Translate this page into:
Mechanistic role of miR-24 and miR-34 in progression in myocardial infarction
⁎Corresponding author. vesslike@yahoo.com (Zhihong Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
CVD are a major human health problem across the globe. Acute myocardial infarction (MI) is a leading cause of cardiovascular diseases. Studies indicate that micro RNAs (miRs) play an important role in the development of cardiovascular disease. The present finding was used to explore the role of miR-24 andmiR-34expression in rat MI. Sprague–Dawley rats were used for developing a MI (MI) model. The pathology of MI was confirmed by histological analysis. The miRs expression was determined by qRT-PCR. Immunoblotting was applied to determine protein expression ofTGF-β1 and E2F3. The histological analysis revealed the development of MI in rats. The evaluation of miR-24 and miR-34 expression showed that miR-24 and miR-34 were upregulated in rats with MI. Moreover, the expression of these microRNAs increased considerably during the progression of MI from 1st to the 5th week. Target Scan analysis showed that miR-34 and miR-24 exert their effects by targeting transforming growth factor beta 1 (TGF-β1) and E2F transcription factor 3 (E2F3), respectively. Therefore, the expression of both E2F3 and TGF-β1 was also examined by Western blot analysis and qRT-PCR. Results revealed that the expression of TGF-β1 and E2F3 were downregulated after the 3rd and 4th week of infarction. The results suggest that miR-34 and miR-24 could be useful biomarkers for the diagnosis, progression and treatment of cardiovascular diseases.
Keywords
Myocardial infarction
MicroRNA
qRT-PCR
Transcription factor 3
Cardiovascular
1 Introduction
Cardiovascular diseases are leading causes of mortality across the world. The most commonly occurring cardiovascular disease is acute myocardial infarction (AMI), which are mainly due to its lethality and late diagnosis (Kou et al., 2016). Hence, a speedy and precise diagnosis of AMI is required to control AMI. Currently, a diverse range of biochemical markers, such as brain natriuretic peptide, cardiac troponin, lactate dehydrogenase, and creatine kinase MB isoform, are being used for diagnosing acute cardiac diseases (Zakkar et al., 2015; Doehner et al., 2016; De Lemos et al., 2010), but these biomarkers have low specificity and sensitivity, and yield false-positive results owing to the relationship with numerous patho-physiologies (De Winter et al., 1995). Hence, it is essential to seek novel diagnostic biomarkers and innovative therapies to reduce the prevalence of cardiovascular diseases. Recent studies reported that microRNAs (miRNAs) are most promising potential biomarkers because of high specificity and sensitivity for very early prediction of cardiovascular diseases (Yao et al., 2015; Antonisamy et al., 2015). Many studies have shown that miRNAs play very important roles in cellular proliferation, cell differentiation and apoptosis (Moore et al., 2010). miRNAs specifically bind with 3́untranslated region of mRNA and further degrade or inhibit translation, thereby modifying expression of the target gene. Many studies have reported the role of miRNA in wide array cardiovascular diseases. Studies have revealed that miR-29 suppresses collagen biosynthesis and is involved in MI progression (Zhou et al., 2016). By targeting the gene Smad4, miR-34a regulates the fibrosis after MI (Van Rooij et al., 2008). Moreover, expression of miR-24 in elevated level reduces the function of smooth muscle cells (Huang et al., 2014). Given this background, the present investigation was aimed to study the significant role of miR-24 and miR-34 in AMI (Table 1).
Gene
Forward primer (5′-3′)
Reverse primer (5′-3′)
miR-24
TCACACTATATCACATTGCCAGG
TATGGTTGTTCTGCTCTCTGTCTC
miR-34
TGTAACAGCAACTCCATGTGG
GCTGTCAACGATACGCTACG
TGF-β1
CCCGCATCCAGGACCTCTC
CGGGGGACTGGCGA
E2F3
CGCCTTCATTATGGACTGC
AGACCATTCCCTTGACTGC
U6
ATTGGAACGATACAGAGAAGAT
GGAACGCTTCACGAATTTG
β-Actin
AACAGTCCGCCTAGAAGCAC
CGTTGACATCCGTAAAGA
2 Materials and methods
2.1 Rat model
The Sprague-Dawley (SD) of SPF-grade were utilized for experimentation. The study was carried out following standard guidelines and has been approved by the Research Ethics Committee of Qingdao University under approval number QU620/2018.Forty healthy SD rats age group 8–11 weeks and body weight 200–255 g were used in this study. Dark (12 h) and light (12 h) cycle has been provided to the experimental animal. Also water and food were supplied to experimental animal.
