Translate this page into:
Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective
*Corresponding author at: Department of Agricultural Microbiology, Faculty of Agricultural Sciences, Aligarh Muslim University, Aligarh 202 002, UP, India. Tel.: +91 9305021113 muneesmicro@rediffmail.com (Munees Ahemad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Available online 11 May 2013

Abstract
Plant growth promoting rhizobacteria are the soil bacteria inhabiting around/on the root surface and are directly or indirectly involved in promoting plant growth and development via production and secretion of various regulatory chemicals in the vicinity of rhizosphere. Generally, plant growth promoting rhizobacteria facilitate the plant growth directly by either assisting in resource acquisition (nitrogen, phosphorus and essential minerals) or modulating plant hormone levels, or indirectly by decreasing the inhibitory effects of various pathogens on plant growth and development in the forms of biocontrol agents. Various studies have documented the increased health and productivity of different plant species by the application of plant growth promoting rhizobacteria under both normal and stressed conditions. The plant-beneficial rhizobacteria may decrease the global dependence on hazardous agricultural chemicals which destabilize the agro-ecosystems. This review accentuates the perception of the rhizosphere and plant growth promoting rhizobacteria under the current perspectives. Further, explicit outlooks on the different mechanisms of rhizobacteria mediated plant growth promotion have been described in detail with the recent development and research. Finally, the latest paradigms of applicability of these beneficial rhizobacteria in different agro-ecosystems have been presented comprehensively under both normal and stress conditions to highlight the recent trends with the aim to develop future insights.
Keywords
ACC deaminase
Indole acetic acid
Nitrogen fixation
Plant growth promoting rhizobacteria
Phosphate solubilization
Rhizosphere
1 Introduction
Different bacterial genera are vital components of soils. They are involved in various biotic activities of the soil ecosystem to make it dynamic for nutrient turn over and sustainable for crop production (Ahemad et al., 2009; Chandler et al., 2008). They stimulate plant growth through mobilizing nutrients in soils, producing numerous plant growth regulators, protecting plants from phytopathogens by controlling or inhibiting them, improving soil structure and bioremediating the polluted soils by sequestering toxic heavy metal species and degrading xenobiotic compounds (like pesticides) (Ahemad, 2012; Ahemad and Malik (2011); Hayat et al., 2010; Rajkumar et al., 2010; Braud et al., 2009). Indeed, the bacteria lodging around/in the plant roots (rhizobacteria) are more versatile in transforming, mobilizing, solubilizing the nutrients compared to those from bulk soils (Hayat et al., 2010). Therefore, the rhizobacteria are the dominant deriving forces in recycling the soil nutrients and consequently, they are crucial for soil fertility (Glick, 2012). Currently, the biological approaches for improving crop production are gaining strong status among agronomists and environmentalists following integrated plant nutrient management system. In this context, there is an ongoing rigorous research worldwide with greater impetus to explore a wide range of rhizobacteria possessing novel traits like heavy metal detoxifying potentials (Ma et al., 2011a; Wani and Khan, 2010), pesticide degradation/tolerance (Ahemad and Khan, 2012a,b), salinity tolerance (Tank and Saraf, 2010; Mayak et al., 2004), biological control of phytopathogens and insects (Hynes et al., 2008; Russo et al., 2008; Joo et al., 2005; Murphy et al., 2000) along with the normal plant growth promoting properties such as, phytohormone (Ahemad and Khan, 2012c Tank and Saraf, 2010), siderophore (Jahanian et al., 2012; Tian et al., 2009), 1-aminocyclopropane-1-carboxylate, hydrogen cyanate (HCN), and ammonia production, nitrogenase activity (Glick, 2012; Khan, 2005) phosphate solubilization (Ahemad and Khan, 2012c) etc. Hence, diverse symbiotic (Rhizobium, Bradyrhizobium, Mesorhizobium) and non-symbiotic (Pseudomonas, Bacillus, Klebsiella, Azotobacter, Azospirillum, Azomonas), rhizobacteria are now being used worldwide as bio-inoculants to promote plant growth and development under various stresses like heavy metals (Ma et al., 2011a,b; Wani and Khan, 2010), herbicides (Ahemad and Khan, 2011l; Ahemad and Khan, 2010g), insecticides (Ahemad and Khan 2011h,k), fungicides (Ahemad and Khan, 2012f; Ahemad and Khan, 2011j), salinity (Mayak et al., 2004) etc.
Although, the mechanisms of rhizobacteria-mediated plant growth promotion are not completely identified, the so-called plant growth promoting rhizobacteria however, have been reported to exhibit the above mentioned properties to expedite the plant growth and development (Khan et al., 2009; Zaidi et al., 2009). The present review is an effort to elucidate the concept of rhizobacteria in the current scenario and their underlying mechanisms of plant growth promotion with recent updates. The latest paradigms of a wide range of applications of these beneficial rhizobacteria in different agro-ecosystems have been presented explicitly to garner broad perspectives regarding their functioning and applicability.
2 Rhizosphere
The narrow zone of soil directly surrounding the root system is referred to as rhizosphere (Walker et al., 2003), while the term ‘rhizobacteria’ implies a group of rhizosphere bacteria competent in colonizing the root environment (Kloepper et al., 1991). In addition to providing the mechanical support and facilitating water and nutrient uptake, plant roots also synthesize, accumulate, and secrete a diverse array of compounds (Walker et al., 2003). These compounds secreted by plant roots act as chemical attractants for a vast number of heterogeneous, diverse and actively metabolizing soil microbial communities. The chemicals which are secreted by roots into the soils are generally called as root exudates. The exudation of a wide range of chemical compounds (Table 1) modifies the chemical and physical properties of the soil and thus, regulates the structure of soil microbial community in the immediate vicinity of root surface (Dakora and Phillips, 2002). In fact, some of the exudates act as repellants against microorganisms while others act as attractants to lodge the microbes. The composition of these exudates is dependent upon the physiological status and species of plants and microorganisms (Kang et al., 2010). Moreover, these exudates also promote the plant-beneficial symbiotic interactions and inhibit the growth of the competing plant species (Nardi et al., 2000). Also, microbial activity in the rhizosphere affects rooting patterns and the supply of available nutrients to plants, thereby modifying the quality and quantity of root exudates. A fraction of these plant-derived small organic molecules is further metabolized by microorganisms in the vicinity as carbon and nitrogen sources, and some microbe-oriented molecules are subsequently re-taken up by plants for growth and development (Kang et al., 2010). Indeed, carbon fluxes are critical determinants of rhizosphere function. It is reported that approximately 5–21% of photosynthetically fixed carbon is transported to the rhizosphere through root exudation (Marschner, 1995). Thus, the rhizosphere can be defined as any volume of soil specifically influenced by plant roots and/or in association with roots hairs, and plant-produced materials (Dessaux et al., 2009). Largely, three separate but interacting components are recognized in the rhizosphere: the rhizosphere (soil), the rhizoplane, and the root itself. Of these, the rhizosphere is the zone of soil influenced by roots through the release of substrates that affect microbial activity. The rhizoplane, on the other hand, is the root surface including the strongly adhering soil particles while the root itself is a component of the system, because many micro-organisms (like endophytes) also colonize the root tissues (Barea et al., 2005). Microbial colonization of the rhizoplane and/or root tissues is known as root colonization, whereas the colonization of the adjacent volume of soil under the influence of the root is known as rhizosphere colonization (Barea et al., 2005; Kloepper et al., 1991; Kloepper, 1994). Adapted from Dakora and Phillips (2002).
Amino acids
α-Alanine, β-alanine, asparagines, aspartate, cystein, cystine, glutamate, glycine, isoleucine, leucine, lysine, methionine, serine, threonine, proline, valine, tryptophan, ornithine, histidine, arginine, homoserine, phenylalanine, γ-Aminobutyric acid,α-Aminoadipic acid
Organic acids
Citric acid, oxalic acid, malic acid, fumaric acid, succinic acid, acetic acid, butyric acid, valeric acid, glycolic acid, piscidic acid, formic acid, aconitic acid, lactic acid, pyruvic acid, glutaric acid, malonic acid, tetronic acid, aldonic acid, erythronic acid
Sugars
Glucose, fructose, galactose, ribose, xylose, rhamnose, arabinose, desoxyribose, oligosaccharides, raffinose, maltose
Vitamins
Biotin, thiamin, pantothenate, riboflavin, niacin
Purines/nucleosides
Adenine, guanine, cytidine, uridine
Enzymes
Acid/alkaline-phosphatase, invertase, amylase, protease
Inorganic ions and gaseous molecules
, OH−, H+ CO2·H2
3 Plant growth promoting rhizobacteria
The plant growth promoting rhizobacteria (PGPR), are characterized by the following inherent distinctiveness’s: (i) they must be proficient to colonize the root surface (ii) they must survive, multiply and compete with other microbiota, at least for the time needed to express their plant growth promotion/protection activities, and (iii) they must promote plant growth (Kloepper, 1994). About 2–5% of rhizobacteria, when reintroduced by plant inoculation in a soil containing competitive microflora, exert a beneficial effect on plant growth and are termed as plant growth promoting rhizobacteria (Kloepper and Schroth, 1978). In accordance with Vessey (2003), soil bacterial species burgeoning in plant rhizosphere which grow in, on, or around plant tissues stimulate plant growth by a plethora of mechanisms are collectively known as PGPR (plant growth promoting rhizobacteria).
Alternatively, Somers et al. (2004) classified PGPR based on their functional activities as (i) biofertilizers (increasing the availability of nutrients to plant), (ii) phytostimulators (plant growth promotion, generally through phytohormones), (iii) rhizoremediators (degrading organic pollutants) and (iv) biopesticides (controlling diseases, mainly by the production of antibiotics and antifungal metabolites) (Antoun and Prévost, 2005). Furthermore, in most studied cases, a single PGPR will often reveal multiple modes of action including biological control (Kloepper, 2003; Vessey, 2003). Furthermore, Gray and Smith (2005) have recently shown that the PGPR associations range in the degree of bacterial proximity to the root and intimacy of association. In general, these can be separated into extracellular (ePGPR), existing in the rhizosphere, on the rhizoplane, or in the spaces between cells of the root cortex, and intracellular (iPGPR), which exist inside root cells, generally in specialized nodular structures (Figueiredo et al., 2011). Some examples of ePGPR are like, Agrobacterium, Arthrobacter, Azotobacter, Azospirillum, Bacillus, Burkholderia, Caulobacter, Chromobacterium, Erwinia, Flavobacterium, Micrococcous, Pseudomonas and Serratia etc. (Bhattacharyya and Jha, 2012). Similarly, some examples of the iPGPR are Allorhizobium, Azorhizobium, Bradyrhizobium, Mesorhizobium and Rhizobium of the family Rhizobiaceae. Most of rhizobacteria belonging to this group are Gram-negative rods with a lower proportion being Gram-positive rods, cocci or pleomorphic (Bhattacharyya and Jha, 2012). Moreover, numerous actinomycetes are also one of the major components of rhizosphere microbial communities displaying marvelous plant growth beneficial traits (Bhattacharyya and Jha, 2012; Merzaeva and Shirokikh 2006). Among them, Micromonospora sp., Streptomyces spp., Streptosporangium sp., and Thermobifida sp., which have shown an enormous potential as biocontrol agents against different root fungal pathogens, are worthy of mention (Bhattacharyya and Jha, 2012; Franco-Correa et al., 2010).
4 Mechanisms of plant growth promotion
According to Kloepper and Schroth (1981), PGPR mediated plant growth promotion occurs by the alteration of the whole microbial community in rhizosphere niche through the production of various substances (Table 2) (Kloepper and Schroth, 1981). Generally, PGPR promote plant growth directly by either facilitating resource acquisition (nitrogen, phosphorus and essential minerals) or modulating plant hormone levels, or indirectly by decreasing the inhibitory effects of various pathogens on plant growth and development in the forms of biocontrol agents (Glick, 2012) (Fig. 1).
PGPR
Plant growth promoting traits
References
Pseudomonas putida
IAA, siderophores, HCN, ammonia, exo-polysaccharides, phosphate solubilization
Ahemad and Khan (2012a,c) and Ahemad and Khan (2011c)
Pseudomonas aeruginosa
IAA, siderophores, HCN, ammonia, exo-polysaccharides, phosphate solubilization
Ahemad and Khan (2012e), Ahemad and Khan (2011a,k) and Ahemad and Khan (2010d)
Klebsiella sp.
IAA, siderophores, HCN, ammonia, exo-polysaccharides, phosphate solubilization
Ahemad and Khan (2011b,f,g)
Enterobacter asburiae
IAA, siderophores, HCN, ammonia, exo-polysaccharides, phosphate solubilization
Ahemad and Khan (2010a,b)
Rhizobium sp. (pea)
IAA, siderophores, HCN, ammonia, exo-polysaccharides
Ahemad and Khan (2012b), Ahemad and Khan (2011i), Ahemad and Khan (2010c) and Ahemad and Khan (2009b)
Mesorhizobium sp.
IAA, siderophores, HCN, ammonia, exo-polysaccharides
Ahemad and Khan (2012d), Ahemad and Khan (2010e,h) and Ahemad and Khan (2009a)
Acinetobacter spp.
IAA, phosphate solubilization, siderophores
Rokhbakhsh-Zamin et al. (2011)
Rhizobium sp.(lentil)
IAA, siderophores, HCN, ammonia, exo-polysaccharides
Ahemad and Khan (2011e,j) and Ahemad and Khan (2010f,g)
Pseudomonas sp. A3R3
IAA, siderophores
Ma et al. (2011a)
Psychrobacter sp. SRS8
Heavy metal mobilization
Ma et al. (2011b)
Bradyrhizobium sp.
IAA, siderophores, HCN, ammonia, exo-polysaccharides
Ahemad and Khan (2012f) and Ahemad and Khan (2011d,h,l)
Pseudomonas aeruginosa 4EA
Siderophores
Naik and Dubey (2011)
Bradyrhizobium sp. 750, Pseudomonas sp., Ochrobactrum cytisi
Heavy metal mobilization
Dary et al. (2010)
Bacillus species PSB10
IAA, siderophores, HCN, ammonia
Wani and Khan (2010)
Paenibacillus polymyxa
IAA, siderophores
Phi et al. (2010)
Rhizobium phaseoli
IAA
Zahir et al. (2010)
Stenotrophomonas maltophilia
Nitrogenase activity, phosphate solubilization, IAA, ACC deaminase
Mehnaz et al. (2010)
Rahnella aquatilis
Phosphate solubilization, IAA, ACC deaminase
Mehnaz et al. (2010)
Pseudomonas aeruginosa, Pseudomonas fluorescens, Ralstonia metallidurans
Siderophores
Braud et al. (2009)
Proteus vulgaris
Siderophores
Rani et al. (2009)
Pseudomonas sp.
