Translate this page into:
Maslinic acid ameliorate electrolytes, membrane bound ATPases, antioxidants and histopathology in isoprenaline attenuated myocardial toxicity in rats
⁎Corresponding author. salthaf@ksu.edu.sa (Althaf Hussain Shaik)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
This is first report revealed that Maslinic acid ameliorate electrolytes, membrane bound ATPases, antioxidants and histopathology against isoproterenol induced cardiotoxicity which is useful for the drug discovery against heart attack.
Abstract
Objectives
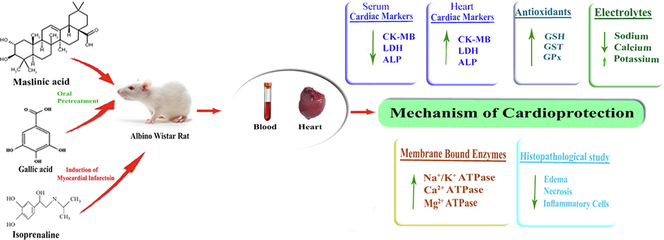
Maslinic acid (MA), a natural compound widely distributed in many fruits and vegetables exhibited vast biological properties. Myocardial infarction (MI) is the most common cause of cardiovascular diseases morbidity and mortality. In this study cardioprotection of MA against isoprenaline (ISO) induced biochemical and histopathological alterations have been evaluated.
Methods
Rats were pretreated with MA (15 mg/kg) for 7 days and MI was induced with administration of ISO (85 mg/kg) on 8th and 9th days. Gallic acid (15 mg/kg) was used as positive control. Blood and heart were collected on 10th day from sacrificed rats and subjected to biochemical and histopathological analysis.
Results
ISO administration significantly increased the enzymes creatine kinase-MB, lactate dehydrogenase, and alkaline phosphatase in serum whereas significantly decreased the marker enzymes in heart homogenate. ISO administration significantly increased the electrolytes sodium and calcium whereas significantly decreased potassium in heart homogenate. ISO showed a significant decrease in membrane bound adenosine triphosphatase (ATPase) enzymes sodium/potassium, calcium and magnesium ATPase in heart homogenate. ISO also significantly decreased the antioxidants reduced glutathione, glutathione S-transferase and glutathione peroxidase in heart homogenate. Furthermore, pretreatment of MA and GA reduced the effect of ISO significantly on all parameters studied. Furthermore, the cardioprotection of MA was also supported by histopathology.
Conclusions
This is the first report revealed that MA attenuates ISO-induced cardiac toxicity by ameliorating biochemical parameters such as cardiac marker enzymes, electrolytes, membrane bound ATPases and increased the antioxidants. The possible cardioprotective mechanism is due to the stabilization of myocardial membrane and antioxidant activity of MA.
Keywords
Maslinic acid
Gallic acid
Isoprenaline
Membrane bound ATPases
Electrolytes
Antioxidant
1 Introduction
Cardiovascular diseases (CVD) are the world’s leading cause of death. Among CVD, Myocardial infarction (MI) is a major cause of mortality worldwide (Prince et al., 2008). MI also familiar as heart attack occurs due to the insufficient coronary blood supply by myocardial oxygen demand, which results to the myocardial necrosis. Hypertension, diabetes mellitus and oxidative stress are the prime risk factors of MI (Khader et al., 2003). Therefore, it is rationale to search for drugs with antihypertensive, antidiabetic and antioxidant properties for the prevention of MI. Hence, the current study planned to investigate the cardioprotection of MA in Isoprenaline (ISO) attenuated myocardial infarcted rats.
ISO is a synthetic catecholamine acts as a beta-adrenergic agonist that causes molecular and pathological changes in animal heart, which are similar to human MI (Nirmala and Puvanakrishnan, 1996). Hence, ISO administered MI is considered as a standardized model in the discovery of cardioprotective drugs. Several mechanisms have been proposed by which ISO causes MI. ISO undergoes autoxidation, generating reactive oxygen species or oxygen free radicals that alter the levels of antioxidants and causes MI (Rona, 1985).