2.2 Establishment of the MI model
The intraperitoneal injection consisting of 10% chloral hydrate was utilized to anesthetize the rats. Before the heart was exposed, tracheal incubation was performed. Next, the MI was developed by the ligation of the descending branch of the left anterior coronary artery. Nonetheless, the sham set of experimental animals underwent surgery without ligation. Randomly the rats were divided in four groups (N = 10). The MI rats were killed at the end of the 1 − 5th weeks. Then the samples were processed analyzed.
2.3 Histological analysis
For histological analysis, the heart tissues were subjected to washing with normal saline and then fixed with formaldehyde. Afterwards the tissues were paraffin-embedded in accordance with journal guidelines. The tissues were cut into 4-µm thick section and then deparaffinized with xylene and subsequently rehydrated. Next the sections were carefully stained with cytoplasmic stain eosin and observed using a microscope.
2.4 RNA isolation from cardiac tissues
TRIzol reagent was employed for the extraction of total RNA in accordance with the guidelines of the manufacturer. The Nano-Drop and agarose gel electrophoresis were used for the assessment of integrity and purity of RNA samples and finally stored at −80 °C for analysis.
2.5 Analyzing the target genes for miR-24 and miR-34
The target genes of miR-24 and miR-34 were determined by bioinformatics tools. The target genes were predicted using Target-Scan (www.targetscan.org) with default parameters.
2.6 Quantitative RT-PCR for E2F3, TGF-β1, miR-34 and miR-24
miRNA and mirVana qRT-PCR analysis kit was employed for the detection of qRT-PCR analysis. The cDNA was synthesized for 30 min at 37 °C and reverse transcriptase has been deactivated for 12 min at 96 °C. The 2−ΔΔCT method was used for expression analysis as described previously.
2.7 Immunoblotting of TGF-β1 and E2F3
mirVana PariS kits has been used for the isolation of protein from the sample. The concentrations of proteins were measured using Lowry’sassay. The proteins were separated using 10% SDS-PAGE and subsequently blotted to PVDF membranes. The 5% BSA was then used to block the membranes followed by incubation with primary antibody for 12 h at 4 °C and further incubated with suitable secondary antibodies. Finally, a chemiluminescent detection system was used to detect the bands of interest.
2.8 Statistical analysis
Three independent experiments were performed to confirm the present data. The values are shown as mean ± SD. In this study Student’s t test was employed and compared the results between two samples. Then one-way ANOVA followed by Tukeys post-hoc test were employed to compare multiple samples. A value of P < 0.05 was considered as statistically significant. Statistical package, SPSS (SPSS, Inc., Chicago, IL) was used to analyze the experimental results.
3 Results
3.1 Progression of MI in rat
Clearalterations in rat myocardial tissues werenoticed within 5 weeks aftercardiac infraction. In control tissues, cellular arrangements with apparent and pointed muscle stripes were found (Fig. 1A). Disappearance and abnormal distribution of cells were noticed at 1 and 3 weeks after MI in the myocardial tissues (Fig. 1B and 1C). After 5 weeks, we noticed a patchy and clumsy tissues arrangement, and intra-myocardial cleft widening was noticed with inflammatory cells and fibroblasts (Fig. 1D).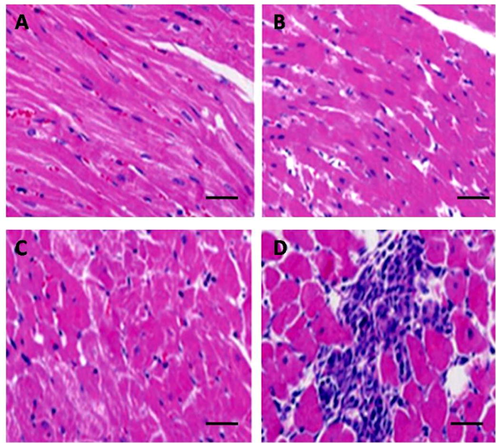
Histological analysis of rat tissues after myocardial infarction. (A) Histology of control rat (sham group) tissue sections. (B) Histology of rat tissue sections 1 week after myocardial infarction. (C) Histology of rat tissue sections 3 weeks after MI. (D) Histology of rat tissue sections 5 weeks after myocardial infarction.