Phosphate solubilization, IAA, siderophore, HCN, biocontrol potentials
Tank and Saraf (2009)
Azospirillum amazonense
IAA, nitrogenase activity
Rodrigues et al. (2008)
Mesorhizobium sp.
IAA, siderophores, HCN, ammonia
Wani et al. (2008)
Pseudomonas sp.
ACC deaminase, IAA, siderophore
Poonguzhali et al. (2008)
Serratia marcescens
IAA, siderophore, HCN
Selvakumar et al. (2008)
Pseudomonas fluorescens
ACC deaminase, phosphate solubilization
Shaharoona et al. (2008)
Acinetobacter sp., Pseudomonas sp.
ACC deaminase, IAA, antifungal activity, N2- fixation, phosphate solubilization
Indiragandhi et al. (2008)
Enterobacter sp.
ACC deaminase, IAA, siderophore, phosphate solubilization
Kumar et al. (2008)
Burkholderia
ACC deaminase, IAA, siderophore, heavy metal solubilization, phosphate solubilization
Jiang et al. (2008)
Pseudomonas jessenii
ACC deaminase, IAA, siderophore, heavy metal solubilization, phosphate solubilization
Rajkumar and Freitas (2008)
Pseudomonas aeruginosa
ACC deaminase, IAA, siderophore, phosphate solubilization
Ganesan (2008)
Pseudomonas sp.
ACC deaminase, IAA, siderophore, heavy metal solubilization, phosphate solubilization
Rajkumar and Freitas (2008)
Azotobacter sp., Mesorhizobium sp., Pseudomonas sp., Bacillus sp.
IAA, siderophore, antifungal activity, ammonia production, HCN
Ahmad et al. (2008)
Bradyrhizobium sp.
IAA, siderophores, HCN, ammonia
Wani et al. (2007a)
Rhizobium sp.
IAA, siderophores, HCN, ammonia
Wani et al. (2007b)
Mesorhizobium ciceri, Azotobacter chroococcum
IAA, siderophores
Wani et al. (2007c)
Pseudomonas, Bacillus
Phosphate solubilization, IAA and siderophores
Wani et al. (2007c)
Klebsiella oxytoca
IAA, phosphate solubilization, nitrogenase activity
Jha and Kumar (2007)
Bacillus spp., Pseudomonas spp., Azotobacter spp., Rhizobium spp.
IAA, ammonia production
Joseph et al. (2007)
Pseudomonas fluorescens
Induced systemic resistance, antifungal activity
Saravanakumar et al. (2007)
Pseudomonas chlororaphis
Antifungal activity
Liu et al. (2007)
Baciilus subtilis
Antifungal activity
Cazorla et al. (2007)
Gluconacetobacter diazotrophicus
Zinc solubilization
Saravanan et al. (2007)
Brevibacillus spp.
Zn resistance, IAA
Vivas et al. (2006)
Bacillus subtilis
IAA, phosphate solubilization
Zaidi et al. (2006)
Pseudomonas sp., Bacillus sp.
IAA, siderophore, phosphate solubilization
Rajkumar et al. (2006)
Pseudomonas putida
Antifungal activity, siderophore, HCN, phosphate solubilization
Pandey et al. (2006)
Bravibacterium sp.
Siderophore
Noordman et al. (2006)
Xanthomonas sp. RJ3, Azomonas sp. RJ4, Pseudomonas sp. RJ10, Bacillus sp. RJ31
IAA
Sheng and Xia (2006)
Bacillus sp.
P-solubilization
Canbolat et al. (2006)
Bradyrhizobium japonicum
IAA
Shaharoona et al. (2006)
Pseudomonas putida
Siderophores, Pb and Cd resistence
Tripathi et al. (2005)
Pseudomonas fluorescens PRS9, Pseudomonas fluorescens GRS1
IAA, siderophores, phosphate solubilization
Gupta et al. (2005)
Variovorax paradoxus, Rhodococcus sp., Flavobacterium
IAA and siderophores
Belimov et al. (2005)
Sphingomonas sp, Mycobacterium sp, Bacillus sp, Rhodococcus sp, Cellulomonas sp., Pseudomonas sp.
IAA
Tsavkelova et al. (2005)
Pseudomonas fluorescens
IAA, siderophores, antifungal activity
Dey et al. (2004)
Bacillus, Azospirillum sp.
IAA, P-solubilization
Yasmin et al. (2004)
Azospirillum brasilense, Azospirillum amazonense
IAA, P solubilization, nitrogenase activity, antibiotic resistance
Thakuria et al. (2004)
Pseudomonas fluorescens
IAA, phosphate solubilization
Jeon et al. (2003)
Rhizobium, Bradyrhizobium
HCN, siderophore, Siderophore, IAA, P-solubilization
Deshwal et al. (2003)
Bacillus, Pseudomons, Azotobacter, Azospirillum, Rhizobium
P-solubilization and IAA
Tank and Saraf (2003)
Mesorhizobium, Bradyrhizobium sp.
Siderophore
Khan et al. (2002)
Azotobacter chroococcum
Gibberellin, kinetin, IAA
Verma et al. (2001)
Azotobacter chroococcum
P-solubilization
Kumar et al. (2001)
Rhizobium meliloti
Siderophore
Arora et al. (2001)
Kluyvera ascorbata
Siderophore
Burd et al. (2000)
Kluyvera ascorbata
ACC deaminase, siderophores, metal resistance
Genrich et al. (1998)
Bradyrhizobium, Rhizobium
Siderophore
Duhan et al. (1998)
Bradyrhizobium, Rhizobium
IAA
Antoun et al. (1998)
Rhizobium ciceri
Siderophopre
Berraho et al. (1997)
Bradyrhizobium japonicum
Siderophore
Wittenberg et al. (1996)
Rhizobium leguminosarum
Cytokinin
Noel et al. (1996)
Rhizobium, Bradyrhizobium
P-solubilization
Abd-Alla (1994)
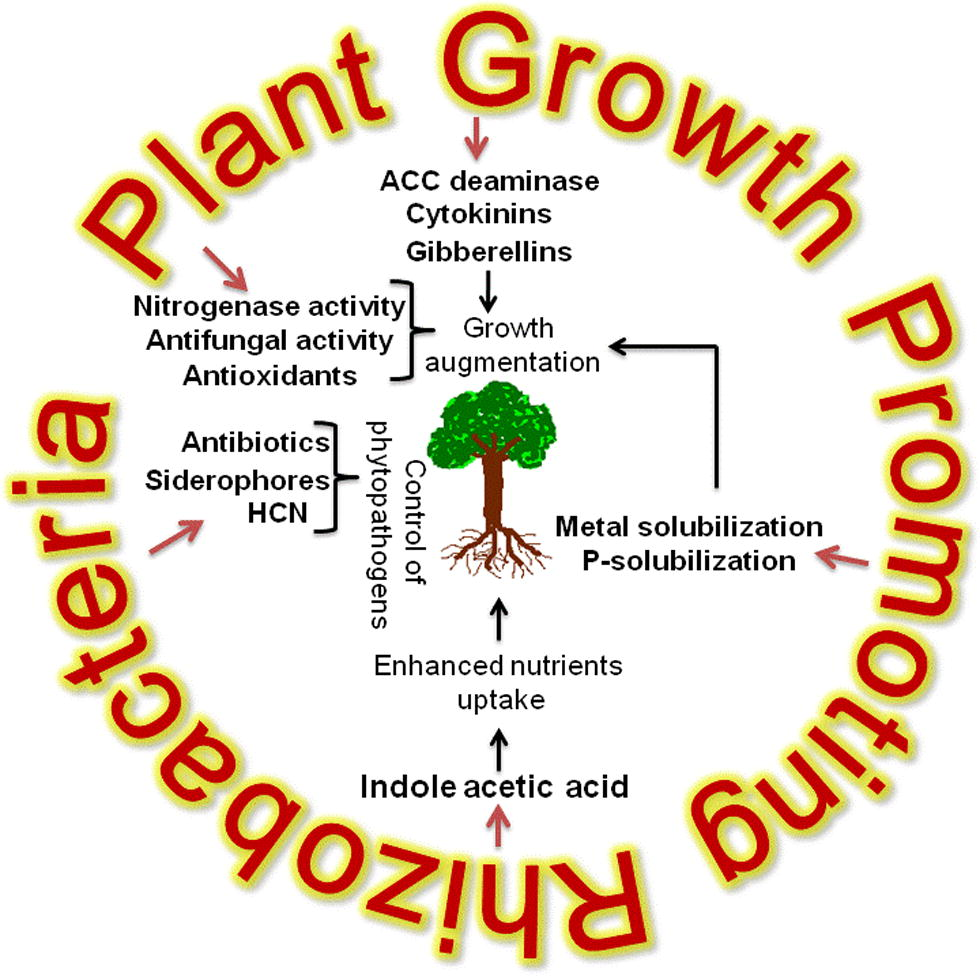
Mechanism of plant growth promotion by rhizobacteria.
4.1 Direct mechanisms
4.1.1 Nitrogen fixation
Nitrogen (N) is the most vital nutrient for plant growth and productivity. Although, there is about 78% N2 in the atmosphere, it is unavailable to the growing plants. The atmospheric N2 is converted into plant-utilizable forms by biological N2 fixation (BNF) which changes nitrogen to ammonia by nitrogen fixing microorganisms using a complex enzyme system known as nitrogenase (Kim and Rees, 1994). In fact, BNF accounts for approximately two-thirds of the nitrogen fixed globally, while the rest of the nitrogen is industrially synthesized by the Haber–Bosch process (Rubio and Ludden, 2008). Biological nitrogen fixation occurs, generally at mild temperatures, by nitrogen fixing microorganisms, which are widely distributed in nature (Raymond et al., 2004). Furthermore, BNF represents an economically beneficial and environmentally sound alternative to chemical fertilizers (Ladha et al., 1997).
Nitrogen fixing organisms are generally categorized as (a) symbiotic N2 fixing bacteria including members of the family rhizobiaceae which forms symbiosis with leguminous plants (e.g. rhizobia) (Ahemad and Khan, 2012d; Zahran, 2001) and non-leguminous trees (e.g. Frankia) and (b) non-symbiotic (free living, associative and endophytes) nitrogen fixing forms such as cyanobacteria (Anabaena, Nostoc), Azospirillum, Azotobacter, Gluconoacetobacter diazotrophicus and Azocarus etc. (Bhattacharyya and Jha, 2012). However, non-symbiotic nitrogen fixing bacteria provide only a small amount of the fixed nitrogen that the bacterially-associated host plant requires (Glick, 2012). Symbiotic nitrogen fixing rhizobia within the rhizobiaceae family (α-proteobacteria) infect and establish symbiotic relationship with the roots of leguminous plants. The establishment of the symbiosis involves a complex interplay between host and symbiont (Giordano and Hirsch, 2004) resulting in the formation of the nodules wherein the rhizobia colonize as intracellular symbionts (Fig. 2). Plant growth-promoting rhizobacteria that fix N2 in non-leguminous plants are also called as diazotrophs capable of forming a non-obligate interaction with the host plants (Glick et al., 1999). The process of N2 fixation is carried out by a complex enzyme, the nitrogenase complex (Kim and Rees, 1994). Structure of nitrogenase was elucidated by Dean and Jacobson (1992) as a two-component metalloenzyme consisting of (i) dinitrogenase reductase which is the iron protein and (ii) dinitrogenase which has a metal cofactor. Dinitrogenase reductase provides electrons with high reducing power while dinitrogenase uses these electrons to reduce N2 to NH3. Based on the metal cofactor three different N fixing systems have been identified (a) Mo-nitrogenase, (b) V-nitrogenase and (c) Fe-nitrogenase. Structurally, N2-fixing system varies among different bacterial genera. Most biological nitrogen fixation is carried out by the activity of the molybdenum nitrogenase, which is found in all diazotrophs (Bishop and Jorerger, 1990).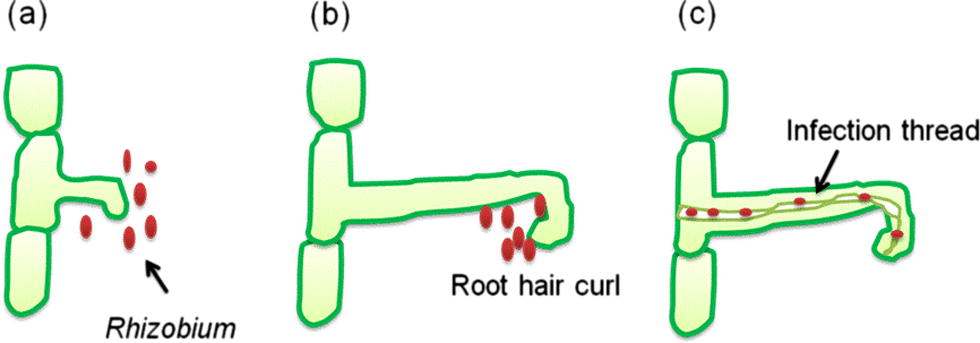
The nodulation process (a) Interaction of rhizobial rhicadhesin with host lectins and rhizobial attachment with root cells. (b) Excretion of nod factors by rhizobia causes root hair curling. (c) Rhizobia penetrate root hair and form an infection thread through which they penetrate the cortical cells and form bacteroid state thereby nodules are formed.
