Mechanical basis of high mobility group box 1 protein in regulating biological damage regards to obesity
⁎Corresponding authors. yanglingchao@xinhuamed.com.cn (Lingchao Yang), liyigang@xinhuamed.com.cn (Yigang Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Obesity accounts for leading epidemic diseases worldwide and it in association with hypertension induce organ damage especially with cardiac and renal damages. In current scenario it is important to understand the genes involved in obesity induced hypertension associated organ damage. Previous studies with HMGB1 shows their role in initiating inflammatory response but its role in hypertension associated organ damage are still obscure. The present study mainly focuses on identifying the role of HMGB1 in cardiac and renal tissue damage following obesity induced hypertension. Following high fat diet and leptin injection the mice are able to develop obesity induced initial hypertension and critical hypertension stages in 4 months and 9 months diet protocol. The histopathological complication in initiating initial stages of cardiac and renal damage as well as critical organ damage are conclude based on the grade of microarchitecture changes. Immunohistochemistry and western blotting studies shows the elevated expression of HMGB1 in cardiac and renal tissue following initial hypertension induced organ damage but its expression is downregulated at critical stages of organ damage of cardiac and renal tissue. But in case of COX2 which is responsible for inflammation, shows gradual upregulated expression pattern as damages progress due to hypertension associated organ damage. Our result conclude that HMGB1 plays a key role in initiating organ damage following obesity induced hypertension and once damage attains saturation its expression is downregulated.
Keywords
Hypertension
HMGB1
COX2
Leptin
Organ damage
Obesity
1 Introduction
Metabolic syndrome is the major challenge that occurs globally that happens due to the risk factors of obesity (Hulthe et al., 2000). Obesity is interrelated with hypertension (Beltowski, 2008), ischemic heart disease (Thomsen and Nordestgaard, 2014), insulin resistance (Kahn et al., 2006), dyslipidemia (Franssen et al., 2011), diabetes (Calle and Kaaks, 2004), cancer (Franssen et al., 2011), damage to liver and kidneys (Noeman et al., 2011). Excess weight together with visceral adiposity accounts for 65–75% of primary hypertension (Hall, 2015). Two major factors that are responsible for epidemic weight gain are driven frequently by lack of physical activity and over nutrition in the form of high calories fast food (McAllister et al., 2009). Emerging studies shows that obesity lowers brain cognitive function (Alarcón et al., 2016) and also they are psychosocially effected in terms of bullying and social isolation (Gunnarsdottir et al., 2012).
Current estimate of obesity condition grows exponentially since 1980 and it cross 1.4 billion populations worldwide (WHO, 2014). Another important correlation is that Body Mass Index (BMI) and hypertension are linearly correlated (Jones et al., 1994) and previous studies clearly shows that in most hypertension cases weight loss helps to reduce the blood pressure (Stevens et al., 2001). Obesity hypertension can contribute to targeted organ injury including failure of renal-pressure natriuresis and development of chronic kidney disease (Hall, 2003). Although obesity hypertension contributes to kidney damage their pathophysiological changes and mechanism governing are more complex to elucidate. Again another organ more effected due to obesity induced hypertension is cardiac system in which the cardiac sympathetic nervous system (SNS) activity is elevated (Rumantir et al., 1999) and it initiate cardiac remodeling (Manrique et al., 2013).
The high mobility group box 1 protein (HMGB1) are chromatin associated protein plays an important role in initiating inflammatory response (Melvin and Edwards, 1999; Erlandson and Anderson, 2004). Other than regulating transcription and nucleosome stabilization, HMGB1 has the ability to secreted outside of cell under stressed condition that plays critical role in certain signal transduction pathway (Mullins et al., 2004). This present study focus on identify the mechanistic role of HMGB1 in developing kidney and cardiac damage in different stages of obesity associated hypertension.
2 Materials and method
2.1 Experimental animals
For the experiment purpose10 months old, male wild type mice of C57BL/6 background was chosen. The mice are fed with high fat diet (ad libitum with 40% calories as fat, 35% content as carbohydrate and 25% calories as protein; Rodent Diet D12451, USA). For control mice low calories diet (with 10% calories as fat, 65% content as carbohydrate and 25% calories as protein; 2014S Rodent Maintenance Diet, Spain) was followed. The diet was followed for continued 4 months for group 1 mice (6 Nos each) and prolonged for another 5 months for group 2 mice (6 Nos each) respectively. At mid stage of 2 months the group 1 and group 2 mice were injected with single dose of leptin (6 µg/mice). The experimental animals are maintained at constant room temperature 23 °C with a relative humidity of 55 ± 5%. The animals used in the experiments are approved by animal research committee of host Institution.