3.2 Expression of miR-24 and miR-34during the development of myocardial infraction
Quantitative RT-PCR analysis showed a moderate increase of miR-24 and miR-34 at 1 and 3 weeks after MI, and elevated expression was noted at 5 weeks after MI. miR-24 and miR-34 levels were progressively upregulated with prolonged time after infarction. The expression of miR-34 was 2.6-fold and 3.8-fold higher after 3 and 4 weeks (Fig. 2), while the expression of miR-24 was 2.8-fold and 4.2-fold higher at 3 and 4 weeksafterMI relative to the control (Fig. 3).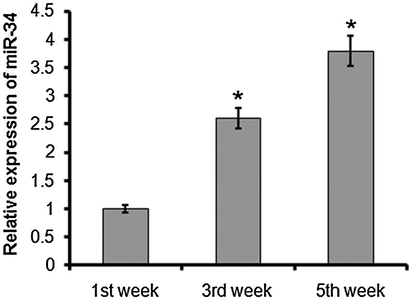
Quantitative RT-PCR analysis of miR-34 1, 3, and 5 weeks after myocardial infarction. The experiment was carried out in triplicate and results are expressed as mean ± SD (*P < 0.05).
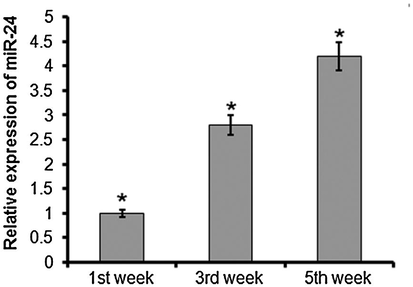
Quantitative RT-PCR analysis of miR-24 at 1, 3, and 5 weeks after rat myocardial infarction. The experiment was carried out in triplicate and results are expressed as mean ± SD (*P < 0.05).
3.3 miR-34 and miR-24 target TGF-β1 and E2F3
To identify the targets of miR-24 and miR-34, these miRs were subjected to TargetScan analysis. The results showed that miR-34 targets TGF-β1 and miR-24 targets E2F3 (Fig. 4). Hence, the expressions ofTGF-β1 and E2F3 were also examined in MImodelrats. The expressions of TGF-β1 and E2F3 were significantly decreased. The expression of miR-34 was 0.62-fold and 0.22-fold higher at 3 and 4 weeks (Fig. 5), and the expression of miR-24 was 0.52-and 0.31-fold higher at 3 and 4 weeks after MI (Fig. 6). The expression of miR-24 and miR-34 and their targets were negatively correlated.
Target Scan analysis showing (A) TGF-β1 as target of miR-34 and (B) E2F3 as target of miR-24.
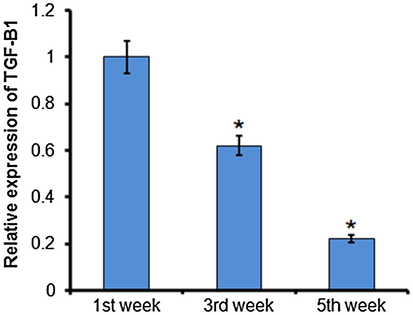
Quantitative RT-PCR analysis of TGF-β1 at 1, 3, and 5 weeks after rat myocardial infarction. The experiment was carried out in triplicate and results are expressed as mean ± SD (*P < 0.05).