The genes for nitrogen fixation, called nif genes are found in both symbiotic and free living systems (Kim and Rees, 1994). Nitrogenase (nif) genes include structural genes, genes involved in activation of the Fe protein, iron molybdenum cofactor biosynthesis, electron donation, and regulatory genes required for the synthesis and function of the enzyme. In diazotrophs, nif genes are typically found in a cluster of around 20–24 kb with seven operons encoding 20 different proteins (Glick, 2012). The molybdenum nitrogenase enzyme complex has two component proteins encoded by the nifDK and the nifH genes. The NifDK component is a heterotetrameric (α2β2) protein formed by two αβ dimers related by a twofold symmetry. NifDK carries one iron molybdenum cofactor (FeMo-co) within the active site in each α-subunit (NifD) (Rubio and Ludden, 2008). The symbiotic activation of nif-genes in the Rhizobium is dependent on low oxygen concentration, which in turn is regulated by another set of genes called fix-genes which are common for both symbiotic and free living nitrogen fixation systems (Kim and Rees, 1994; Dean and Jacobson, 1992). Since nitrogen fixation is a very energy demanding process, requiring at least 16 mol of ATP for each mole of reduced nitrogen, it would be advantageous if bacterial carbon resources were directed toward oxidative phosphorylation, which results in the synthesis of ATP, rather than glycogen synthesis, which results in the storage of energy in the form of glycogen (Glick, 2012). For instance, treatment of legume plants with rhizobia having a deleted gene for glycogen synthase resulted in a considerable augmentation in both the nodule number and plant dry weight with reference to treatment with the wild-type strain (Marroqui et al., 2001).
4.1.2 Phosphate solubilization
Phosphorus (P), the second important plant growth-limiting nutrient after nitrogen, is abundantly available in soils in both organic and inorganic forms (Fig. 3) (Khan et al., 2009). Despite of large reservoir of P, the amount of available forms to plants is generally low. This low availability of phosphorous to plants is because the majority of soil P is found in insoluble forms, while the plants absorb it only in two soluble forms, the monobasic (
) and the diabasic (
) ions (Bhattacharyya and Jha, 2012). The insoluble P is present as an inorganic mineral such as apatite or as one of several organic forms including inositol phosphate (soil phytate), phosphomonesters, and phosphotriesters (Glick, 2012). To overcome the P deficiency in soils, there are frequent applications of phosphatic fertilizers in agricultural fields. Plants absorb fewer amounts of applied phosphatic fertilizers and the rest is rapidly converted into insoluble complexes in the soil (Mckenzie and Roberts, 1990). But regular application of phosphate fertilizers is not only costly but is also environmentally undesirable. This has led to search for an ecologically safe and economically reasonable option for improving crop production in low P soils. In this context, organisms coupled with phosphate solubilizing activity, often termed as phosphate solubilizing microorganisms (PSM), may provide the available forms of P to the plants and hence a viable substitute to chemical phosphatic fertilizers (Khan et al., 2006). Of the various PSM(s) inhabiting the rhizosphere, phosphate-solubilizing bacteria (PSB) are considered as promising biofertilizers since they can supply plants with P from sources otherwise poorly available by various mechanisms (Fig. 4) (Zaidi et al., 2009). Bacterial genera like Azotobacter, Bacillus, Beijerinckia, Burkholderia, Enterobacter, Erwinia, Flavobacterium, Microbacterium, Pseudomonas, Rhizobium and Serratia are reported as the most significant phosphate solubilizing bacteria (Bhattacharyya and Jha, 2012). Typically, the solubilization of inorganic phosphorus occurs as a consequence of the action of low molecular weight organic acids which are synthesized by various soil bacteria (Zaidi et al., 2009). Conversely, the mineralization of organic phosphorus occurs through the synthesis of a variety of different phosphatases, catalyzing the hydrolysis of phosphoric esters (Glick, 2012). Importantly, phosphate solubilization and mineralization can coexist in the same bacterial strain (Tao et al., 2008).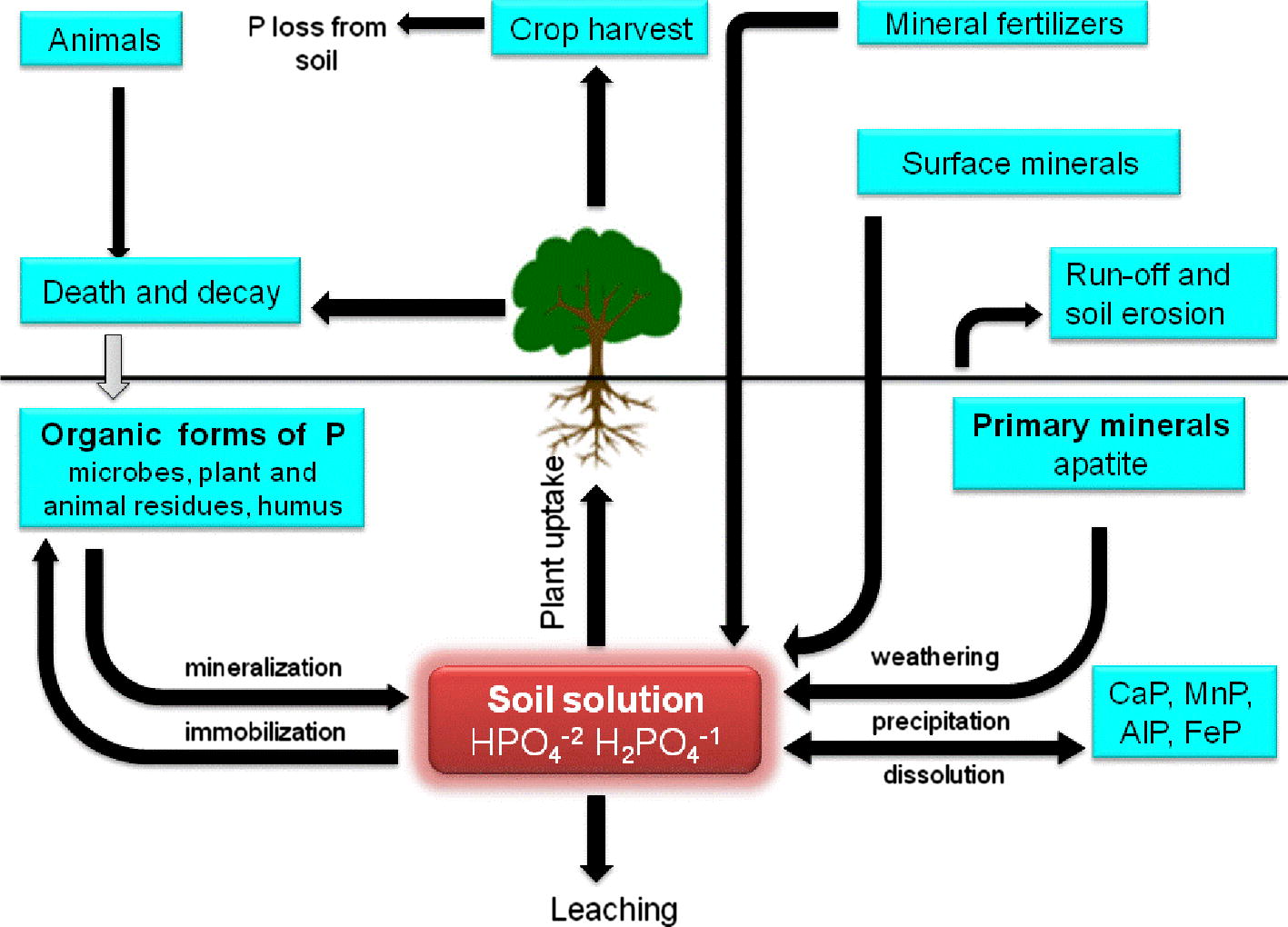
Movement of phosphorus in soils.
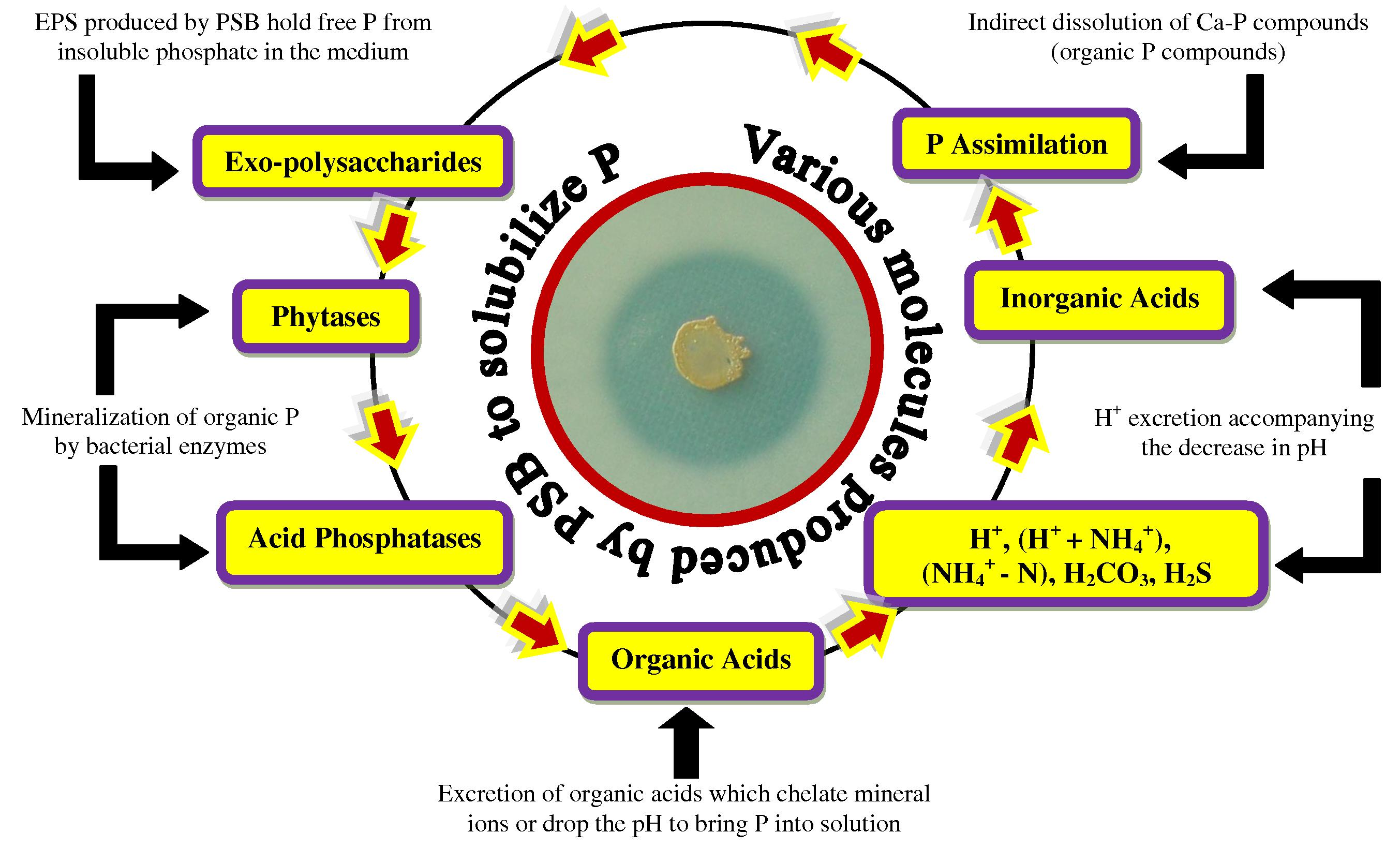
Various organic/inorganic substances produced by PSB responsible for phosphate solubilization in soils.
Though, PSB are commonly found in most soils; their establishment and performances are severely affected by environmental factors especially under stress conditions (Ahemad and Khan, 2012a,e; Ahemad and Khan, 2010a,b). However, the beneficial effects of the inoculation with PSB used alone (Ahemad and Khan, 2012e; Ahemad and Khan, 2011k; Ahemad and Khan, 2010d; Poonguzhali et al., 2008; Chen et al., 2008) or in combination with other rhizospheric microbes have been reported (Zaidi and Khan, 2005; Vikram and Hamzehzarghani, 2008). Besides providing P to the plants, the phosphate solubilizing bacteria also augment the growth of plants by stimulating the efficiency of BNF, enhancing the availability of other trace elements by synthesizing important plant growth promoting substances (Suman et al., 2001; Ahmad et al., 2008; Zaidi et al., 2009) (Table 2).
4.1.3 Siderophore production
Iron is a vital nutrient for almost all forms of life. All microorganisms known hitherto, with the exception of certain lactobacilli, essentially require iron (Neilands, 1995). In the aerobic environment, iron occurs principally as Fe3+ and is likely to form insoluble hydroxides and oxyhydroxides, thus making it generally inaccessible to both plants and microorganisms (Rajkumar et al., 2010). Commonly, bacteria acquire iron by the secretion of low-molecular mass iron chelators referred to as siderophores which have high association constants for complexing iron. Most of the siderophores are water-soluble and can be divided into extracellular siderophores and intracellular siderophores. Generally, rhizobacteria differs regarding the siderophore cross-utilizing ability; some are proficient in using siderophores of the same genus (homologous siderophores) while others could utilize those produced by other rhizobacteria of different genera (heterologous siderophores) (Khan et al., 2009). In both Gram-negative and Gram-positive rhizobacteria, iron (Fe3+) in Fe3+-siderophore complex on bacterial membrane is reduced to Fe2+ which is further released into the cell from the siderophore via a gating mechanism linking the inner and outer membranes. During this reduction process, the siderophore may be destroyed/recycled (Rajkumar et al., 2010; Neilands, 1995). Thus, siderophores act as solubilizing agents for iron from minerals or organic compounds under conditions of iron limitation (Indiragandhi et al., 2008). Not only iron, siderophores also form stable complexes with other heavy metals that are of environmental concern, such as Al, Cd, Cu, Ga, In, Pb and Zn, as well as with radionuclides including U and Np (Neubauer et al., 2000; Kiss and Farkas, 1998). Binding of the siderophore to a metal increases the soluble metal concentration (Rajkumar et al., 2010). Hence, bacterial siderophores help to alleviate the stresses imposed on plants by high soil levels of heavy metals.
Plants assimilate iron from bacterial siderophores by means of different mechanisms, for instance, chelate and release of iron, the direct uptake of siderophore-Fe complexes, or by a ligand exchange reaction (Schmidt, 1999). Numerous studies of the plant growth promotion vis-à-vis siderophore-mediated Fe-uptake as a result of siderophore producing rhizobacterial inoculations have been reported (Rajkumar et al., 2010). For example, Crowley and Kraemer (2007) revealed a siderophore mediated iron transport system in oat plants and inferred that siderophores produced by rhizosphere microorganisms deliver iron to oat, which has mechanisms for using Fe-siderophore complexes under iron-limited conditions. Similarly, the Fe-pyoverdine complex synthesized by Pseudomonas fluorescens C7 was taken up by Arabidopsis thaliana plants, leading to an increase of iron inside plant tissues and to improved plant growth (Vansuyt et al., 2007). Recently, Sharma et al. (2003) assessed the role of the siderophore-producing Pseudomonas strain GRP3 on iron nutrition of Vigna radiate. After 45 days, the plants showed a decline in chlorotic symptoms and iron, chlorophyll a and chlorophyll b content increased in strain GRP3 inoculated plants compared to control.