2.2 Histology
Dissected heart and renal tissue from control, group 1 and group 2 mice are made into small pieces. After initial washing with distilled water the dissected tissue is subjected to formalin solution for 36 h at 50 °C. Following fixation, the tissues are transferred to 60% isopropyl alcohol solution for 1 h. Then the tissues are gradually transferred to increasing concentration of isopropyl alcohol solution. After complete dehydration, the tissues are transferred to clearing agent, xylene for 50 min. Finally, the tissues are transferred to wax for impregnation, the tissues are molded into small blocks which can be mounted on the microtome to obtain thin sectioning (5 µm size). The processed slides are stained with hematoxylin & eosin for visualized under the microscope.
2.3 Immunohistochemistry
The paraffin-embedded, heart and renal tissues are subjected to sectioning (6 µm size) and placed on a slide which is overlaid with water. The slides are placed in the hot plate at 60 °C for 5 min, which helps to stretch out the sections. After the wax melted out, the slide is immediately washed with xylene which helps to remove wax from sections. Following isopropyl alcohol rinse and finally with water, antigen retrieval was carried out using trypsin solution for 10 min. The endogenous peroxidase activity of cells is inhibited by treating the slides with 4% H2O2 solution for 20 min. After washing the slides, the sections are incubated with blocking solution (4% BSA (Bovine Serum Albumin) in 1X TBST (Tris-buffered saline, 0.1% Tween 20) for 2 h at RT. After that the slides are incubated with primary antibodies, anti-HMGB1 (catalog number: SAB2108675; Sigma-Aldrich, Hong Kong, China) or anti-COX2 antibody (catalog number: SAB4502491; Sigma-Aldrich, Hong Kong, China) (diluted in 1X TBST) for 6 h at 4 °C. After incubation the slides are washed with 1X PBS (Phosphate Buffer Saline) for two to three times. The washed slides are incubated with HRP (Horseradish Peroxidase) conjugated specific secondary antibody for 1 h at room temperature. Following incubation time, the secondary antibody is washing throughout with 1X PBS and overlaid with DAB (3,3′-diaminobenzidine) substrate for 5 min which helps to develop the specific signals.
2.4 Western blotting
Following dissection, the heart and renal tissue are washed using ice cold 1X PBS solution and cut into smaller pieces. For protein sample preparation, the tissue samples are homogenizing along with ice cold 2X protein sample buffer. The prepared cell lysate is heated at 100 °C for 5 min and cooled to RT, centrifuged to separate the supernatant. The protein concentration in each samples are determined using Lowry methods and each wells of SDS-PAGE gel are loaded with equal concentration and run for 5 h at 50 V. Following protein separation, the gel is transferred to PVDF membrane and further blocking with 4% BSA in TBST solution for 2 h. After blocking step the membrane is incubated with primary anti-HMGB1 (catalog number: SAB2108675; Sigma-Aldrich, Hong Kong, China) or anti-COX2 antibody (catalog number: SAB4502491; Sigma-Aldrich, Hong Kong, China) antibody for 6 h at 4 °C. After incubation, the membrane is washed with 1X TBST buffer and incubated with specific secondary antibody at RT for 2 h. Following final washing the membrane are developed with DAB solution kit to obtain the specific signals.
2.5 Statistical analysis
The experimental data are repeated for three times and statistical data are represented as mean ± SEM. The significant differences between the groups were analyzed using student’s t test. The statistical results were considered as significant level when the p value < 0.05.