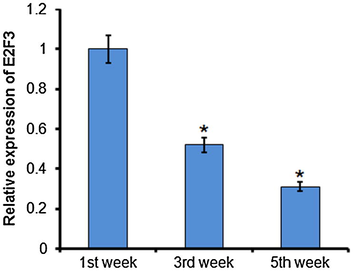
Quantitative RT-PCR analysis of E2F3 at 1, 3, and 5 weeks after rat myocardial infarction. The experiment was carried out in triplicate and results are expressed as mean ± SD (*P < 0.05).
3.4 Protein level of TGF-β1 and E2F3 in myocardial tissues
To validate the regulation of target genes, immunoblotting was also performed. Also, expression of TGF-β1 and E2F3 wassignificantly reduced (Fig. 7). It is well known that as the disease progresses, miR-24 and miR-34 bind with their specific target genes, TGF-β1 and E2F3, and thereby degrade their mRNA, their expression is thus progressively downregulated, leading to development of MI.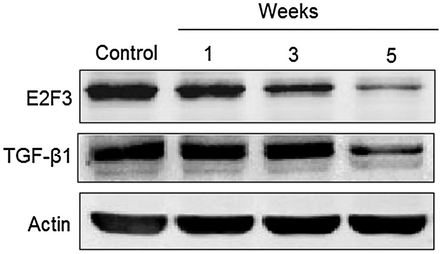
Western blot analysis showing the expression of TGF-β1 and E2F3 1, 3, and 5 weeks after myocardial infarction. The experiment was carried out in triplicate and results are expressed as mean ± SD (*P < 0.05).
4 Discussion
AMI is a cardiovascular disease which is the most widespread cause of heart failure (Fiedler et al., 2014). Myocardial fibroblasts are the mostabundantly distributed cell type found within the cardiac tissues. Under pathological conditions, these cells secrete collagen, which in turn damages heart function (Chen et al., 2005). In addition, previous results revealed that abnormal expression of miRNAs critically influence in the progression of many diseases. Many studies have shown that miRNAs can be used as biomarkers for numerous diseases, including cardiovascular diseases (Schmittgen et al., 2004). In this study, a rat model of MI was successfully established, which was initially validated by histological studies and other experiments were performed. In the AMI rats, the expression of miR-34 and miR-24 were performed and it was revealed that the pattern of expression of these miRNAs was significantly elevated as compared with the sham group.
To validate this expression, the targets for miR-24 and mir-34 were identified by bioinformatics, showing that miR-34 targets TGF-β1 and miR-24 targets E2F3. Interestingly, our results revealed that elevated expression of miR-24 and miR-34 was associated with downregulation of the expression of target genes, confirming that mir-34 and miR-24 specifically control the expression of their target genes E2F3T and GF-β1 by degrading their specific mRNAs and thereby inhibiting the translation process. Further, expression analysis using western blot revealed that the target genes expression remained down regulated long after initiation of MI. TGF-β1 and E2F3 are important signaling pathways for MI. One study validated that elevated levels of miR-24 during MI influence the production of collagen (Lim et al., 2016; Chen et al., 2008). Moreover, earlier investigation revealed that activation of E2F3 in the tissues of myocardium caused a rapid recovery from cardiac fibrosis (Gilad et al., 2008; Wang et al., 2012). It was previously reported that Smad is an important signaling pathways active in MI (McMahon et al., 2003; Yoshimoto et al., 2008; Sun et al., 2008; Zeng et al., 2014). TGF-β1 and E2F3 are also involved in this process, but further studies are necessary. The present study suggests a new strategy by suggesting the utility of miR-24 and miR-34 as prospective biomarkers for progression of cardiac diseases.