4.1.4 Phytohormone production
Microbial synthesis of the phytohormone auxin (indole-3-acetic acid/indole acetic acid/IAA) has been known for a long time. It is reported that 80% of microorganisms isolated from the rhizosphere of various crops possess the ability to synthesize and release auxins as secondary metabolites (Patten and Glick, 1996). Generally, IAA secreted by rhizobacteria interferes with the many plant developmental processes because the endogenous pool of plant IAA may be altered by the acquisition of IAA that has been secreted by soil bacteria (Glick, 2012; Spaepen et al., 2007). Evidently, IAA also acts as a reciprocal signaling molecule affecting gene expression in several microorganisms. Consequently, IAA plays a very important role in rhizobacteria-plant interactions (Spaepen and Vanderleyden, 2011). Moreover, down-regulation of IAA as signaling is associated with the plant defense mechanisms against a number of phyto-pathogenic bacteria as evidenced in enhanced susceptibility of plants to the bacterial pathogen by exogenous application of IAA or IAA produced by the pathogen (Spaepen and Vanderleyden, 2011). IAA has been implicated in virtually every aspect of plant growth and development, as well as defense responses. This diversity of function is reflected by the extraordinary complexity of IAA biosynthetic, transport and signaling pathways (Santner et al., 2009). Generally, IAA affects plant cell division, extension, and differentiation; stimulates seed and tuber germination; increases the rate of xylem and root development; controls processes of vegetative growth; initiates lateral and adventitious root formation; mediates responses to light, gravity and florescence; affects photosynthesis, pigment formation, biosynthesis of various metabolites, and resistance to stressful conditions. IAA produced by rhizobacteria likely, interfere the above physiological processes of plants by changing the plant auxin pool. Moreover, bacterial IAA increases root surface area and length, and thereby provides the plant greater access to soil nutrients. Also, rhizobacterial IAA loosens plant cell walls and as a result facilitates an increasing amount of root exudation that provides additional nutrients to support the growth of rhizosphere bacteria (Glick, 2012). Thus, rhizobacterial IAA is identified as an effector molecule in plant–microbe interactions, both in pathogenesis and phytostimulation (Spaepen and Vanderleyden, 2011).
An important molecule that alters the level of IAA synthesis is the amino acid tryptophan, identified as the main precursor for IAA and thus plays a role in modulating the level of IAA biosynthesis (Zaidi et al., 2009). Strangely, tryptophan stimulates IAA production while, anthranilate, a precursor for tryptophan, reduces IAA synthesis. By this mechanism, IAA biosynthesis is fine-tuned because tryptophan inhibits anthranilate formation by a negative feedback regulation on the anthranilate synthase, resulting in an indirect induction of IAA production (Spaepen et al., 2007). However, supplementation of culture media with tryptophan increases the IAA production by most of the rhizobacteria (Spaepen and Vanderleyden, 2011). Biosynthesis of tryptophan starts from the metabolic node chorismate in a five-step reaction encoded by the trp genes. The branch point compound chorismate is synthesized starting from phosphoenolpyruvate and erythrose 4-phosphate in the shikimate pathway, a common pathway for the biosynthesis of aromatic amino acids and many secondary metabolites (Spaepen and Vanderleyden, 2011; Merino et al., 2008; Dosselaere and Vanderleyden, 2001). Starting with tryptophan, at least five different pathways have been described for the synthesis of IAA, and most pathways show similarity to those described in plants, although some intermediates can differ (Fig. 5) (Spaepen and Vanderleyden, 2011; Patten and Glick, 1996): (1) IAA formation via indole-3-pyruvic acid and indole-3-acetic aldehyde is found in a majority of bacteria like, Erwinia herbicola; saprophytic species of the genera Agrobacterium and Pseudomonas; certain representatives of Bradyrhizobium, Rhizobium, Azospirillum, Klebsiella, and Enterobacter, (2) The conversion of tryptophan into indole-3-acetic aldehyde may involve an alternative pathway in which tryptamine is formed as in pseudomonads and azospirilla and (3) IAA biosynthesis via indole-3-acetamide formation is reported for phytopathogenic bacteria Agrobacterium tumefaciens, Pseudomonas syringae, and E. herbicola; saprophytic pseudomonads like (e.g. Pseudomonas putida and P. fluorescens). (4) IAA biosynthesis that involves tryptophan conversion into indole-3-acetonitrile is found in the cyanobacterium (Synechocystis sp.) and (5) the tryptophan-independent pathway, more common in plants, is also found in azospirilla and cyanobacteria.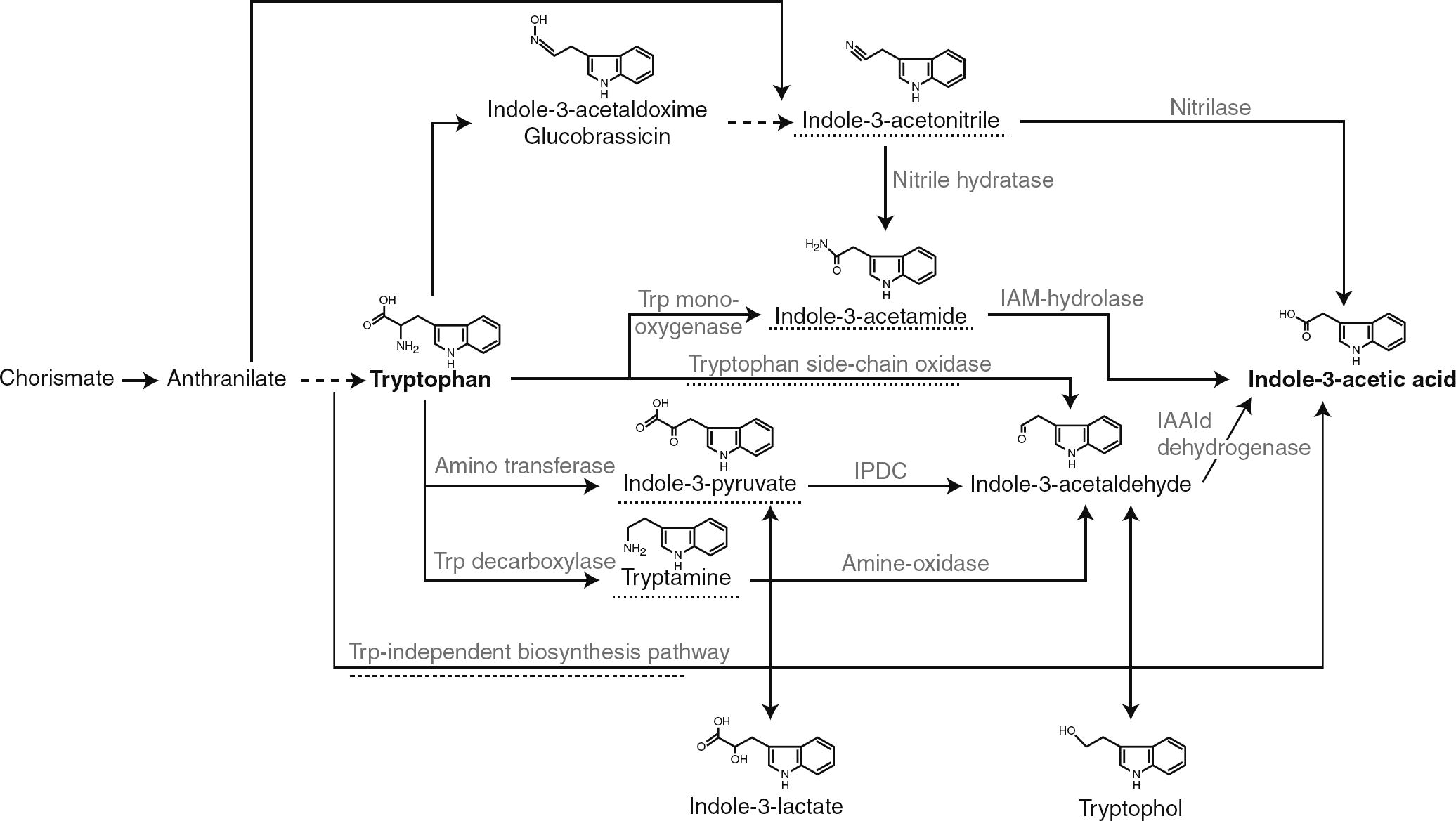
Overview of the different pathways to synthesize IAA in bacteria. The intermediate referring to the name of the pathway or the pathway itself is underlined with a dashed line. IAAld, indole-3-acetaldehyde; IAM, indole-3-acetamide; IPDC, indole-3-pyruvate decarboxylase; Trp, tryptophan (Adapted from Spaepen et al. (2007)).
Most Rhizobium species have been shown to produce IAA (Ahemad and Khan, 2012b,d,f; Ahemad and Khan, 2011e, j). Since, IAA is involved in multiple processes including cell division, differentiation and vascular bundle formation, these three processes are also essential for nodule formation. Hence, it seems likely that auxin levels in the host legume plants are necessary for nodule formation (Glick, 2012; Spaepen et al., 2007). It is also reported that the inoculation with Rhizobium leguminosarum bv. viciae wherein the IAA biosynthetic pathway had been introduced, produced potential nitrogen fixing root nodules containing up to 60-fold more IAA than nodules formed by the wild-type counterpart in Vicia hirsute (Camerini et al., 2008). Environmental stress factors which modulate the IAA biosynthesis in different bacteria include acidic pH, osmotic and matrix stress, and carbon limitation (Spaepen et al., 2007). Among genetic factors, both the location of auxin biosynthesis genes in the bacterial genome (either plasmid or chromosomal) and the mode of expression (constitutive vs. induced) have been shown to affect the level of IAA production. The location of auxin biosynthesis genes can affect the IAA level, as plasmids are mostly present in multiple copies. This can be illustrated by the difference in the IAA level between the rhizobacterial strains, Pseudomonas savastanoi pv. savastanoi and P. syringae pv. syringae. In the former strain, the genes for auxin biosynthesis genes are present on a plasmid, while in the latter one the corresponding genes are located on the chromosomal DNA, resulting in a lower IAA production. The IAA production in P. syringae pv. Syringae could be increased many fold by introducing a low-copy plasmid, carrying the IAA biosynthetic operon (Spaepen and Vanderleyden, 2011; Spaepen et al., 2007; Brandl and Lindow, 1997; Patten and Glick, 1996).
4.1.5 1-Aminocyclopropane-1-carboxylate (ACC) deaminase
Generally, ethylene is an essential metabolite for the normal growth and development of plants (Khalid et al. 2006). This plant growth hormone is produced endogenously by approximately all plants and is also produced by different biotic and abiotic processes in soils and is important in inducing multifarious physiological changes in plants. Apart from being a plant growth regulator, ethylene has also been established as a stress hormone (Saleem et al., 2007). Under stress conditions like those generated by salinity, drought, water logging, heavy metals and pathogenicity, the endogenous level of ethylene is significantly increased which negatively affects the overall plant growth. For instance, the high concentration of ethylene induces defoliation and other cellular processes that may lead to reduced crop performance (Saleem et al., 2007; Bhattacharyya and Jha, 2012). Plant growth promoting rhizobacteria which possess the enzyme, 1-aminocyclopropane-1-carboxylate (ACC) deaminase, facilitate plant growth and development by decreasing ethylene levels, inducing salt tolerance and reducing drought stress in plants (Nadeem et al., 2007; Zahir et al., 2008). Currently, bacterial strains exhibiting ACC deaminase activity have been identified in a wide range of genera such as Acinetobacter, Achromobacter, Agrobacterium, Alcaligenes, Azospirillum, Bacillus, Burkholderia, Enterobacter, Pseudomonas, Ralstonia, Serratia and Rhizobium etc. (Shaharoona et al., 2007a,b; Nadeem et al., 2007; Zahir et al., 2008; Zahir et al., 2009; Kang et al., 2010). Such rhizobacteria take up the ethylene precursor ACC and convert it into 2-oxobutanoate and NH3 (Arshad et al., 2007) (Fig. 6). Several forms of stress are relieved by ACC deaminase producers, such as effects of phytopathogenic microorganisms (viruses, bacteria, and fungi etc.), and resistance to stress from polyaromatic hydrocarbons, heavy metals, radiation, wounding, insect predation, high salt concentration, draft, extremes of temperature, high light intensity, and flooding (Glick, 2012; Lugtenberg and Kamilova, 2009). As a result, the major noticeable effects of seed/ root inoculation with ACC deaminase-producing rhizobacteria are the plant root elongation, promotion of shoot growth, and enhancement in rhizobial nodulation and N, P and K uptake as well as mycorrhizal colonization in various crops (Nadeem et al., 2007; Shaharoona et al., 2008; Nadeem et al., 2009; Glick, 2012).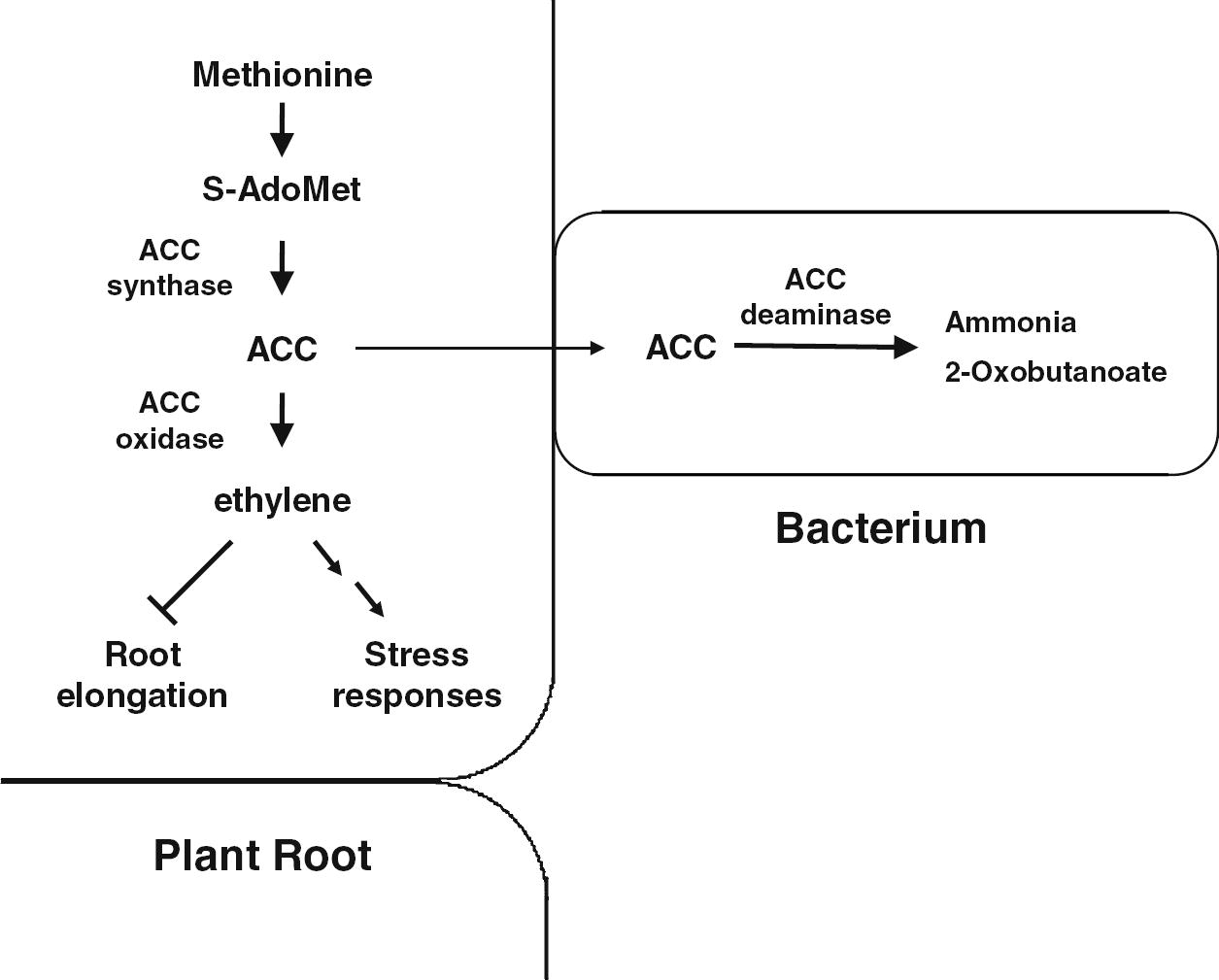
A possible mechanism of how stress controller bacteria reduce ethylene levels in the plant root using bacterial ACC deaminase. ACC synthesized in plant tissues by ACC synthase is thought be exuded from plant roots and be taken up by neighboring bacteria. Subsequently, the bacteria hydrolyze ACC to ammonia and 2-oxobutanoate. This ACC hydrolysis maintains ACC concentrations low in bacteria and permits continuous ACC transfer from plant roots to bacteria. Otherwise, ethylene can be produced from ACC and then cause stress responses including growth inhibition. S-AdoMet: S-adenosyl-l-methionine; ACC: 1-aminocyclopropane-1-carboxylate (Adapted from Kang et al. (2010)).