3 Results
3.1 Physical conditions of obesity associated hypertension induction mice
Obesity in association with hypertension is one of the major reasons for developing heart and renal disease. Supplementing with high fat diet for prolonged duration are able to induce cardiac remodeling in experimental animals (Sibouakaz et al., 2018). In order to enhance the body weight of C57BL/6 background male mice, high fat diet was provided along with one-time leptin injection as described in materials and method section. The mice continuously feed for 4 months developed obesity whereas mice feed for continuous 9 months develop severe form of obesity along with extreme hypertension. As expected, weight gain together with a loss of physical activity was observed in the mice fed with high fat diet for consecutive 4 months. The mice develop 1.8 (63 ± 3 2) times more weight than the control mice (35 ± 1 g) which are fed with normal diet. To determine the further effect of obesity the mice are fed with high fat diet for continuous 9 months and we observed morbid obesity of weight gain (142 ± 2 g) when compared with similar age control mice (41 ± 3 g). From physical observation, the mice show weakness and fatigue together with breathing difficulties. The symptoms occur due to lipids deposition in heart, which makes them to feel harder to pump the blood throughout the body and thereby initiating hypertension associated organ damage. As a symptom of renal damage we also observed decreased in urine output volume (40% reduction) and swelling of hind legs which are able to predict the renal damage.
3.2 Remodeling of cardiac and renal tissue following obesity associated hypertension
The obesity burden and leptin injection for a continuous period induce cardiac remodeling and renal damage, and it was clearly observed in the histological image of initial and advanced stage of obesity associated hypertension (Fig. 1A-F). The basic histological observation of the control heart tissue (12 month old mice) shows that the myocardium fibers are arranged in very compact pattern with a clear nucleus (Fig. 1A). The mice with initial stage of obesity associated hypertension develops cardiac remodeling with early degeneration of heart tissue with loosen structures (Fig. 1B). The pathology of the cardiac tissue adverse as the diet, prolonged for continuous 9 months with more degeneration and tissue hardening (Fig. 1C). Similarly, the control kidney cells are more compactly arranged (Fig. 2D) but soon it develops initial obesity condition, the fibrosis starts to appears (Fig. 2E) and in advanced stages the renal tissues are subjected to significant injury (Fig. 2F).
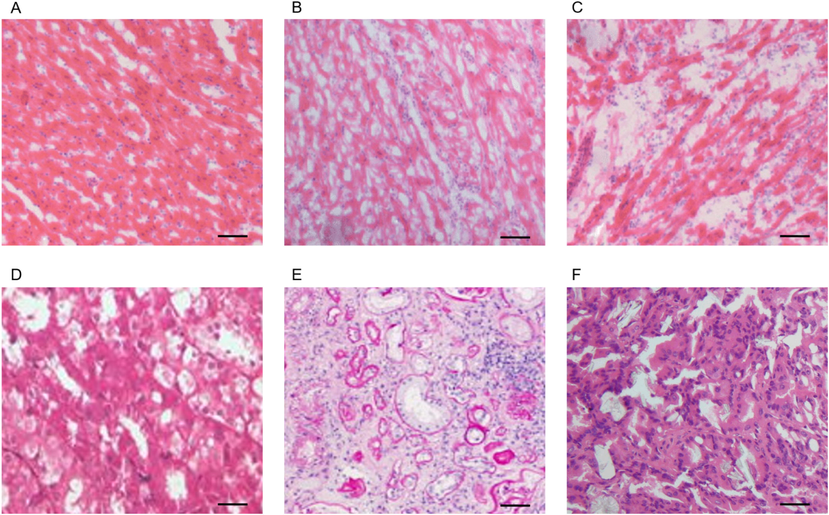
- Remodeling of heart and renal tissue following obesity induced hypertension: A.) histological section of control heart tissue with compact packing of cells with nucleus close to each other. B.) Initial obesity induced cardiac tissue degeneration. C.) Damage of cardiac tissue with tissue hardening following critical hypertension. D.) Histological section of control renal tissue with regular arrangement of renal cells. E.) Initial obesity induced renal tissue degeneration. F.) Damage of renal tissue with tissue hardening following critical obesity condition. Scale = 50 µm.
