Translate this page into:
Maslinic acid and gallic acid protective efficacy on lipids, lipoproteins and lipid metabolizing enzymes against isoproterenol administered cardiotoxicity: An in vivo and in silico molecular docking evidences
⁎Corresponding author. salthaf@ksu.edu.sa (Althaf Hussain Shaik)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Objective
The current study designed to investigate the protective efficacy of maslinic acid (MA) and gallic acid (GA) against isoproterenol (ISO) administered cardiac toxicity in rats by exploring in vivo and in silico approaches.
Methods
The animals were divided into five different groups. The individual animal groups were orally pretreated with MA (15 mg/kg) and GA ((15 mg/kg) for 7 days and ISO administered subcutaneously on 8th and 9th days to induce MI. Blood, heart and liver were collected from sacrificed animals and used for biochemical analysis.
Results
The results represented significant decrease in the levels of high density lipoprotein cholesterol, lipoprotein lipase and lecithin cholesterol acyl transferase whereas significant increase in the levels of total cholesterol, triglycerides, low density lipoprotein cholesterol, very low density lipoprotein cholesterol and HMG-CoA reductase in ISO (85 mg/kg) treated rats. However, pretreatment of ISO treated rats with MA and GA markedly brought all the parameters such as lipids, lipoproteins and lipid metabolic marker enzymes to near normal level indicating the ameliorating effect of MA and GA against MI. Further, the investigation extended to in silico analysis to study the interaction of MA and GA with the key lipid metabolizing enzymes by using Gold 3.0.1 software. The molecular docking studies revealed that HMG-CoA reductase, LPL and LCAT formed strong enzyme ligand complexes with MA and GA.
Conclusions
MA and GA exhibited protective efficacy against ISO administered cardiotoxicity and the results further supported by molecular docking studies.
Keywords
Maslinic acid
Gallic acid
Isoproterenol
Lipids
Lipid metabolic marker enzymes
Molecular docking
1 Introduction
Myocardial infarction (MI) also known as heart attack, is the world’s population leading cause of death. It occurs due to the improper blood supply to the heart muscle that implies to the necrosis of myocardium. Multiple risk factors like sedentary lifestyle, stress, smoking, alcohol consumption, obesity, diabetes and blood pressure are suitable causes of MI. Asides, hypercholesterolemia, hyperlipidemia and hypertriglyceridemia together lead to the development of atherosclerosis and MI (Yang et al., 2008). It is also evidenced that the key lipid metabolizing enzymes HMG-CoA reductase and lipoprotein lipase (LPL) takes a crucial part in the progression of MI. Lecithin cholesterol acyl transferase (LCAT) is an enzyme that esterifies the cholesterol on the surface of high density lipoprotein cholesterol (HDL-C) and leads to the formation of giant HDL-C particles which protects from cardiovascular diseases (CVD) (Shaik et al., 2018).
Isoproterenol (ISO) is a powerful potent β-adrenergic agonist that has intense chronotropic and inotropic properties. It causes severe stress in cardiac tissue and develops necrosis of heart muscle (Liu et al., 2009). Several mechanisms have been proposed by which ISO causes MI. ISO enhances the free radical formation which causes increased lipid peroxidation (LPO). This increased LPO causes infarct-like necrosis of the heart muscle (Prince and Rajadurai, 2005). The heart failure subsequent to MI has been proved to be connected with the deficiency of antioxidants and augmented myocardial oxidative stress (Hill and Singal, 1996).
Bioinformatics is the computational technique applied to solve the biological difficulties by structural prediction and molecular docking studies of biomolecules like genes, nucleic acids and proteins. Docking software is useful in the evaluation of drug design and in the prediction study of drug binding to the active site of enzyme. Depending on the molecular docking results, designers have the feasibility to refine the molecules of drugs.
CVD prevention is associated with the utilization of fruits and green vegetables that enriched with natural antioxidants. The ameliorative property of plants may be due to triterpenoids. The triterpene compounds represented different physiological activities including anti-atherosclerotic, anti-inflammatory, anti-cancer, anti-diabetic, antiulcerogenic, hypolipidaemic and hepatoprotective (Dzubak et al., 2006). Maslinic acid (MA), a natural triterpenoid extensively distributed in aerial parts of medicinal plants such as leaves and fruits (Montilla et al., 2003). MA has been reported for many pharmacological properties like antioxidant, anti-hyperlipidaemic, anti-hyperglycemic and anti-inflammatory activities. In our earlier studies we reported the cardioprotection of MA and Gallic acid (GA) on antioxidant enzyme paraoxonase (Hussain Shaik et al., 2012) and myocardial membrane bound ATPase enzymes (Shaik et al., 2020). This study has been planned to discover the protective efficacy of MA and GA on lipid metabolizing enzymes against ISO administered cardiotoxicity by analyzing in vivo and in silico approaches.