Modern drugs are effective in the control of CVD, but due to their side effects those drugs utilization is limited. So, there is an urgent need for the discovery of natural phytochemicals as these drugs have no toxic side reactions. Maslinic acid (MA) compound belongs to pentacyclic triterpenes group which has widely distributed in many fruits and vegetables like olive (Guillen et al., 2009) and basil (Guinda et al., 2010). MA exhibited various beneficial effects such as antihyperlipidemic (Jun et al., 2007), antihyperglycemic, antidiabetic (Tang et al., 2008), antioxidant (Montilla et al., 2003), anticancer (Reyes-Zurita et al., 2011), anti-inflammatory (Aladedunye et al., 2008), hepatoprotective (Sheng-lei et al., 2014) activities. MA resembles the structural similarity with oleanolic acid, which has been proved as cardioprotective in ISO-induced cardiotoxicity in rats (Senthil et al., 2007). In our previous preliminary study, we reported the cardioprotective activity of MA on paraoxonase enzyme in ISO administered myocardial necrotic rats (Shaik et al., 2012). In continuation of our research, the present study has been planned to evaluate the cardioprotective effect of MA on membrane ATPase enzymes, antioxidants and electrolytes in ISO administered MI.
2 Material and methods
2.1 Animals
Rats (Male Albino Wistar) of weighing 130–170 g were housed in cages on a light–dark schedule (12/12 h). The animals were accustomed to the local conditions for one week. Animals were provided with standard pellet diet along with water ad libitum. The animal experiments were approved (Registration number 470/01/a/CPCSEA) by the institutional animal ethical committee of S.K. University, India.
2.2 Chemicals & reagents
MA was purchased from Cayman chemicals company, United States of America. ISO was obtained from Sigma chemical company, United States of America. Gallic acid (GA) was procured from SRL Pvt. Ltd., India. Other reagents in this study used were of analytical grade.
2.3 Experimental design
MA dose was fixed by preliminary dose-dependent test. Two doses of MA such as 7.5 and 15 mg/kg were screened in ISO administered animals. The dose 15 mg/kg of MA was effective in lowering the increased levels of serum cardiac marker enzymes. Hence the higher dose 15 mg/kg of MA was selected in the present study. GA was used as positive control. The animals were categorized into 5 groups with 8 in each group.
-
Control animals
-
MA (15 mg/kg) treated animals
-
ISO (85 mg/kg) treated animals
-
MA (15 mg/kg) pretreated animals + ISO
-
GA (15 mg/kg) pretreated animals + ISO
Sodium carboxymethyl cellulose (0.5%) was used to dissolve MA and saline was used to dissolve GA. The compounds were treated to the animals for 7 days by oral gavage. Distilled water was used to dissolve ISO and subcutaneously injected to the rats for two consecutive days. Rats were sacrificed on 10th day by cervical dislocation. Immediately blood was obtained from heart then serum was separated. After procuring the blood, heart homogenate was prepared. The homogenate centrifuged and supernatant collected. The supernatant and serum samples were used for analysis.
2.4 Biochemical assays
Creatine kinase-MB (CK-MB), lactate dehydrogenase (LDH) and alkaline phosphatase (ALP) in serum and heart homogenate were assayed by using diagnostic kits. Sodium and potassium, and calcium electrolytes in heart homogenate were estimated by the method of Trinder (1951), Tietz (1995) respectively. Sodium/Potassium (Na+/K+), Calcium (Ca2+) and Magnesium (Mg2+) ATPase enzymes in heart homogenate were analyzed as described by Bonting (1970), Hjerton and Pan (1983), and Ohnishi et al. (1982) respectively. The antioxidants reduced glutathione (GSH), glutathione-S-transferase (GST) and glutathione peroxidase (GPx) in heart homogenate were determined by the method of Ellman (1959), Habig et al. (1974) and Rotruck et al. (1973) respectively. The protein concentration was analyzed as described by Lowry et al. (1951).