5 Conclusions
In conclusion, target-Scan analysis showed that miR-34 and miR-24 exert their effects by targeting transforming growth factor beta 1 (TGF-β1) and E2F transcription factor 3 (E2F3), respectively. Therefore, the expression of both E2F3 and TGF-β1 was also examined by Western blot analysis and qRT-PCR. Results revealed that the expression of TGF-β1 and E2F3 were downregulated after the 3rd and 4th week of infarction. The results suggest that miR-34 and miR-24 could be useful biomarkers for the diagnosis, progression, and treatment of cardiovascular diseases. The present study revealed the aberrant miR-24 and miR-34 expression in MI and this may play a remarkable role in diagnosis of MI and in development of target drugs for treating it.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anti-diarrhoeal activity of friedelin isolated from Azima tetracantha Lam. in wistar rats. South Ind. J. Biol. Sci.. 2015;1:34-37.
- [Google Scholar]
- Real-time quantification of microRNAs by stem–loop RT–PCR. Nucleic acids Res.. 2005;33:e179
- [Google Scholar]
- Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res.. 2008;18:997-1006.
- [Google Scholar]
- Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503-2512.
- [Google Scholar]
- Value of myoglobin, troponin T, and CK-MB mass in ruling out an acute MI in the emergency room. Circulation. 1995;92:3401.
- [Google Scholar]
- Influence of core body temperature on Tryptophan metabolism, kynurenines, and estimated IDO activity in critically ill patients receiving target temperature management following cardiac arrest. Resuscitation. 2016;107:107-114.
- [Google Scholar]
- Functional microRNA library screening identifies the hypoxamir miR-24 as a potent regulator of smooth muscle cell proliferation and vascularization. Antioxid. Redox. Signal.. 2014;21:1167-1176.
- [Google Scholar]
- MicroRNA-34a regulates cardiac fibrosis after myocardial infarction by targeting Smad4. Expert Opin. Ther. Targets. 2014;18:1355-1365.
- [Google Scholar]
- Inhibition of miR-23 protects myocardial function from ischemia-reperfusion injury through restoration of glutamine metabolism. Eur. Rev. Med. Pharmacol. Sci.. 2016;20:4286-4293.
- [Google Scholar]
- Incidence, implications, and predictors of stent thrombosis in acute MI. Am J Cardiol.. 2016;117:1562-1568.
- [Google Scholar]
- Alternative pathway for the role of furin in tumor cell invasion process. Enhanced MMP-2 levels through bioactive TGFbeta. Exp Cell Res.. 2003;291:326-339.
- [Google Scholar]
- Biomarkers of acute kidney injury in anesthesia, intensive care and major surgery: From the bench to clinical research to clinical practice. Minerva Anestesiol.. 2010;76:425-440.
- [Google Scholar]
- A high-throughput method to monitor the expression of microRNA precursors. Nucleic acids Res.. 2004;32 e43-e43
- [Google Scholar]
- Transforming growth factor-beta-regulated miR-24 promotes skeletal muscle differentiation. Nucleic Acids Res.. 2008;36:2690-2699.
- [Google Scholar]
- Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. USA. 2008;105:13027-13032.
- [Google Scholar]
- MicroRNA-24 regulates cardiac fibrosis after myocardial infarction. J. Cell Mol. Med.. 2012;16:2150-2160.
- [Google Scholar]
- Mitochondrial ROS induces cardiac inflammation via a pathway through mtDNA damage in a pneumonia-related sepsis model. PLoS One. 2015;10:1-28.
- [Google Scholar]
- 2-Methylene 19-nor-25-dehydro1alpha-hydroxyvitamin D3 26,23-lactones: Synthesis, biological activities and molecular basis of passive antagonism. Bioorg. Med. Chem.. 2008;16:457-473.
- [Google Scholar]
- Inflammation, oxidative stress and postoperative atrial fibrillation in cardiac surgery. Pharmacol. Ther.. 2015;154:1320.
- [Google Scholar]
- Upregulation of E2F transcription factor 3 is associated with the prognosis of cardiac fibrosis. Eur. Rev. Med. Pharmacol. Sci.. 2014;31:1139-1146.
- [Google Scholar]
- Up-regulation of miRNA-21 expression promotes migration and proliferation of Sca-1+ cardiac stem cells in mice. Med. Sci. Monitor.. 2016;22:1724.
- [Google Scholar]