2 Material and methods
2.1 Chemicals and drugs
The active compound MA was purchased from Cayman chemical company, United States of America. ISO and GA were procured from Sigma chemicals, United States of America. All other chemicals and reagents used were analytical grade.
2.2 Animals and experimental procedure
The rats of Albino Wistar strain weighing around 150 g were acclimatized to standard animal house conditions for one week. Standard food provided and water ad libitum. The animal experimental study got approval from Institutional Animal Ethics Committee, Sri Krishnadevaraya University, India with registration number “Ref.No. 470/01/a/CPCSEA”.
The rats categorized into 5 groups contained 8 in each group.
-
Control animals
-
Animals fed with 15 mg/kg MA
-
Animals subcutaneously injected with 85 mg/kg ISO
-
Animals fed with 15 mg/kg MA + ISO
-
Animals fed with 15 mg/kg GA + ISO
The rats orally pretreated with MA for 7 days by dissolved in sodium carboxymethyl cellulose (0.05%), whereas saline used to dissolve the positive control GA. The control group and ISO group rats were treated with sodium carboxymethyl cellulose. ISO was dissolved in distilled water and subcutaneously injected on 8th and 9th days to induce MI. All animals sacrificed on 10th day by cervical decapitation. Blood, heart and liver samples were collected for biochemical analysis.
2.3 Biochemical estimations
The lipids and lipoproteins such as total cholesterol (TC), triglycerides (TG), and HDL-C in heart tissue were assayed by using standard diagnostic kits. The following formulae used to calculate the levels of very low density lipoprotein cholesterol (VLDL-C) and low density lipoprotein cholesterol (LDL-C).
LCAT activity was assayed in serum (Nagasaki and Akanuma, 1977). Lipoprotein lipase (LPL) activity was assayed in heart and liver tissue homogenate (Shirai and Jackson, 1982). 3-hydroxy-3-methylglutaryl-coenzyme-A (HMG-CoA) reductase enzyme activity was assayed in heart, liver and serum (Rao and Ramakrishnan, 1975). The protein level was measured by Lowry et al. (n.d.).
2.4 Molecular docking studies
Molecular docking of MA and GA with HMG-CoA reductase, LPL and LCAT was performed. Structures prepared by obtained the crystal structure from protein data bank (HMG-CoA-1HW9 Istvan and Deisenhofer, 2001; LPL-6E7K Birrane et al., 2019; LCAT-4X96 Glukhova and Tesmer, 2015). For docking, active sites of these enzymes were predicted using Castp server (Andrew et al., 2003). MA and GA compound structures constructed and optimized in Chemsketch software (ChemSketch, 2020). The statins occupied in the binding sites of enzymes were eliminated. The chain A of enzymes opted and hydrogen atoms were incorporated into the enzymes for molecular docking studies. The binding orientation of MA and GA in enzyme structures were studied by performing molecular docking method using GOLD (Genetic Optimization of Ligand Docking) version 3.0.1 program (Jones et al. 1997). The best energetically conformation of the compound was identified and after docking, individual binding poses were calculated the interactions with the protein. All the protein and ligand structural images were generated using PyMol (http://www.pymol.org) (DeLano, 2006).
2.5 Statistical analysis
All results data was statistically analyzed by one way analysis of variance (ANOVA) and Duncan’s Multiple Range (DMR) test. The comparison among groups considered statistically significant at p < 0.05.
3 Results
Table 1 represents MA and GA protective effect on cardiac lipids and lipoproteins in control and ISO treated rats. ISO significantly increased (P < 0.05) the concentrations of TC, TG, LDL-C, and VLDL-C whereas significantly decreased (p < 0.05) the concentrations of heart HDL-C when compared with those of normal rats. Pretreatment with MA (15 mg/kg) to ISO injected rats decreased the levels of heart TC, TG, LDL-C, and VLDL-C, whereas the levels of HDL-C in heart of ISO-induced myocardial infarcted rats increased when compared with untreated ISO-induced myocardial infarcted rats. GA (15 mg/kg) also decreased the levels of heart TC, TG, LDL-C, and VLDL-C, whereas the levels of heart HDL-C increased significantly (p < 0.05). MA (15 mg/kg) alone not exhibited any significant change in lipids and lipoproteins in comparison with control rats. Values are mean ± S.D. (n = eight rats). Values not shared a common superscript (a, b, c and d) differ significantly from each other (p < 0.05, Duncan’s multiple range test).