2.5 Histopathology of heart
Formalin (10%) was used for the fixation of heart tissues from all the groups. The samples were processed and paraffin wax was used for embedding. Hematoxylin and eosin stain was applied on 5 µm sized sections then screened light microscopic analysis.
2.6 Statistical study
Data was statistically analyzed by using one way analysis of variance and Duncan’s multiple range test. Result considered statistical significant at p < 0.05.
3 Results
3.1 Effects of MA on cardiac markers
Table 1 illustrates the effects of MA on the levels of marker enzymes CK-MB, LDH and ALP in serum and heart of control and ISO groups. ISO treatment significantly (P < 0.05) elevated the levels of marker enzymes in serum whereas significantly (P < 0.05) reduced the levels of these enzymes in heart homogenate when compared to control rats. The levels of CK-MB, LDH and ALP were decreased significantly (P < 0.05) in serum whereas increased significantly (P < 0.05) in heart homogenate with MA (15 mg/kg) and GA (15 mg/kg) pretreatment when compared to ISO group. Treatment with MA (15 mg/kg) alone did not show significant (P < 0.05) change on cardiac markers.
Groups
CK-MB (U/L)
LDH (U/L)
ALP (U/L)
Serum
Heart
Serum
Heart
Serum
Heart
Control
327.4 ± 26.4a
294.7 ± 6.5a
814.7 ± 11.8a
1980.5 ± 16.4a
168.8 ± 15.1a
160.9 ± 2.7a
MA (15 mg/Kg bw)
320.9 ± 9.1a
298.4 ± 4.4a
800.5 ± 19.4a
1985.1 ± 29. 8a
161.6 ± 13.5a
163.4 ± 2.9a
ISO (85 mg/Kg bw)
625.0 ± 16.1b
156.5 ± 5.3b
1634.2 ± 35.5b
1259.7 ± 46.9b
286.5 ± 25.3b
90.2 ± 1.2b
MA (15 mg/Kg bw) + ISO
373.6 ± 12.8c
259.8 ± 4.8c
892.8 ± 17.2c
1879.1 ± 21.7c
184.4 ± 22.6a
146.9 ± 3.2c
GA (15 mg/Kg bw) + ISO
400.0 ± 13.1d
235.9 ± 4.0d
963.5 ± 29.4d
1736.9 ± 24.5d
209.4 ± 17.1c
131.8 ± 4.2d
3.2 Effects of MA on electrolytes
Table 2 explains the effects of MA on the levels of myocardial electrolytes in control and ISO groups. ISO administration significantly (p < 0.05) increased the electrolytes sodium and calcium, and significantly (p < 0.05) decreased potassium with compared to control group. Animals pretreated with MA (15 mg/kg) showed significant (p < 0.05) decrease in the levels of sodium and calcium, and significant increase in potassium levels when compared to ISO administered group. GA (15 mg/kg) pretreatment also showed significant (p < 0.05) decrease in sodium and calcium, and significant increase in potassium levels when compared to ISO treated rats. MA (15 mg/kg) pretreatment in ISO treated rats significantly (p < 0.05) increased the levels of potassium to near normal. Alone treatment of MA (15 mg/kg) did not show significant (P < 0.05) effect on the levels of electrolytes.