4.2 Indirect mechanisms
The application of microorganisms to control diseases, which is a form of biological control, is an environment-friendly approach (Lugtenberg and Kamilova, 2009). The major indirect mechanism of plant growth promotion in rhizobacteria is through acting as biocontrol agents (Glick, 2012). In general, competition for nutrients, niche exclusion, induced systemic resistance and antifungal metabolites production are the chief modes of biocontrol activity in PGPR (Lugtenberg and Kamilova, 2009). Many rhizobacteria have been reported to produce antifungal metabolites like, HCN, phenazines, pyrrolnitrin, 2,4-diacetylphloroglucinol, pyoluteorin, viscosinamide and tensin (Bhattacharyya and Jha, 2012). Interaction of some rhizobacteria with the plant roots can result in plant resistance against some pathogenic bacteria, fungi, and viruses. This phenomenon is called induced systemic resistance (ISR) (Lugtenberg and Kamilova, 2009). Moreover, ISR involves jasmonate and ethylene signaling within the plant and these hormones stimulate the host plant’s defense responses against a variety of plant pathogens (Glick, 2012). Many individual bacterial components induce ISR, such as lipopolysaccharides (LPS), flagella, siderophores, cyclic lipopeptides, 2,4-diacetylphloroglucinol, homoserine lactones, and volatiles like, acetoin and 2,3-butanediol (Lugtenberg and Kamilova, 2009).
5 Applications of PGPR as multifunctional agents
The effect of PGPR in crop productivity varies under laboratory, greenhouse and field trials. Because, soil is an unpredictable environment and an intended result is sometimes difficult to achieve. Climatic variations also have a large impact on the effectiveness of PGPR but sometimes unfavorable growth conditions in the field are to be expected as normal functioning of agriculture (Zaidi et al., 2009). Plant growth promoting traits do not work independently of each other but additively as it was suggested in the “additive hypothesis,” that multiple mechanisms, such as phosphate solubilization, dinitrogen fixation, ACC deaminase and antifungal activity, IAA and siderophore biosynthesis etc. are responsible for the plant growth promotion and increased yield (Bashan and Holguin, 1997). Under both natural agro-ecological niches and controlled soil environments, significant increase in yields of different crop plants has been observed following PGPR applications (Table 3). Due to the existing reluctance worldwide to embrace foods produced by genetically modified plants, PGPR may be advantageous as a means of promoting plant growth. The wide scale application of PGPR may decrease the global dependence on agricultural chemicals. Furthermore, it is a technology which is readily accessible to farmers in both developed and developing countries (Gamalero et al., 2009).
PGPR
Plant
Conditions
Results of addition of bacteria to plants
Reference
Pseudomonas putida, Azospirilium, Azotobacter
Artichoke (Cynara scolymus)
In vitro
Phosphate solublizing bacteria along with nitrogen fixing bacteria led to significant increase in radicle and shoot length, shoot weight, coefficient of velocity of germination, seedling vigority index, and significant decrease in mean time of germination
Jahanian et al. (2012)
Pseudomonas sp. PS1
Greengram (Vigna radiata (L.) wilczek)
Pots
Significantly increased plant dry weight, nodule nubers, total chlorophyll content, leghaemoglobin, root N, shoot N, root P, shoot P, seed yield and seed protein
Ahemad and Khan (2012e), Ahemad and Khan (2011k) and Ahemad and Khan (2010d)
Bradyrhizobium MRM6
Greengram (Vigna radiata (L.) wilczek)
Pots
When herbicide tolerant Rhizobium strain MRP1 was used with herbicide, it increased the growth parameters at all tested concentrations of herbicides (quizalafop-p-ethyl and clodinafop)
Ahemad and Khan (2012f) and Ahemad and Khan (2011h,l)
Pseudomonas sp. A3R3
Alyssum serpyllifolium, Brassica juncea
Pots
Increased significantly the biomass (B. juncea) and Ni content (A. serpyllifolium) in plants grown in Ni-stressed soil
Ma et al. (2011a)
Pseudomonas sp.
Soybean, wheat
Fields
Significantly increased soil enzyme activities, total productivity, and nutrient uptake
Sharma et al. (2011)
Psychrobacter sp. SRS8
Ricinus communis, Helianthus annuus
Pots
Stimulated plant growth and Ni accumulation in both plant species with increased plant biomass, chlorophyll, and protein content
Ma et al. (2011b)
Rhizobium strain MRP1
Pea (Pisum sativum)
Pots
Significantly increased the growth, symbiotic properties (nodulation and leghaemoglobin content), amount of N and P nutrients in plant organs, seed yield and seed protein of pea plants
Ahemad and Khan (2011i), Ahemad and Khan (2010c) and Ahemad and Khan (2009b)
Rhizobium phaseoli
Vigna radiata L.
Pots
In the presence of tryptophan, Rhizobium mitigated the adverse effects of salinity and increased the plant height, number of nodules per plant, plant biomass, grain yield, and grain nitrogen concentration significantly.
Zahir et al. (2010)
Bacillus species PSB10
Chickpea (Cicer arietinum)
Pots
Significantly improved growth, nodulation, chlorophyll, leghaemoglobin, seed yield and grain protein; reduced the uptake of chromium in roots, shoots and grains
Wani and Khan (2010)
Mesorhizobium strain MRC4
Chickpea (Cicer arietinum)
Pots
Significantly increased symbiotic properties (nodulation and leghaemoglobin content), root N, shoot N, root P, shoot P, seed yield and seed protein
Ahemad and Khan (2010e,h) and Ahemad and Khan (2009a)
Rhizobium strain MRL3
Lentil (Lens esculentus)
Pots
Significantly increased symbiotic properties (nodulation and leghaemoglobin content), root N, shoot N, root P, shoot P, seed yield and seed protein
Ahemad and Khan (2011j), Ahemad and Khan (2010f,g)
Bradyrhizobium sp. 750, Pseudomonas sp., Ochrobactrum cytisi
Lupinus luteus
Fields
Increased both biomass, nitrogen content, accumulation of metals (improved phytostabilisation potential)
Dary et al. (2010)
Paenibacillus polymyxa
Pepper
Gnotobiotic conditions
Significantly increased the biomass of plants and elicited induced systemic resistance against bacterial spot pathogen Xanthomonas axonopodis pv. vesicatoria untreated plants.
Phi et al. (2010)
Pseudomnas putida strain R-168, Pseudomonas fluorescens strain R-93, Pseudomonas fluorescens DSM 50090, Pseudomonas putida DSM291, Azospirillum lipoferum DSM 1691, Azospirillum brasilense DSM 1690
Maize (Zea mays L.)
Fields
Plant height, seed weight, number of seed per ear and leaf area, shoot dry weight significantly increased
Gholami et al. (2009)
Pseudomonas sp. SRI2, Psychrobacter sp. SRS8, Bacillus sp. SN9
Brassica juncea, Brassica oxyrrhina
Pots
Increased the biomass of the test plants and enhanced Ni accumulation in plant tissues
Ma et al. (2009a)
Psychrobacter sp. SRA1, Bacillus cereus SRA10
Brassica juncea, Brassica oxyrrhina
Pots
Enhanced the metal accumulation in plant tissues by facilitating the release of Ni from the non-soluble phases in the soil
Ma et al. (2009b)
Achromobacter xylosoxidans strain Ax10
Brassica juncea
Pots
Significantly improved Cu uptake by plants and increased the root length, shoot length, fresh weight and dry weight of plants
Ma et al. (2009c)
Pseudomonas aeruginosa, Pseudomonas fluorescens, Ralstonia metallidurans
Maize
Pots
Promoted plant growth, facilitated soil metal mobilization, enhanced Cr and Pb uptake
Braud et al. (2009)
Klebsiella pneumonia
Triticum aestivum
Pots
Significantly increased the root length and shoot length
Sachdev et al. (2009)
Pseudomonas sp.
Chickpea
Pots
Enhanced fresh and dry weight of plants even at 2 mM nickel concentration
Tank and Saraf (2009)
Azospirillum amazonense
Rice (Oryza sativa L.)
Greenhouse
Grain dry matter accumulation (7–11.6%), the number of panicles (3–18.6%) and nitrogen accumulation at grain maturation (3.5–18.5%) increased
Rodrigues et al. (2008)
Pseudomonas species
Rice (Oryza sativa), maize (Zea mays)
In vitro
Pseudomonad isolated from rice showed a higher ability to control bacterial and fungal root pathogens than that obtained from maize
Lawongsa et al. (2008)
Pseudomonas aeruginosa strain MKRh3
Black gram
Pots
Plants showed lessened cadmium accumulation, extensive rooting, and enhanced plant growth
Ganesan (2008)
Mesorhizobium sp. RC3
Chickpea (Cicer arietinum)
Pots
Increased the dry matter accumulation, number of nodules, seed yield and grain protein by 71%, 86%, 36% and 16%, respectively, compared to noninoculated plants. Nitrogen in roots and shoots increased by 46% and 40%, respectively, at 136 mg Cr/kg
Wani et al. (2008)
Pseudomonas aeruginosa
Indian mustard and pumpkin
Pots
Stimulated plant growth, reduced Cd uptake
Sinha and Mukherjee (2008)
Bacillus weihenstephanensis strain SM3
Helianthus annuus
Pots
Increased plant biomass and the accumulation of Cu and Zn in the root and shoot systems, also augmented the concentrations of water soluble Ni, Cu and Zn in soil with their metal mobilizing potential
Rajkumar et al. (2008)
Azospirillum brasilense Sp245
Common bean (Phaseolus vulgaris L.)
Greenhouse
Root growth increased
Remans et al. (2008)
Bacillus sp. Paenibacillus sp.
Rice
Pots
Promoted significantly the root and shoot growth
Beneduzi et al. (2008)
Bacillus edaphicus
Indian mustard (Brassica juncea)
Pots
Stimulated plant growth, facilitated soil Pb mobilization, enhanced Pb accumulation
Sheng et al. (2008)
Bacillus subtilis, Pseudomonas aeruginosa
Solanum lycopersicum L. (tomato), Abelmoschus esculentus (okra), Amaranthus sp. (African spinach)
Greenhouse
Dry biomass increased 31% for tomato, 36% for okra, and 83% for African spinach
Adesemoye et al. (2008)
Pseudomonas tolaasii ACC23, Pseudomonas fluorescens ACC9, Alcaligenes sp. ZN4, Mycobacterium sp. ACC14
Brassica napus
Pots
Protected canola plant against the inhibitory effects of cadmium
Dell’Amico et al. (2008)
Azotobacter chroococcum, Azospirillum lipoferum
Cotton (Gossypium hirsutum L.)