Groups
TC (mg/dL)
TG (mg/dL)
LDL (mg/dL)
VLDL (mg/dL)
HDL (mg/dL)
Control
5.4 ± 0.4a
4.3 ± 0.3a
2.0 ± 0.4a
0.8 ± 0.07a
2.5 ± 0.3a
MA (15 mg/Kg bw)
5.1 ± 0.4a
4.2 ± 0.3a
1.7 ± 0.3a
0.8 ± 0.06a
2.5 ± 0.1a
ISO (85 mg/Kg bw)
10.2 ± 0.4b
7.3 ± 0.4b
7.2 ± 0.6b
1.4 ± 0.08b
1.5 ± 0.1b
MA (15 mg/Kg bw) + ISO
6.3 ± 0.2c
5.0 ± 0.2c
2.9 ± 0.4c
1.0 ± 0.04c
2.3 ± 0.2a
GA (15 mg/Kg bw) + ISO
7.0 ± 0.2d
5.9 ± 0.1d
3.5 ± 0.1d
1.1 ± 0.03d
2.2 ± 0.1a
Fig. 1 shows the effect of MA and GA on serum LCAT in control and ISO administered rats. Serum LCAT levels decreased significantly (p < 0.05) in ISO administered rats when compared with control rats, while MA (15 mg/kg) pretreatment and GA (15 mg/kg) pretreatment increased the levels of LCAT significantly (p < 0.05) as compared to ISO-induced myocardial infarcted rats.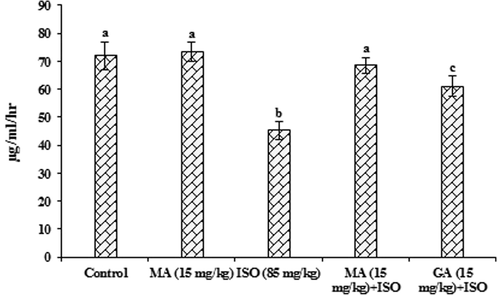
Protective effect of maslinic acid (MA) and gallic acid (GA) on lecithin cholesterol acyl transferase (LCAT) in heart of isoproterenol (ISO) induced cardiac toxicity in rats. Values are mean ± S.D. (n = eight rats). Values not shared a common superscript (a, b and c) differ significantly from each other (p < 0.05, Duncan’s multiple range test).
Fig. 2 represents the effect of MA and GA on the activity of LPL in heart and liver of control and ISO administered rats. Animals injected with ISO exhibited significant (p < 0.05) decrease in the activity of LPL when compared to control rats. Oral administration of MA (15 mg/kg) and GA (15 mg/kg) to ISO-induced MI rats increased the activity of LPL significantly (p < 0.05) when compared to ISO alone administered rats.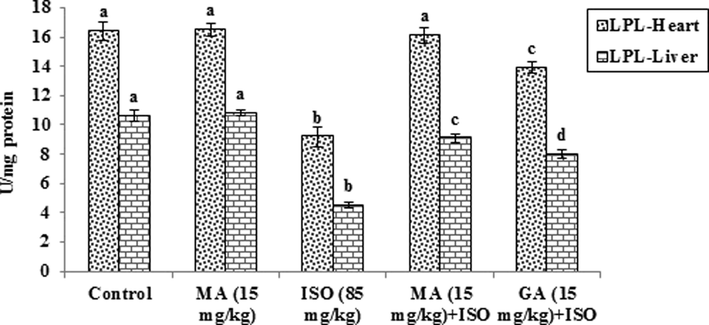
Protective effect of maslinic acid (MA) and gallic acid (GA) on lipoprotein lipase enzyme in heart and liver of isoproterenol (ISO) induced cardiac toxicity in rats Values are mean ± S.D. (n = eight rats). Values not shared a common superscript (a, b, c and d) differ significantly from each other (p < 0.05, Duncan’s multiple range test).