Groups
Na+ (nmol/mg protein)
K+ (nmol/mg protein)
Ca2+ (nmol/mg protein)
Control
4.8 ± 0.3a
6.0 ± 0.3a
7.6 ± 0.3a
MA (15 mg/Kg bw)
4.7 ± 0.3a
6.1 ± 0.1a
7.5 ± 0.1a
ISO (85 mg/Kg bw)
7.8 ± 0.4b
3.7 ± 0.4b
10.9 ± 0.6b
MA (15 mg/Kg bw) + ISO
5.5 ± 0.2c
5.6 ± 0.3a*
8.5 ± 0.5c
GA (15 mg/Kg bw) + ISO
6.4 ± 0.1d
5.0 ± 0.1c
8.9 ± 0.1c
3.3 Effects of MA on membrane ATPases
Fig. 1 depicts the effects of MA on heart membrane bound transport enzymes of control and experimental rats. Na+/K+ ATPase, Ca2+ ATPase and Mg2+ ATPase activities significantly (p < 0.05) decreased in the myocardial homogenate of ISO administered group when compared with the control group. MA (15 mg/kg) pretreatment to ISO treated rats exhibited significant (p < 0.05) increase and GA (15 mg/kg) pretreatment also significantly (p < 0.05) increase in the activities of Na+/K+ ATPase, Ca2+ ATPase and Mg2+ ATPase enzymes when compared to ISO alone administered rats. Pretreatment with MA (15 mg/kg) in ISO administered group significantly (p < 0.05) increased the activity of Na+/K+ ATPase and Ca2+ ATPase enzymes to near normal. Alone treatment of MA (15 mg/kg) did not show significant (P < 0.05) change on the activities of any ATPase enzymes.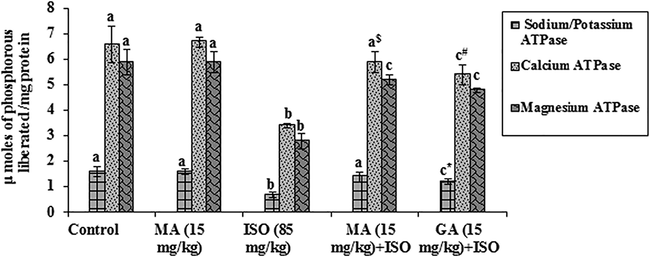
Effects of MA on membrane bound ATPases in heart. Values are mean ± S.D. (n = eight rats). Values not shared a common superscript (a, b and c) differ significantly from each other (p<0.05, Duncan’s multiple range test). * Group not significantly differs with MA (15mg/Kg) + ISO treated group. $Group significantly differs with MA (15mg/Kg) treated group. # Group not significantly differs with MA (15mg/Kg) + ISO treated group.
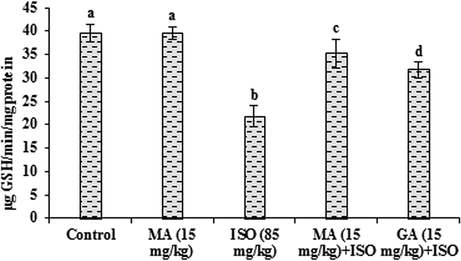
3.4 Effects of MA on antioxidants
The data presented in Figs. 2–4 represent the effects of MA on antioxidants in control and ISO heart homogenate of rats. The content of GSH along with the activities of GST and GPx were lowered significantly (P < 0.05) in ISO administered group when compared to control group. MA (15 mg/kg) pretreatment significantly (P < 0.05) increased the level of GSH and the activities of these antioxidant enzymes and the data of GA (15 mg/kg) is reversed with compared to ISO treated rats. Significant (P < 0.05) alterations not observed in any antioxidants when treated with MA (15 mg/kg) alone.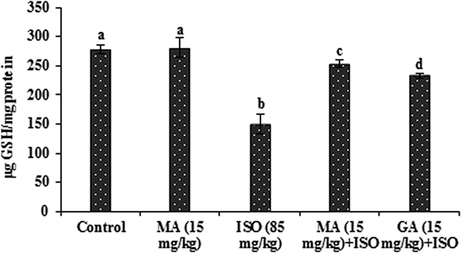
Effects of MA on GSH in heart. Values are mean ± S.D. (n = eight rats). Values not shared a common superscript (a, b, c and d) differ significantly from each other (p<0.05, Duncan’s multiple range test).