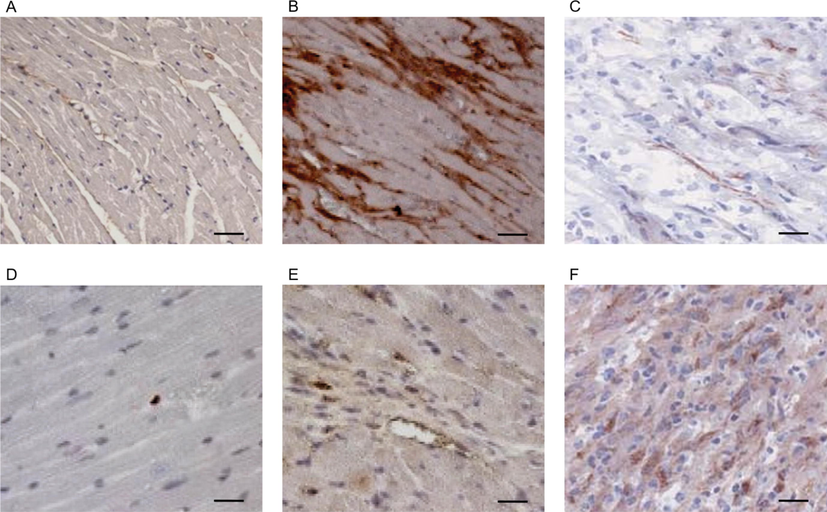
- Expression level of HMGB1 and COX2 in different pathological stages of obesity induced hypertension in cardiac tissue: A.) control heart tissue of age 9 months’ mice with limited expression of HMGB1. B.) High fat diet-induced initial obesity stage shows upregulated expression of HMGB1 in cardiac tissue. C.) Regular intake of high fat diet for 9 months results with critical obesity which shows down regulated expression of HMGB1 in cardiac tissue. D.) Control mice with 9 months old heart tissue show optimum expression of COX2. E.) Initial obesity mice shows a moderate expression pattern of COX2 in cardiac tissue. F.) Critical stage of obesity mice showing elevated expression of COX2. Scale = 50 µm.
3.3 Interplay betweenHMGB1expression and organ damage
HMGB1plays a key role in initiating inflammatory response and in the present study, we observed the expression of HMGB1 in the control mice (9 month old mice), which shows very minimal expression (Fig. 2A). But we observed strong overexpression pattern of HMGB1 in initial stages of obesity induced hypertension which shows the diet with high fat for the continuous 4 months, initiate inflammatory response (Fig. 2B) which in overall changes the cardiac microarchitecture. Surprisingly, the expression of HMGB1 is highly reduced in serve damage form of cardiac tissue which are followed with 9 months of high fat diet (Fig. 2C). To confirm it further we analyzed the expression of COX2 in different stages of obesity associated cardiac tissue damage. In control tissue, COX2 expression is undetectable but when damage progress, the expression of COX2 increases depending on the magnitude of damage occurs (Fig. 2D-F).
In order to confirm HMGB1 expression which act similar function in kidney tissue we analyzed its expression level in control, initial and advanced damage stages of obesity induced hypertension. The expression of HMGB1 shows minimal expression in control tissue (Fig. 3A) but like that of in cardiac tissue it shows overexpression pattern in initial damage due to obesity induced hypertension (Fig. 3B). In over damage condition the HMGB1 expression is certainly downregulated (Fig. 3C) but the expression of COX2 shows gradual overexpression pattern following obesity induced hypertension associated kidney damage (Fig. 3D-F).
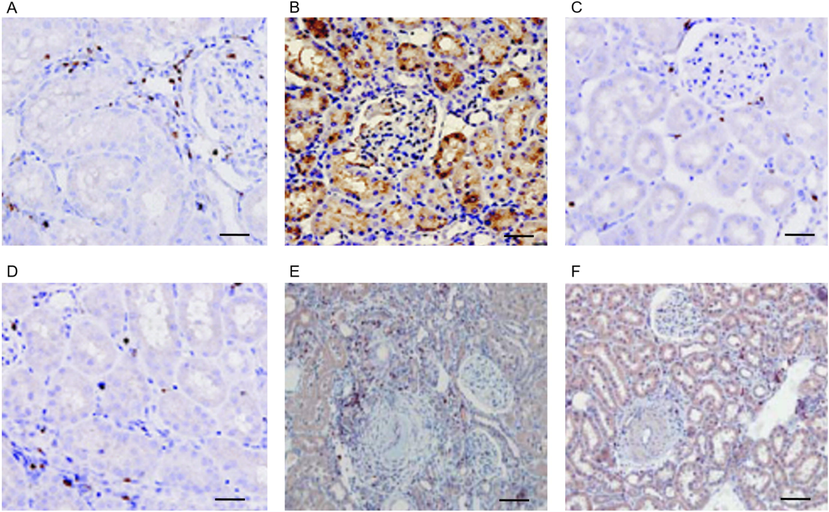
- HMGB1 and COX2 expression studies in obesity induced hypertension associated renal tissue damage: A.) control renal tissue of 9 months old mice showing limited expression pattern of HMGB1. B.) High fat diet-induced initial obesity stage mice shows increased expression of HMGB1 in renal tissue. C.) Regular intake of high fat diet for 9 months followed by high renal damage shows down regulated expression of HMGB1. D.) Control mice with 9 months old renal tissue show optimum expression of COX2. E.) Initial obesity mice shows a moderate expression pattern of COX2 in renal tissue. F.) High damage of renal tissue of critical obesity mice showing elevated expression of COX2. Scale = 50 µm.