Fig. 3 reveals the effect of MA and GA on HMG-CoA reductase activity in heart, liver and plasma of rats. The enzyme in heart, liver and plasma decreased significantly (p < 0.05) in ISO administered rats when compared to control rats. MA (15 mg/kg) pretreatment to ISO group showed significant (p < 0.05) rise and GA (15 mg/kg) pretreatment to ISO group also showed significant (p < 0.05) rise in the activity of HMG-CoA reductase when compared with ISO alone administered group. The low ratio of HMG-CoA/mevalonate represents the high activity of HMG CoA reductase enzyme and vice versa.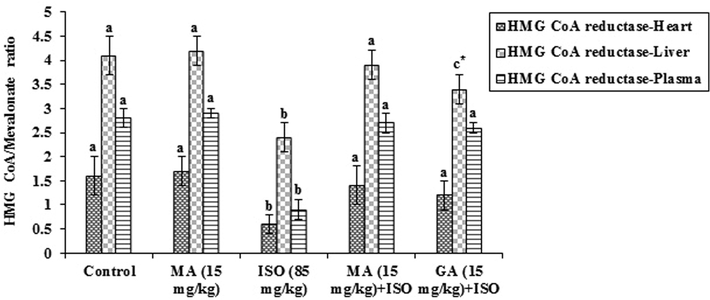
Protective effect of maslinic acid (MA) and gallic acid (GA) on HMG-CoA reductase enzyme in heart, liver and plasma of isoproterenol (ISO) induced cardiac toxicity in rats. Values are mean ± S.D. (n = eight rats). Values not shared a common superscript (a, b and c) differ significantly from each other (p < 0.05, Duncan’s multiple range test). * Group not significantly differs with MA (15 mg/Kg) + ISO treated group.
Fig. 4 A and B illustrated the molecular docking studies of the active compounds MA and GA with the enzyme HMG-CoA reductase. From the molecular docking of MA into active site of HMG-CoA reductase (Table 2), hydrogen bond observed between the hydroxyl group (H44) of MA and oxygen atom of ARG452, and other hydrogen bond noticed between hydroxyl group of LEU509 and oxygen atom (O5) of MA. In docking of GA with HMG-CoA reductase (Table 2), hydrogen bond observed between the hydroxyl group of ARG86 and oxygen atom (O3) GA, and other hydrogen bond noticed between hydroxyl group of LYS88 and oxygen atom (O4) of GA.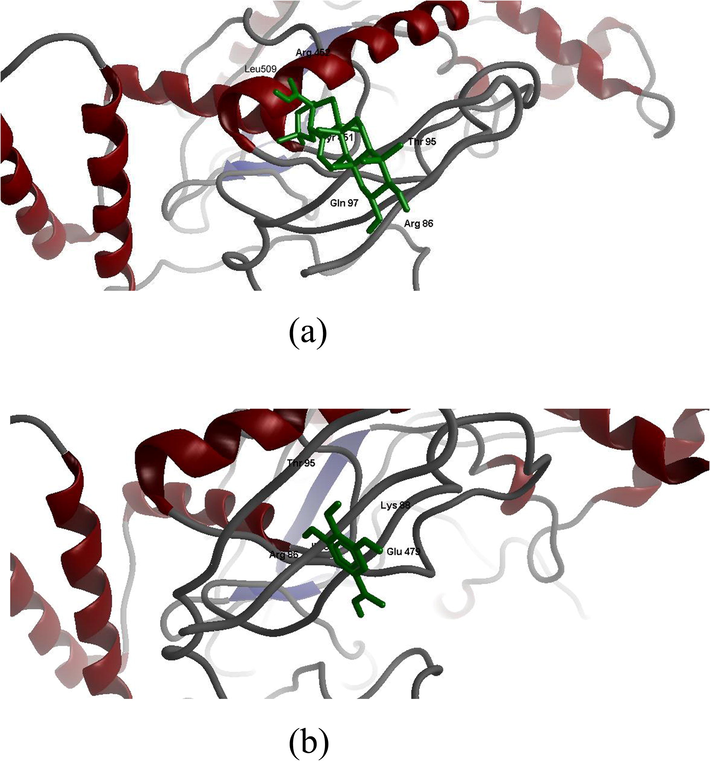
A and B. Molecular Docking of maslinic acid (MA) and gallic acid (GA) with HMG-CoA reductase enzyme.