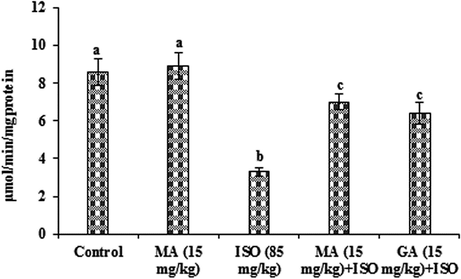
Effects of MA on GST in heart. Values are mean ± S.D. (n = eight rats). Values not shared a common superscript (a, b and c) differ significantly from each other (p<0.05, Duncan’s multiple range test).
Effects of MA on GPx in heart. Values are mean ± S.D. (n = eight rats). Values not shared a common superscript (a, b, c and d) differ significantly from each other (p<0.05, Duncan’s multiple range test).
3.5 Effects of MA on histopathology of heart tissue
Fig. 5 demonstrates the effects of MA on the histopathological changes of control and ISO administered heart tissues. Fig. 5A reveals the normal architecture of control heart group. Fig. 5C represents the ISO treated group shows the infarction of cardiac fibers with infiltrated inflammatory cells and edema. MA (15 mg/kg) + ISO administered group represents mild edema without necrosis. The fiber of myocardium represents normal architecture (Fig. 5D). In GA (15 mg/kg) treated group there was minimal edema and myonecrosis with less inflammatory cells (Fig. 5E). Alone treatment of MA (15 mg/kg) did not alter histopathology in heart tissue (Fig. 5B).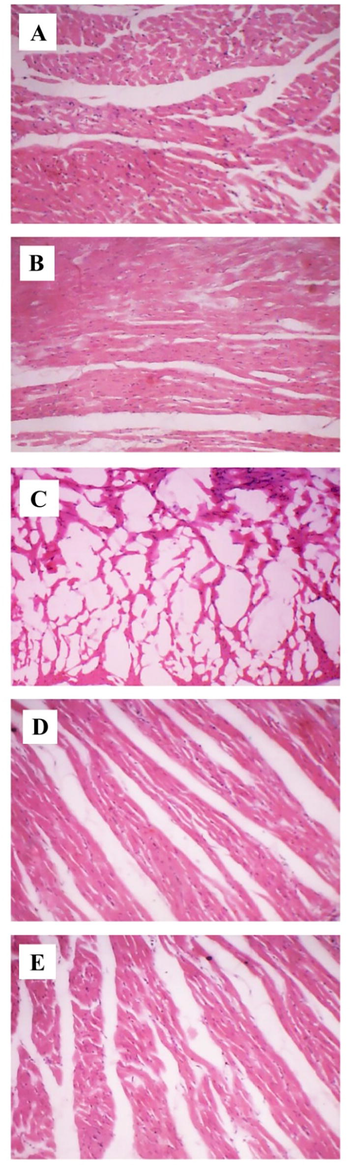
Effects of MA on histopathology of heart (H&E, 10x). A) Control, B) MA (15 mg/kg) treated, C) ISO (85 mg/kg) administered, D) MA (15 mg/kg) pretreated + ISO (85 mg/kg) administered, E) GA (15 mg/kg) pretreated + ISO (85 mg/kg) administered.
4 Discussion
In our present investigation, we studied the cardioprotective effective of MA on ISO administered cardio-toxicity in rats. Our results reveal the evidence that MA offers significant cardioprotection on ISO-induced biochemical changes such as cardiac marker enzymes, electrolytes, membrane bound ATPases, and non-enzymatic and enzymatic antioxidants in myocardium.
ISO administration induces myocardial necrosis resulting in altered cell membrane integrity and enhanced cell membrane permeability, which causes the leakage of marker enzymes from heart into the blood (Derbali et al., 2015). In this study, the myocardial enzymes CK-MB, LDH and ALP were abundantly increased in serum and decreased in the heart of ISO administered rats. Pretreatment with MA drastically reversed the toxic effects of ISO and restored all the marker enzymes. This protection of MA may be due to the preservation of myocardial membrane integrity and prevention of the cardiac enzymes leakage into the blood circulation. This report is in accordance with the previous reports (Asaikumar et al., 2019).
