesterol insoluble complex responsible for the nematotoxic effects. It reduces the level of cholesterol in nematode eggs and larvae of M. javanica (Ibrahim and Srour, 2013).
4 Concluding remarks and future perspectives
The host plant develops complex molecular strategies to evade the invasion of RKNs. In these interactions, the elicitor is a specific signal component that the host recognizes and induces gene expression and related proteins in the defence response. In the last decades, the metabolic changes that occur during the formation of giant cells have not been well understood. Recently, researchers have been working on analyzing and gaining a better understanding of host-nematode interactions. Changes in the molecules profiling during infection with RKNs may play an important role in how host plants adapt to nematode parasitism stress. Metabolomics analysis of this interaction allows us to study changes in metabolite synthesis during infection, and alteration in metabolite profiling could influence nematode parasitism and its development within plants. Nematode effector molecules in plant-nematode interactions are still evolving, and much is needed in this field. The recent advances in metabolic approaches have provided new insights into the role of plant metabolites in nematode defence responses and the identification of novel compounds that may be associated with defence-related metabolites. Determining the specific metabolic pathways and related compounds involved in nematode defence responses may control nematode infestations and improve plant resistance to pests, including RKNs. Comprehensive understanding and a holistic view of the molecular events during complex host-nematode interaction will be possible by integrating state-of-the-art omics technologies, including transcriptomics, proteomics, metabolomics, and genomes.
Furthermore, researching nematode effector proteins will aid in our understanding of how these nematodes influence host reactions. These advancements in molecular research have deepened our knowledge about host-nematode interactions and hold promise for developing more effective strategies to control nematode infections in agriculture and medicine. Therefore, technological advances such as metabolomics, bioinformatics, and gene editing could facilitate the discovery of molecules and their new mechanisms involved in interactions or repellence.
Acknowledgements
The facility and support provided by the Department of Botany, AMU, are gratefully acknowledged.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abd-Elgawad, M.M.M., Askary, T.H., 2015. Impact of phytonematodes on agriculture economy. In Biocontrol Agents of Phytonematodes, Askary, T.H., Martinelli, P.R.P., Eds.; CABI: Wallingford, UK, pp 3–49. https://doi.org/10.1079/9781780643755.0003.
- Management of root-knot nematode infection by using fly ash and Trichoderma harzianum in Capsicum annum plants by modulating growth, yield, photosynthetic pigments, biochemical substances, and secondary metabolite profiles. Notulae. Botanicae. Horti. Agrob. Cluj-Napoca. 2022;50:12591.
- [CrossRef] [Google Scholar]
- Molecular changes concomitant with vascular system development in mature galls induced by root-knot nematodes in the model tree host Populus tremula × P. alba. Int. J. Mol. Sci.. 2020;21(2):406.
- [CrossRef] [Google Scholar]
- Nematode development and changes in enzymatic defensive activity in rice plants upon Meloidogyne graminicola infection for preliminary screening of resistant cultivars. Songklanakarin J. Sci. Technol.. 2022;44:26-31.
- [CrossRef] [Google Scholar]
- Plant-mediated systemic interactions between pathogens, parasitic 416 nematodes, and herbivores above-and belowground. Annu. Rev. Phytopath.. 2016;54:499-527.
- [CrossRef] [Google Scholar]
- Host-specific signatures of the cell wall changes induced by the plant parasitic nematode, Meloidogyne incognita. Sci. Rep.. 2018;8:1-13.
- [CrossRef] [Google Scholar]
- Essential Oils as nematicides in plant protection—a review. Plants. 2023;12:1418.
- [CrossRef] [Google Scholar]
- A novel Meloidogyne graminicola effector, MgMO237, interacts with multiple host defence-related proteins to manipulate plant basal immunity and promote parasitism. Mol. Plant Pathol.. 2018;19(8):1942-1955.
- [CrossRef] [Google Scholar]
- Functions of flavonoids in plant–nematode interactions. Plants. 2018;7:85.
- [CrossRef] [Google Scholar]
- Nematicidal potential of Artemisia annua and its main metabolites. Eur. J. Plantol.. 2013;137:295-304.
- [CrossRef] [Google Scholar]
- Nematicidal activity of essential oil from lavandin (Lavandula× intermedia Emeric ex Loisel.) as related to chemical profile. Molecules. 2021;26:6448.
- [CrossRef] [Google Scholar]
- A reliable protocol for in situ micro RNAs detection in feeding sites induced by root-knot nematodes. Front. Plant Sci.. 2016;7:966.