Enzyme
Molecule/Ligand
Number of Hydrogen Bonds
Atoms
Bond Length (Å)
Docking Score (Kj/mol)
Protein
Molecule
HMG-CoA reductase
MA
1
ARG452(H)
LEU-509(O)1(O)
5(O)1.820
1.45−108.65
−107.26
GA
2
ARG86(H)
LYS88 (H)3(O)
4(O)2.350
2.365−105.45
−105.65
LPL
MA
2
CYS54(H)
3(O)
1.125
−118.35
GA
2
CYS54(H)
3(O)
1.426
−111.32
LCAT
MA
1
ARG99 (H)
GLU37 (H)3 (O)
3 (O)1.342
1.242−128.43
−118.35
GA
1
GLU37 (H)
LYS105(H)3 (O)
5(O)1.272
1.284−126.40
−123.58
Fig. 5 A and B showed the docking studies of the active compounds MA and GA with LPL enzyme. MA docked with hydrogen bond forming between the hydroxyl group of CYS54 and oxygen atom (O3) of LPL enzyme. Also Molecular docking of GA into active site of LPL showed hydrogen bond between the hydroxyl group of CYS54 and oxygen atom (O3) of GA as MA.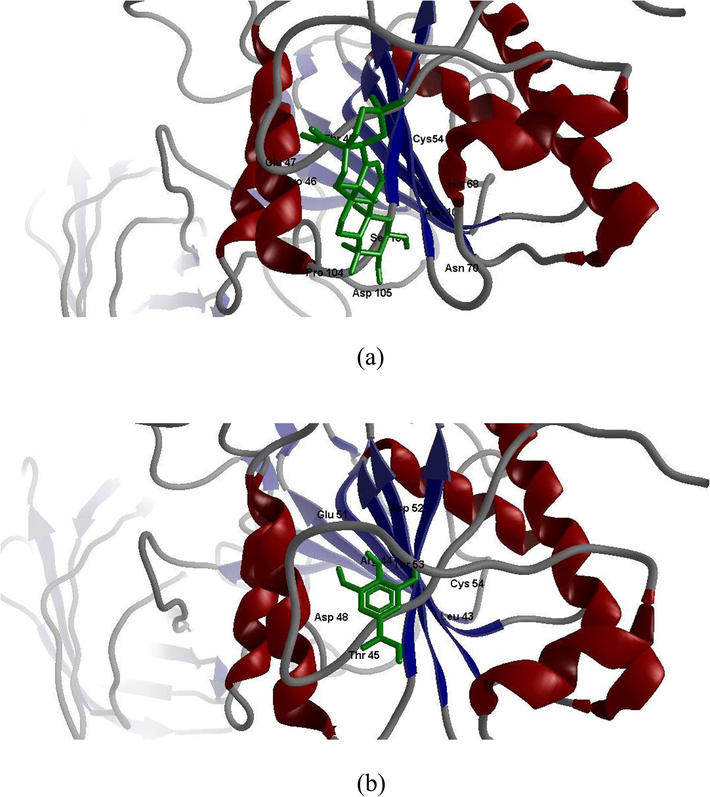
A and B. Molecular Docking of maslinic acid (MA) and gallic acid (GA) with lipoprotein lipase (LPL) enzyme.
From the Fig. 6 A and B, it was confirmed that MA and GA are the active inhibitor compounds of the enzyme LCAT. In docking studies of MA with LCAT, hydrogen bond is observed between the hydroxyl group of ARG99 and oxygen atom (O3) of MA. Another hydrogen bond formed between oxygen atom of GLU37 and oxygen atom (O3) of MA. In docking studies of GA with LCAT, two hydrogen bonds observed between the hydroxyl groups of GLU37 and oxygen atom (O3) of GA. Another hydrogen bond formed between hydroxyl group of LYS105 and oxygen atom (O5) of GA.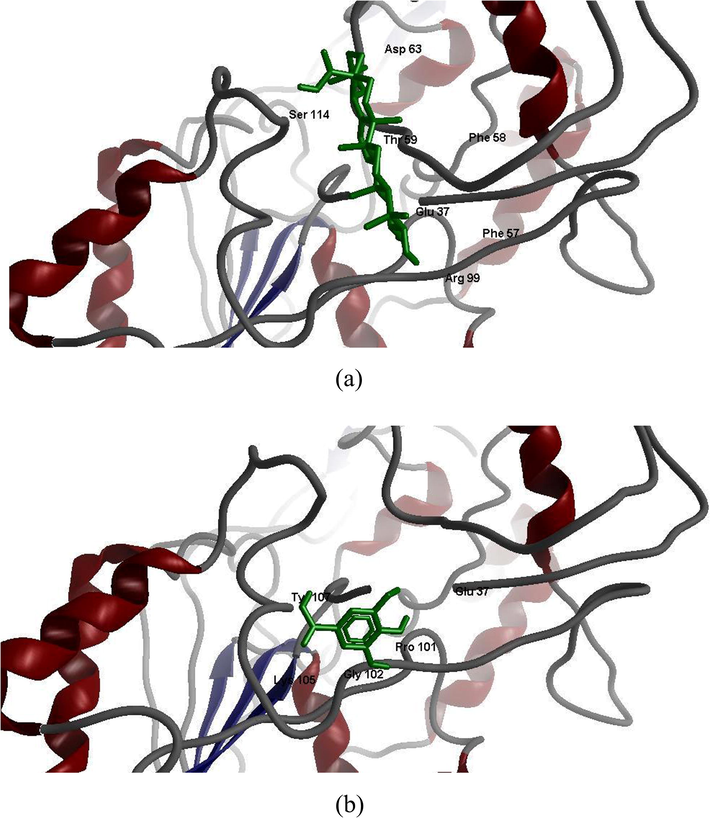
A and B. Molecular Docking of maslinic acid (MA) and gallic acid (GA) with lecithin cholesterol acyl transferase (LCAT) enzyme.