Fields
Seed yield (21%), plant height (5%) and microbial population in soil (41%) increased over their respective controls while boll weight and staple length remained statistically unaffected
Anjum et al. (2007)
Bradyrhizobium sp. (vigna) RM8
Greengram (Vigna radiate)
Pots
Enhanced the nodule numbers by 82%, leghaemoglobin by 120%, seed yield by 34%, grain protein by 13%, root N by 41% and shoot N by 37% at 290 mg Ni/kg soil
Wani et al. (2007a)
Rhizobium sp. RP5
Pea (Pisum sativum)
Pots
Enhanced the dry matter, nodule numbers, root N, shoot N, leghaemoglobin, seed yield, and grain protein by 19%, 23%, 26%, 47%, 112%, 26%, and 8%, respectively, at 290 mg Ni/kg
Wani et al. (2007b)
Pseudomonas putida CC-R2-4, Bacillus subtilis CC-pg104
Lectuca sativa L.
Gnotobiotic conditions
Significant increase in shoot length and root length achieved through encapsulated inoculant
Rekha et al. (2007)
Methylobacterium oryzae, Berkholderia sp.
Lycopersicon esculentom
Gnotobiotic conditions, pots
–
Madhaiyan et al. (2007)
Bacillus spp.
Barley (Hordeum vulgare)
Greenhouse
Increased root weight up to 16.7% and shoot weight up to 347%
Canbolat et al. (2006)
Sinorhizobium sp. Pb002
Brassica juncea
Microcosms
Increased the efficiency of lead phytoextraction by B. juncea plants
Di Gregorio et al. (2006)
Brevibacillus
Trifolium repens
Pots
Enhanced plant growth and nutrition of plants and decreased zinc concentration in plant tissues
Vivas et al. (2006)
Xanthomonas sp. RJ3, Azomonas sp. RJ4, Pseudomonas sp. RJ10, Bacillus sp. RJ31
Brassica napus
Pots
Stimulated plant growth and increased cadmium accumulation
Sheng and Xia (2006)
Pseudomonas sp, Bacillus sp.
Mustard
Pots
Stimulated plant growth and decreased Cr (VI) content
Rajkumar et al. (2006)
Ochrobactrum, Bacillus cereus
Mungbean
Pots
Lowers the toxicity of chromium to seedlings by reducing Cr (VI) to Cr (III)
Faisal and Hasnain (2006)
Pseudomonas jessenii PS06, Mesorhizobium ciceri C-2/2
Cicer arietinum (chickpea)
Greenhouse, fields
The co-inoculation treatment increased the seed yield (52% greater than the uninoculated control treatment) and nodule fresh weight
Valverde et al. (2006)
Azotobacter chroococcum HKN-5, Bacillus megaterium HKP-1, Bacillus mucillaginosus HKK-1
Brassica Juncea
Greenhouse
Protected plant from metal toxicity, stimulated plant growth
Wu et al. (2006)
Bacillus subtilis SJ-101
Brassica Juncea
Growth chamber
Facilitated Ni accumulation
Zaidi et al. (2006)
Pseudomonas putida KNP9
Mung bean
Greenhouse
Stimulated the plant growth, reduced Pb and Cd uptake
Tripathi et al. (2005)
Rhizobacterial strains A3 and S32
Brassica juncea
Pots
Promoted the plant growth under chromium stress
Rajkumar et al. (2005)
Pseudomas fluorescens
Soybean
Greenhouse
Increased plant growth
Gupta et al. (2005)
Ochrobactrum intermedium
Sunflower
Pots
Increased plant growth and decreased Cr(VI) uptake
Faisal and Hasnain (2005)
Variovox paradoxus, Rhodococcus sp, Flavobacterium
Brassica juncea
In vitro
Stimulating root elongation
Belimov et al. (2005)
Azospirillum brasilense, Bacillus pantothenticus, Pseudomonas pieketti
Rice (Oryza sativa)
Micro-plots
Increased rice grain yield maximum up to 76.9%
Thakuria et al. (2004)
Pseudomonas fluorescens PGPR1, PGPR2, PGPR4
Peanut (Arachis hypogaea L.)
Pots, fields
Significantly enhanced pod yield, haulm yield and nodule dry weight over the control
Dey et al. (2004)
Unidentified PGPR isolate
Wheat
Gnotobiotic conditions
Increases in root elongation (up to 17.3%), root dry weight (up to 13.5%), shoot elongation (up to 37.7%) and shoot dry weight (up to 36.3%) in inoculated wheat seedlings
Khalid et al. (2004)
Pseudomonas fluorescens Avm, Rhizobium leguminosarum bv phaseoli CPMex46
Alfalfa
Growth chamber
Improved Cu and Fe translocation from root to shoot
Carrillo-Castaneda et al. (2003)
Enterobacter sakazakii 8MR5, Pseudomonas sp. 4MKS8, Klebsiella oxytoca 10MKR7
Zea mays L. (maize)
Pots
Inoculation increased growth parameters
Babalola et al. (2003)
Pseudomonas sp.
Soybean, mungbean, wheat
Pots
Promotes growth of plants
Gupta et al. (2002)
Enterobacter cloacae
Brassica napus
Pots
Both root and shoot length significantly increased.
Saleh and Glick (2001)
Brevundimonas Kro13
–
Culture media
Sequestered cadmium directly from solution
Robinson et al. (2001)
Kluyvera ascorbata SUD165
Indian mustard, canola, tomato
Growth chamber
Both strains decreased some plant growth inhibition by heavy metals, No increase of metal uptake with either strain over non-inoculated plants
Burd et al. (2000)
Pseudomonas putida
Vigna radiata L. (mungbean)
Pots
The ethylene production inhibited in the inoculated plants
Mayak et al. (1999)
6 Conclusion
Plant growth promoting rhizobacteria, having multiple activities directed toward plant growth promotion vis-à-vis exhibiting bioremediating potentials by detoxifying pollutants like, heavy metals and pesticides and controlling a range of phytopathogens as biopesticides, have shown spectacular results in different crop studies. The productive efficiency of a specific PGPR may be further enhanced with the optimization and acclimatization according to the prevailing soil conditions. In future, they are expected to replace the chemical fertilizers, pesticides and artificial growth regulators which have numerous side-effects to sustainable agriculture. Further research and understanding of mechanisms of PGPR mediated-phytostimulation would pave the way to find out more competent rhizobacterial strains which may work under diverse agro-ecological conditions.
References
- Solubilization of rock phosphates by Rhizobium and Bradyrhizobium. Folia Microbiol.. 1994;39:53-56.
- [Google Scholar]
- Comparison of plant growth-promotion with Pseudomonas aeruginosa and Bacillus subtilis in three vegetables. Braz. J. Microbiol.. 2008;39:423-426.
- [Google Scholar]
- Implications of bacterial resistance against heavy metals in bioremediation: a review. IIOABJ. 2012;3:39-46.
- [Google Scholar]
- Effect of insecticide-tolerant and plant growth promoting Mesorhizobium on the performance of chickpea grown in insecticide stressed alluvial soils. J. Crop Sci. Biotechnol.. 2009;12:213-222.
- [Google Scholar]
- Toxicity assessment of herbicides quizalafop-p-ethyl and clodinafop towards Rhizobium pea symbiosis. Bull. Environ. Contam. Toxicol.. 2009;82:761-766.
- [Google Scholar]
- Influence of selective herbicides on plant growth promoting traits of phosphate solubilizing Enterobacter asburiae strain PS2. Res. J. Microbiol.. 2010;5:849-857.
- [Google Scholar]
- Plant growth promoting activities of phosphate-solubilizing Enterobacter asburiae as influenced by fungicides. Eurasia. J. Biosci.. 2010;4:88-95.
- [Google Scholar]
- Comparative toxicity of selected insecticides to pea plants and growth promotion in response to insecticide-tolerant and plant growth promoting Rhizobium leguminosarum. Crop Prot.. 2010;29:325-329.
- [Google Scholar]
- Phosphate-solubilizing and plant-growth-promoting Pseudomonas aeruginosa PS1 improves greengram performance in quizalafop-p-ethyl and clodinafop amended soil. Arch. Environ. Contam. Toxicol.. 2010;58:361-372.
- [Google Scholar]
- Ameliorative effects of Mesorhizobium sp. MRC4 on chickpea yield and yield components under different doses of herbicide stress. Pestic. Biochem. Physiol.. 2010;98:183-190.
- [Google Scholar]
- Insecticide-tolerant and plant-growth promoting Rhizobium improves the growth of lentil (Lens esculentus) in insecticide-stressed soils. Pest Manag. Sci.. 2010;67:423-429.
- [Google Scholar]
- Growth promotion and protection of lentil (Lens esculenta) against herbicide stress by Rhizobium species. Ann. Microbiol.. 2010;60:735-745.
- [Google Scholar]
- Improvement in the growth and symbiotic attributes of fungicide-stressed chickpea plants following plant growth promoting fungicide-tolerant Mesorhizobium inoculation. Afr. J. Basic Appl. Sci.. 2010;2:111-116.
- [Google Scholar]
- Toxicological assessment of selective pesticides towards plant growth promoting activities of phosphate solubilizing Pseudomonas aeruginosa. Acta Microbiol. Immunol. Hung.. 2011;58:169-187.
- [Google Scholar]
- Effects of insecticides on plant-growth-promoting activities of phosphate solubilizing rhizobacterium Klebsiella sp. strain PS19. Pestic. Biochem. Physiol.. 2011;100:51-56.
- [Google Scholar]
- Assessment of plant growth promoting activities of rhizobacterium Pseudomonas putida under insecticide-stress. Microbiol. J.. 2011;1:54-64.
- [Google Scholar]
- Effect of pesticides on plant growth promoting traits of greengram-symbiont, Bradyrhizobium sp. strain MRM6. Bull. Environ. Contam. Toxicol.. 2011;86:384-388.
- [Google Scholar]
- Ecotoxicological assessment of pesticides towards the plant growth promoting activities of Lentil (Lens esculentus)-specific Rhizobium sp. strain MRL3. Ecotoxicology. 2011;20:661-669.
- [Google Scholar]
- Biotoxic impact of fungicides on plant growth promoting activities of phosphate-solubilizing Klebsiella sp. isolated from mustard (Brassica compestris) rhizosphere. J. Pest Sci 2011
- [CrossRef] [Google Scholar]
- Toxicological effects of selective herbicides on plant growth promoting activities of phosphate solubilizing Klebsiella sp. strain PS19. Curr. Microbiol.. 2011;62:532-538.
- [Google Scholar]
- Insecticide-tolerant and plant growth promoting Bradyrhizobium sp. (vigna) improves the growth and yield of greengram [Vigna radiata (L.) Wilczek] in insecticide-stressed soils. Symbiosis. 2011;54:17-27.
- [Google Scholar]
- Effect of tebuconazole-tolerant and plant growth promoting Rhizobium isolate MRP1 on pea-Rhizobium symbiosis. Sci. Hortic.. 2011;129:266-272.
- [Google Scholar]
- Plant growth promoting fungicide-tolerant Rhizobium improves growth and symbiotic characteristics of lentil (Lens esculentus) in fungicide-applied soil. J. Plant Growth Regul.. 2011;30:334-342.
- [Google Scholar]
- Pseudomonas aeruginosa strain PS1 enhances growth parameters of greengram [Vigna radiata (L.) Wilczek] in insecticide-stressed soils. J. Pest Sci.. 2011;84:123-131.
- [Google Scholar]
- Response of greengram [Vigna radiata (L.) Wilczek] grown in herbicide-amended soil to quizalafop-p-ethyl and clodinafop tolerant plant growth promoting Bradyrhizobium sp. (vigna) MRM6. J. Agric. Sci. Technol.. 2011;13:1209-1222.
- [Google Scholar]
- Effect of fungicides on plant growth promoting activities of phosphate solubilizing Pseudomonas putida isolated from mustard (Brassica compestris) rhizosphere. Chemosphere. 2012;86:945-950.
- [Google Scholar]
- Ecological assessment of biotoxicity of pesticides towards plant growth promoting activities of pea (Pisum sativum)-specific Rhizobium sp. strain MRP1. Emirates J. Food Agric.. 2012;24:334-343.
- [Google Scholar]
- Evaluation of plant growth promoting activities of rhizobacterium Pseudomonas putida under herbicide-stress. Ann. Microbiol.. 2012;62:1531-1540.
- [Google Scholar]
- Effects of pesticides on plant growth promoting traits of Mesorhizobium strain MRC4. J. Saudi Soc. Agric. Sci.. 2012;11:63-71.
- [Google Scholar]
- Alleviation of fungicide-induced phytotoxicity in greengram [Vigna radiata (L.) Wilczek] using fungicide-tolerant and plant growth promoting Pseudomonas strain. Saudi J. Biol. Sci.. 2012;19:451-459.
- [Google Scholar]
- Productivity of greengram in tebuconazole-stressed soil, by using a tolerant and plant growth-promoting Bradyrhizobium sp. MRM6 strain. Acta Physiol. Plant.. 2012;34:245-254.
- [Google Scholar]
- Bioaccumulation of heavy metals by zinc resistant bacteria isolated from agricultural soils irrigated with wastewater. Bacteriol. J.. 2011;2:12-21.
- [Google Scholar]
- Remediation of herbicides contaminated soil using microbes. In: Khan M.S., Zaidi A., Musarrat J., eds. Microbes in Sustainable Agriculture. New York, USA: Nova Science Publishers; 2009.
- [Google Scholar]
- Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol. Res.. 2008;163:173-181.
- [Google Scholar]
- Response of cotton to plant growth promoting rhizobacteria (PGPR) inoculation under different levels of nitrogen. J. Agric. Res.. 2007;45:135-143.
- [Google Scholar]
- Antoun, H., Prévost, D., 2005. Ecology of plant growth promoting rhizobacteria. In: Siddiqui, Z.A. (Ed.), PGPR: biocontrol and biofertilization, Springer, Dordrecht, pp. 1–38.
- Potential of Rhizobium and Bradyrhizobium species as plant growth promoting rhizobacteria on non-legumes: effects on radishes (Raphanus sativus L.) Plant Soil. 1998;204:57-67.
- [Google Scholar]
- Isolation of siderophore producing strains of Rhizobium meliloti and their biocontrol potential against Macrophomina phaseolina that causes charcoal rot of groundnut. Curr. Sci.. 2001;81:673-677.
- [Google Scholar]
- Perspectives of bacterial ACC deaminase in phytoremediation. Trends Biotechnol.. 2007;25:356-362.
- [Google Scholar]
- Amplification of 1-aminocyclopropane-1-carboxylic (ACC) deaminase from plant growth promoting rhizobacteria in Striga-infested soils. Afr. J. Biotechnol.. 2003;2:157-160.