Extensive literature survey divulged that limited research work has been accomplished on electrolytes in relation with ISO-induced MI. Hence, this study focused to analyze the role of ISO on electrolytes and ATPases. ATPases are the membrane bound enzymes that participate in the transportation of electrolytes such as Na+, K+, Ca2+ and Mg2+. Electrolytes imbalance may play a crucial role in metabolic disorders of heart. In the current investigation ISO administered rats showed decreased levels of Na+/K+, Ca2+ and Mg2+ ATPases. Inactivation of ATPases is due to the oxidation of –SH groups present in the active sites of protein, which leads to the conformational changes of these enzymes (Jayachandran et al., 2009). ISO treated rats also showed increased concentrations of Na+ and Ca2+ along with decreased concentration of K+ which might be due to the altered activities of membrane associated ATPase enzymes as a result of ISO accelerated lipid peroxidation. Treatment with MA increased the activities of ATPases and ameliorated the levels of electrolytes in ISO treated rats, which may be accredited to the direct antioxidant activity of MA. The protective effect of MA might be due to the prevention of –SH group oxidation by blocking the peroxidation of membrane lipids, which denotes the membrane stability action of MA. These results are in accordance with earlier results (Khan et al., 2018).
Antioxidants such as GSH, GST and GPx comprise the primary defense system, and scavenge the free radicals. The non-enzymatic antioxidant GSH involves in the protection of proteins that contains –SH groups from the injury of free radicals, also assists as the substrate for the antioxidant enzymes GST and GPx. ISO treatment in rats decreased in the levels GSH, GST and GPx in the myocardium. The decrease in antioxidants may be due to the formation of alkoxy, hydroxyl and superoxide radicals at the site of injury. MA pretreatment restored all antioxidants in ISO treated rats, which might be reduced cardiac necrosis caused by free radicals. The study is in concurrent with previous results (Yu et al., 2018).
The cardioprotection of MA on ISO administered MI has been further supported by light microscopic investigation. The histopathological photomicrograph of ISO treated heart exhibited severe infarction with edema and more inflammatory cells and with degenerated myocardial fibers. However, pretreatment with MA has exhibited resistance towards myocardial injury by reduced edema and necrosis. MA pretreatment preserved the structural integrity of myocardium and maintained the normal cardiac fibers with normal cardiomyocytes morphology. The result is in accordance with previous report (Asaikumar et al., 2019).
5 Conclusion
In conclusion, our study clearly reveals the cardioprotection of MA against ISO-induced myocardial toxicity in rats. The cardioprotective effect may be attributed to the ability of MA to ameliorate the cardiac marker enzymes, electrolytes, antioxidants, and to stabilize the membrane bound ATPase enzymes and to preserve the histo-architecture of heart. Considering all our results together, it may be recommended that MA could be used as a promising therapeutic cardioprotective compound to treat MI.
Acknowledgement
The authors express their gratitude to the Deanship of Scientific Research (DSR), King Saud University, Saudi Arabia for supporting this work through the fund of Research Group (RG- 1438- 058).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anti-inflammatory and antioxidant activities and constituents of Platostoma africanum P. Beauv. Nat. Prod. Res.. 2008;22:1067-1073.
- [CrossRef] [Google Scholar]
- Preventive effect of nerolidol on isoproterenol induced myocardial damage in Wistar rats: evidences from biochemical and histopathological studies. Drug. Dev. Res. 2019
- [CrossRef] [Google Scholar]
- Presence of enzyme system in mammalian tissues: membrane and ion transport. London: Wiley Interscience; 1970. p. :257-263.