4 Discussion
ISO induced MI considered to be highly advantageous when compared with physical occlusion of coronary artery where ISO exerts intense inotropic and chronotropic actions to induce MI and also there is a possibility of reperfusion after ISO administration (Saeed and Ahmed, 2006). ISO induced MI in rats is a well-established model to analyze to protective effect of cardioprotective drugs (Zhou et al., 2008).
Lipids and lipoproteins are independent risk factors for CVD. Lipid profile is a crucial factor in the progression and pathogenesis of MI. Hypercholesterolemia and hypertriglyceridemia are considered to be the major risk factors in developing the MI. In our results the levels of TC, TG, LDL-C and VLDL-C were increased in heart whereas the levels of HDL-C in heart were decreased in ISO treated rats. Previously described that rise in cardiac cholesterol in ISO administered rats is due to augmented absorption of LDL-C by myocardial membranes from the blood circulation (Anandan et al., 2007). The noticed rise in TGs might be due the reduced activity of LPL and reduced uptake of TGs from blood circulation (Sushama Kumari et al., 1990). Rats pretreated with MA and GA showed decreased content of heart TC, TG, LDL-C, VLDL-C and increased content of HDL-C indicating the beneficial effects MA and GA in reducing hyperlipidemia caused by ISO administration which may be due to MA and GA anti-hypercholesterolemic and anti-hyperlipidemic activity.
The results represented that MA and GA raised the activity of LCAT in ISO treated rats. It is proposed that LCAT promotes the process Reverse Cholesterol Transport (RCT) which considered as antiatherogenic mechanism (Glomset et al., 1966). In this process HDL molecules removed the excess amount of cholesterol from the cells and excreted by delivered to liver (Glomset, 1968). MA and GA raised the levels of LCAT which directly raises the levels of HDL-C in ISO treated rats. HDL molecule is heterogeneous and contains several different lipoprotein particles. HDL-C accommodating apoA-I considered as more effective in RCT (Barbaras et al., 1988). LCAT activity is highly enhanced by consisting the potent cofactor ApoA-I, and the activity of LCAT is sensitive with modulating the size of apoA-I. The diet induced atherosclerosis has been prevented by the overexpression of LCAT in transgenic rabbits (Hoeg et al., 1996). MA and GA pretreatment accelerated the activity of LCAT in ISO administered rats. Thus, the noticed acceleration of LCAT might be due to the obstacle of LPO in MA and GA pretreated ISO induced rats.
The TGs like chylomicrons and VLDL-C in plasma lipoproteins hydrolyzed by LPL and causes changes in lipoprotein metabolism which stimulates hepatic removal of lipoproteins. Based on the earlier reports it is considered as, an increased levels in LPL are anti-atherogenic and the decreased levels in LPL are atherogenic (Miesenbock et al., 1993; Reymer et al., 1995). It is reported that several LPL-deficient patients have developed relatively advanced atherosclerosis (Kareem et al., 2009). In our report pretreatment with MA and GA enriched the activity of LPL in ISO treated rats. This is due to the acceleration of LPL activity by MA and GA.