- [Google Scholar]
- Azospirillum-plant relationships: Environmental and physiological advances (1990–1996) Can. J. Microbiol.. 1997;43:103-121.
- [Google Scholar]
- Cadmium-tolerant plant growth promoting rhizobacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.) Soil Biol. Biochem.. 2005;37:241-250.
- [Google Scholar]
- Evaluation of genetic diversity and plant growth promoting activities of nitrogen-fixing Bacilli isolated from rice fields in South Brazil. Appl. Soil Ecol.. 2008;39:311-320.
- [Google Scholar]
- Iron requirement and siderophore production in Rhizobium ciceri during growth on an iron-deficient medium. World J. Microbiol. Biotechnol.. 1997;13:501-510.
- [Google Scholar]
- Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J. Microbiol. Biotechnol.. 2012;28:1327-1350.
- [Google Scholar]
- Genetics and molecular biology of an alternative nitrogen fixation system. Plant Mol. Biol.. 1990;41:109-125.
- [Google Scholar]
- Environmental signals modulate the expression of an indole-3-acetic acid biosynthetic gene in Erwinia herbicola. Mol. Plant Microbe Interact.. 1997;10:450-499.
- [Google Scholar]
- Enhanced phytoextraction of an agricultural Cr-, Hg- and Pb-contaminated soil by bioaugmentation with siderophoreproducing bacteria. Chemosphere. 2009;74:280-286.
- [Google Scholar]
- Plant growth promoting bacteria that decrease heavy metal toxicity in plants. Can. J. Microbiol.. 2000;46:237-245.
- [Google Scholar]
- Introduction of a novel pathway for IAA biosynthesis to rhizobia alters vetch root nodule development. Arch. Microbiol.. 2008;190:67-77.
- [Google Scholar]
- Effect of plant growth-promoting bacteria and soil compaction on barley seedling growth, nutrient uptake, soil properties and rhizosphere microflora. Biol. Fertil. Soils. 2006;42:350-357.
- [Google Scholar]
- Plant growth-promoting bacteria promote copper and iron translocation from root to shoot in alfalfa seedlings. J. Plant Nutr.. 2003;26:1801-1814.
- [Google Scholar]
- Isolation and characterization of antagonistic Bacillus subtilis strains from the avocado rhizoplane displaying biocontrol activity. J. Appl. Microbiol.. 2007;103:1950-1959.
- [Google Scholar]
- Microbial biopesticides for integrated crop management: an assessment of environmental and regulatory sustainability. Trends Food Sci. Tech.. 2008;19:275-283.
- [Google Scholar]
- Studies on phosphorus solubilizing activity of a strain of phosphobacteria isolated from chestnut type soil in China. Biores. Technol.. 2008;99:6702-6707.
- [Google Scholar]
- Function of siderophores in the plant rhizosphere. In: Pinton R., ed. The Rhizosphere, Biochemistry and Organic Substances at the Soil-Plant Interface. CRC Press; 2007. p. :73-109.
- [Google Scholar]
- Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil. 2002;245:35-47.
- [Google Scholar]
- “In situ” phytostabilisation of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth promoting rhizobacteria. J. Hazard. Mater.. 2010;177:323-330.
- [Google Scholar]
- Biochemical genetics of nitrogenase. In: Stacey G., Burris R.H., Evans H.J., eds. Biological Nitrogen Fixation. New York: Chapman and Hall; 1992. p. :763-834.
- [Google Scholar]
- Improvement of Brassica napus growth under cadmium stress by cadmium resistant rhizobacteria. Soil Biol. Biochem.. 2008;40:74-84.
- [Google Scholar]
- Rhizobia as a biological control agent against soil borne plant pathogenic fungi. Indian J. Exp. Biol.. 2003;41:1160-1164.
- [Google Scholar]
- Rhizosphere: so many achievements and even more challenges. Plant Soil. 2009;321:1-3.
- [Google Scholar]
- Growth promotion and yield enhancement of peanut (Arachis hypogaea L.) by application of plant growth-promoting rhizobacteria. Microbiol. Res.. 2004;159:371-394.
- [Google Scholar]
- Combined application of Triton X-100 and Sinorhizobium sp. Pb002 inoculum for the improvement of lead phytoextraction by Brassica juncea in EDTA amended soil. Chemosphere. 2006;63:293-299.
- [Google Scholar]
- A metabolic node in action: chorismate-utilizing enzymes in microorganisms. Crit. Rev. Microbiol.. 2001;27:75-131.
- [Google Scholar]
- Siderophore production in relation to N2 fixation and iron uptake in pigeon pea-Rhizobium symbiosis. Folia Microbiol.. 1998;43:421-426.
- [Google Scholar]
- Bacterial Cr (VI) reduction concurrently improves sunflower (Helianthus annuus L.) growth. Biotechnol. Lett.. 2005;27:943-947.
- [Google Scholar]
- Growth stimulatory effect of Ochrobactrum intermedium and Bacillus cereus on Vigna radiata plants. Lett. Appl. Microbiol.. 2006;43:461-466.
- [Google Scholar]
- Plant growth promoting rhizobacteria:fundamentals and applications. In: Maheshwari D.K., ed. Plant Growth and Health Promoting Bacteria. Berlin, Heidelberg: Springer-Verlag; 2011. p. :21-42.
- [Google Scholar]
- Evaluation of actinomycete strains for key traits related with plant growth promotion and mycorrhiza helping activities. Appl. Soil Ecol.. 2010;45:209-217.
- [Google Scholar]
- The use of microorganisms to facilitate the growth of plants in saline soils. In: Khan M.S., Zaidi A., Musarrat J., eds. Microbial Strategies for Crop Improvement. Berlin, Heidelberg: Springer; 2009.
- [Google Scholar]
- Rhizoremediation of cadmium soil using a cadmium-resistant plant growth-promoting rhizopseudomonad. Curr. Microbiol.. 2008;56:403-407.
- [Google Scholar]
- A plant growth-promoting bacterium that decreases nickel toxicity in seedlings. Appl. Environ. Microbiol.. 1998;64:3663-3668.
- [Google Scholar]
- The effect of plant growth promoting rhizobacteria (PGPR) on germination, seedling growth and yield of maize. Int. J. Biol. Life Sci.. 2009;1:35-40.
- [Google Scholar]
- The expression of MaEXP1, a Melilotus alba expansin gene, is upregulated during the sweet clover-Sinorhizobium meliloti interaction. MPMI. 2004;17:613-622.
- [Google Scholar]
- Plant Growth-Promoting Bacteria: Mechanisms and Applications. Hindawi Publishing Corporation, Scientifica; 2012.
- Biochemical and Genetic Mechanisms Used by Plant Growth Promoting Bacteria. London: Imperial College Press; 1999.
- Intracellular and extracellular PGPR: commonalities and distinctions in the plant–bacterium signaling processes. Soil Biol. Biochem.. 2005;37:395-412.
- [Google Scholar]
- Development of heavy metal resistant mutants of phosphate solubilizing Pseudomonas sp. NBRI4014 and their characterization. Curr. Microbiol.. 2002;45:323-332.
- [Google Scholar]
- In situ characterization of mercury resistant growth promoting fluorescent pseudomonads. Microbiol. Res.. 2005;160:385-388.
- [Google Scholar]
- Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol.. 2010;60:579-598.
- [Google Scholar]
- Isolation, selection, and characterization of beneficial rhizobacteria from pea, lentil and chickpea grown in Western Canada. Can. J. Microbiol.. 2008;54:248-258.
- [Google Scholar]
- Characterization of plant growth-promoting traits of bacteria isolated from larval guts of diamondback moth Plutella xylostella (Lepidoptera: Plutellidae) Curr. Microbiol.. 2008;56:327-333.
- [Google Scholar]
- The effect of plant growth promoting rhizobacteria (pgpr) on germination and primary growth of artichoke (Cynara scolymus) Int. J. Agric. Crop Sci.. 2012;4:923-929.
- [Google Scholar]
- Plant growth promotion in soil by some inoculated microorganisms. J. Microbiol.. 2003;41:271-276.
- [Google Scholar]
- Endophytic colonization of Typha australis by a plant growth-promoting bacterium Klebsiella oxytoca strain GR-3. J. Appl. Microbiol.. 2007;103:1311-1320.
- [Google Scholar]
- Isolation and characterization of a heavy metal-resistant Burkholderia sp. from heavy metal-contaminated paddy field soil and its potential in promoting plant growth and heavy metal accumulation in metal-polluted soil. Chemosphere. 2008;72:157-164.
- [Google Scholar]
- Gibberellins-producing rhizobacteria increase endogenous gibberellins content and promote growth of red peppers. J. Microbiol.. 2005;43:510-515.
- [Google Scholar]
- Characterization of plant growth promoting rhizobacteria associated with chickpea (Cicer arietinum L.) Int. J. Plant Prod.. 2007;2:141-152.
- [Google Scholar]
- Use of plant growth-promoting rhizobacteria to control stress responses of plant roots. Plant Biotechnol. Rep.. 2010;4:179-183.
- [Google Scholar]
- Screening plant growth-promoting rhizobacteria for improving growth and yield of wheat. J. Appl. Microbiol.. 2004;96:473-480.
- [Google Scholar]
- Effect of substrate-dependent microbial ethylene production on plant growth. Microbiology. 2006;75:231-236.
- [Google Scholar]
- Role of soil microbes in the rhizospheres of plants growing on trace metal contaminated soils in phytoremediation. J. Trace Elem. Med. Biol.. 2005;18:355-364.
- [Google Scholar]
- Biocontrol of fungal pathogens by the use of plant growth promoting rhizobacteria and nitrogen fixing microorganisms. Ind. J. Bot. Soc.. 2002;81:255-263.
- [Google Scholar]
- Role of phosphate-solubilizing microorganisms in sustainable agriculture – a review. Agron. Sustain. Dev.. 2006;27:29-43.
- [Google Scholar]
- Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ. Chem. Lett.. 2009;7:1-19.
- [Google Scholar]
- Metal-binding ability of desferrioxamine B. J. Inclusion Phenom. Mol. Recognit. Chem.. 1998;32:385-403.
- [Google Scholar]
- Plant growth-promoting rhizobacteria (other systems) In: Okon Y., ed. Azospirillum/Plant Associations. Boca Raton, FL, USA: CRC Press; 1994. p. :111-118.
- [Google Scholar]
- Kloepper, J.W., 2003. A review of mechanisms for plant growth promotion by PGPR In: Reddy, M.S., Anandaraj, M., Eapen, S.J., Sarma, Y.R., Kloepper, J.W. (Eds.), Abstracts and Short Papers. 6th International PGPR Workshop, 5–10 October 2003, Indian Institute of Spices Research, Calicut, India, pp. 81–92.
- Kloepper, J.W., Schroth, M.N., 1978. Plant growth-promoting rhizobacteria on radishes. In: Proceedings of the 4th International Conference on Plant Pathogenic Bacteria, vol. 2. Station de Pathologie Végétale et de Phytobactériologie, INRA, Angers, France, pp. 879–882.
- Relationship of in vitro antibiosis of plant growth promoting rhizobacteria to plant growth and the displacement of root microflora. Phytopathology. 1981;71:1020-1024.
- [Google Scholar]
- Plant growth promotion mediated by bacterial rhizosphere colonizers. In: Keister D.L., Cregan P.B., eds. The Rhizosphere and Plant Growth. Dordrecht, Netherlands: Kluwer Academic Publishers; 1991. p. :315-326.
- [Google Scholar]
- Establishment of phosphate solubilizing strains of Azotobacter chroococcum in the rhizosphere and their effect on wheat cultivars under greenhouse conditions. Microbiol. Res.. 2001;156:87-93.
- [Google Scholar]
- Influence of plant growth promoting bacteria and its mutant on heavy metal toxicity in Brassica juncea grown in fly ash amended soil. Chemosphere. 2008;72:678-683.
- [Google Scholar]
- Introduction: assessing opportunities for nitrogen fixation in rice-a frontier project. Plant Soil. 1997;124:1-10.
- [Google Scholar]
- Molecular and phenotypic characterization of potential plant growth-promoting Pseudomonas from rice and maize rhizospheres. World J. Microbiol. Biotechnol.. 2008;24:1877-1884.
- [Google Scholar]
- Characterization of a phenazine-producing strain Pseudomonas chlororaphis GP72 with broad-spectrum antifungal activity from green pepper rhizosphere. Curr. Microbiol.. 2007;54:302-306.
- [Google Scholar]
- Isolation and characterization of Ni mobilizing PGPB from serpentine soils and their potential in promoting plant growth and Ni accumulation by Brassica spp. Chemosphere. 2009;75:719-725.
- [Google Scholar]
- Improvement of plant growth and nickel uptake by nickel resistant-plant-growth promoting bacteria. J. Hazard. Mater.. 2009;166:1154-1161.
- [Google Scholar]
- Inoculation of plant growth promoting bacterium Achromobacter xylosoxidans strain Ax10 for the improvement of copper phytoextraction by Brassica juncea. J. Environ. Manage.. 2009;90:831-837.
- [Google Scholar]
- Inoculation of endophytic bacteria on host and non-host plants-effects on plant growth and Ni uptake. J. Hazard. Mater.. 2011;195:230-237.
- [Google Scholar]
- Inoculation of Ni-resistant plant growth promoting bacterium Psychrobacter sp. strain SRS8 for the improvement of nickel phytoextraction by energy crops. Int. J. Phytoremediation. 2011;13:126-139.
- [Google Scholar]
- Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.) Chemosphere. 2007;69:220-228.
- [Google Scholar]
- Enhanced symbiotic performance by Rhizobium tropici glycogen synthase mutants. J. Bacteriol.. 2001;183:854-864.
- [Google Scholar]
- Mineral Nutrition of Higher Plants. London: Academic Press; 1995.
- Effect of wild-type and mutant plant growth-promoting rhizobacteria on the rooting of mung been cuttings. J. Plant Growth Regul.. 1999;18:49-53.
- [Google Scholar]
- Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem.. 2004;42:565-572.
- [Google Scholar]
- McKenzie, R.H., Roberts, T.L., 1990. Soil and fertilizers phosphorus update. In: Proceedings of Alberta Soil Science Workshop Proceedings, Feb. 20–22, Edmonton, Alberta, pp. 84–104.