3.4 Overexpression of COX2 is not correlated with HMGB1expression
To further conclude our results, the quantitative expression of HMGB1 and COX2 are further cross-checked using western blotting (Fig. 4). The results show that the expression of HMGB1 in initial obesity-induced cardiac and renal tissue shows 4.3 and 3.8 times higher expression than control mice tissue but it is down regulated in advanced stage obesity with only 1.2 times higher expression than control cardiac tissue. Similarly, COX2 shows an upregulated expression pattern in initial obesity-induced cardiac and renal tissue (1.8and 2.1 times higher than control), but as the obesity reach critical stage its expression is gradually upregulated 3.8 and 4.1 times higher expression than control mice of 9 months old as shown in Fig. 4.
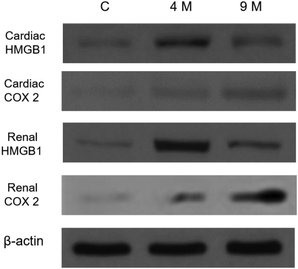
- Validating HMGB1 and COX2 expression level using Western blotting: Lane 1represent the expression level of HMGB1 and COX2 in control heart and renal tissue. Lane 2 shows expression of HMGB1 and COX2 in initial obesity condition. Lane 3 represents the expression of HMGB1 and COX2in cardiac and renal tissue following high fat diet for continuous 9 months. For loading control β-actin was used.
4 Discussion
Obesity and hypertension are interlinked and in both combinations the risk factors for strokes, diabetes, heart disease, organ damages, cancer risks and reproductive disorders are more high. In long term obesity linked heart disease, the remodeling of cardiac tissue occurs with aberrant expression of many genes like Smad6 and TGF-β (Niu and Liu, 2017). Although the regulatory role of HMGB1 are understood in general its specific role in a different pathological stage of obesity-induced cardiac tissue damage is still difficult to address.
Previous studies has an evidence in induced cardiac remodeling following obesity induced hypertension conditions (Pei et al., 2013). In our investigation, we observed that high fat diet of C57BL/6 mice for 4 months, results with the development of cardiac and tissue remodeling along with early degeneration of cardiac tissue (Fig. 1B& E). As the high fat diet, continuous for unto 9 months it develops lethal obesity with significant changes in cardiac and renal tissue with tissue hardening (Fig. 1C& F). The changes that occur during obesity induced hypertension are due to deregulation of many genes and inflammatory mediated chemicals (Lundgren et al., 1996).
Our result with HMGB1 clearly confirms that in the initial stage of obesity-associated cardiac stress the HMGB1 shows increased expression which initiate cardiac and renal remodeling by initiating tissue degeneration (Fig. 2B& B). But after the complete remodeling of cardiac tissue with tissue hardening (Fig. 2C & C) their expression is down regulated, which implies its initial role in cardiac remodeling and renal damage. The expression of HMGB1at different condition shows variant expression pattern with increased expression in developing heart and shows minimal expression in adult stages, but in adult heart failure they are upregulated. Taken together our data shows at the extreme end of heart damage its expression is controlled, but in the initial stages of cardiac damage which is associated with obesity induced hypertension, HMGB1 shows higher expression together with cardiac remodeling by activating many genes involved with it.
The expression of COX2 is well documented in inflammatory condition and in the current study the experimental and organ damage initiated with obesity induced hypertension are cope up with gradual upregulation of COX2. The data obtained are crosschecked along with COX2 which directly aid in confirming the role of HMGB1 in organ damage. Similarly, it was reported that the upregulated expression of COX2 occurs with increased production of H2S which worsen the ischemic injury (Hu et al., 2008). Our results also support their findings that its expression in initial obesity is elevated, which impair the cardiac tissue condition that results in tissue remodeling.