HMG-CoA reductase is a key metabolic enzyme of cholesterol biosynthesis. The significant increased activity of HMG-CoA reductase in heart, liver and plasma of ISO treated rats was noticed in this study. The increased activity leads to the development of atherosclerosis by accumulating cholesterol in the form of foam cells (Berliner and Heinecke, 1996). Cellular cholesterol homeostasis is an important factor in the eradication of MI. Our results showed that pretreatment of MA and GA regulated the biosynthesis of cholesterol by the inhibition of HMG-CoA reductase in ISO treated rats. Thus, the reduced quantity of cholesterol in MA and GA treatment might be correlated to the reduced HMG-CoA reductase activity in ISO treated rats. The potent antioxidant property of MA and GA (Shaik et al., 2020) indirectly supports to the reduction of lipid levels, by the inhibition of LPO.
The present in vivo studies are also supported with molecular docking results of in silico studies where MA and GA showed the effective binding with the enzymes HMG-CoA reductase, LPL and LCAT by which it regulates the activity of these enzymes. Molecular docking study conveyed that MA and GA are the excellent ligand molecules towards HMG-CoA reductase, LPL and LCAT enzymes. The inhibition of HMG-CoA reductase enzyme by MA and GA might be due to strong hydrogen bonds formation between ligand and amino acids of enzyme which insisted to the inhibition of enzyme. It is evidenced from this study that, the inhibition of HMG-CoA reductase and regulation of LPL, LCAT by MA and GA leads to reduction in the biosynthesis of cholesterol. The RMSD value of the best hit (with the lowest L_RMSD) based on shape complementarity. The root mean squares deviation (RMSD) between the C-alpha atom of the template and the model was 0.89 Å, indicating close homology. The generated pdb file analyzed for their binding conformations. Analysis was based on E total or free energy of binding, lowest docked energy, and calculated RMSD values. Free binding energy between the model and template was 0.92 Å, which again showed the close homology. The results obtained from protein–ligand docking algorithms are in general satisfactory. The total clusters of docking conformations, with the top 30 docked molecules showed negative binding energies. Cluster one shows the following results, Energy −1.873230e + 02, RMS −1.00. IC50 Values were all within range. Among all docking conformations cluster rank 10 i.e., solution16 gave the best predicted binding free energy of −1.6083 kcal mol − 1 of HMG-CoA reductase and regulation of LPL, LCAT by MA and GA.
5 Conclusions
In conclusion, MA and GA revealed protective efficacy of anti-hypercholesterolemic, anti-hyperlipidemic and lipid metabolism regulatory activities. Furthermore, the molecular docking studies confirmed that the compounds MA and GA effectively act on the key lipid metabolizing enzymes and offered cardioprotection.
Acknowledgements
The authors express gratitude to the Deanship of Scientific Research (DSR), King Saud University, Saudi Arabia for supporting the present work through the fund of Research Group (RG- 1438-058).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Protective effect of n-3 polyunsaturated fatty acids concentrate on isoproterenol-induced myocardial infarction in rats. Prostaglandins Leukot. Essent. Fat. Acids. 2007;76:153-158.
- [CrossRef] [Google Scholar]
- Computed Atlas of Surface Topography of proteins. Nucleic Acids Res. Jul 1. 2003;31(13):3352-3355.
- [CrossRef] [Google Scholar]
- Barbaras, R., Puchois, P., Grimaldi, P., Barkia, A., Fruchart, J.C., Ailhaud, G., 1988. HDL receptor and reverse cholesterol transport in adipose cells. pp. 271–277. https://doi.org/10.1007/978-1-4613-0733-4_34
- J.A. Berliner J.W. Heinecke The role of oxidized lipoproteins in atherogenesis 1996 Biol. Med Free Radic 10.1016/0891-5849(95)02173-6.
- Structure of the lipoprotein lipase-GPIHBP1 complex that mediates plasma triglyceride hydrolysis. Proc. Natl. Acad. Sci. USA. 2019;116:1723-1732.
- [CrossRef] [Google Scholar]
- ChemSketch/ ACD version 2020.1.2, Advanced Chemistry Development, Inc., Toronto, ON, Canada, www.acdlabs.com, 2020.
- The PyMOL Molecular Graphics System. SanCarlos, CA, USA: DeLano Scientific; 2006. http://www.pymol.org
- Pharmacological activities of natural triterpenoids and their therapeutic implications. Prod. Rep. Nat. 2006
- [CrossRef] [Google Scholar]
- The plasma lecithins:cholesterol acyltransferase reaction. J. Lipid Res.. 1968;9:155-167.
- [Google Scholar]
- Role of plasma lecithin:cholesterol acyltransferase in the metabolism of high density lipoproteins. J. Lipid Res.. 1966;7:638-648.