- Genetic and phenotypic diversity of plant growth promoting rhizobacteria isolated from sugarcane plants growing in Pakistan. J. Microbiol. Biotechnol.. 2010;20:1614-1623.
- [Google Scholar]
- Evolution of bacterial trp operons and their regulation. Curr. Opin. Microbiol.. 2008;11:78-86.
- [Google Scholar]
- Colonization of plant rhizosphere by actinomycetes of different genera. Microbiology. 2006;75:226-230.
- [Google Scholar]
- Plant growth-promoting rhizobacterial mediated protection in tomato against tomato mottle virus. Plant Dis.. 2000;84:779-784.
- [Google Scholar]
- Preliminary investigations on inducing salt tolerance in maize through inoculation with rhizobacteria containing ACC deaminase activity. Can. J. Microbiol.. 2007;53:1141-1149.
- [Google Scholar]
- Rhizobacteria containing ACC-deaminase confer salt tolerance in maize grown on salt-affected fields. Can. J. Microbiol.. 2009;55:1302-1309.
- [Google Scholar]
- Lead-enhanced siderophore production and alteration in cell morphology in a Pb-resistant Pseudomonas aeruginosa strain 4EA. Curr. Microbiol.. 2011;62:409-414.
- [Google Scholar]
- Siderophores: structure and function of microbial iron transport compounds. J. Biol. Chem.. 1995;270:26723-26726.
- [Google Scholar]
- Siderophores, NTA, and citrate: potential soil amendments to enhance heavy metal mobility in phytoremediation. Int. J. Phytoremediation. 2000;2:353-368.
- [Google Scholar]
- Rhizobium leguminosarum as a plant growth promoting rhizobacterium: direct growth promotion of canola and lettuce. Can. J. Microbiol.. 1996;42:279-283.
- [Google Scholar]
- Growth stimulation of Brevibacterium sp. by siderophores. J. Appl. Microbiol.. 2006;101:637-646.
- [Google Scholar]
- Characterization of a phosphate solubilizing and antagonistic strain of Pseudomonas putida (B0) isolated from a Sub-Alpine Location in the Indian Central Himalaya. Curr. Microbiol.. 2006;53:102-107.
- [Google Scholar]
- Bacterial biosynthesis of indole-3-acetic acid. Can. J. Microbiol.. 1996;42:207-220.
- [Google Scholar]
- Assessment of root-associated Paenibacillus polymyxa groups on growth promotion and induced systemic resistance in pepper. J. Microbiol. Biotechnol.. 2010;20:1605-1613.
- [Google Scholar]
- Isolation and identification of phosphate solubilizing bacteria from chinese cabbage and their effect on growth and phosphorus utilization of plants. J. Microbiol. Biotechnol.. 2008;18:773-777.
- [Google Scholar]
- Effects of inoculation of plant growth promoting bacteria on Ni uptake by Indian mustard. Bioresour. Technol.. 2008;99:3491-3498.
- [Google Scholar]
- Growth of Brassica juncea under chromium stress: influence of siderophores and indole-3-acetic acid producing rhizosphere bacteria. J. Environ. Biol.. 2005;26:693-699.
- [Google Scholar]
- Influence of plant growth promoting bacteria and Cr (VI) on the growth of Indian mustard. Chemosphere. 2006;62:741-748.
- [Google Scholar]
- Characterization of metal-resistant plant-growth promoting Bacillus weihenstephanensis isolated from serpentine soil in Portugal. J. Basic Microbiol.. 2008;48:500-508.
- [Google Scholar]
- Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol.. 2010;28:142-149.
- [Google Scholar]
- Comparative assessment of in situ bioremediation potential of cadmium resistant acidophilic Pseudomonas putida 62BN and alkalophilic Pseudomonas monteilli 97AN strains on soybean. Int. Biodet. Biodegrad.. 2009;63:62-66.
- [Google Scholar]
- Effect of free and encapsulated Pseudomonas putida CC-R2-4 and Bacillus subtilis CC-pg104 on plant growth under gnotobiotic conditions. Biores. Technol.. 2007;98:447-451.
- [Google Scholar]
- Physiological and genetic analysis of root responsiveness to auxin-producing plant growth-promoting bacteria in common bean (Phaseolus vulgaris L.) Plant Soil. 2008;302:149-161.
- [Google Scholar]
- Cadmium adsorption by rhizobacteria: implications for New Zealand Pastureland. Agric. Ecosyst. Environ.. 2001;87:315-321.
- [Google Scholar]
- Azospirillum amazonense inoculation: effects on growth, yield and N2 fixation of rice (Oryza sativa L.) Plant Soil. 2008;302:249-261.
- [Google Scholar]
- Characterization of plant-growth-promoting traits of Acinetobacter species isolated from rhizosphere of Pennisetum glaucum. J. Microbiol. Biotechnol.. 2011;21:556-566.
- [Google Scholar]
- Biosynthesis of the iron-molybdenum cofactor of nitrogenase. Annu. Rev. Microbiol.. 2008;62:93-111.
- [Google Scholar]
- Enhanced micropropagation response and biocontrol effect of Azospirillum brasilense Sp245 on Prunus cerasifera L. clone Mr.S 2/5 plants. J. Biotechnol.. 2008;134:312-319.
- [Google Scholar]
- Isolation and characterization of indole acetic acid (IAA) producing Klebsiella pneumoniae strains from rhizosphere of wheat (Triticum aestivum) and their effect on plant growth. Indian J. Exp. Biol.. 2009;47:993-1000.
- [Google Scholar]
- Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J. Indian Microbiol. Biotechnol.. 2007;34:635-648.
- [Google Scholar]
- Involvement of gacS and rpoS in enhancement of the plant growth-promoting capabilities of Enterobacter cloacae CAL2 and Pseudomonas putida UW4. Can. J. Microbiol.. 2001;47:698-705.
- [Google Scholar]
- Plant hormones are versatile chemical regulators of plant growth. Nature Chem. Biol.. 2009;5:301-307.
- [Google Scholar]
- PGPR-induced defense responses in the tea plant against blister blight disease. Crop Prot.. 2007;26:556-565.
- [Google Scholar]
- Solubilization of zinc compounds by the diazotrophic, plant growth promoting bacterium Gluconacetobacter diazotrophicus. Chemosphere. 2007;66:1794-1798.
- [Google Scholar]
- Mechanisms and regulation of reduction-based iron uptake in plants. New Phytol.. 1999;141:1-26.
- [Google Scholar]
- Cold tolerance and plant growth promotion potential of Serratia marcescens strain SRM (MTCC 8708) isolated from flowers of summer squash (Cucurbita pepo) Lett. Appl. Microbiol.. 2008;46:171-175.
- [Google Scholar]
- Effect of plant growth promoting rhizobacteria containing ACC-deaminase on maize (Zea mays L.) growth under axenic conditions and on nodulation in mung bean. Lett. Appl. Microbiol.. 2006;42:155-159.
- [Google Scholar]
- Differential response of etiolated pea seedlings to inoculation with rhizobacteria capable of utilizing 1-aminocyclopropane-1-carboxylate or L-methionine. J. Microbiol.. 2007;45:15-20.
- [Google Scholar]
- Effectiveness of various Pseudomonas spp. and Burkholderia caryophylli containing ACC-deaminase for improving growth and yield of wheat (Triticum aestivum L.) J. Microbiol. Biotechnol.. 2007;17:1300-1307.
- [Google Scholar]
- Fertilizer-dependent efficiency of Pseudomonads for improving growth, yield, and nutrient use efficiency of wheat (Triticum aestivum L.) Appl. Microbiol. Biotechnol.. 2008;79:147-155.
- [Google Scholar]
- Plant growth-promoting bacterium Pseudomonas sp. strain GRP3 influences iron acquisition in mung bean (Vigna radiata L. Wilzeck) Soil Biol. Biochem.. 2003;35:887-894.
- [Google Scholar]
- Selection of plant growth-promoting Pseudomonas spp. that enhanced productivity of soybean-wheat cropping system in central India. J. Microbiol. Biotechnol.. 2011;21:1127-1142.
- [Google Scholar]
- Improvement of rape (Brassica napus) plant growth and cadmium uptake by cadmium-resistant bacteria. Chemosphere. 2006;64:1036-1042.
- [Google Scholar]
- Characterization of plant growth-promoting Bacillus edaphicus NBT and its effect on lead uptake by Indian mustard in a lead-amended soil. Can. J. Microbiol.. 2008;54:417-422.
- [Google Scholar]
- Cadmium-induced siderophore production by a high Cd-resistant bacterial strain relieved Cd toxicity in plants through root colonization. Curr. Microbiol.. 2008;56:55-60.
- [Google Scholar]
- Rhizosphere bacterial signalling: a love parade beneath our feet. Crit. Rev. Microbiol. 2004;30:205-240.
- [Google Scholar]
- Auxin and plant-microbe interactions. Cold Spring Harb. Perspect. Biol. 2011
- [CrossRef] [Google Scholar]
- Indole- 3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev.. 2007;31:425-448.
- [Google Scholar]
- Molecular assessment of diversity among endophytic diazotrophs isolated from subtropical Indian sugarcane. World J. Microbiol. Biotechnol.. 2001;17:39-45.
- [Google Scholar]
- Phosphate solubilization, exopolysaccharide production and indole acetic acid secretion by rhizobacteria isolated from Trigonella graecum. Indian J. Microbiol.. 2003;43:37-40.
- [Google Scholar]
- Enhancement of plant growth and decontamination of nickel-spiked soil using PGPR. J. Basic Microbiol.. 2009;49:195-204.
- [Google Scholar]
- Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. J. Plant Interact.. 2010;5:51-58.
- [Google Scholar]
- Phosphate solubilizing and -mineralizing abilities of bacteria isolated from. Pedosphere. 2008;18:515-523.
- [Google Scholar]
- Characterization and screening of bacteria from rhizosphere of rice grown in acidic soils of Assam. Curr. Sci.. 2004;86:978-985.
- [Google Scholar]
- Genetic diversity of siderophore-producing bacteria of tobacco rhizosphere. Braz. J. Microbiol.. 2009;40:276-284.
- [Google Scholar]
- Isolation and functional characterization of siderophore-producing lead- and cadmium-resistant Pseudomonas putida KNP9. Curr. Microbiol.. 2005;5:233-237.
- [Google Scholar]
- Auxin production by bacteria associated with orchid roots. Microbiology. 2005;74:46-53.
- [Google Scholar]
- Differential effects of coinoculations with Pseudomonas jessenii PS06 (a phosphate-solubilizing bacterium) and Mesorhizobium ciceri C-2/2 strains on the growth and seed yield of chickpea under greenhouse and field conditions. Plant Soil. 2006;287:43-50.
- [Google Scholar]
- Iron acquisition from Fe-pyoverdine by Arabidopsis thaliana. Mol. Plant Microbe Interact.. 2007;20:441-447.
- [Google Scholar]
- In vitro production of plant growth regulators (PGRs) by Azorobacter chroococcum. Indian J. Microbiol.. 2001;41:305-307.
- [Google Scholar]
- Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003;255:571-586.
- [Google Scholar]
- Effect of phosphate solubilizing bacteria on nodulation and growth parameters of greengram (Vigna radiate L. Wilczec) Res. J. Microbiol.. 2008;3:62-72.
- [Google Scholar]
- Two bacterial strains isolated from a Zn-polluted soil enhance plant growth and mycorrhizal efficiency under Zn toxicity. Chemosphere. 2006;52:1523-1533.
- [Google Scholar]
- Bacillus species enhance growth parameters of chickpea (Cicer arietinum L.) in chromium stressed soils. Food Chem. Toxicol.. 2010;48:3262-3267.
- [Google Scholar]
- Effect of metal tolerant plant growth promoting Bradyrhizobium sp. (vigna) on growth, symbiosis, seed yield and metal uptake by greengram plants. Chemosphere. 2007;70:36-45.
- [Google Scholar]
- Co inoculation of nitrogen fixing and phosphate solubilizing bacteria to promote growth, yield and nutrient uptake in chickpea. Acta Agron. Hung.. 2007;55:315-323.
- [Google Scholar]
- Synergistic effects of the inoculation with nitrogen fixing and phosphate solubilizing rhizobacteria on the performance of field grown chickpea. J. Plant Nutr. Soil Sci.. 2007;170:283-287.
- [Google Scholar]
- Chromium-reducing and plant growth-promoting Mesorhizobium improves chickpea growth in chromium-amended soil. Biotechnol. Lett.. 2008;30:159-163.
- [Google Scholar]
- Siderophore bound iron in the peribacteroid space of soybean root nodules. Plant Soil. 1996;178:161-169.
- [Google Scholar]
- Engineering plant-microbe symbiosis for rhizoremediation of heavy metals. Appl. Environ. Microbiol.. 2006;72:1129-1134.
- [Google Scholar]
- Isolation, characterization and beneficial effects of rice associated plant growth promoting bacteria from Zanzibar soils. J. Basic Microbiol.. 2004;44:241-252.
- [Google Scholar]
- Effectiveness of rhizobacteria containing ACC-deaminase for growth promotion of pea (Pisum sativum) under drought conditions. J. Microbiol. Biotechnol.. 2008;18:958-963.
- [Google Scholar]
- Comparative effectiveness of Pseudomonas and Serratia sp. containing ACC-deaminase for improving growth and yield of wheat (Triticum aestivum L.) under salt-stressed conditions. Arch. Microbiol.. 2009;191:415-424.
- [Google Scholar]
- Substrate-dependent auxin production by Rhizobium phaseoli improves the growth and yield of Vigna radiata L. under salt stress conditions. J. Microbiol. Biotechnol.. 2010;20:1288-1294.
- [Google Scholar]
- Rhizobia from wild legumes: diversity, taxonomy, ecology, nitrogen fixation and biotechnology. J. Biotechnol.. 2001;91:143-153.
- [Google Scholar]
- Interactive effect of rhizospheric microorganisms on growth, yield and nutrient uptake of wheat. J. Plant Nutr.. 2005;28:2079-2092.
- [Google Scholar]
- Significance of Bacillus subtilis strain SJ 101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere. 2006;64:991-997.
- [Google Scholar]
- Plant growth promotion by phosphate solubilizing bacteria. Acta Microbiol. Immunol. Hung.. 2009;56:263-284.
- [Google Scholar]