5 Conclusions
In overall, the results studies conclude that the expression of HMGB1 and COX2 shows increased expression in initial obesity induced hypertension associated cardiac and renal stress which pays way for disease progression. After attaining overall organ damagesHMGB1 expression is down regulated, which shows its critical role in initiating cardiac and renal damage.
Funding support
This work was supported by The Natural Science Foundation of China (No. 81600266 to LC Yang), Shanghai Committee of Science and Technology of China (16ZR1422300 to LC Yang)
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Lower working memory performance in overweight and obese adolescents is mediated by white matter microstructure. J. Int. Neuropsychol. Soc.. 2016;22:281-292.
- [Google Scholar]
- Central vs. peripheral leptin excess in the pathogenesis of obesity-associated hypertension. J. Hypertens.. 2008;26:827-828.
- [Google Scholar]
- Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer. 2004;4:579.
- [Google Scholar]
- The nuclear protein HMGB1 as a proinflammatory mediator. Eur. J. Immunol.. 2004;34:1503-1512.
- [Google Scholar]
- Teasing and social rejection among obese children enrolling in family-based behavioural treatment: effects on psychological adjustment and academic competencies. Int. J. Obes.. 2012;36:35.
- [Google Scholar]
- Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ. Res.. 2015;116:991-1006.
- [Google Scholar]
- Cyclooxygenase-2 mediates the delayed cardioprotection induced by hydrogen sulfide preconditioning in isolated rat cardiomyocytes. Pflügers Arch. J. Physiol.. 2008;455:971-978.
- [Google Scholar]
- The metabolic syndrome, LDL particle size, and atherosclerosis: the Atherosclerosis and Insulin Resistance (AIR) study. Arterioscler. Thromb. Vasc. Biol.. 2000;20:2140-2147.
- [Google Scholar]
- Body mass index and blood pressure in Korean men and women: the Korean National Blood Pressure Survey. J. Hypertens.. 1994;12:1433-1437.
- [Google Scholar]
- Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840.
- [Google Scholar]
- Elaboration of type-1 plasminogen activator inhibitor from adipocytes: a potential pathogenetic link between obesity and cardiovascular disease. Circulation. 1996;93:106-110.
- [Google Scholar]
- Obesity and insulin resistance induce early development of diastolic dysfunction in young female mice fed a Western diet. Endocrinology. 2013;154:3632-3642.
- [Google Scholar]
- Ten putative contributors to the obesity epidemic. Crit. Rev. Food Sci. Nutr.. 2009;49:868-913.
- [Google Scholar]
- Coregulatory proteins in steroid hormone receptor action: the role of chromatin high mobility group proteins HMG-1 and-2. Steroids. 1999;64:576-586.
- [Google Scholar]
- Activation of human umbilical vein endothelial cells leads to relocation and release of high-mobility group box chromosomal protein 1. Scand. J. Immunol.. 2004;60:566-573.
- [Google Scholar]
- The aberrant expression of Smad6 and TGF-β in obesity linked cardiac disease. Eur. Rev. Med. Pharmacol. Sci.. 2017;21:138-142.
- [Google Scholar]
- Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol. Metab. Syndr.. 2011;3:17.
- [Google Scholar]
- Cardiac-derived adiponectin induced by long-term insulin treatment ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic mice via AMPK signaling. Basic Res. Cardiol.. 2013;108:322.
- [Google Scholar]
- Neural mechanisms in human obesity-related hypertension. J. Hypertens.. 1999;17:1125-1133.
- [Google Scholar]
- Biochemical and ultrastructural cardiac changes induced by high-fat diet in female and male prepubertal rabbits. Anal. Cell. Pathol.. 2018;2018
- [Google Scholar]
- Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann. Intern. Med.. 2001;134:1-11.
- [Google Scholar]
- Myocardial infarction and ischemic heart disease in overweight and obesity with and without metabolic syndrome. JAMA Intern Med. 2014;174:15-22.
- [Google Scholar]
- World Health Organization: Obesity and overweight. Fact Sheet N 311. 2014. Ref Type Online Source, 2016.