- [Google Scholar]
- Low resolution crystal structure of Lecithin: Cholesterol Acyltransferase (LCAT; residues 21–397) Nat. Commun.. 2015;6 6250 6250
- [Google Scholar]
- Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292:1160-1164.
- [CrossRef] [Google Scholar]
- Antioxidant and oxidative stress changes during heart failure subsequent to myocardial infarction in rats. Am. J. Pathol.. 1996;148:291-300.
- [Google Scholar]
- Overexpression of lecithin:cholesterol acyltransferase in transgenic rabbits prevents diet-induced atherosclerosis. Proc. Natl. Acad. Sci. USA. 1996;93:11448-11453.
- [CrossRef] [Google Scholar]
- Maslinic acid protects against isoproterenol-induced cardiotoxicity in albino Wistar rats. J. Med. Food. 2012;15
- [CrossRef] [Google Scholar]
- Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol.. 1997;267:727-748.
- [CrossRef] [Google Scholar]
- Effect of aqueous extract of nutmeg on hyperglycaemia, hyperlipidaemia and cardiac histology associated with isoproterenol-induced myocardial infarction in rats. Trop. J. Pharm. Res.. 2009;8
- [CrossRef] [Google Scholar]
- Protective effects of N-acetylcysteine in isoproterenol-induced myocardium injury in rats. Mol. Biol. Rep.. 2009;36:761-765.
- [CrossRef] [Google Scholar]
- Lowry, Randall, R.J., Lewis, A., n.d. Méthode de Lowry. Protein measurement with the Folin phenol reagent, J. Biol. Chem. 1951;193(1):265-75.
- Heterozygous lipoprotein lipase deficiency due to a missense mutation as the cause of impaired triglyceride tolerance with multiple lipoprotein abnormalities. J. Clin. Invest.. 1993;91:448-455.
- [CrossRef] [Google Scholar]
- Antioxidant activity of maslinic acid, a triterpene derivative obtained from Olea europaea. Planta Med.. 2003;69:472-474.
- [CrossRef] [Google Scholar]
- A new colorimetric method for the determination of plasma lecithin-cholesterol acyltransferase activity. Clin. Chim. Acta. 1977;75:371-375.
- [CrossRef] [Google Scholar]
- Preventive effect of Aegle marmelos leaf extract on isoprenaline-induced myocardial infarction in rats: biochemical evidence. J. Pharm. Pharmacol.. 2005;57:1353-1357.
- [CrossRef] [Google Scholar]
- Indirect assessment of hydroxymethylglutaryl-CoA reductase (NADPH) activity in liver tissue. Clin. Chem.. 1975;21:1523-1525.
- [Google Scholar]
- A lipoprotein lipase mutation (Asn291Ser) is associated with reduced HDL cholesterol levels in premature atherosclerosis. Nat. Genet.. 1995;10:28-34.
- [CrossRef] [Google Scholar]
- Role of cyclooxygenase-2 in myocardial infarction and ischaemia. J. Coll. Physicians Surg. Pak.. 2006;16:324-328. https://doi.org/5.2006/JCPSP.324328
- [Google Scholar]
- Maslinic acid ameliorate electrolytes, membrane bound ATPases, antioxidants and histopathology in isoprenaline attenuated myocardial toxicity in rats. J. King Saud Univ. - Sci.. 2020;32:1055-1059.
- [CrossRef] [Google Scholar]
- Terminalia pallida fruit ethanolic extract ameliorates lipids, lipoproteins, lipid metabolism marker enzymes and paraoxonase in isoproterenol-induced myocardial infarcted rats. Saudi J. Biol. Sci.. 2018;25:431-436.
- [CrossRef] [Google Scholar]
- Lipoprotein lipase-catalyzed hydrolysis of p-nitrophenyl butyrate. Interfacial activation by phospholipid vesicles. J. Biol. Chem.. 1982;257:1253-1258.
- [Google Scholar]
- Protective action of aspirin in experimental myocardial infarction induced by isoproterenol in rats and its effect on lipid peroxidation. Indian J. Exp. Biol.. 1990;28:480-485.
- [Google Scholar]
- Increasing oxidative stress with progressive hyperlipidemia in human: Relation between malondialdehyde and atherogenic index. J. Clin. Biochem. Nutr.. 2008;43:154-158.
- [CrossRef] [Google Scholar]
- Cardioprotective effect of fluvastatin on isoproterenol-induced myocardial infarction in rat. Eur. J. Pharmacol.. 2008;586:244-250.
- [CrossRef] [Google Scholar]