- Cardioprotective effect of linseed oil against isoproterenol-induced myocardial infarction in Wistar rats: a biochemical and electrocardiographic study. J. Physiol. Biochem.. 2015;71:281-288.
- [CrossRef] [Google Scholar]
- Apolipoprotein E determines the hepatic transcriptional profile of dietary maslinic acid in mice. J. Nutr. Biochem.. 2009;20:882-893.
- [CrossRef] [Google Scholar]
- Pentacyclic triterpenoids from olive fruit and leaf. J. Agric. Food Chem.. 2010;58:9685-9691.
- [CrossRef] [Google Scholar]
- Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem.. 1974;249:7130-7139.
- [Google Scholar]
- Purification and characterization of two forms of low affinity Ca2+ ATPase from erythrocyte membrane. Biochim. Biophys. Acta. 1983;728:281-288.
- [CrossRef] [Google Scholar]
- Antilipoperoxidative and membrane stabilizing effect of diosgenin, in experimentally induced myocardial infarction. Mol. Cell. Biochem.. 2009;327:203-210.
- [CrossRef] [Google Scholar]
- Effects of oleanolic acid and maslinic acid on hyperlipidemia. Drug Dev. Res.. 2007;68:261-266.
- [CrossRef] [Google Scholar]
- Oral contraceptives use and the risk of myocardial infarction: a meta-analysis. Contraception. 2003;68:11-17.
- [CrossRef] [Google Scholar]
- Raspberry ketone protects against isoproterenol-induced myocardial infarction in rats. Life Sci.. 2018;194:205-212.
- [CrossRef] [Google Scholar]
- Antioxidant activity of maslinic acid, a triterpene derivative obtained from Olea europaea. Planta Med.. 2003;69:472-474.
- [CrossRef] [Google Scholar]
- Protective role of curcumin against isoproterenol induced myocardial infarction in rats. Mol. Cell Biochem.. 1996;159:85-93.
- [CrossRef] [Google Scholar]
- A comparative study of plasma membrane Mg2+ ATPase activities in normal, regenerating and malignant cells. Biochim. Biophys. Acta.. 1982;684:67-74.
- [CrossRef] [Google Scholar]
- Cardioprotective effect of ‘Marutham’ a polyherbal formulation on isoproterenol-induced myocardial infarction in Wistar rats. Fitoterapia. 2008;79:433-438.
- [CrossRef] [Google Scholar]
- The natural triterpene maslinic acid induces apoptosis in HT29 colon cancer cells by a JNKp53-dependent mechanism. BMC Cancer. 2011;11:154.
- [CrossRef] [Google Scholar]
- Selenium, biochemical role as a component of glutathione peroxidase. Science. 1973;179:588-590.
- [CrossRef] [Google Scholar]
- Cardioprotective effect of oleanolic acid on isoproterenol-induced myocardial ischemia in rats. Toxicol. Pathol.. 2007;35:418-423.
- [CrossRef] [Google Scholar]
- Maslinic acid protects against isoproterenol-induced cardiotoxicity in Albino Wistar rats. J. Med. Food.. 2012;15:741-746.
- [CrossRef] [Google Scholar]
- Effects of maslinic acid as a novel glycogen phosphorylase inhibitor on blood glucose and hepatic glycogen in mice. Chin. J. Nat. Med.. 2008;6:53-56.
- [CrossRef] [Google Scholar]
- Clinical guide to laboratory tests (3rd edition). Philadelphia: WB Saunders and company; 1995.
- A rapid method for the determination of sodium in serum. Analyst.. 1951;76:596-599.
- [CrossRef] [Google Scholar]
- Protective effects of maslinic acid against alcohol-induced acute liver injury in mice. Food Chem Toxicol.. 2014;74:149-155.
- [CrossRef] [Google Scholar]
- Cardioprotective effect of rosuvastatin against isoproterenol-induced myocardial infarction injury in rats. Int. J. Mol. Med.. 2018;41:3509-3516.
- [CrossRef] [Google Scholar]