- [CrossRef] [Google Scholar]
- Divergent expression of cytokinin biosynthesis, signalling and catabolism genes underlying differences in feeding sites induced by cyst and root-knot nematodes. The Plant J.. 2017;92:211-228.
- [CrossRef] [Google Scholar]
- Rhizosphere microbiomes modulated by pre-crops assisted plants in defense against plant-parasitic nematodes. Front. Microbiol.. 2018;9:1133.
- [CrossRef] [Google Scholar]
- Untargeted metabolomics of tomato plants after root-knot nematode infestation. J. Agric. Food Chem.. 2016;64:5963-5968.
- [CrossRef] [Google Scholar]
- Gall-forming root-knot nematodes hijack key plant cellular functions to induce multinucleate and hypertrophied feeding cells. J. Insect Physiol.. 2016;84:60-69.
- [CrossRef] [Google Scholar]
- Oxylipins are implicated as communication signals in tomato–root-knot nematode (Meloidogyne javanica) interaction. Sci. Rep.. 2021;11:1-16.
- [CrossRef] [Google Scholar]
- Rice susceptibility to root-knot nematodes is enhanced by the Meloidogyne incognita MSP18 effector gene. Planta. 2019;250(4):1215-1227.
- [CrossRef] [Google Scholar]
- Host-mediated RNAi for simultaneous silencing of different functional groups of genes in Meloidogyne incognita using fusion cassettes in Nicotiana tabacum. Plant Cell Rep.. 2021;40:2287-2302.
- [CrossRef] [Google Scholar]
- Comparative efficacy of essential oil nanoemulsions and bioproducts as alternative strategies against root-knot nematode, and its impact on the growth and yield of Capsicum annuum L. J. Saudi Soc. Agric. Sci.. 2023;22(1):47-53.
- [CrossRef] [Google Scholar]
- Efficiency of some plant extracts in the control of root-knot nematodes Meloidogyne spp. Fayoum J. Agri. Res. Dev.. 2019;33:78-89.
- [CrossRef] [Google Scholar]
- Root endodermal barrier system contributes to defence against plant-parasitic cyst and root-knot nematodes. The Plant J.. 2019;100:221-236.
- [CrossRef] [Google Scholar]
- Thiamine-induced priming against root-knot nematode infection in rice involves lignification and hydrogen peroxide generation. Mol. Plant Pathol.. 2016;17:614-624.
- [CrossRef] [Google Scholar]
- Saponins suppress nematode cholesterol biosynthesis and inhibit root knot nematode development in tomato seedlings. Nat. Prod. Chem. Res.. 2013;2:1-6.
- [CrossRef] [Google Scholar]
- In planta secretion of a calreticulin by migratory and sedentary stages of root-knot nematode. Mol. Plant Microbe. Interact.. 2005;18:1277-1284.
- [CrossRef] [Google Scholar]
- MicroRNAs, new players in the plant–nematode interaction. Front. Plant Sci.. 2019;10:1180.
- [CrossRef] [Google Scholar]
- Compounds associated with infection by the root-knot nematode, Meloidogyne javanica, influence the ability of infective juveniles to recognize host plants. J. Agric. Food Chem.. 2020;68(34):9100-9109.
- [CrossRef] [Google Scholar]
- The root-knot nematode Meloidogyne incognita produces a functional mimic of the Arabidopsis inflorescence deficient in abscission signalling peptide. J. Exp Bot.. 2018;69:3009-3021.
- [CrossRef] [Google Scholar]
- The root-knot nematode effector MiPFN3 disrupts plant actin filaments and promotes parasitism. PLoS Pathogens. 2018;14(3):e1006947.
- [CrossRef] [Google Scholar]
- Conserved nematode signalling molecules elicit plant defenses and pathogen resistance. Nat. Commu.. 2015;6(1)
- [CrossRef] [Google Scholar]
- Proteomics unravels new candidate genes for Meloidogyne resistance in wild Arachis. J. Proteomics. 2020;217:103690
- [CrossRef] [Google Scholar]
- The root-knot nematode effector MiEFF18 interacts with the plant core spliceosomal protein SmD1 required for giant cell formation. New Phytologist.. 2021;229(6):3408-3423.
- [CrossRef] [Google Scholar]
- The Mi-EFF1/Minc17998 effector interacts with the soybean GmHub6 protein to promote host plant parasitism by Meloidogyne incognita. Physio. Mol. Plant Pathol.. 2021;114:101630.
- [CrossRef] [Google Scholar]
- Apoplastic interactions between plants and plant root intruders. Front. Plant Sci.. 2015;6:617.
- [CrossRef] [Google Scholar]
- Minc03328 effector gene downregulation severely affects Meloidogyne incognita parasitism in transgenic Arabidopsis thaliana. Planta. 2022;255:1-16.
- [CrossRef] [Google Scholar]
- The Meloidogyne graminicola effector Mg16820 is secreted in the apoplast and cytoplasm to suppress plant host defense responses. Mol. Plant Pathol.. 2018;19(11):2416-2430.
- [CrossRef] [Google Scholar]
- Effect of geraniol, a plant-based alcohol monoterpene oil, against Meloidogyne javanica. Euro. J. Plant Pathol.. 2018;152:701-710.
- [CrossRef] [Google Scholar]
- Transcriptome analysis of resistant and susceptible alfalfa cultivars infected with root-knot nematode Meloidogyne incognita. PloS One. 2015;10(2):e0118269.
- [CrossRef] [Google Scholar]
- Members of the Meloidogyne avirulence protein family contain multiple plant ligand-like motifs. Phytopathol.. 2014;104:879-885.
- [CrossRef] [Google Scholar]
- Transcriptomic analysis of resistant and susceptible responses in a new model root-knot nematode infection system using Solanum torvum and Meloidogyne arenaria. Front. Plant Sci.. 2021;12:680151
- [CrossRef] [Google Scholar]
- Impacts of root metabolites on soil nematodes. Front. Plant Sci.. 2020;10:1792.
- [CrossRef] [Google Scholar]
- The Meloidogyne javanica effector Mj2G02 interferes with jasmonic acid signalling to suppress cell death and promote parasitism in Arabidopsis. Mol. Plant Pathol.. 2021;22:1288-1301.
- [CrossRef] [Google Scholar]
- Brassinosteroids act as a positive regulator for resistance against root-knot nematode involving RESPIRATORY BURST OXIDASE HOMOLOG-dependent activation of MAPKs in tomato. Plant, Cell Environ.. 2018;41:1113-1125.
- [CrossRef] [Google Scholar]
- Transcriptomic changes in sweet potato peroxidases in response to infection with the root-knot nematode Meloidogyne incognita. Mol. Bio. Rep.. 2019;46:4555-4564.
- [CrossRef] [Google Scholar]
- Identification of genes involved in Meloidogyne incognita-induced gall formation processes in Arabidopsis thaliana. Plant Biotechnol.. 2021;38:1-8.
- [Google Scholar]
- The Meloidogyne incognita nuclear effector MiEFF1 interacts with Arabidopsis cytosolic glyceraldehyde-3-phosphate dehydrogenases to promote parasitism. Front. Plant Sci.. 2021;12:641480
- [CrossRef] [Google Scholar]
- Plant-parasitic nematode effectors—insights into their diversity and new tools for their identification. Curr. Opin. Plant Biol.. 2019;50:37-43.
- [CrossRef] [Google Scholar]
- Root-knot nematode parasitism suppresses host RNA silencing. Mol. Plant Microbe Interact.. 2017;30:295-300.
- [CrossRef] [Google Scholar]
- Responses of Heterodera glycines and Meloidogyne incognita infective juveniles to root tissues, root exudates, and root extracts from three plant species. Plant Dis.. 2018;102:1733-1740.
- [CrossRef] [Google Scholar]
- A distinct role of pectate lyases in the formation of feeding structures induced by cyst and root-knot nematodes. Mol. Plant Microbe Interact.. 2014;27:901-912.
- [CrossRef] [Google Scholar]
- Transcriptome analysis of resistant and susceptible tobacco (Nicotiana tabacum) in response to root-knot nematode Meloidogyne incognita infection. Biochem. Biophys. Res. Commun.. 2017;482:1114-1121.
- [CrossRef] [Google Scholar]
- Identification of jasmonic acid-associated microRNAs and characterization of the regulatory roles of the miR319/TCP4 module under root-knot nematode stress in tomato. J. Exp. Bot.. 2015;66:4653-4667.
- [CrossRef] [Google Scholar]
- The root-knot nematode effector MiPDI1 targets a stress-associated protein (SAP) to establish disease in Solanaceae and Arabidopsis. New Phyto.. 2020;228(4):1417-1430.
- [CrossRef] [Google Scholar]
- Plant immunity: danger perception and signalling. Cell. 2020;181:978-989.
- [CrossRef] [Google Scholar]
- A Meloidogyne graminicola C-type lectin, Mg01965, is secreted into the host apoplast to suppress plant defence and promote parasitism. Mol. Plant Pathol.. 2019;20(3):346-355.
- [CrossRef] [Google Scholar]







